Abstract
Background and Aims
Standard coagulation tests such as prothrombin time, activated partial thromboplastin time, and international normalized ratio are determined by liver-synthesized coagulation factors. Despite an increased international normalized ratio, patients with cirrhosis are in a “rebalanced” state of hemostasis as the concomitant effect of reduced protein C, protein S, and thrombomodulin is not evaluated in standard coagulation tests. The cell-based model of hemostasis indicates additional mechanisms such as systemic inflammation, sepsis, and organ failures tip the delicate coagulation balance to an anticoagulant type in acute-on-chronic liver failure. In acute liver failure, thrombin generation and platelet function remain intact despite a marked prolongation in prothrombin time. We aimed to explain the principles, application, and utility of viscoelastic tests such as thromboelastography, rotational thromboelastometry, and Sonoclot.
Methods
We reviewed the available literature from MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trial with the search terms 'coagulation', 'cirrhosis', 'acute-on-chronic liver failure', 'thromboelastography', 'thromboelastometry' and 'sonoclot' for cross sectional studies, cohort studies and randomized trials
Results
The point-of-care viscoelastic tests provide actionable targets for correcting the coagulation defect in a patient with bleeding and provide evidence-based algorithms for use in liver disease. A limitation of these tests is the inability to assess vessel injury and endothelial elements.
Conclusion
Global coagulation tests provide a comprehensive estimate of coagulation in vitro; however, their use has only been validated in the setting of liver transplantation. Newer guidelines for hemostatic resuscitation are now accepting these POC tests, but additional data are required to validate their use as standard of care.
Keywords: ROTEM, thromboelastography, sonoclot, coagulation, cirrhosis
Abbreviations: ACT, activated clotting time; ALF, acute liver failure; ACLF, acute-on-chronic liver failure; aPTT, activated partial thromboplastin time; CR, clot rate; INR, international normalized ratio; MA, maximum amplitude; R, reaction time; ROTEM, rotational thromboelastometry; SCT, standard coagulation tests; TEG, thromboelastography; VWF, von Willebrand factor
Patients with liver disease have a rebalanced coagulation status, with both procoagulant and anticoagulant mechanisms affecting the cellular elements of hemostasis.1 Additional critical scenarios supervene in patients with acute-on-chronic liver failure (ACLF), including variceal bleeding, invasive diagnostic and therapeutic procedures, organ failures, systemic inflammation and sepsis, risk of portal vein, and deep vein thromboses. Standard coagulation tests (SCTs), namely prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), fibrinogen levels, and platelet count are not predictive of either bleeding or clotting in liver disease.3,4 This is because these tests do not account for the cellular elements of hemostasis and assess coagulation as an enzymatic pathway. Therefore, the conventional use of injudicious blood component transfusions to attempt to correct these parameters, especially before procedures, is ineffectual and results in transfusion-related circulatory overload (TACO) or transfusion-related acute lung injury (TRALI).5 Global coagulation tests are based on the viscoelastic (VE) strength of clot formation and lysis in a calibrated graphical format. These include thromboelastography (TEG), rotational thromboelastometry (ROTEM), and Sonoclot and offer the hepatologist, interventional radiologist, or surgeon a better means of assessing procoagulant and anticoagulant pathways, clot strength, and fibrinolysis as a point-of-care (POC) test. In this review, we will discuss the types of VE tests, their interpretation, and algorithms for use in liver disease.
Cell-based model of hemostasis in liver disease
Cirrhosis results in a rebalanced coagulation profile due to reduction in procoagulant proteins in the setting of thrombocytopenia and portal hypertension. Factors inhibiting platelet function (PF) include low platelet count and increased nitric oxide and prostacyclin, and the competing factors such as higher von Willebrand factor (VWF) and factor VIII (FVIII) favor platelet aggregation.3 Specific coagulation factors V, VII, IX, X, XI, prothrombin, protein C, and protein S are reduced with concomitant increased FVIII and VWF activity in cirrhosis.2 Increased FVIII levels in cirrhosis are due to two factors. First an increased biosynthesis of VWF bioprotein in the liver and increased release of FVIII from the liver sinusoidal endothelial cells with reduced expression of low-density lipoprotein receptor–related protein. Both cause increased expression of FVIII and VWF in cirrhosis.6
Understanding the role of the anticoagulant protein C is relevant, as synthesis is reduced in liver disease. The protein C system modulates FVIII and factor V, which are the co-factors for activation of factor X and prothrombin in the final steps of thrombin generation. Protein C is activated on the endothelium by the thrombin-thrombomodulin-endothelial protein C receptor complex. Activated protein C (APC) cleaves FVIII on the phospholipid surface of platelets and requires protein S and factor V as co-factors. APC has anti-inflammatory and anti-apoptotic activity, and genetic defects in the protein C are associated with venous thromboses.7
Thrombin generation is intact in acute liver failure (ALF), as although factors II, V, VII, and X are decreased, concomitant reduction of proteins C, Z, and S; antithrombin; heparin co-factor II; and α2-macroglobulin rebalances the system ensuring adequate hemostasis. Also, FVIII is increased in ALF as an acute phase reactant. However, fibrinogen, a glycoprotein cleaved by thrombin to fibrin, is affected qualitatively and quantitatively, resulting in hypofibrinolysis.8
