Abstract
Background
Spontaneous bacterial peritonitis (SBP) is a bacterial infection associated with a high mortality rate in cirrhotic patients. The gold standard for the detection of SBP is a manual cell count from ascitic fluid; however, alternative screening methods are under investigation. In particular, leukocyte esterase reagent strips (LERS) has been studied as an alternative method to detect SBP with a low cost and instant turnaround time. Therefore, this study aims to evaluate the performance of LERS in the detection of SBP.
Methods
A literature search was performed for studies evaluating LERS for the detection of SBP on PubMed, Embase, Scopus, Cochrane, and clinical trial registries. Summary sensitivity, specificity, log diagnostic odds ratio (LDOR), and the area under the summary receiver operating curve (AUC) were calculated according to the respective manufacturer.
Results
In total, 31 studies were evaluated. The summary sensitivity of Aution Sticks, Combur, Multistix, Periscreen reagent strips was 0.962 (95% confidence interval [CI] 0.926, 0.998), 0.892 (95% CI 0.846, 0.938), 0.806 (95% CI 0.738, 0.874), and 0.939 (95% CI 0.900, 0.979), respectively. The summary specificity of Aution Sticks, Combur, Multistix, and Periscreen reagent strips was 0.940 (95% CI 0.904, 0.976), 0.922 (95% CI 0.874, 0.970), 0.974 (95% CI 0.962, 0.985), and 0.672 (95% CI 0.381, 0.963), respectively.
Conclusion
LERS appears to have a notable overall performance for the detection of SBP. LERS appeared to be an acceptable alternative to diagnose SBP in facilities without ability to perform cell count. However, there were significant differences in performance between each manufacturer.
Keywords: reagent strips, leukocyte esterase, ascites, spontaneous bacterial peritonitis, cirrhosis
Abbreviations: AUC, area under the summary receiver operating curve; LDOR, log diagnostic odds ratio; LERS, leukocyte esterase reagent strips; PMN, polymorphonuclear; SBP, Spontaneous bacterial peritonitis; SROC, summary receiver operating characteristic
Spontaneous bacterial peritonitis (SBP) is an infection of ascitic fluid, in the absence of an apparent source, such as an intra-abdominal inflammatory focus, contiguous source of infection, or malignancy.1, 2, 3 The prevalence of SBP often varies, ranging from 3.5% to 30% based on the setting of diagnosis (inpatient vs. outpatient).1,2 Although some patients may experience fever or abdominal pain, others are often asymptomatic.1Therefore, early diagnosis is crucially important, as the mortality of untreated SBP is high.2
Currently, the gold standard for diagnosis of SBP is the detection of a polymorphonuclear (PMN) cell count from ascitic fluid ≥250 cells/mm3.3, 4, 5This is performed by a manual cell count, which can often be time-consuming.3Therefore, utilization of rapid, easy to operate, and inexpensive methods to diagnose SBP at the bedside may offer early diagnosis and prompt antibiotic administration.6 Leukocyte esterase reagent strips (LERS) are a commonly used test to diagnose urinary tract infection. This method detects leukocyte esterase activity resulting in a color change of the reagent strip.2,3,7 Apart from urine, LERS has been used to detect the presence of PMNs in various body fluids such as pleural fluid, cerebrospinal fluid, and synovial fluid.8, 9, 10 Several studies within recent years have examined the use of LERS for the bedside diagnosis of SBP. The recommendations that LERS suffice as a stand-alone method for SBP detection have been inconsistent because of a variety of manufacturers and differences in cutoff values. Therefore, the aim of this meta-analysis study is to examine the performance of LERS for the diagnosis of SBP based on manufacturers and cutoff values.
Materials and methods
This meta-analysis was conducted in accordance to Cochrane's manual of diagnostic test accuracy, and the manuscript was prepared with consideration to the preferred reporting items for systematic reviews and meta-analysis of diagnostic test accuracy (PRISMA-DTA) guidelines.11,12 A literature search of PubMed, Embase, Scopus, Google Scholar, Cochrane, ClinicalTrials.gov, and the European Clinical Trial Registry was performed from inception through March 2020 by a systemic review specialized librarian (C.S.). Search terms for this meta-analysis included (1) SBP and (2) leukocyte esterase, leukocyte esterase, reagent strip, Aution Sticks, Combur, Multistix, Periscreen, Uriscan, Uri-Plus, Choiceline, Elastase Dip, Combina, Combi, or Nephur. The titles and abstracts of the resulting search were reviewed by two independent authors (P.K. and J.G.). Discrepancies were resolved via discussion between the mentioned two independent authors and their senior author (W.M.). Both independent authors compiled the extracted data from each study, including study characteristics, study population characteristics, and study results. Study characteristics included author, year of publication, start and end dates, country, and the type of study design performed. Study population characteristics included the total number of adult patients, total number of paracenteses performed, mean age of patients, the setting of paracentesis (inpatient vs. outpatients), Child-Pugh scores, and criteria for SBP diagnosis. SBP was defined by a PMN ≥250 cells/mm3 regardless of ascitic fluid culture. Exclusion criteria included other secondary causes of neutrocytic ascites, such as malignancy, different intra-abdominal infection(s), recent abdominal surgeries, or recent exposure to antibiotics. Abstracted data also included the prevalence of SBP, LERS manufacturers in each study, grading systems for each LERS, cutoff values, method of detection (visual comparison vs. analyzer), sensitivity, specificity, true positive, false positive, false negative, and true negative. This information was either obtained directly from the data provided by each study or calculated using other available information. Quality assessment for the studies included was performed using QUADAS-2 by both independent authors (P.K. and J.G.). Any discrepancies noted by both were again resolved through discussion between the independent authors and their senior author (W.M.).
