Abstract
Partial splenic embolization (PSE) is a minimally invasive endovascular procedure for the treatment of hypersplenism. This procedure is normally done by catheterization of the splenic artery, which if ligated during surgery, makes the procedure very challenging. We present a case where the splenic artery had been ligated during liver transplant surgery, and the only major endovascular route to reach the spleen was through the hypertrophied tortuous gastroepiploic artery.
Successful PSE in these patients suggests the feasibility of this procedure after splenic artery ligation and can thus help to spare a patient from the otherwise more invasive surgical splenectomy.
Keywords: partial splenic embolization, hypersplenism, splenic artery ligation, liver transplant
Abbreviations: GEA, Gastroepiploic artery; LT, Liver transplant; PSE, Partial splenic embolization
Hypersplenism is a clinical disorder characterized by splenomegaly and a significant reduction in one or more of the cellular elements of the blood in the presence of normocellular or hypercellular bone marrow.1 It can occur due to many underlying conditions, including infiltrative disorders, malignancies, and liver cirrhosis.2 In patients with cirrhosis and portal hypertension, the incidence of hypersplenism has been described in between 11% and 55%.1
After liver transplant (LT), the size of the spleen reduces, and hypersplenism improves, although persistent splenomegaly may be seen in some patients.3 Although splenectomy is the definitive treatment for hypersplenism, it is avoided after liver transplantation due to the potential risk of overwhelming sepsis following splenectomy.1
Another treatment option in patients suffering from hypersplenism is partial splenic embolization (PSE). PSE is a minimally invasive endovascular procedure, which reduces the splenic size and improves blood counts.1 This procedure is normally done via femoral artery access followed by catheterization of celiac axis and splenic artery. However, if splenic artery has been ligated during a surgery, the catheterization of the splenic artery can be very challenging. We present a case where the splenic artery had been ligated during LT surgery, and the only major available endovascular route to reach the spleen was through the long, tortuous gastroepiploic artery (GEA).
Case
A 43-year-old man who had received a living donor right lobe liver graft 6 months earlier for Hepatitis C-related cirrhosis presented with clinical features of hypersplenism. He had massive splenomegaly (Span = 19.2 cm), anemia, thrombocytopenia, and leukocytopenia (Table 1). His immunosuppressants consisted of the standard triple medication as per our institutional protocol consisting of Calcineurin Inhibitor (Tacrolimus), an antimetabolite (Mycophenolate mofetil), and steroids, which are tapered over a duration of 3 months. Bone marrow biopsy was normal, which ruled out bone marrow suppression/aplasia. There was no evidence of recurrence of portal hypertension (no evidence of portal vein stenosis on Doppler ultrasound and contrast CT, normal hepatic venous pressure gradient, normal hepatic vein flow pattern on Doppler ultrasound and normal hepatic vein to IVC pressure gradient on invasive pressure measurements). Hence a diagnosis of hypersplenism was made.
Table 1.
Lab Parameters Before and After PSE.
| 1 day before embolization | 2 days | 3 days | 10 days jan | 2 weeks | 1 month | 2 months | 5 months | |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin g/dl | 11.5 | 10.7 | 10 | 9.9 | 9.8 | 12 | 12 | 12 |
| TLC (109/L) | 2.73 | 10.6 | 14.3 | 14.7 | 11.48 | 8.8 | 8.0 | 7.1 |
| Platelet count (109/L) | 70 | 150 | 150 | 242 | 270 | 188 | 130 | 130 |
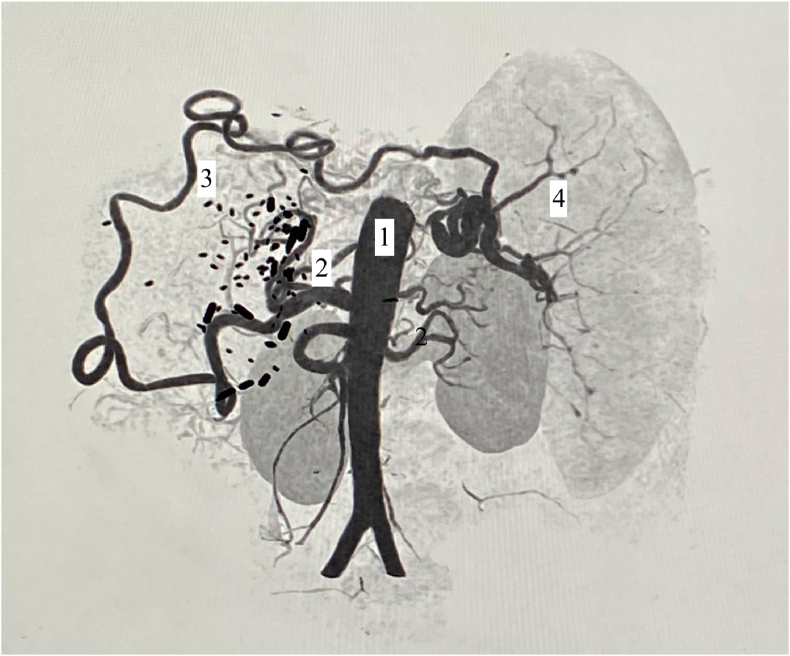
Contrast-enhanced CT scan revealed occluded main splenic artery (surgical ligation) but distal opacification of the splenic artery lumen near splenic hilum with collaterals from the gastroepiploic artery (GEA) and left gastric artery. A careful study of the CT scan suggested that majority of the blood supply was coming from hypertrophied GEA and a minor contribution from smaller left gastric artery collaterals. A decision was taken to embolize the distal splenic artery branches via the long and tortuous GEA (Figure 1).