Most cases of bleeding in cirrhosis relate to the presence of portal hypertension similar to variceal hemorrhage, portal hypertensive gastropathy–related bleeds, or gastric antral vascular ectasia. Other cases such as paracentesis site hematoma or post-biopsy bleeding are related to vessel injury rather than a hypocoagulable state. To add to the confusion, SCTs such as PT are elevated, and thrombocytopenia, related to hypersplenism, suggests that “the patient may bleed.” It is a surprise to the clinician to discover that cirrhosis is a prothrombotic state. Thrombocytopenia is dependent on the stage of hepatic decompensation, severity of portal hypertension, and splenomegaly.9,10 In cirrhosis, predominant etiology of bleeding is portal hypertension rather than due to coagulopathy per se.11 The traditional chemical enzymatic pathways are now superseded by the cell-based model of hemostasis. Endothelial injury, a crucial event, is the driver for bleeding and thrombosis, which precedes the activation of the inflammatory cascade. The positive feedback loops are for platelet aggregation and thrombin generation and creation of the fibrin mesh by accelerated thrombogenesis. The third step is the retraction of the clot and fibrinolysis. Sometimes, even though a clot forms in cirrhosis, a defect in the third step leads to accelerated clot lysis and can cause delayed bleeding. Inherently the enzymes of the coagulation cascade in cirrhosis are also inflammatory enzymes, and a good clot requires an environment free from antiplatelet agents, anticoagulants, or excessive lysis (Supplementary Figure 1). This model of hemostasis that incorporates cellular elements, i.e., platelets, endothelial cells, inflammatory cells, and the coagulation enzymes, explains why local vessel injury in cirrhosis do not override the systemic coagulation balance, and SCTs remain the same in patients with cirrhosis without dynamic response to transfusion of fresh frozen plasma and platelet concentrates.1,2 The current evidence suggests that cirrhosis is, in fact, a prothrombotic state. Procoagulant proteins such as VWF and FVIII are increased in cirrhosis, and anticoagulants such as antithrombin and protein C are reduced. Therefore, cirrhosis is a hypercoagulable state with a consequent risk of thromboembolism, portal, and splenic vein thrombosis. This, in turn, increases portal hypertension and related complications.12 The rate of portal vein thrombosis is about 8% per year with significant impact on morbidity and mortality.13 Therefore, anticoagulation, both prophylactic and therapeutic, has been proposed in different studies for portal vein and mesenteric vein thromboses.14, 15, 16, 17 The risk of venous thromboembolism is 0.5–2% annually and an associated increased risk of arterial stroke13,17 Low-molecular-weight heparin is considered safest for therapeutic anticoagulation and is not associated with increased risk of variceal hemorrhage.18
Current role of Standard coagulation tests in clinical practice
SCTS such as PT, aPTT, and platelet count are not predictive of bleeding risk as they exclude the cellular elements of hemostasis and are unable to assess the effect of thrombomodulin and cannot assess the stage of the coagulation pathway which is affected.6,19 Hence, these SCTs cannot be used to demonstrate the thrombomodulin-mediated normal thrombin generation potential in cirrhosis.11 SCTs can recognize the inability to clot but cannot aid in diagnosing the level (stage) of impairment in the coagulation cascade. It takes around 40–90 min for the SCTs to complete, and results vary with time, such as fluctuating levels of platelet count, PT, and so on, which can be partially corrected by means of transfusion but have low clinical predictability of either bleeding or thromboses. On the contrary, VE tests take <30 min to complete and diagnose the exact stage of alteration in global coagulation cascade and allow dose titration of fresh frozen plasma, platelet concentrate, or the use of antifibrinolytic drugs. Hence, correction of VE tests may lead to lesser incidence of circulatory overload and lung injury.
Evidence-based coagulation monitoring with viscoelastic testing in liver disease
POC global coagulation testing devices are increasingly being used in clinical practice to diagnose the coagulation defect and prescribe blood component therapy during liver transplantation and other surgeries.20,21 Some studies have reported the use of VE tests in ACLF and nonvariceal bleeding in cirrhosis.3,6,22 The three main devices include TEG, ROTEM, and Sonoclot. The initial stages of clotting can be assessed as the reaction/clot formation time and clot rate (CR) in the first 10–15 min, but the fibrinolytic stage requires 30–40 min. VE devices are created on the principle that when a pin oscillates in a fluid blood sample, the movement of the pin is dampened as the clot thickens, and again as fibrinolysis occurs, the movement is free. This change in the VE property of blood is assessed and translated to a vector graph. VE tests can be performed serially and intraoperatively to see if the blood component has corrected the coagulation defect. The biggest advantage of VE tests is that they show whether the bleeding patients have platelet dysfunction, a fibrinogen defect, or clotting factor deficiency, all three scenarios requiring individual blood components. It also provides a means of excluding the presence of exogenous heparin or antiplatelet drugs. VE tests can also advise on the role of antifibrinolytics.
POC testing using TEG and ROTEM has been described in new research and is the basis for incorporation of these tests for transfusion management in liver transplantation.22
The limitations of VE tests are that they are in vitro, the endogenous activators are not being assessed, and essentially, they determine the coagulation process in the provided tube of blood, and not what is occurring at the bleeding site in vivo. Applying information from the interpretation of these tests is the most useful currently available diagnostic tool for both procoagulant and anticoagulant defects.