Statistical Analysis
This study used the R version 3.2.4 (R Core team 2013) with Metafor and Mada packages for statistical analysis.13,14 An inter-observer agreement was measured and evaluated by Cohen's Kappa coefficient. The summary sensitivities, specificities, and log diagnostic odds ratios (LDOR) were calculated via bivariate meta-analysis as described by Reitasma.15 The hierarchical summary receiver operating characteristic model was used to calculate the summary receiver operating characteristic (SROC), as outlined by Rutter and Gatsonis.16 Lastly, the area under SROC curves (AUC) was calculated for inclusion in this study. Subgroup analyses were conducted by stratifying the data based on LERS manufacturer, cutoff values, and method of detection (visual vs. analyzer). Study heterogeneity was evaluated by the I2 statistic; a range between 0% and 40%, 30% and 60%, 50% and 90%, and 75% and 100% suggested low, moderate, substantial, and considerable heterogeneity, respectively.17 Publication biases were assessed by Deeks' funnel plot, where a P-value <0.1 suggests publication bias.18
Results
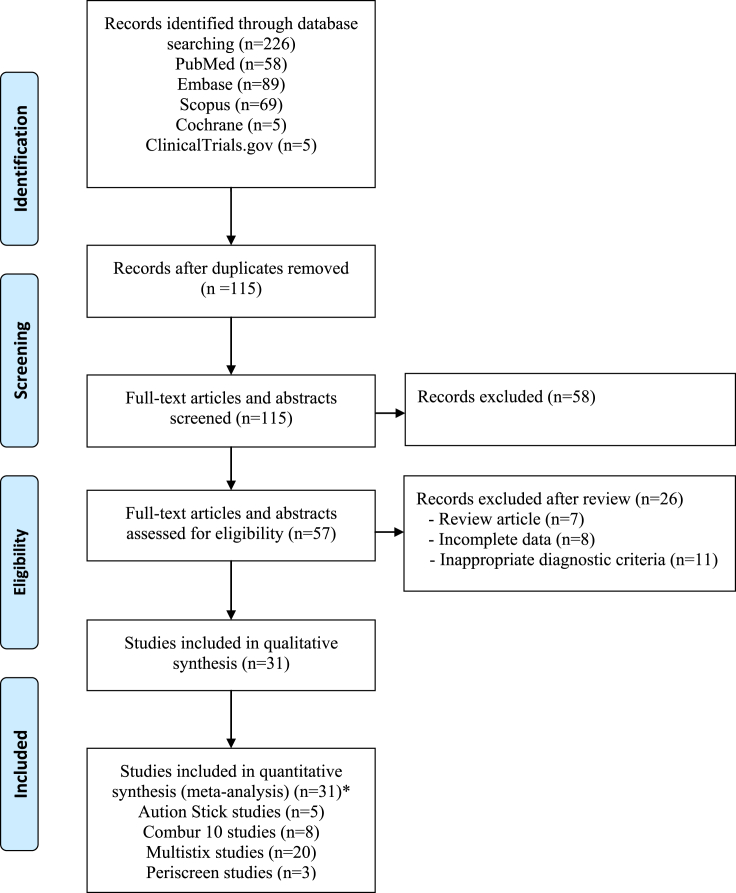
Figure 1 highlights the screening and selection process performed for this meta-analysis. After the removal of duplicate articles, a total of 115 studies were found using the search criteria previously mentioned. Of these, 42 articles were discovered to be relevant. Thereafter, two studies were excluded because of the absence of information regarding LERS manufacturers, and nine studies were excluded because of an inadequate amount of data associated with specific LERS manufacturers for use in this meta-analysis. For example, these included two studies with Medi-Test Combi, a study with Choiceline, a study with Combi-screen 10SL, a study with Fagnos Elastase Dip, a study with Human-Test Combina, a study with Uri-Plus, a study with Uri-Quick, and a study with Uriscan. Ultimately, 31 studies (4446 patients), including four abstracts and 27 full articles, were used in this meta-analysis. Of these, five studies evaluated the performance of Aution Sticks, eight studies evaluated Combur, 20 studies evaluated Multistix, and three studies evaluated Periscreen. Table 1 demonstrates the characteristics of each study evaluating the use of LERS in the detection of SBP, whereas Table 2 demonstrated extracted data.
Figure 1.
A flowchart demonstrating the study search and selection process for this meta-analysis according to the PRISMA statement. (∗Four studies evaluated two LERS, whereas one study evaluated 3 LERS for the detection of SBP).
Table 1.
Characteristics of Studies Evaluating Reagent Strips for the Detection of SBP.
| Study | Type | Country | Study type | Strip type | Analyze method | Start date | End date | Mean age (years) | Sample size (n) | Most common etiology of cirrhosis | Location | Child-Pugh score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (n) | B (n) | C (n) | ||||||||||||
| Ahmed et al.19 | Full paper | Egypt | Prospective | Aution Sticks | Visual | NR | NR | NR | 100 | NR | IP | NR | NR | NR |
| Al Sawaf et al.20 | Full paper | Egypt | Prospective | Combur 10 | Visual | Jan 2010 | Dec 2012 | 51 ± 11 | 168 | Hepatitis C | IP | 0 | 16 | 152 |
| Braga et al.21 | Full paper | Brazil | Prospective | Combur 10 | Visual | Mar 2004 | Aug 2004 | 51.7 | 42 | Alcohol | IP, OP | 0 | 23 | 19 |
| Butani et al.22 | Full paper | USA | NR | Multistix | Visual | NR | NR | NR | 75 | NR | NR | NR | NR | NR |
| Campillo et al.23 | Full paper | France | NR | Multistix Combur 10 | Visual | Dec 2003 | Feb 2005 | 57 ± 12 | 116 | Alcohol | IP | 0 | 38 | 78 |
| Castellote et al.24 | Full paper | NR | Prospective | Aution Sticks | Visual | NR | NR | 59 ± 13 | 128 | Alcohol | IP | 0 | 46 | 82 |
| Chinnock et al.25 | Full paper | India | Prospective | Peri-screen | Visual | Apr 2016 | Jan 2018 | 56 ± 10 | 282 | Alcohol | ER | NR | NR | NR |
| Chugh et al.26 | Full paper | India | NR | Multistix | Visual | NR | NR | 44 ± 15 | 103 | Alcohol | NR | 0 | 48 | 55 |
| De Araujo et al.27 | Full paper | Brazil | Prospective | Multistix | Visual | Jun 2006 | Apr 2007 | 55 ± 11 | 71 | Hepatitis C | IP, OP | 0 | 27 | 39 |
| Gaya et al.28 | Full paper | United Kingdom | Cross-Sectional | Multistix | Analyzer | NR | NR | 57 ± 11 | 105 | Alcohol | IP, OP | NR | NR | 80 |
| Jha et al.29 | Full paper | India | Prospective | Multistix | Visual | Apr 2005 | Jun 2007 | 44 ± 13 | 100 | Alcohol | IP | 0 | 27 | 73 |
| Jothimani et al.30 | Abstract | India | NR | Peri-screen | Visual | June 2016 | Mar 2017 | 52 | 72 | NR | NR | NR | NR | NR |
| Khatwani et al.31 | Full paper | Pakistan | Cross-Sectional | Multistix | Visual | Jan 2009 | Jun 2009 | 45 ± 15 | 94 | Hepatitis C | IP | NR | NR | NR |
| Kim et al.32 | Full paper | Korea | Prospective | Multistix | Visual | Dec 2003 | Mar 2004 | 57 ± 9 | 53 | Hepatitis B | IP | 0 | 21 | 32 |
| Li et al.33 | Abstract | NR | NR | Multistix | Visual | NR | NR | 46 | 84 | NR | IP | NR | NR | NR |
| Nalmpantidis et al.34 | Abstract | Greece | Prospective | Aution Sticks | NR | NR | NR | 70 | 43 | NR | NR | 14 | 17 | 12 |
| Nousbaum et al.35 | Full paper | France | Prospective | Multistix | Visual | Jan 2004 | May 2004 | 60 | 1041 | Alcohol | IP, OP | 0 | 470 | 571 |
| Oey et al.36 | Full paper | Netherlands | Prospective | Combur 10 | Visual Analyzer | Jul 2006 | Jul 2007 | 51 ± 10 | 52 | Alcohol | IP, OP | NR | NR | NR |
| Rerknimitr et al. (2006)37 | Full paper | Thailand | NR | Combur 10 | Visual | Jul 2004 | Jul 2007 | 57 ± 13 | 127 | Hepatitis B | NR | 3 | 34 | 90 |
| Rerknimitr et al. (2010)38 | Full paper | Thailand | Prospective | Aution Sticks Combur 10 Multistix | Visual | Sep 2006 | Dec 2008 | 59 ± 12 | 143 | Hepatitis B | NR | 0 | 58 | 85 |
| Ribeiro et al.39 | Full paper | Brazil | Prospective | Multistix | Visual | Apr 2003 | Dec 2004 | 54 ± 12 | 106 | Alcohol | IP, OP | 10 | 89 | 101 |
| Sapey (JGH-1) et al.40 | Full paper | France, USA | Prospective | Multistix | Visual | NR | NR | 60 ± 11 | 53 | Alcohol | OP | 3 | 18 | 32 |
| Sapey (JGH-2) et al.40 | Full paper | France, USA | Prospective | Multistix | Visual | NR | NR | 51 ± 13 | 23 | Alcohol | IP | 0 | 10 | 13 |
| Sapey (LI) et al.41 | Full paper | France | Prospective | Multistix | Visual | NR | NR | 55 ± 11 | 51 | Alcohol | IP, OP | 6 | 20 | 25 |
| Sarwar et al.42 | Full paper | Pakistan | Cross-Sectional | Combur 10 | Visual | Nov 2003 | Aug 2004 | 52 ± 10 | 214 | NR | ER, IP, OP | NR | NR | NR |
| Tellez et al.43 | Full paper | Mexico | Prospective | Multistix | Visual | Mar 2005 | Feb 2007 | 54 | 138 | Hepatitis C | ER | 4 | 51 | 83 |
| Thévenot et al. (2004)44 | Full paper | France | Prospective | Combur 10 Multistix | Visual | Apr 2002 | Apr 2003 | 59 ± 10 | 31 | Alcohol | IP, OP | 0 | 10 | 21 |
| Thévenot et al. (2016)45 | Full paper | France | Prospective | Peri-screen | Visual | Mar 2014 | Aug 2015 | 61 ± 10 | 649 | Alcohol | IP, OP | NR | NR | NR |
| Torun et al.46 | Full paper | Turkey | NR | Aution Sticks | Visual | Apr 2005 | Jul 2006 | 59 ± 12 | 63 | Hepatitis B | IP | 0 | 25 | 38 |
| Vanbiervliet et al.47 | Full paper | France | Prospective | Multistix | Visual | Sep 2000 | May 2001 | 62 ± 14 | 72 | Alcohol | IP | 0 | 28 | 44 |
| Wisniewski et al.48 | Abstract | France | NR | Multistix | Visual | Jun 2003 | May 2004 | NR | 47 | NR | IP | NR | NR | NR |
ELISA, enzyme-linked immunosorbent assay; ER, emergency room; IP, inpatient; OP, outpatient; NR, not reported; PMN, polymorphonuclear leukocytes; SBP, spontaneous bacterial peritonitis.