Figure 1.
Reconstructed image fom CT angiography: 1 = abdominal aorta, 2 = celiac axis, 3 = Gastroepiploic artery, 4 = splenic artery branches.
IV antibiotics were given (Cefoperazone 500 mg + Sulbactam 500 mg, IV twice daily for 3 days) starting from the morning of the day of the procedure. No prior Haemophilus, Pneumococcal, or Meningococcal vaccination was deemed necessary. Under all aseptic precautions, femoral artery access was taken by routine Seldinger technique and a 5F introducer sheath was placed. The celiac axis was cannulated with a Cobra-2 catheter (5F, 65 cm long, Cook Medical, Bloomington, IN) and advanced into the gastroduodenal artery and GEA as distally as possible. A microcatheter (2.7F, 130 cm long, Progreat, Terumo Corp, Japan) was advanced coaxially through the lumen of the Cobra-2 catheter and advanced as distally as possible till the whole length of the microcatheter entered onto the Cobra-2 catheter. The microcatheter tip was now lying in the distal part of GEA near the splenic hilum beyond the origin of small gastric branches. Polyvinyl alcohol particles (PVA 500-700 microns, Contour, Boston Scientific, Natick, MA) were injected through the microcatheter till the flow in the splenic artery branches was sluggish, and the splenic blush was significantly reduced.
Postprocedure, no major complication was observed. The patient was discharged on the third day of the procedure in a stable condition. At five months follow up, the patient is doing well, and the platelets showed a sustained improvement after the PSE (Table 1).
Discussion
Hypersplenism generally improves following LT. Initially, platelet counts falls due to sequestration in the microcirculation of the new liver, which is followed by an increase in platelet numbers as thrombopoietin is produced by the graft liver.1 Intriguingly, in our case, despite splenic artery ligation during liver transplant, hypersplenism persisted, perhaps due to reformation of splenic artery from GEA and left gastric artery.
PSE is an established method of treatment for hypersplenism with proven safety and efficacy. PSE increases platelet counts via two mechanisms: (1) by reducing the splenic size, thereby reducing the trapping of thrombocytes in the spleen; and (2) by decreasing levels of platelet-associated immunoglobulin G (IgG).4
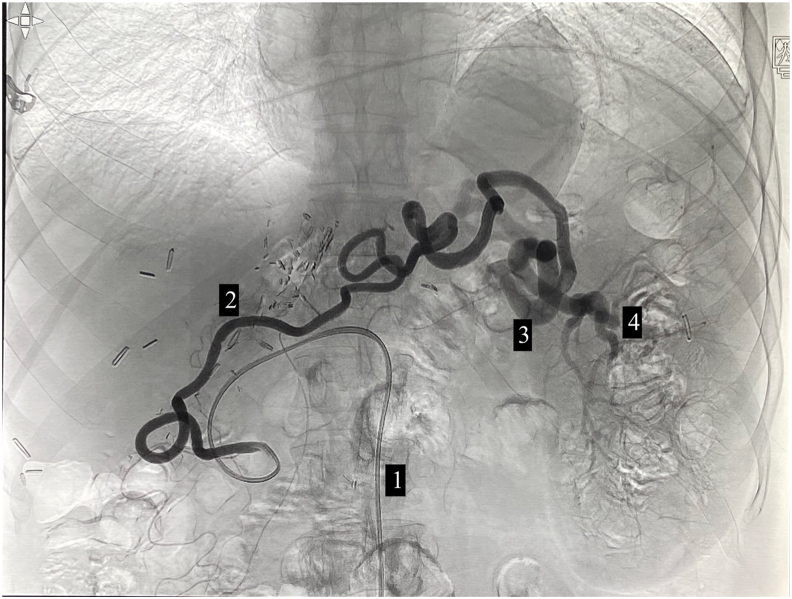
PSE is an endovascular procedure and requires patent splenic artery for its catheterization. However, in this particular patient, splenic artery catheterization was not possible due to splenic artery ligation. The splenic artery ligation had been done during the liver transplant to modulate the excessive portal blood flow.5 Collateral circulation had developed feeding spleen via hypertrophied GEA and short gastric arteries as demonstrated on contrast CT (Figure 1). As such alternative route for catheterization was planned via celiac axis to GDA to GEA to hilar part of the splenic artery (Figure 2).
Figure 2.