Thromboelastography
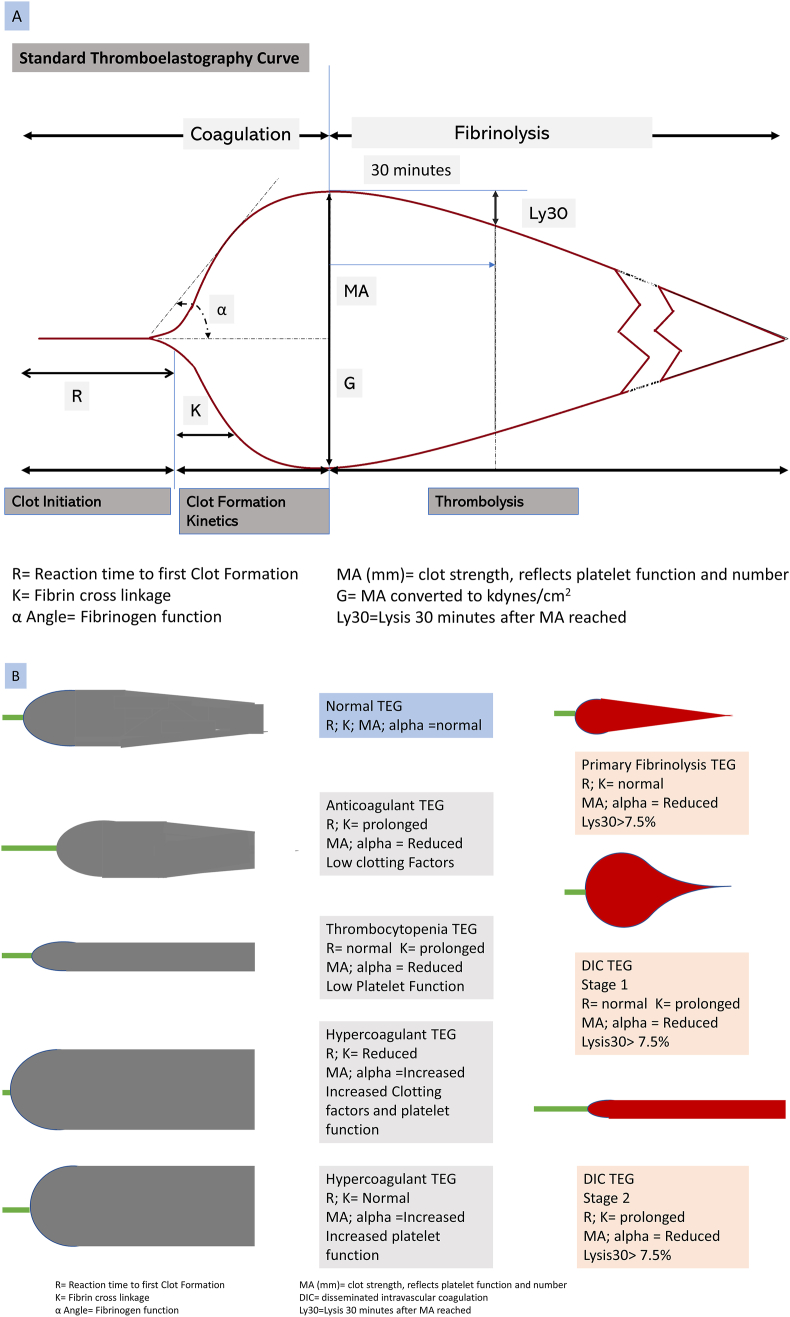
TEG is the POC test performed using the TEG® 5000 analyzer (Haemonetics Corporation, Chicago, IL; www.haemonetics.com). TEG assesses the rate of clot formation, the maximum clot strength, and clot lysis.23,24 The TEG provides information of PF and clot lysis within 30 min. A combination of several activators/inhibitors of the native TEG tests is used to determine the specific coagulation defect (Supplementary Table 1). The TEG graph yields the following parameters, reaction time (R time) prolongation,which is predictive of clotting factor deficiency, clot formation time (K), which reflects on fibrinogen function and rate of clot formation, maximum amplitude (MA), which is an indicator of PF and clot strength and clot lysis at 30 min and 60 min after the MA peak, which pertains to fibrinolysis.3,25,26
Figure 1a shows the normal TEG curve, and the computed variables with corresponding SCT correlates at each phase of coagulation. Figure 1b shows recognizable TEG traces which correspond to a clinical scenario such as hypercoagulable and hypocoagulable states, primary hyperfibrinolysis, PF, disseminated intravascular coagulation, and so on, which can be identified by the pattern of the curve.
Figure 1.
(A) Normal thromboelastography (TEG) trace showing coagulation variables. (B) Recognizable TEG traces which correspond to specific coagulation defects.
Sonoclot Coagulation and Platelet Function Analyzer
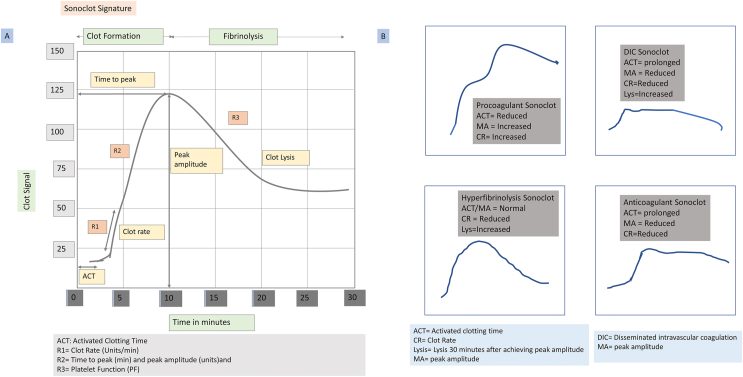
Another VE test is the Sonoclot coagulation analyzer (Sienco Inc., Arvada, CO), first introduced in 1975 by von Kualla.27 The Sonoclot-derived curve provides data on coagulation enzymes, fibrin gel formation, fibrinolysis, and PF. The Sonoclot can use either whole blood, citrated blood, or plasma samples. The test sample, 330–360 μl (blood or plasma) is added to the cuvette.28 The Sonoclot probe moves up and down along the vertical axis in the cuvette containing the activator reagents. As the sample starts to clot, changes in impedance to movement are measured. Quantitative measures of activated clotting time (ACT), CR, and the PF are generated from the graphical output.29 In addition, the time to peak (TP) and peak amplitude (PA) are also calculated. The ACT produced by Sonoclot reflects initial fibrin formation (Figures 2a and b). Clinically useful data on the use of FFP and cryoprecipitate (ACT and CR) can be generated within 15 min. However, information to guide the use of platelet concentrates (TP and PA) takes 20–30 min, and clot lysis takes 30–45 min (Supplementary Table 2).