Table 2.
Extracted Data From Studies Evaluating Reagent Strips for the Detection of SBP.
| Study | Number of paracenteses (n) | Prevalence of SBP (%) | Cutoff value | Grade scale | Sensitivity (%) | Specificity (%) | True positive(n) | False positive (n) | False negative (n) | True negative (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aution Sticks | ||||||||||
|
100 | 42 | ≥3 | 5 | 100 | 91.38 | 42 | 5 | 0 | 53 |
|
228 | 22.81 | ≥2 | 5 | 96.49 | 88.89 | 55 | 19 | 2 | 152 |
|
108 | 13.95 | NR | 5 | 81.25 | 95.65 | 13 | 4 | 3 | 88 |
|
250 | 12 | ≥1 | 5 | 90 | 93.18 | 27 | 15 | 3 | 205 |
|
63 | 23.81 | ≥2 | 5 | 93.33 | 100 | 14 | 0 | 1 | 48 |
| Combur 10 | ||||||||||
|
168 | 29.17 | ≥1 | 4 | 89.80 | 81.51 | 44 | 22 | 5 | 97 |
|
100 | 21.43 | ≥2 | 4 | 100 | 98.86 | 12 | 1 | 0 | 87 |
|
443 | 21.55 | ≥1 | 4 | 80.22 | 90.27 | 73 | 32 | 18 | 297 |
|
157 | 7.64 | NR | 4 | 75 | 99.31 | 9 | 1 | 3 | 144 |
|
200 | 21 | ≥1 | 4 | 88.10 | 81.01 | 37 | 30 | 5 | 128 |
|
250 | 12 | ≥1 | 4 | 90 | 93.2 | 27 | 15 | 3 | 205 |
|
214 | 17.76 | ≥2 | 4 | 94.74 | 92.05 | 36 | 14 | 2 | 162 |
|
100 | 25.81 | ≥2 | 4 | 87.50 | 100 | 7 | 0 | 1 | 23 |
| Multistix | ||||||||||
|
136 | 8.82 | ≥2 | 5 | 83.33 | 99.19 | 10 | 1 | 2 | 123 |
|
443 | 21.55 | ≥1 | 5 | 69.23 | 94.83 | 63 | 17 | 28 | 312 |
|
103 | 19.42 | ≥2 | 5 | 95 | 96.39 | 19 | 3 | 1 | 80 |
|
159 | 23.94 | ≥1 | 5 | 80 | 98.46 | 20 | 2 | 5 | 128 |
|
173 | 8.09 | ≥1 | 5 | 100 | 91.19 | 14 | 14 | 0 | 145 |
|
100 | 18 | ≥2 | 5 | 77.77 | 95.12 | 16 | 4 | 4 | 88 |
|
94 | 55.32 | ≥1 | 5 | 92.31 | 95.24 | 48 | 2 | 4 | 40 |
|
75 | 24 | ≥3 | 5 | 50 | 100 | 7 | 0 | 7 | 48 |
|
84 | 29.76 | NR | 5 | 92 | 84.75 | 23 | 9 | 2 | 50 |
|
108 | 13.95 | NR | 5 | 64.3 | 100 | 10 | 0 | 5 | 93 |
|
2123 | 5.51 | ≥3 | 5 | 45.3 | 99.25 | 53 | 15 | 64 | 1991 |
|
250 | 12 | ≥1 | 5 | 80 | 94.55 | 24 | 12 | 6 | 208 |
|
200 | 13.21 | ≥2 | 5 | 85.19 | 95.95 | 23 | 7 | 4 | 166 |
|
151 | 11.32 | ≥1 | 5 | 100 | 100 | 6 | 0 | 0 | 47 |
|
33 | 21.74 | ≥1 | 5 | 83 | 96 | 4 | 1 | 1 | 17 |
|
245 | 23.53 | ≥1 | 5 | 64.71 | 99.56 | 11 | 1 | 6 | 227 |
|
229 | 21.97 | ≥3 | 5 | 77.55 | 97.71 | 38 | 4 | 11 | 171 |
|
100 | 25.81 | ≥3 | 5 | 87.5 | 100 | 7 | 0 | 1 | 23 |
|
144 | 12.50 | ≥1 | 5 | 100 | 100 | 9 | 0 | 0 | 63 |
|
47 | 12.77 | ≥1 | 5 | 83.33 | 82.93 | 5 | 7 | 1 | 34 |
| Periscreen | ||||||||||
|
330 | 7.09 | ≥ trace | 5 | 95 | 48.07 | 38 | 309 | 2 | 286 |
|
NR | 20.83 | NR | 4 | 100 | 96.49 | 15 | 2 | 0 | 55 |
|
1402 | 5.99 | ≥ trace | 4 | 91.67 | 57.13 | 77 | 565 | 7 | 753 |
NR, no reported; SBP, spontaneous bacterial peritonitis.
Study Characteristics
Across the included studies evaluating Aution sticks, there were 749 patients with a total of 749 paracenteses performed. The overall prevalence of SBP was 22.91%, with individual studies ranging from 12% to 42%. Of the five studies, three included only inpatient data, whereas two studies did not have information regarding the setting of paracentesis. Four studies used visual comparison and one study did not specify the analysis method.
