Abstract
Background
End-stage liver disease (ESLD) is not considered a risk factor for atherosclerotic cardiovascular disease (ASCVD). However, lifestyle characteristics commonly associated with increased ASCVD risk are highly prevalent in ESLD. Emerging literature shows a high burden of asymptomatic coronary artery disease (CAD) in patients with ESLD and a high ASCVD risk in liver transplantation (LT) recipients. Coronary artery calcium score (CAC) is a noninvasive test providing reliable CAD risk stratification. We implemented an LT evaluation protocol with CAC playing a central role in triaging and determining the need for further CAD assessment. Here, we inform our results from this early experience.
Methods
Patients with ESLD referred for LT evaluation were prospectively studied. We compared accuracy of CAC against that of CAD risk factors/scores, troponin I, dobutamine stress echocardiogram (DSE), and single-photon emission computed tomography (SPECT) to detect coronary stenosis ≥70 (CAD ≥ 70) per left heart catheterization (LHC). Thirty-day post-LT cardiac outcomes were also analyzed.
Results
One hundred twenty-four of 148 (84%) patients underwent CAC, 106 (72%) DSE/SPECT, and 50 (34%) LHC. CAC ≥ 400 was found in 35 (28%), 100 to 399 in 17 (14%), and <100 in 72 (58%). LHC identified CAD ≥ 70% in 8 of 29 (28%), 2 of 9 (22%), and 0 of 4, respectively. Two acute coronary syndromes occurred after LT in a patient with CAC 811 (CAD < 70%), and one with CAC 347 (CAD ≥ 70%). No patients with CAC < 100 presented with acute coronary syndrome after LT. When using CAD ≥ 70% as primary endpoint of LT evaluation, CAC ≥ 346 was the only test showing predictive usefulness (negative predictive value 100%).
Conclusions
CAC is a promising tool to guide CAD risk stratification and need for LHC during LT evaluation. Patients with a CAC < 100 can safely undergo LT without the need for LHC or cardiac stress testing, whereas a CAC < 346 accurately rules out significant CAD stenosis (≥70%) on LHC, outperforming other CAD risk-stratification strategies.
Keywords: agatston score, cardiac stress test, angiogram, cirrhosis, end-stage liver disease
Abbreviations: ACS, Acute coronary syndromes; ALD, alcoholic liver disease; ASCVD, Atherosclerotic cardiovascular disease; ASCVD, atherosclerosis cardiovascular disease risk; BMI, Body mass index; CABG, Coronary angioplasty bypass surgery; CAC, Coronary calcium score; CAD, Coronary artery disease; CKD, chronic kidney disease; DSE/SPECT, Dobutamine stress echocardiogram or single-photon emission computed tomography; ESLD, End-stage liver disease; HCV, hepatitis C virus; IQR, Interquartile range; LCx, left circumflex; LT, liver transplantation; LHC, Left heart catheterization; METs, Metabolic equivalents; MELD, model for end stage liver disease; MESA, Multi-Ethnic Study of Atherosclerosis; NPV, negative predictive value; OPTN, Organ Procurement and Transplantation Network; OM, obtuse marginal; PCI, Percutaneous coronary intervention; PDA, posterior descending artery; POBA, plain old balloon angioplasty; PPV, positive predictive value; ROC, Receiver operating characteristic; RCA, right coronary artery; RI, ramus intermedius; RPL, right posterolateral; SD, Standard deviation; VT, Ventricular tachycardia
In the setting of elective moderate- to high-risk surgery, clinical guidelines on perioperative cardiovascular evaluation recommend performing cardiac stress testing to asymptomatic patients with poor or unknown functional capacity (those achieving <4 metabolic equivalents [METs]), particularly in the presence of 3 or more atherosclerotic cardiovascular disease (ASCVD) risk factors.1 This should be followed by left heart catheterization (LHC) if cardiac stress testing results are markedly positive and revascularization if the extent of coronary disease is clinically relevant (defined as ≥70% luminal stenosis) — mostly percutaneous coronary intervention (PCI) with stent placement. Liver transplantation (LT) is deferred until cardiology clearance because of the need for dual antiplatelet treatment (for 6 weeks to 6 months, depending on stent characteristics) to prevent stent thrombosis, which is more frequent at early stages after PCI. However, evidence for CAD risk-stratification for LT is limited, and most transplant centers proceed with mandatory cardiac stress testing or LHC in spite of lack of clear and evidence-based guidelines.