Figure 2.
(A) Classical Sonoclot signature and stage of coagulation. (B) Abnormal Sonoclot signatures and coagulation scenarios such as hypercoagulable states, disseminated intravascular coagulation, and hyperfibrinolysis.
Rotational Thromboelastometry (ROTEM Delta)
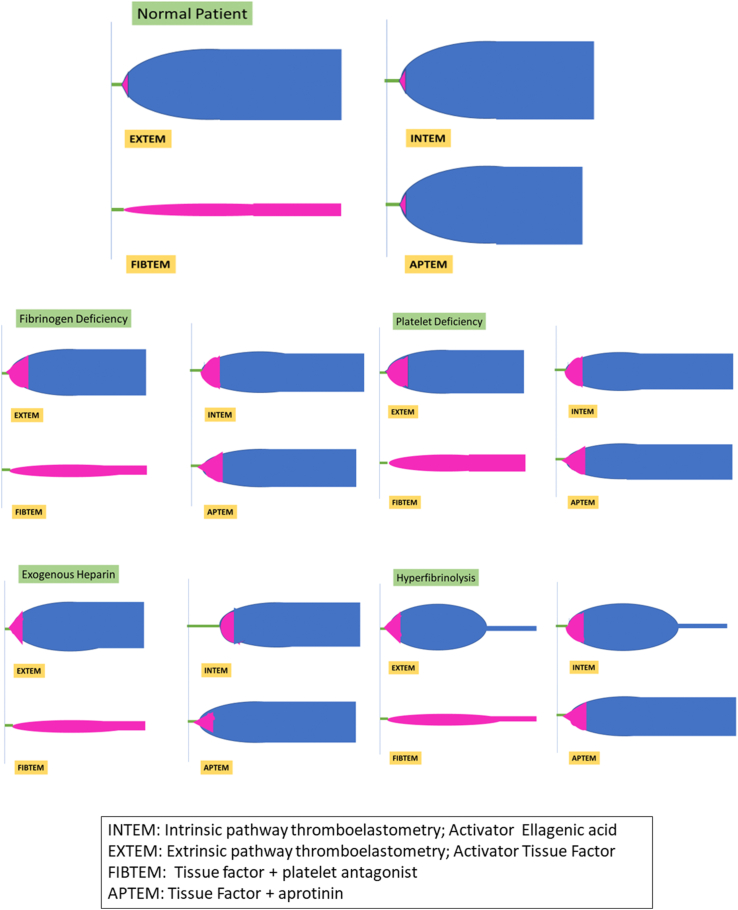
The ROTEM Delta (Tem International GmbH; www.rotem.de) is a POC device based on the thromboelastometry principle to assess whole blood coagulation.27 In the ROTEM device, the pin oscillates rather than the cup (TEG), but essentially all 3 devices measure impedance because of clot formation. The derived output components of the ROTEM assay are shown in Table 1.30 ROTEM gives a holistic view of coagulation using simultaneously performed tests. Initial test is performed using the dual tests for intrinsic pathway (INTEM) and the extrinsic pathway (EXTEM). If normal, then bleeding is unlikely due to a coagulation defect, and one must look for vessel injury in a bleeding field during surgery or endoscopy. Modifications of the test give us more information. The differential for reduced clot firmness is due to platelet dysfunction or fibrinogen defect, and this can be differentiated using fibrinogen mapping (FIBTEM).31,32
Table 1.
Interpretation of ROTEM Data Variables.
| Variable | Abbreviated | Definition | Interpretation |
|---|---|---|---|
| Clotting time | CT | Time interval between addition of reagents till initiation of clot formation | Prolonged CT indicates factor deficiency in INTEM/EXTEM |
| Clot formation time | CFT | Time interval from CT until a clot firmness of 20 mm point has been reached | Rate of clot formation |
| Alpha angle | α | Angle of tangent to the slope of the curve | Speed at which the clot is forming and mainly influenced by platelet function but is also affected by fibrinogen and coagulation factors |
| Amplitude A10 | A10 | Amplitude 10 min after CT→ predicts the maximum clot firmness | Gives early idea about platelet function. |
| Maximum clot firmness | MCF | Maximum vertical amplitude | Low MCF suggests reduced platelet number or function or fibrinogen defect |
| Lysis | Maximum lysis >15% | Fibrinolysis >15% | APTEM and EXTEM help diagnose fibrinolysis |
Abbreviations: ROTEM, rotational thromboelastometry; INTEM, intrinsic pathway thromboelastometry; EXTEM, extrinsic pathway thromboelastometry; APTEM, aprotinin thromboelastometry.
Supplementary Table 3 shows the parameters assessed during the parallel ROTEM assays and the modifiers of the reaction. A 340-μl citrated whole blood sample was added to the ROTEM cuvette, and a sensor slowly oscillates back and forth suspended in the blood sample. The dampened signal is detected, and parallel tests for INTEM and EXTEM are started using the specific reagents described in Supplementary Table 3. Results of clot formation time and CR are available within 5–10 min, and the complete assay results are available in 30 min. The classical ROTEM graphical output and the alterations in disease states are shown in Figure 3.
Figure 3.
Classical rotational thromboelastometry graphical output and variations.