Among the studies evaluating Combur, there were 1544 patients with a total of 1632 paracenteses performed. The overall prevalence of SBP was 19.55%, with individual studies ranging from 7.64% to 29.17%. Of the eight studies, two included inpatient data only, four included both inpatient and outpatient samples, and two did not specify the location paracentesis was performed.
Of the studies evaluating Multistix, there were 2581 patients with a total of 5179 paracenteses performed. The overall prevalence of SBP was 19.50%, with individual studies ranging from 5.51% to 55.32%. Of the 19 studies, eight included inpatient data only, six included both inpatient and outpatient samples, one included emergency room samples only, one included outpatient data only, and three did not specify the location paracentesis was performed.
Among the studies evaluating Periscreen, there were 2111 patients with a total of 1732 paracenteses performed. The overall prevalence of SBP was 19.50%, with individual studies ranging from 5.99% to 20.83%. Of the three studies analyzing Periscreen, one included both inpatient and outpatient, one included emergency room samples only, and one study did not specify the location paracentesis was performed.
Performance of Each LERS in the Detection of SBP
Aution Sticks
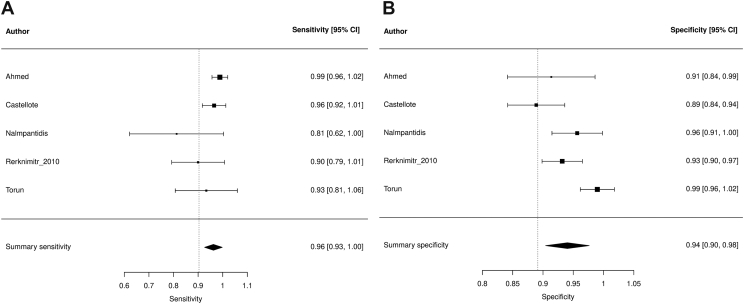
Summary sensitivity, specificity, and LDOR were 0.962 (95% confidence interval [CI] 0.926, 0.998), 0.940 (95% CI 0.904, 0.976), 4.963 (95% CI 4.230, 5.696), and 0.962, respectively (see Table 3). Figure 2A and B highlights a graphical representation of summary sensitivity and specificity across Aution Stick studies. SROC is shown in Supplementary Figure 1 (AUC 0.962). The level of heterogeneity for summary sensitivity, specificity, and LDOR were low (26.86%), considerable (74.04%), and low (0%), respectively. By visual detection method, sensitivity, specificity, LDOR, and AUC were 0.973 (95% CI 0.945, 1.008), 0.935 (95% CI 0.890, 0.981), 5.518 (95% CI 4.320, 5.997), and 0.964, respectively. The level of heterogeneity for sensitivity, specificity, and LDOR by visual detection were low (8.27%), considerable (79.59%), and low (0%), respectively.
Table 3.
Results of Summary Statistic From Meta-analyses.
| Index tests and subgroup analyses | Number of studies (n) | Sensitivity (95% CI) | I2 (%) | Specificity (95% CI) | I2 (%) | LDOR (95% CI) | I2 (%) | AUC |
|---|---|---|---|---|---|---|---|---|
| Aution Sticks | ||||||||
|
5 | 0.962 (0.926, 0.998) | 26.86 | 0.940 (0.904, 0.976) | 74.04 | 4.963 (4.230, 5.696) | 0 | 0.962 |
|
4 | 0.973 (0.945, 1.008) | 8.27 | 0.935 (0.890, 0.981) | 79.59 | 5.518 (4.320, 5.997) | 0 | 0.964 |
| Combur 10 | ||||||||
|
8 | 0.892 (0.846, 0.938) | 34.89 | 0.922 (0.874, 0.970) | 94.60 | 4.247 (3.579, 4.914) | 15.54 | 0.903 |
|
4 | 0.866 (0.815, 0.917) | 18.79 | 0.871 (0.811, 0.931) | 86.07 | 3.741 (3.288, 4.195) | 0.65 | 0.915 |
|
6 | 0.814 (0.689, 0.939) | 86.78 | 0.970 (0.949, 0.991) | 71.25 | 4.715 (3.976, 5.454) | 0 | 0.964 |
| Multistix | ||||||||
|
20 | 0.806 (0.738, 0.874) | 78.84 | 0.938 (0.962, 0.985) | 81.39 | 4.577 (4.204, 4.950) | 0 | 0.943 |
|
18 | 0.801 (0.729, 0.872) | 78.27 | 0.976 (0.965, 0.986) | 74.56 | 4.546 (4.159, 4.933) | 0 | 0.942 |
|
10 | 0.847 (0.771, 0.923) | 64.98 | 0.964 (0.944, 0.985) | 81.51 | 4.549 (3.826, 5.273) | 6.13 | 0.933 |
|
5 | 0.775 (0.598, 0.952) | 88.78 | 0.976 (0.962, 0.990) | 39.09 | 4.766 (3.778, 5.754) | 0 | 0.974 |
|
7 | 0.622 (0.492, 0.753) | 76.92 | 0.992 (0.989, 0.996) | 0 | 4.789 (4.305, 5.272) | 0 | 0.971 |
| Periscreen | ||||||||
|
3 | 0.939 (0.900, 0.979) | 0 | 0.672 (0.381, 0.963) | 99.45 | 3.229 (1.814, 4.644) | 46.63 | 0.936 |
AUC, area under the summary receiver operating curve; CI, confidence interval; LDOR, log diagnostic odds ratio.
Figure 2.
Forest plots demonstrating individual and summary sensitivity and specificity of each study evaluating Aution Sticks for the detection of SBP: sensitivity of Aution sticks (A) and specificity of Aution Sticks (B).
Combur
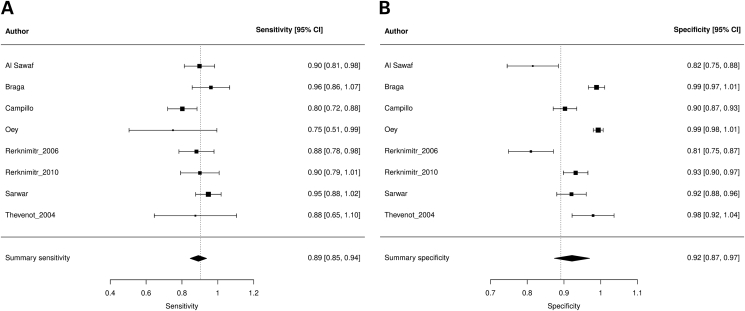
Summary sensitivity, specificity, and LDOR were 0.892 (95% CI 0.846, 0.938), 0.922 (95% CI 0.874, 0.970), and 4.247 (95% CI 3.579, 4.914), respectively (see Table 3). Figure 3A and B highlights a graphical representation of summary sensitivity and specificity across Combur studies. SROC is shown in Supplementary Figure 2 (AUC 0.903). The level of heterogeneity for summary sensitivity, specificity, and LDOR were low (34.89%), considerable (94.60%), and low (15.54%), respectively. At the cutoff value of ≥1, sensitivity, specificity, LDOR, and AUC were 0.866 (95% CI 0.815, 0.917), 0.871 (95% CI 0.811, 0.931), 3.741 (95% CI 3.288, 4.195), and 0.915, respectively. At the cutoff value of ≥2, sensitivity, specificity, LDOR, and AUC were 0.814 (95% CI 0.689, 0.939), 0.970 (95% CI 0.949, 0.991), 4.715 (95% CI 3.976, 5.454), and 0.964, respectively. The heterogeneity for different cutoff values is outlined in Table 3.