Invasive angiography showing angiographic catheter (1) tip at the origin of the gastroepiploic artery and opacification of the hypertrophied tortuous gastroepiploic artery (2) and subsequent opacification of the distal splenic artery (3) and its branches (4).
Although navigation through the long, tortuous GEA was a challenge, the embolization procedure was done uneventfully. The routine microcatheter length (130 cm) was barely sufficient to reach the distal target. Also, the main angiographic catheter (Cobra-2 in this case) may not provide ample support while navigating the microcatheter in the tortuous GEA. As such, a guiding catheter may be needed to support the angiography catheter, which, in turn, will let the microcatheter to pass further, thus making a triaxial system.
Technically, PSE can be done by two methods; proximal embolization or distal embolization. In the proximal embolization, the catheter is placed immediately distal to the origins of the pancreatic and short gastric arteries, and an embolic agent is released. Embolic agent is dispersed throughout the spleen, and small, diffuse, randomized infarcts occur. In the distal embolization method, the catheter is advanced into a distal segmental branch of the splenic artery. The entire distal splenic segment is then embolized.6 Essentially, both methods result in infarction of a significant volume of the spleen usually one-half to two-thirds. In this case we chose proximal embolization technique.
PSE has been proposed as an alternative to surgical splenectomy. PSE presents several advantages over splenectomy, including reduced invasiveness of the procedure, short procedure time, early postprocedural ambulation, lack of a need for blood transfusion, and preservation of a part of splenic tissue function to protect against infections.2
PSE, however, can have its complications, such as postembolization syndrome, splenic abscess, rupture of the spleen, pneumonia, pleural effusion, and septicemia. Postembolization syndrome is the most common side effect of PSE. It consists of left-sided abdominal pain, fever, malaise, and gastrointestinal symptoms in the absence of a positive blood culture or other evidence of infection. It is a self-limiting, benign phenomenon that usually indicates extensive tissue necrosis and/or local intravascular thrombosis.4
Xu reported a case of cirrhosis and hypersplenism who had undergone ligation of the splenic artery (not liver transplant). Partial splenic embolization (PSE) was performed through the compensatory left gastroepiploic and the dorsal pancreatic artery.7
To the best of our knowledge, this is the first case of its kind to be reported in the literature where splenic embolization was attempted via GEA in a liver transplant recipient who had also undergone prior splenic artery ligation. Successful accomplishment of the procedure in these patients suggests the feasibility of PSE after splenic artery ligation and thus helped to spare these patients from the otherwise more invasive surgical splenectomy.
Credit authorship contribution statement
Shahnawaz Bashir: Writing – original draft, Reviewing and Editing. Subhash Gupta: Conceptualization, Reviewing and Editing, Supervision. Shaleen Agarwal: Reviewing and Editing, Supervision. Sanjiv Saigal: Reviewing and Editing, Supervision.
Conflicts of interest
The authors have none to declare.
Acknowledgement
Nil.
Funding
None.
References
- 1.McCormick P.A., Murphy K.M. Splenomegaly, hypersplenism and coagulation abnormalities in liver disease. Baillieres Best Pract Res Clin Gastroenterol. 2000 Dec;14:1009–1031. doi: 10.1053/bega.2000.0144. [DOI] [PubMed] [Google Scholar]
- 2.Talwar A., Gabr A., Riaz A., et al. Adverse events related to partial splenic embolization for the treatment of hypersplenism: a systematic review. J Vasc Interv Radiol. 2020 Jul;31:1118–1131. doi: 10.1016/j.jvir.2019.08.015. e6. [DOI] [PubMed] [Google Scholar]
- 3.Tutar N.U., Isiklar I., Ulu E.M., Haberal M. Spleen size changes in pediatric liver transplant recipients with functioning grafts. Transplant Proc. 2007 Dec;39:3199–3201. doi: 10.1016/j.transproceed.2007.08.105. [DOI] [PubMed] [Google Scholar]
- 4.Amin M.A., el-Gendy M.M., Dawoud I.E., Shoma A., Negm A.M., Amer T.A. Partial splenic embolization versus splenectomy for the management of hypersplenism in cirrhotic patients. World J Surg. 2009 Aug;33:1702–1710. doi: 10.1007/s00268-009-0095-2. [DOI] [PubMed] [Google Scholar]
- 5.Vasavada B.B., Chen C.L., Zakaria M. Portal flow is the main predictor of early graft dysfunction regardless of the GRWR status in living donor liver transplantation - a retrospective analysis of 134 patients. Int J Surg. 2014;12:177–180. doi: 10.1016/j.ijsu.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Hadduck T.A., McWilliams J.P. Partial splenic artery embolization in cirrhotic patients. World J Radiol. 2014;6:160–168. doi: 10.4329/wjr.v6.i5.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z.J., Zheng L.Q., Pan X.N. Partial embolization as re-treatment of hypersplenism after unsuccessful splenic artery ligation. World J Gastroenterol. 2015;21:1365–1370. doi: 10.3748/wjg.v21.i4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]