Comparison of the TEG, Sonoclot, and ROTEM systems
In all three systems, the blood sample is added to a disposable cup with test reagent, and dampening of oscillations of a sensor pin or wire is assessed relative to the cuvette. When the blood is liquid, the trace is a straight line (ACT, R time, or CFT). As the blood starts clotting, increased resistance is seen as a widened curve, and numerical values are computed as shown in the three classical traces (Figure 1, Figure 2, Figure 4). The differences in the 3 systems are summarized in Table 2. The chemical reagents, activators and inhibitors, are different, and the ROTEM assay specifically differentiates between the intrinsic and extrinsic pathways. The TEG has an additional platelet mapping function, which cannot be assessed using ROTEM.33
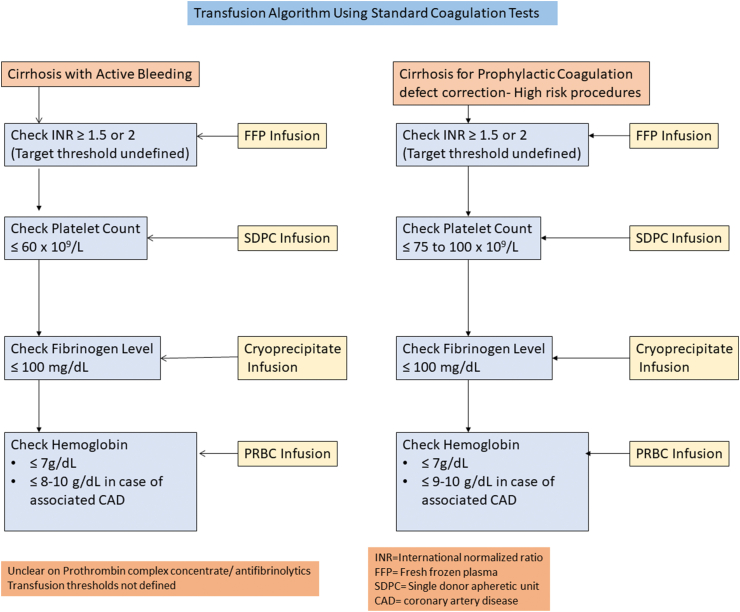
Figure 4.
Transfusion algorithm based on standard coagulation tests.
Table 2.
Comparison of the Three Viscoelastic Point-of-Care Tests.
| Step of Clot Formation | Factors Affecting Clot Stability | TEG | Sonoclot | ROTEM | Therapeutic Options |
|---|---|---|---|---|---|
| Initial clot formation/fibrin formation | Factors XII and XI of the intrinsic pathway or factors VII and VIII if a tissue factor activator is used | R | SonACT | CT | Administration of plasma, coagulation factors |
| Clot kinetics | Factor II and VIII activity, platelet, and fibrinogen function | Kinetics (K) and angle α | CR | Clot formation time (CFT) and angle α | Fresh frozen plasma (FFP) Cryoprecipitate |
| Maximum clot strength | Platelet functionand number, thrombin generation, fibrinogen | Maximum amplitude (MA) | Peak amplitude | Maximum clot firmness (MCF) | Cryoprecipitate/platelet concentrate |
| Maximum lysis | Fibrinolysis | LY30, LY45, LY60 | R3 curve | CL30, CL45, CL60 | Antifibrinolytic drugs Assessment of disseminated intravascular coagulation |
| Advantages | Rapid interpretation at 30 min; rapid TEG yields results even earlier and is useful in transplantation | Rapid results in 15–20 min, decision for FFP and platelet concentrate use can be taken early. | Stable system as movement does not impair the graphical output. Can clearly differentiate between fibrinogen defects and platelet dysfunction. |
All 3 systems provide good guidance for FFP usage. | |
| Disadvantages | Decision pathways for platelet dysfunction and fibrinogen defects show overlap. (K time, α angle, and maximum amplitude). Movement of the system impairs results |
Differentiation of the contribution by platelets and fibrinogen defects to clot rate is difficult to assess. Movement of the system impairs results |
Expensive as multiple tests are run simultaneously | All 3 systems are validated for use in liver transplantation with increasing evidence for restricted use in intensive care practice in acute variceal bleeding. |
Abbreviations: TEG, thromboelastography; ROTEM, rotational thromboelastometry; Ly, lysis; ACT, activated clotting time; CT, clotting time; CL, clot lysis.