Figure 3.
Forest plots demonstrating individual and summary sensitivity and specificity of each study evaluating Combur for the detection of SBP: sensitivity of Combur (A) and specificity of Combur (B).
Multistix
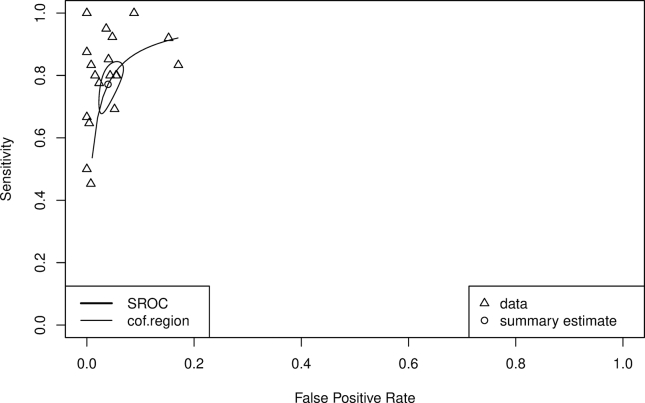
Summary sensitivity, specificity, and LDOR were 0.806 (95% CI 0.738, 0.874), 0.974 (95% CI 0.962, 0.985), and 4.577 (95% CI 4.204, 4.950), respectively (see Table 3). Figure 4A and B highlights a graphical representation of summary sensitivity and specificity across Multistix studies. SROC is shown in Supplementary Figure 3 (AUC 0.943). The level of heterogeneity for summary sensitivity, specificity, and LDOR were considerable (78.84%), considerable (81.39%), and low (0%), respectively. For visual detection method, sensitivity, specificity, LDOR, and AUC were 0.801 (95% CI 0.729, 0.872), 0.976 (95% CI 0.965, 0.986), 4.546 (95% CI 4.159, 4.933), and 0.942, respectively. The level of heterogeneity for sensitivity, specificity, and LDOR by visual detection were considerable (78.27%), considerable (74.56%), and low (0%), respectively. At the cutoff value of ≥1, sensitivity, specificity, LDOR, and AUC were 0.847 (95% CI 0.771, 0.923), 0.964 (95% CI 0.944, 0.985), 4.549 (95% CI 3.826, 5.273), and 0.933, respectively. At the cutoff value of ≥2, sensitivity, specificity, LDOR, and AUC were 0.775 (95% CI 0.598, 0.952), 0.976 (95% CI 0.962, 0.990), 4.766 (95% CI 3.778, 5.754), and 0.974, respectively. At the cutoff value of ≥3, sensitivity, specificity, LDOR, and AUC were 0.622 (95% CI 0.492, 0.753), 0.992 (95% CI 0.989, 0.996), 4.789 (95% CI 4.305, 5.272), and 0.971, respectively. The heterogeneity for different cutoff values is also outlined in Table 3.
Figure 4.
Forest plots demonstrating individual and summary sensitivity and specificity of each study evaluating Multistix for the detection of SBP: sensitivity of Multistix (A) and specificity of Multistix (B).
Periscreen
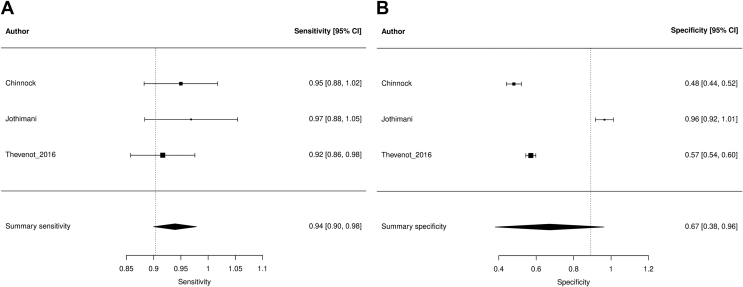
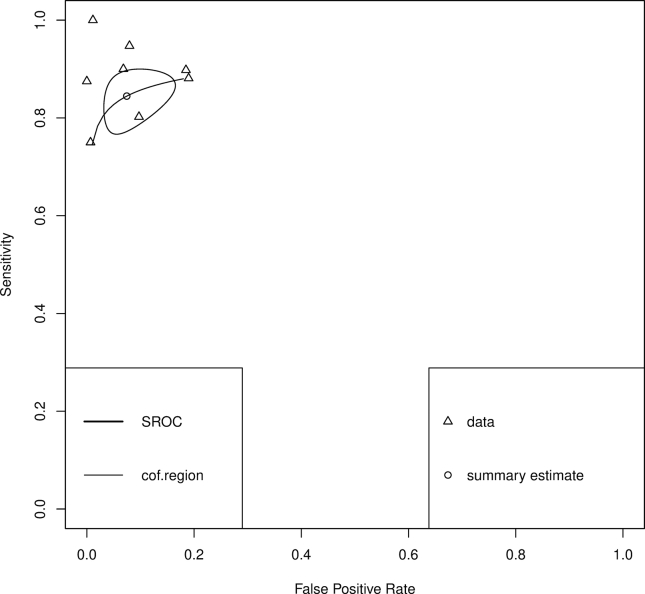
Summary sensitivity, specificity, and LDOR were 0.939 (95% CI 0.900, 0.979), 0.672 (95% CI 0.381, 0.963), and 3.229 (95% CI 1.814, 4.644), respectively (see Table 3). Figure 5A and B highlights a graphical representation of summary sensitivity and specificity across Periscreen studies. SROC is shown in Supplementary Figure 4 (AUC 0.936). The level of heterogeneity for summary sensitivity, specificity, and LDOR was low (0%), considerable (99.45%), and moderate (46.63%), respectively.
Figure 5.
Forest plots demonstrating individual and summary sensitivity and specificity of each study evaluating Periscreen for the detection of SBP: sensitivity of Periscreen (A) and specificity of Periscreen (B).
Quality Assessment and Publication Bias
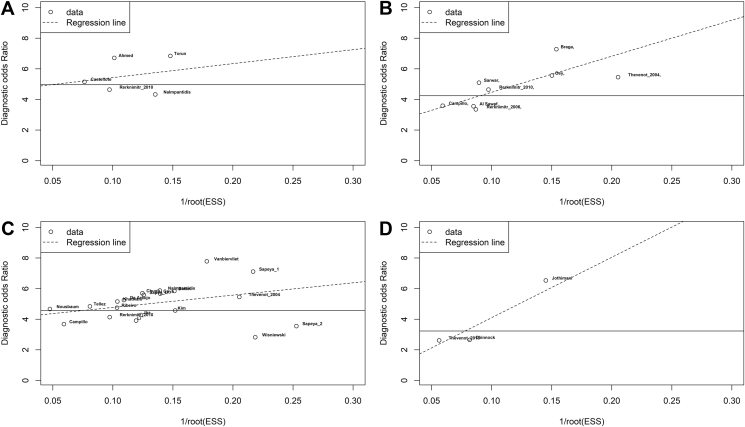
Generally, the level of heterogeneity was low to considerable throughout for summary sensitivity but moderate to considerably high for summary specificity. At the time of article screening and the selection process, there was a high degree of agreement between the two authors, as demonstrated by Cohen's kappa coefficient of 0.93. A simplified QUADAS-2 for each study investigating LERS is shown in Table 4, whereas a complete QUADAS-2 is shown in Supplementary Table 1. Overall, the concern for patient selection bias, general applicability, and the ability to conduct or interpret the index test was thought to be low. Using Deeks' funnel plot, no publication bias was found among studies evaluating Aution Sticks, Combur, Multistix, and Periscreen (P = 0.40, 0.48, 0.58, and 0.25, respectively; see Figure 6A–D).