Dobutamine stress echocardiography (DSE) is recommended to assess CAD as part of the evaluation of LT candidates.2 The main caveat of DSE in patients with end-stage liver disease (ESLD) is that it is less likely to reach target heart rate during chemically induced stress.3 Single-photon emission computed tomography (SPECT) has decreased accuracy in patients with ESLD as well, given the chronic vasodilatory state with inherent reduced response to vasodilators observed in this population.4 Therefore, LHC is the most reliable method to evaluate CAD in LT candidates for ESLD; however, it is an invasive test and carries the risk of bleeding and contrast-induced nephropathy among other serious complications.5, 6, 7
The coronary artery calcium score (CAC) from computed tomography is the noninvasive surrogate to LHC for the assessment of CAD, showing promising results in patients with liver disease and LT candidates.4,8, 9, 10, 11, 12 The CAC measures the total calcium burden in the coronary arteries, and the amount of calcification is expressed as the Agatston score. A calcium score of 0 is indicative of the absence of coronary artery calcification and a low likelihood of CAD. In contrast, a positive test (Agatston score > 100) reflects the presence of calcified plaque in the coronary arteries (i.e., atherosclerotic plaques), which has been associated with an increased risk for CAD.4,11, 12, 13, 14
In an attempt to better triage CAD evaluation for LT candidates, we implemented a standardized evaluation protocol including the use of CAC, along with DSE/SPECT and other parameters commonly used for ASCVD risk-stratification for patients with ESLD referred for LT. Here, we inform our results from this early experience.
Patients and methods
This is a retrolective analysis of prospectively collected data after implementation of a standardized CAD evaluation protocol at a single transplant center between 2014 and 2016. All consecutive patients older than 18 years undergoing LT evaluation were included. Patients with acute liver failure, history of coronary angioplasty bypass surgery (CABG), or PCI were excluded. All demographic, clinical, and biochemical data were recorded from the LT evaluation visit, and patients were followed up until the end of January 2017. Our institutional review board approved this study protocol.
CAC to Triage Coronary Artery Disease Evaluation Protocol
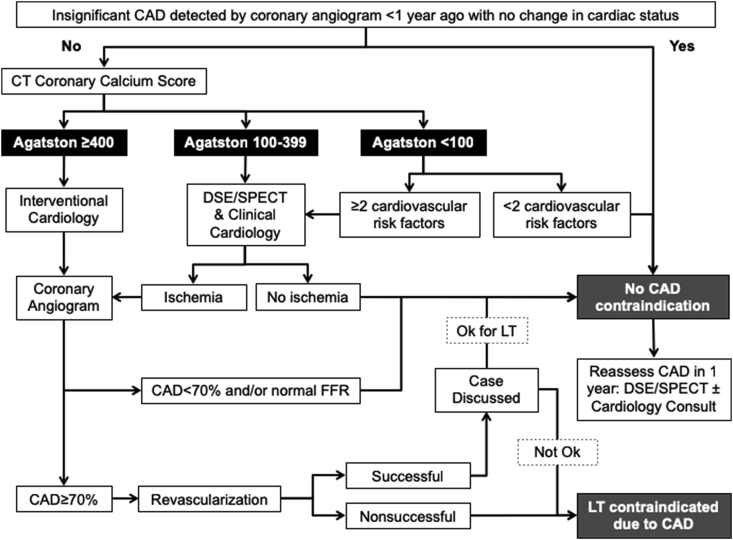
Patients with a heart rate >70 bpm (and no hypotension) were given 25 mg of metoprolol on the night before and the day of CAC testing. Patients with an Agatston score <100 could proceed directly to LT if no more than 1 of the following risk factors were noted during history and physical: age >50 years, diabetes mellitus, dyslipidemia, hypertension, chronic kidney disease (glomerular filtration rate <30 mL/min), hemochromatosis, grade 2 obesity (body mass index ≥35 kg/m2), stroke, smoking >20 pack-years, functional capacity estimated at <4 METs, abnormal electrocardiogram (ischemia changes), or elevated troponin I (>0.07 ng/mL). However, if ≥2 risk factors were documented, they would undergo cardiac stress testing and were evaluated by a cardiologist. The same approach was followed for patients with an Agatston score between 100 and 399, whereas those with a score ≥400 were sent directly to LHC (Figure 1). Except for patients with prior PCI and CABG, CAC was mandatory in all cases. Although DSE was favored, SPECT was used at the discretion of our cardiologists. We collected data from all evaluated patients, focusing on the ones that underwent our CAC-based protocol. Fractional flow reserve was performed during LHC to better ascertain stenosis’ hemodynamic significance and need for PCI. Transthoracic echocardiogram, right heart catheterizations, and brain natriuretic peptide were performed routinely to investigate heart failure and portopulmonary hypertension. All studies were performed within a period of 0.5–3 months.
Figure 1.