Advantages of using VE tests in liver disease treatment algorithms
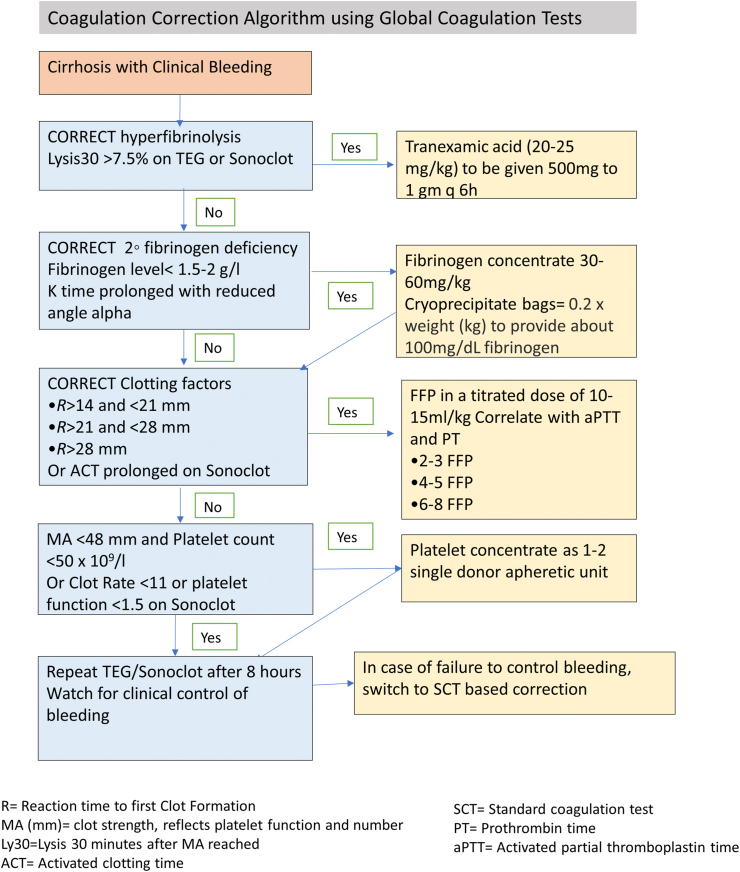
VE tests can be repeatedly performed during liver transplantation and can diagnose the coagulation defect at the bedside. The role of VE in the care pathway in other conditions has been evaluated in recent studies.34, 35, 36 Usual clinical practice algorithms are shown in Figure 4 based on conventional coagulation tests and show that there is ill-defined evidence to support current practice. The scenarios for prophylactic correction of the coagulation defects for high-risk and low-risk invasive procedures in cirrhosis remain undefined. Currently, VE tests provide complementary findings to SCTs such as PT/INR and fibrinogen levels, and usually, they are performed in addition to SCTs as POC tests but have the potential to replace some or all the SCTs.37 Figure 5 suggests an algorithm using TEG and Sonoclot for guiding evidence-based transfusions in cirrhosis with clinically evident coagulopathic bleeding.38 VE tests offer four main benefits over SCTs. First, these are POC tests and can be used during surgery to determine causes of on-table blood loss. Second, results are available in minutes, and targets are actionable. Third, the coagulation defect can be compartmentalized and treated without blanket orders of unnecessary plasma or cryoprecipitate. Low PF can be treated with platelet concentrate alone and hyperfibrinolysis with antifibrinolytic agents such as tranexamic acid. Finally, the variables are dynamic, and appropriate correction can result in a normal coagulation trace if the correct components are used. The use of TEG, ROTEM, and Sonoclot has been described in clinical states of cirrhosis, ALF, acute decompensation of cirrhosis, and ACLF. VE tests are being mentioned in newer guidelines for hemostatic resuscitation, but more validation is needed before they can be recommended as a standard of care.3,5,39,40
Figure 5.
Transfusion algorithm based on global coagulation tests.
Recombinant factor VIIa, a repurposed drug used for bleeding in acquired hemophilia, has been studied for management of variceal bleeding in patients in cirrhosis, but the two randomized controlled trials and subsequent meta-analysis did not suggest any mortality or transfusion reduction benefit in either the whole group with cirrhosis and portal hypertensive bleeding or in the subset with advanced cirrhosis.41, 42, 43 This is likely because most bleeding occurs because of high portal pressures and vessel injury rather than primary or secondary coagulation failure in cirrhosis.
In patients who are undergoing liver transplantation or hepatic resection, the Consensus European Guidelines on the use of rFVII as an adjunctive therapy in severe bleeding may have limited use but have stated that prophylactic use is not recommended.44
Another available repurposed drug is the use of 4-factor prothrombin complex concentrate (PCC) which has factors II, VII, IX, and X and may have additional proteins C and S. PCCs are low-volume products with 25 times the amount of the aforementioned factors which can be infused over 10 min to rapidly correct the INR without additional risk of TRALI/TACO. However, the high cost, low-quality evidence of use, and risk of thromboembolic phenomena limit wider application in cirrhosis and liver failure. Most data have been reported during transplant surgery to manage uncontrolled bleeding as an alternative to blood component therapy.45
Limitations of viscoelastic tests
The main limitation of these tests is that the clinician must extrapolate information taken from clotting of a small volume of blood in vitro, minus the assessment of local site injury. As most bleeding in cirrhosis and ACLF is due to vessel injury, i.e., variceal rupture, biopsy needle site injury, and so on, the defect in global coagulation cannot predict if a patient will bleed. Contrariwise, the global tests do explain the coagulation defect in a patient with clinically apparent bleeding and inform the clinician what dose and type of blood component to transfuse, or whether an antifibrinolytic agent should be administered. SCTS lack the predictive ability and do not change much even after blood component transfusion. The conventional practice of trying to correct an INR to an arbitrary target of <1.5 and platelet count to >75,000/μl is challenged by all evidence to the contrary. In any case, the use of VE tests to limit preprocedural blood components such as FFP and platelet concentrate is a useful application with increasing validation.40 Thrombin generation is unaffected by FFP transfusion.46,47
Currently, avoidance of vessel injury, reduction of portal pressure, and minimal and optimal use of blood components guided by VE testing protocols appear to be the best bet for coagulation defect management. Procoagulant states in the setting of cirrhosis and liver failure such as PVT and deep vein thromboses are often undiagnosed or dismissed as insignificant and remain unrecorded. More prospective data are needed on the natural history of thrombotic states in cirrhosis such as venous thromboembolism, arterial stroke, and local portal vein thromboses. Further research on the role of anticoagulation and transfusion algorithms to improve patient care in patients with liver disease is warranted.
Credit authorship contribution statement
M.P., A.V.K., S.D., and K.K. were all involved in the manuscript preparation. All the authors have read and approved the manuscript.
Conflicts of interest
The authors have none to declare.