Table 4.
Simplified QUADAS Form.
| Study |
Patient selection |
Index test |
Reference standard |
Flow and timing |
|||
|---|---|---|---|---|---|---|---|
| Could the selection of patients have introduced bias? | Is there concern the included patients do not match the review question? | Could the conduct or interpretation of the index test have introduced bias? | Is there concern that the index test, its conduct, or interpretation differ from the review question? | Could the reference standard, its conduct, or its, interpretation have introduced bias? | Is there concern the target condition as defined by the reference standard does not match the review question? | Could the patient flow have introduced bias? | |
| Ahmed et al. | L | L | L | L | L | L | L |
| Al Sawaf et al. | L | L | L | L | L | L | L |
| Braga et al. | L | L | L | L | L | L | L |
| Butani et al. | L | L | L | L | L | L | L |
| Campillo et al. | L | L | L | L | L | L | L |
| Castellote et al. | L | L | L | L | L | L | L |
| Chinnock et al. | L | L | L | L | L | L | L |
| Chugh et al. | L | L | L | L | L | L | L |
| De Araujo et al. | L | L | L | L | L | L | L |
| Gaya et al. | L | L | L | L | L | L | L |
| Jha et al. | L | L | L | L | L | L | L |
| Jothimani et al. | L | L | L | L | L | L | L |
| Khatwani et al. | L | L | L | L | L | L | L |
| Kim et al. | L | L | L | L | L | L | L |
| Li et al. | H | L | H | L | H | L | H |
| Nalmpantidis et al. | L | L | L | L | L | L | L |
| Nousbaum et al. | L | L | L | L | L | L | L |
| Oey et al. | L | L | L | L | L | L | L |
| Rerknimitr et al. (2006) | L | L | L | L | L | L | L |
| Rerknimitr et al. (2010) | L | L | L | L | L | L | L |
| Ribeiro et al. | L | L | L | L | L | L | L |
| Sapey et al. (JGH 1 & 2) | L | L | L | L | L | L | L |
| Sapey et al. (LI) | L | L | L | L | L | L | L |
| Sarwar et al. | L | L | L | L | L | L | L |
| Tellez et al. | L | L | L | L | L | L | L |
| Thevenot et al. (2004) | L | L | L | L | L | L | L |
| Thevenot et al. (2016) | L | L | L | L | L | L | L |
| Torun et al. | L | L | L | L | L | L | L |
| Vanbiervliet et al. | L | L | L | L | L | L | L |
| Wisniewki et al. | L | L | L | L | L | L | L |
H, high; L, low, N, no; R, random; U, unclear; Y, yes.
Figure 6.
Deeks' funnel plots of studies evaluating Aution Sticks (A), Combur (B), Multistix (C), and Periscreen (D). ESS, effective sample size.
Discussion
Our study evaluated the performance of various types of LERS for ascitic fluid testing in the detection of SBP from a total of 31 studies consisting of 4446 patients. The Aution Sticks, Combur, Multistix, and Periscreen had a modest overall performance, as reflected by AUC of 0.962, 0.903, 0.943, and 0.936, respectively. The summary sensitivity of Aution Sticks, Combur, Multistix, and Periscreen was 0.962, 0.892, 0.806, and 0.939, respectively. The summary specificity of Aution Sticks, Combur, Multistix, and Periscreen was 0.940, 0.922, 0.922, and 0.672, respectively.
Currently, SBP is diagnosed by the presence of PMNs ≥250 cells/mm3 in an ascitic fluid regardless of culture. Although automated cell count has been studied, the manual cell count method remains the standard practice and is universally available.49 Furthermore, the manual cell count is laborious, time-consuming, and relatively expensive. In addition, both automated and manual cell counts are also subject to errors from cell lysis and specimen handling. Unfortunately, medical facilities in rural areas or developing countries may have limited access to laboratories capable of performing cell count. More frequently, the cell count is often not routinely possible in the setting of therapeutic paracentesis. Therefore, scientists have searched for an instant, convenient, and inexpensive method to diagnose SBP in these previously mentioned circumstances. In the past decade, previous studies have suggested that LERS may be a potential solution. However, the reported accuracy of LERS for the detection of SBP has been inconsistent, with sensitivities ranging from 45% to 100% and specificities ranging from 90% to 100%.3 Across 17 studies, Koulaouzidis et al. supported a potential for screening for SBP by noting a negative predictive value between 87% and 100%.50 In addition, Nguyen-Khac et al. described that the diagnosis of SBP via LERS solely is not a surrogate to the gold standard but may serve a purpose in the detection and management of SBP.51
In this present study, all types of LERS had a good performance for the detection of SBP with AUC varying from 0.903 to 0.962. However, there were notable differences in performance between each manufacturer. Regarding sensitivity, overall sensitivity was good ranging from 0.806 to 0.962; however, Aution Sticks and Periscreen appeared to be significantly superior to Multistix (0.962 [95% CI 0.926, 0.998] and 0.939 [95% CI 0.90, 0.979] vs. 0.806 [95% CI 0.738, 0.874]). Similarly, the overall specificity of LERS was good, ranging from 0.922 to 0.940 except for Periscreen, which had a specificity of 0.672, although this was not statistically significant.
In the subgroup analyses of different cutoffs, only Combur 10 and Multistix had adequate data for the analyses. The threshold effect was apparent in these analyses, as evident by a decrease in sensitivity and an increase in specificity as cutoff increases. However, specificity at the lowest cutoff value (≥1) remained good (0.871 [95% CI 0.811 0.931] and 0.964 [0.944, 0.985]). These findings suggest that LERS is a potential alternative for the cell count method despite the differences between manufacturers. In addition, LERS can serve as a screening tool for SBP as it has good overall sensitivity at the lowest cutoff without sacrificing specificity. Other advantages of LERS include its low cost, simplicity, and instant turnaround time; therefore, LERS could be a perfect tool in facilities without the capability of cell count or when there is suspicion of SBP during a therapeutic paracentesis.
In this study, we also attempted to evaluate the effect of the visual and analyzer method; however, there was only one study in the Multistix group, which used the analyzer method, and one study in the Aution Sticks group without specification of the comparison method. Regardless, when analyzing the studies using visual comparison alone, there was no significant difference in the performance. Although the analyzer method could potentially eliminate human error during visual comparison of LERS, the performance of LERS using the visual method appeared to good. Moreover, analyzer machines are costly and not universally available, which defeats the purpose of LERS.
Despite the potential clinical application of LERS as suggested by the data in this meta-analysis, there are several notable limitations. Firstly, most of the studies in this meta-analysis evaluated patients in the inpatient or emergency room setting, which has a much higher prevalence of SBP than the outpatient setting or in the setting of therapeutic paracentesis. Therefore, this should be considered when applying these findings in clinical practice. Unfortunately, subgroup analysis according to these settings was not possible because of inadequate data. Secondly, the differences in the manufacturers and grading scale of LERS may impact the performance of LERS in clinical practice. Finally, heterogeneity of specificity appeared to be universally high across all types of LERS. Although the heterogeneity of sensitivity appeared to be universally low, the heterogeneity of sensitivity for Multistix appeared to be substantial to considerable.