Coronary artery disease evaluation protocol in liver transplant candidates. CAD, coronary artery disease; CT, computed tomography; DSE, dobutamine stress echocardiogram; FFR, fractional flow reserve; LT, liver transplantation; SPECT, single-photon emission computed tomography.
Study Endpoints
Our main endpoint was detection of clinically relevant CAD with a luminal stenosis ≥70% identified during LHC. As a secondary outcome, we investigated early adverse cardiovascular events (within the initial 30 days after transplant), paying attention to acute coronary syndromes (ACS), and presence of luminal stenosis ≥50%. We also tested the usefulness of various ASCVD stratification strategies, including presence of traditional CAD risk factors (including ASCVD risk scoring), troponin I levels, DSE/SPECT, and CAC to identify asymptomatic luminal stenosis ≥70% by LHC (used as gold standard). DSE was considered indeterminate whenever target heart rate was not reached (≥85% of maximum predicted), and either DSE or SPECT could be classified as indeterminate in the presence of poor-quality imaging or artifacts. Indeterminate results were not taken into account for analysis. Calculated ASCVD risk scores were the Pooled Cohort Equations and the Multi-Ethnic Study of Atherosclerosis (MESA).15,16 High 10-year predicted ASCVD was defined as a Pooled Cohort Equations or MESA ≥ 7.5%. Two cardiologists (S.B. and Z.A.) reviewed all cardiac tests for accuracy. They evaluated each cardiac test separately without access to all cardiac test results at any given time.
Statistical Analysis
Data are presented as means with standard deviation, medians with interquartile range, or as frequency (percentage), as appropriate. Continuous variables were compared with Student's t test or Mann-Whitney rank sum test, whereas categorical were compared with Fisher's exact test, chi-square, or McNemar's test. Spearman's rho was used for correlations. Receiver operating characteristic (ROC) curve was constructed to identify the best Agatston cutoff point identifying clinically relevant CAD. A P value < 0.05 was considered statistically significant, and all statistical tests were two-tailed. Analyses were performed using Stata 12 (StataCorp, College Station, TX).
Results
We reviewed 148 unique transplant evaluations during the study period (Figure 2). However, the CAC protocol could not be followed in 24 (16%) cases because of insurance denial. The main baseline characteristics of the patients are shown in Table 1. There were no major differences between patients with and those without CAC, except for the former being older and less likely to undergo LT, in spite of similar severity of liver disease, frequency of traditional cardiovascular risk factors, and possibility of successfully completing evaluation. Reasons for not listing were noncompliance in 11 (32%), psychosocial issues in 4 (12%), cardiovascular disease in 4 (12%; 3 with CAD), noncardiovascular medical contraindication in 4 (12%), MELD score < 15 in 5 (15%), and deceased while undergoing LT evaluation in 6 (17%). Notably, when cardiovascular risk factors were aggregated in scores, ASCVD risk was significantly higher among CAC patients. The MESA score significantly predicted a lower cardiovascular risk than the ASCVD risk score, classifying a higher proportion of CAC patients as low risk and, thus, attenuating predicted cardiovascular risk (P < 0.001).
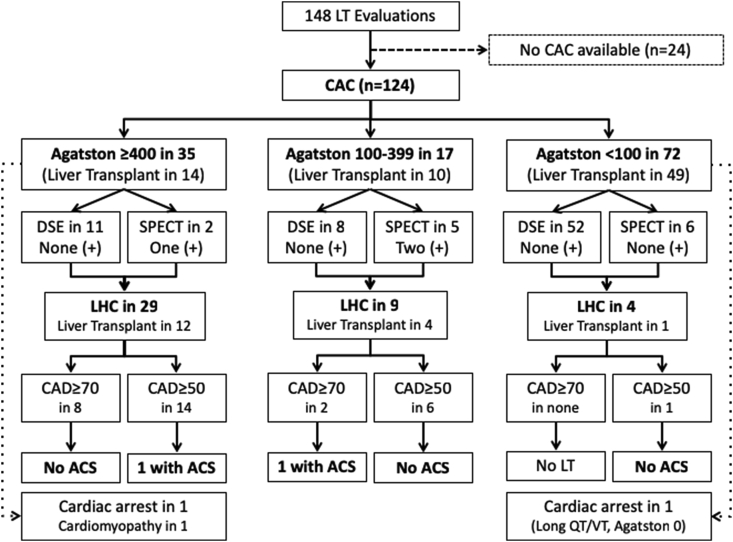
Figure 2.
Flow diagram showing coronary artery disease evaluation stratified by coronary calcium score. ACS, acute coronary syndrome; CAD, coronary artery disease; CAC, computed tomography coronary calcium score; DSE, dobutamine stress echocardiogram; LHC, left heart catheterization; LT, liver transplant; SPECT, single-photon emission computed tomography; VT, ventricular tachycardia.