Acknowledgement
The review article was supported by an Institutional grant PGIMER project ID 6701 awarded to MP.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.05.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lisman T., Caldwell S.H., Burroughs A.K., et al. Coagulation in Liver Disease Study Group.Hemostasis and thrombosis in patients with liver disease: the ups and downs. J Hepatol. 2010;53:362–371. doi: 10.1016/j.jhep.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 2.Tripodi A., Primignani M., Mannucci P.M., Caldwell S.H. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112:274–281. doi: 10.1038/ajg.2016.498. [DOI] [PubMed] [Google Scholar]
- 3.Premkumar M., Saxena P., Rangegowda D., et al. Coagulation failure is associated with bleeding events and clinical outcome during systemic inflammatory response and sepsis in acute-on-chronic liver failure: an observational cohort study. Liver Int. 2019;39:694–704. doi: 10.1111/liv.14034. [DOI] [PubMed] [Google Scholar]
- 4.Segal J.B., Dzik W.H. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413–1425. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 5.Case J.J., Khan N., Delrahim M., et al. Association of massive transfusion for resuscitation in gastrointestinal bleeding with transfusion-related acute lung injury. Indian J Crit Care Med. 2017;21:506–513. doi: 10.4103/ijccm.IJCCM_380_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollestelle M.J., Geertzen H.G., Straatsburg I.H., van Gulik T.M., van Mourik J.A. Factor VIII expression in liver disease. Thromb Haemostasis. 2004 Feb;91:267–275. doi: 10.1160/TH03-05-0310. PMID: 14961153. [DOI] [PubMed] [Google Scholar]
- 7.Dahlbäck B., Villoutreix B.O. The anticoagulant protein C pathway. FEBS Lett. 2005 Jun 13;579:3310–3316. doi: 10.1016/j.febslet.2005.03.001. Epub 2005 Mar 13. PMID: 15943976. [DOI] [PubMed] [Google Scholar]
- 8.Lisman T., Bakhtiari K., Adelmeijer J., Meijers J.C., Porte R.J., Stravitz R.T. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemostasis. 2012 Jul;10:1312–1319. doi: 10.1111/j.1538-7836.2012.04770.x. PMID: 22568491. [DOI] [PubMed] [Google Scholar]
- 9.Kumar M., Ahmad J., Maiwall R., et al. Thromboelastography-guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology. 2020;71:235–246. doi: 10.1002/hep.30794. [DOI] [PubMed] [Google Scholar]
- 10.Northup P.G., Caldwell S.H. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064–1074. doi: 10.1016/j.cgh.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Tripodi A., Salerno F., Chantarangkul V., et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553–558. doi: 10.1002/hep.20569. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal B., Wright G., Gatt A., et al. Evaluation of coagulation abnormalities in acute liver failure. J Hepatol. 2012;57:780–786. doi: 10.1016/j.jhep.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Gulley D., Teal E., Suvannasankha A., et al. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53:3012–3017. doi: 10.1007/s10620-008-0265-3. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosino P., Tarantino L., Di M.G., et al. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta-analysis. Thromb Haemostasis. 2017;117:139–148. doi: 10.1160/TH16-06-0450. [DOI] [PubMed] [Google Scholar]
- 15.Nery F., Chevret S., Condat B., et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61:660–667. doi: 10.1002/hep.27546. [DOI] [PubMed] [Google Scholar]
- 16.Faccia M., Ainora M.E., Ponziani F.R., et al. Portal vein thrombosis in cirrhosis: why a well-known complication is still matter of debate. World J Gastroenterol. 2019;25:4437–4451. doi: 10.3748/wjg.v25.i31.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh N.S., Navi B.B., Schneider Y., Jesudian A., Kamel H. Association between cirrhosis and stroke in a nationally representative cohort. JAMA Neurol. 2017;74:927–932. doi: 10.1001/jamaneurol.2017.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villa E., Cammà C., Marietta M., et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–1260. doi: 10.1053/j.gastro.2012.07.018. e1-e4. [DOI] [PubMed] [Google Scholar]
- 19.Tripodi A., Caldwell S.H., Hoffman M., Trotter J.F., Sanyal A.J. The prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther. 2007;26:141–148. doi: 10.1111/j.1365-2036.2007.03369.x. [DOI] [PubMed] [Google Scholar]
- 20.Senzolo M., Coppell J., Cholangitas E., et al. The effects of glycosaminoglycans on coagulation: a thromboelastographic study. Blood Coagul Fibrinolysis. 2007;18:227–236. doi: 10.1097/MBC.0b013e328010bd3d. [DOI] [PubMed] [Google Scholar]
- 21.Krzanicki D., Sugavanam A., Mallett S. Intraoperative hypercoagulability during liver transplantation as demonstrated by thromboelastography. Liver Transplant. 2013;19:852–861. doi: 10.1002/lt.23668. [DOI] [PubMed] [Google Scholar]
- 22.Northup P.G., Friedman L.S., Kamath P.S. AGA clinical practice update on surgical risk assessment and perioperative management in cirrhosis: expert review. Clin Gastroenterol Hepatol. 2019;17:595–606. doi: 10.1016/j.cgh.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Haemonetics Corporation . Haemonetics Corporation; Braintree, MA, USA: 2013. The TEG System Provides Visual Representation of Your Patient's Hemostasis in: TEG®5000: Hemostasis Analyzer System.https://teg.haemonetics.com/en/teg-5000-thrombelastograph [Internet] [Google Scholar]
- 24.Luddington R.J. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 25.De Pietri L., Bianchini M., Montalti R., et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63:566–573. doi: 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Donald P., Vasudevan A., Angus P., et al. Coagulation in acutely ill patients with severe chronic liver disease: insights from thromboelastography. J Crit Care. 2017;38:215–224. doi: 10.1016/j.jcrc.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Sienco Inc. Sonoclot® Coagulation & Platelet Function Analyzer with Graphics Printer. For in Vitro Diagnostic Use: Operator's Manual. For Firmware Version 4.0. Sonoclot Analyzer, DP-2951, User manual 020-1001 Rev. 4.0.1 [Internet]. Arvada, CO, USA: Sienco Inc., [accessed10.05.2020]. 96p. Available from: https://www.sienco.com/sonoclot-usa/sonoclot-usa/user-manuals/020-1001-user-manual-r401.pdf.