In summary, our data suggested an excellent overall performance of all LERS. Considering a good sensitivity, LERS has the potential to become a rapid screening tool for diagnosing SBP alternative to the cell count method. LERS is especially useful in certain circumstances with limited resources such as rural settings, developing countries, or facilities with no capacity for the cell count. A standardization of LERS for manufacturers and grading scales may help improve its performance.
Availability of data and material
This study includes all additional materials used in the supplementary section for data transparency purposes.
Code availability
R version 3.2.4 (R Core team 2013) with Metafor and Mada packages was used for the statistical analysis in this study.
CRediT authorship contribution statement
Kishan P. Patel: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. John P. Gallagher: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Parker M. Korbitz: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Cynthia Schmidt: Investigation, Writing – original draft, Writing – review & editing. Thammasin Ingviya: Writing – original draft, Writing – review & editing. Tomoki Sempokuya: Writing – original draft, Writing – review & editing. Wuttiporn Manatsathit: Conceptualization, Methodology, Validation, Formal analysis, Supervision.
Conflicts of interest
The authors have none to declare.
Acknowledgement
The authors report no acknowledgements for this study.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.05.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Summary receiver operating characteristic (SROC) curves of studies evaluating Aution Sticks for the detection of SBP.
Supplementary Figure 2.
Summary receiver operating characteristic (SROC) curves of studies evaluating Combur for the detection of SBP.
Supplementary Figure 3.
Summary receiver operating characteristic (SROC) curves of studies evaluating Multistix for the detection of SBP.
Supplementary Figure 4.
Summary receiver operating characteristic (SROC) curves of studies evaluating Periscreen for the detection of SBP.
References
- 1.Setoyama H., Tanaka M., Sasaki Y. Springer Singapore; Singapore: 2019. Diagnosis of Spontaneous Bacterial Peritonitis in Clinical Investigation of Portal Hypertension; pp. 511–516. [Google Scholar]
- 2.Gad A., El-Nemr N., Saad M. The diagnostic value of leukocyte esterase reagent dip-stick in spontaneous bacterial peritonitis diagnosis in patients with liver cirrhosis. Suez Canal Univ Med J. 2019;22:56–63. [Google Scholar]
- 3.Shizuma T. Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: a literature review. World J Hepatol. 2018;10:254–266. doi: 10.4254/wjh.v10.i2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H., Shim J.H., An J., et al. Reappraisal of the accuracy of diagnostic criteria for spontaneous bacterial peritonitis in cirrhotic patients with or without hepatocellular carcinoma: a preliminary result. Gut. 2018;67:A1–A118. [Google Scholar]
- 5.Fiore M., Maraolo A.E., Leone S., et al. Spontaneous peritonitis in critically ill cirrhotic patients: a diagnostic algorithm for clinicians and future perspectives. Therapeut Clin Risk Manag. 2017;13:1409–1414. doi: 10.2147/TCRM.S144262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allam A., Eltaras S., Hussin M., et al. Diagnosis of spontaneous bacterial peritonitis in children using leukocyte esterase reagent strips and granulocyte elastase immunoassay. Clin Exp Hepatol. 2018;4:247–252. doi: 10.5114/ceh.2018.80126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chugh K., Agrawal Y., Goyal V., Khatri V., Kumar P. Diagnosing bacterial peritonitis made easy by use of leukocyte esterase dipsticks. Int J Crit Illn Inj Sci. 2015;5:32–37. doi: 10.4103/2229-5151.152337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azoulay E., Fartoukh M., Galliot R., et al. Rapid diagnosis of infectious pleural effusions by use of reagent strips. Clin Infect Dis. 2000;31:914–919. doi: 10.1086/318140. [DOI] [PubMed] [Google Scholar]
- 9.Bortcosh W., Siedner M., Carroll R.W. Utility of the urine reagent strip leucocyte esterase assay for the diagnosis of meningitis in resource-limited settings: meta-analysis. Trop Med Int Health. 2017;22:1072–1080. doi: 10.1111/tmi.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C., Li R., Wang Q., Duan J., Wang B. Leukocyte esterase as a biomarker in the diagnosis of periprosthetic joint infection. Med Sci Monit. 2017;23:353–358. doi: 10.12659/MSM.899368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P., Green S. John Wiley & Sons; 2011. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36:1–48. [Google Scholar]
- 14.Doebler P., Holling H. Meta-analysis of diagnostic accuracy with mada. R Packag. 2015;1:15. doi: 10.1007/s11336-014-9430-0. [DOI] [PubMed] [Google Scholar]
- 15.Reitsma J.B., Glas A.S., Rutjes A.W., Scholten R.J., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Rutter C.M., Gatsonis C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865–2884. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 17.Deeks J.J., Higgins J.P.T., Altman D.G. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020) Higgins J.P.T., Thomas J., Chandler J., et al., editors. Cochrane; 2020. Chapter 10: analysing data and undertaking meta-analyses. [Google Scholar]
- 18.Deeks J.J., Macaskill P., Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed E., Fouad M., Aldahrouty A.H., et al. Urine strips test and high sensitive C - reactive protein in diagnosis of spontaneous bacterial peritonitis in Egyptian cirrhotic patients with ascites. Res J Pharmaceut Biol Chem Sci. 2019;10:326–332. [Google Scholar]
- 20.Al Sawaf Y., Kassem G., Assem M., Taha A., El Sorogy H. Towards reliable and rapid bed-side diagnosis of spontaneous bacterial peritonitis in cirrhotic patients: multi center study. Hepatol Int. 2013;7:750. [Google Scholar]
- 21.Braga L., Souza M., Barbosa A., et al. Diagnosis of spontaneous bacterial peritonitis in cirrhotic patients in northeastern Brazil by use of rapid urine-screening test. São Paulo Med J = Rev Paul Med. 2006;124:141–144. doi: 10.1590/S1516-31802006000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butani R., Shaffer R., Szyjkowksi R., et al. Rapid diagnosis of infected ascitic fluid using leukocyte esterase dipstick testing. Am J Gastroenterol. 2004;99:532–537. doi: 10.1111/j.1572-0241.2004.04084.x. [DOI] [PubMed] [Google Scholar]
- 23.Campillo B., Richardet J.P., Dupeyron C. Diagnostic value of two reagent strips (Multistix® 8 SG and Combur® 2 LN) in cirrhotic patients with spontaneous bacterial peritonitis and symptomatic bacterascites. Gastroentérol Clin Biol. 2006;30:446–452. doi: 10.1016/s0399-8320(06)73201-8. [DOI] [PubMed] [Google Scholar]
- 24.Castellote J., Lopez C., Gornals J., et al. Rapid diagnosis of spontaneous bacterial peritonitis by use of reagent strips. Hepatology. 2003;37:893–896. doi: 10.1053/jhep.2003.50120. [DOI] [PubMed] [Google Scholar]