- 28.von Kaulla K.N., Ostendorf P., von Kaulla E. The impedance machine: a new bedside coagulation recording device. J Med. 1975;6:73–88. [PubMed] [Google Scholar]
- 29.Premkumar M., Saxena P., Mirza P., et al. Heparin like effect increases the risk of mortality and bleeding in patients with severe alcoholic hepatitis. Clin Gastroenterol Hepatol. 2020;18:486–495. doi: 10.1016/j.cgh.2019.04.057. [DOI] [PubMed] [Google Scholar]
- 30.Tem Innovations GmbH . Tem Innovations GmbH, c; Munich, Germany: 2013. Welcome to ROTEM®.http://rotem.de [Internet] Available from: [Google Scholar]
- 31.Gorlinger K., Bhardwaj V., Kapoor P.M. Simulation in coagulation testing using rotational thromboelastometry: a fast emerging, reliable point of care technique. Ann Card Anaesth. 2016;19:516–520. doi: 10.4103/0971-9784.185546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchner C., Dirkmann D., Treckmann J.W., et al. Coagulation management with factor concentrates in liver transplantation: a single-center experience. Transfusion. 2014;54:2760–2768. doi: 10.1111/trf.12707. [DOI] [PubMed] [Google Scholar]
- 33.Hunt H., Hyde C., Stanworth S., et al. Thromboelastography (TEG) and thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding [Diagnostic Test Accuracy Protocol] Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD010438.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blasi A., Calvo A., Prado V., et al. Coagulation failure in patients with acute-on-chronic liver failure (ACLF) and decompensated cirrhosis: beyond INR. Hepatology. 2018;68:2325–2337. doi: 10.1002/hep.30103. [DOI] [PubMed] [Google Scholar]
- 35.Fisher C., Patel V.C., Stoy S.H., et al. Balanced haemostasis with both hypo- and hyper-coagulable features in critically ill patients with acute-on-chronic-liver failure. J Crit Care. 2018;43:54–60. doi: 10.1016/j.jcrc.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 36.Bedreli S., Sowa J.P., Gerken G., Saner F.H., Canbay A. Management of acute-on-chronic liver failure: rotational thromboelastometry may reduce substitution of coagulation factors in liver cirrhosis. Gut. 2016;65:357–358. doi: 10.1136/gutjnl-2015-309922. [DOI] [PubMed] [Google Scholar]
- 37.Premkumar M., Sarin S.K. Current concepts in coagulation profile in cirrhosis and acute-on-chronic liver failure. Clin Liver Dis. 2020 Nov 3;16:158–167. doi: 10.1002/cld.976. PMID: 33163169; PMCID: PMC7609701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stravitz R.T. Algorithms for managing coagulation disorders in liver disease. Hepatol Int. 2018;12:390–401. doi: 10.1007/s12072-018-9886-6. [DOI] [PubMed] [Google Scholar]
- 39.Intagliata N.M., Argo C.K., Stine J.G., et al. Faculty of the 7th international coagulation in liver disease. Concepts and controversies in haemostasis and thrombosis associated with liver disease: proceedings of the 7th international coagulation in liver disease conference. Thromb Haemostasis. 2018;118:1491–1506. doi: 10.1055/s-0038-1666861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Premkumar M., Mehtani R., Divyaveer S., et al. Clinical validation of global coagulation tests to guide blood component transfusions in cirrhosis and ACLF. J Clin Transl Hepatol. 2021 doi: 10.14218/JCTH.2020.00121. Published online: Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch J., Thabut D., Bendtsen F. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123–1130. doi: 10.1053/j.gastro.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Bosch J., Thabut D., Albillos A., et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized controlled trial. Hepatology. 2008;47:1604–1614. doi: 10.1002/hep.22216. [DOI] [PubMed] [Google Scholar]
- 43.Marti-Carvajal A.J., Salanti G., Marti-Carvajal P.J. Human recombinant activated factor VII for upper gastrointestinal bleeding in patients with liver diseases. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD004887.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Vincent J.L., Rossaint R., Riou B., Ozier Y., Zideman D., Spahn D.R. Recommendations on the use of recombinant activated factor VII as an adjunctive treatment for massive bleeding--a European perspective. Crit Care. 2006;10:R120. doi: 10.1186/cc5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall S.V., Noble J., Flores A.S. Prothrombin complex concentrate in liver transplant surgery: correction of therapeutic anticoagulation and the coagulopathy of end-stage liver disease: case series. Front Pharmacol. 2020;11:566433. doi: 10.3389/fphar.2020.566433. Published 2020 Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rassi A.B., d'Amico E.A., Tripodi A., et al. Fresh frozen plasma transfusion in patients with cirrhosis and coagulopathy: effect on conventional coagulation tests and thrombomodulin-modified thrombin generation. J Hepatol. 2020 Jan;72:85–94. doi: 10.1016/j.jhep.2019.09.008. Epub 2019 Sep 16. PMID: 31536747. [DOI] [PubMed] [Google Scholar]
- 47.Müller M.C., Straat M., Meijers J.C., et al. Fresh frozen plasma transfusion fails to influence the hemostatic balance in critically ill patients with a coagulopathy. J Thromb Haemostasis. 2015 Jun;13:989–997. doi: 10.1111/jth.12908. Epub 2015 Apr 18. PMID: 25809519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.