- 25.Chinnock B., Woolard R., Hendey G.W., et al. Sensitivity of a bedside reagent strip for the detection of spontaneous bacterial peritonitis in ED patients with ascites. Am J Emerg Med. 2019;37:2155–2158. doi: 10.1016/j.ajem.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 26.Chugh K., Agrawal Y., Goyal V., et al. Diagnosing bacterial peritonitis made easy by use of leukocyte esterase dipsticks. Int J Crit Illness Inj Sci. 2015;5:32–37. doi: 10.4103/2229-5151.152337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Araujo A., De Barros Lopes A., Michalczuk M.T., et al. Is there yet any place for reagent strips in diagnosing spontaneous bacterial peritonitis in cirrhotic patients? An accuracy and cost-effectiveness study in Brazil. J Gastroenterol Hepatol. 2008;23:1895–1900. doi: 10.1111/j.1440-1746.2008.05571.x. [DOI] [PubMed] [Google Scholar]
- 28.Gaya D.R., Lyon T.D., Clarke J., et al. Bedside leucocyte esterase reagent strips with spectrophotometric analysis to rapidly exclude spontaneous bacterial peritonitis: a pilot study. Eur J Gastroenterol Hepatol. 2007;19:289–295. doi: 10.1097/MEG.0b013e328013e991. [DOI] [PubMed] [Google Scholar]
- 29.Jha A.K., Kumawat D.C., Yasvant B.K., Goenka M.K. Multistix 10 SG leukocyte esterase dipstick testing in rapid bedside diagnosis of spontaneous bacterial peritonitis: a prospective study. J Clin Exp Hepatol. 2012;2:224–228. doi: 10.1016/j.jceh.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jothimani D., Arasan E., Patil V., Rela M. Periscreen strip test—rapid diagnosis of spontaneous bacterial peritonitis. J Clin Exp Hepatol. 2017;7:S54. [Google Scholar]
- 31.Khatwani N., Chhutto M., Abro H., Habib-Ur R., Shaikh M. Diagnostic validity of leukocyte esterase dipstick test for diagnosis of spontaneous bacterial peritonitis in cirrhotic patients. J Ayub Med Coll Abbottabad : JAMC. 2012;23:51–54. [PubMed] [Google Scholar]
- 32.Kim D.K., Suh D.J., Kim G.D., et al. Usefulness of reagent strips for the diagnosis of spontaneous bacterial peritonitis. Kor J Hepatol. 2005;11:243–249. [PubMed] [Google Scholar]
- 33.Li J., Pan Y., Bao W.G., Niu J., Wang F. Multistix10SG urine test in diagnosing spontaneous bacterial peritonitis. Chin J Hepatol. 2006;14:784–785. [PubMed] [Google Scholar]
- 34.Nalmpantidis G., Kapetanos D., Taloumtzis H., et al. Prevalence of spontaneous bacterial peritonitis in patients with cirrhosis of the liver and evaluation of reagent strips in its diagnosis and follow-up. Arch Hellenic Med. 2012;29:87–94. [Google Scholar]
- 35.Nousbaum J.B., Cadranel J.F., Nahon P., et al. Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology. 2007;45:1275–1281. doi: 10.1002/hep.21588. [DOI] [PubMed] [Google Scholar]
- 36.Oey R., Kuiper J.J., Burren H., Man R. Reagent strips are efficient to rule out spontaneous bacterial peritonitis in cirrhotics. Neth J Med. 2016;74:257–261. [PubMed] [Google Scholar]
- 37.Rerknimitr R., Rungsangmanoon W., Kongkam P., Kullavanijaya P. Efficacy of leukocyte esterase dipstick test as a rapid test in diagnosis of spontaneous bacterial peritonitis. World J Gastroenterol. 2006;12:7183–7187. doi: 10.3748/wjg.v12.i44.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rerknimitr R., Limmathurotsakul D., Bhokaisawan N., Kongkam P., Treeprasertsuk S., Kullavanijaya P. A comparison of diagnostic efficacies among different reagent strips and automated cell count in spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2010;25:946–950. doi: 10.1111/j.1440-1746.2009.06153.x. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro T.C., Kondo M., Amaral A.C., et al. Evaluation of reagent strips for ascitic fluid leukocyte determination: is it a possible alternative for spontaneous bacterial peritonitis rapid diagnosis? Braz J Infect Dis. 2007;11:70–74. doi: 10.1590/s1413-86702007000100017. [DOI] [PubMed] [Google Scholar]
- 40.Sapey T., Mena E., Fort E., et al. Rapid diagnosis of spontaneous bacterial peritonitis with leukocyte esterase reagent strips in a European and in an American center. J Gastroenterol Hepatol. 2005;20:187–192. doi: 10.1111/j.1440-1746.2004.03554.x. [DOI] [PubMed] [Google Scholar]
- 41.Sapey T., Kabissa D., Fort E., Laurin C., Mendler M.H. Instant diagnosis of spontaneous bacterial peritonitis using leukocyte esterase reagent strips: Nephur-Test® vs. MultistixSG®. Liver Int. 2005;25:343–348. doi: 10.1111/j.1478-3231.2005.01086.x. [DOI] [PubMed] [Google Scholar]
- 42.Sarwar S., Alam A., Izhar M., et al. Bedside diagnosis of spontaneous bacterial peritonitis using reagent strips. J Coll Phys Surg–Pakistan: JCPSP. 2005;15:418–421. [PubMed] [Google Scholar]
- 43.Tellez-Avila F., Chavez-Tapia N., Franco-Guzman A., Uribe M., Vargas-Vorackova F. Rapid diagnosis of spontaneous bacterial peritonitis using leukocyte esterase reagent strips in Emergency Department: Uri-Quick Clini-10SG® vs. Multistix 10SG®. Ann Hepatol. 2012;11:696–699. [PubMed] [Google Scholar]
- 44.Thévenot T., Cadranel J.F., Nguyen-Khac E., et al. Diagnosis of spontaneous bacterial peritonitis in cirrhotic patients by use of two reagent strips. Eur J Gastroenterol Hepatol. 2004;16:579–583. doi: 10.1097/00042737-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Thévenot T., Briot C., Mace V., et al. The periscreen strip is highly efficient for the exclusion of spontaneous bacterial peritonitis in cirrhotic outpatients. Am J Gastroenterol. 2016;111:1402–1409. doi: 10.1038/ajg.2016.344. [DOI] [PubMed] [Google Scholar]
- 46.Torun S., Dolar E., Yilmaz Y., et al. Evaluation of leukocyte esterase and nitrite strip tests to detect spontaneous bacterial peritonitis in cirrhotic patients. World J Gastroenterol. 2007;13:6027–6030. doi: 10.3748/wjg.v13.45.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanbiervliet G., Rakotoarisoa C., Filippi J., et al. Diagnostic accuracy of a rapid urine-screening test (Multistix8SG) in cirrhotic patients with spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2002;14:1257–1260. doi: 10.1097/00042737-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Wisniewski B., Rautou P.E., Al Sirafi Y., et al. Diagnosis of spontaneous ascites infection in patients with cirrhosis: reagent strips. Presse Med. 2005;34:997–1000. doi: 10.1016/s0755-4982(05)84098-9. [DOI] [PubMed] [Google Scholar]
- 49.Lippi G., Danese E., Cervellin G., et al. Laboratory diagnostics of spontaneous bacterial peritonitis. Clin Chim Acta. 2014;430:164–170. doi: 10.1016/j.cca.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Koulaouzidis A., Leontiadis G.I., Abdullah M., et al. Leucocyte esterase reagent strips for the diagnosis of spontaneous bacterial peritonitis: a systematic review. Eur J Gastroenterol Hepatol. 2008;20:1055–1060. doi: 10.1097/MEG.0b013e328300a363. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen-Khac E., Cadranel J.F., Thevenot T., et al. Review article: the utility of reagent strips in the diagnosis of infected ascites in cirrhotic patients. Aliment Pharmacol Ther. 2008;28:282–288. doi: 10.1111/j.1365-2036.2008.03735.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study includes all additional materials used in the supplementary section for data transparency purposes.