Table 1.
Baseline Characteristics.
| Variable | All | CAC | No CAC | P |
|---|---|---|---|---|
| Age (years)a | 57 ± 9 | 58 ± 8 | 53 ± 12 | 0.02 |
| Sex (male)c | 89 (60%) | 73 (59%) | 16 (67%) | 0.47 |
| Ethnicity (non-Hispanic)c | 142 (96%) | 120 (97%) | 22 (92%) | 0.24 |
| Racec | ||||
|
128 (87%) | 108 (87%) | 20 (84) | 0.92 |
|
11 (7%) | 9 (7%) | 2 (8) | |
|
9 (6%) | 7 (6%) | 2 (8) | |
| BMI (kg/m2)a | 29 ± 5 | 29 ± 5 | 30 ± 6 | 0.38 |
| Cirrhosis etiologyc | ||||
|
33 (22%) | 30 (24%) | 3 (13%) | 0.12 |
|
44 (30%) | 36 (29%) | 8 (33%) | |
|
31 (21%) | 27 (22%) | 4 (17%) | |
|
13 (9%) | 12 (10%) | 1 (4%) | |
|
12 (8%) | 10 (8%) | 2 (8%) | |
|
15 (10%) | 9 (7%) | 6 (25%) | |
| Liver disease and liver transplant evaluation outcome | ||||
| MELDa | 17 ± 7 | 17 ± 6 | 17 ± 7 | 0.85 |
| MELD-Naa | 20 ± 7 | 20 ± 7 | 20 ± 7 | 0.83 |
| Hepatocellular carcinomac | 39 (26%) | 34 (27%) | 5 (21%) | 0.50 |
| Variceal bleedingc | 45 (30%) | 38 (31%) | 7 (29%) | 0.88 |
| Hepatic encephalopathyc | 92 (62%) | 77 (62%) | 15 (63%) | 0.97 |
| Ascitesc | 118 (80%) | 102 (82%) | 16 (67%) | 0.08 |
| Waitlistedc | 114 (77%) | 93 (75%) | 21 (88%) | 0.18 |
| Transplantedc | 94 (64%) | 73 (59%) | 21 (88%) | 0.008 |
| Cardiovascular risk factors | ||||
| Troponin > 0.07 | 7 (6%) | 6 (6%) | 1 (7%) | 0.86 |
| Smokingc | 74 (51%) | 61 (50%) | 13 (54%) | 0.70 |
| Obesityc | 66 (45%) | 53 (43%) | 13 (54%) | 0.31 |
| Hypertensionc | 28 (19%) | 24 (19%) | 4 (17%) | 0.75 |
| Diabetesc | 43 (29%) | 35 (28%) | 8 (35%) | 0.52 |
| Strokec | 8 (5%) | 7 (6%) | 1 (4%) | 0.76 |
| Dyslipidemiac | 31 (21%) | 27 (22%) | 4 (17%) | 0.57 |
| CKD DOQI 4/5c | 10 (7%) | 8 (6%) | 2 (8%) | 0.73 |
| Smoking >20 pack/yearc | 48 (33%) | 42 (34%) | 6 (25%) | 0.39 |
| Prior CADc | 10 (7%) | 7 (6%) | 3 (13%) | 0.22 |
| >4 METsc | 23 (16%) | 18 (15%) | 5 (21%) | 0.92 |
| Total CV risk factorsb | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.70 |
| ≥2 CV risk factorsc | 103 (70%) | 85 (69%) | 18 (75%) | 0.52 |
| ≥3 CV risk factorsc | 68 (46%) | 58 (47%) | 10 (42%) | 0.64 |
| ASCVD scoreb | 8.2 (4.4–15.8) | 8.3 (5.0–16.9) | 4.7 (1.9–11.9) | 0.05 |
| 10-year ASCVD riskc | ||||
|
41 (29%) | 29 (24%) | 12 (52%) | 0.02 |
|
26 (18%) | 24 (20%) | 2 (9%) | |
|
75 (53%) | 66 (55%) | 9 (39%) | |
| MESA scoreb | N/A | 7.1 (2.5–16.3) | N/A | N/A |
| 10-year MESA riskc | ||||
| • Low (<5%) | N/A | 48 (43%) | N/A | N/A |
|
10 (9%) | |||
|
54 (48%) | |||
ASCVD, atherosclerosis cardiovascular disease risk; ALD, alcoholic liver disease; BMI, body mass index; CAD, coronary artery disease; CAC, coronary calcium score; CKD, chronic kidney disease; HCV, hepatitis C virus; MELD, model for end-stage liver disease; MESA, Multi-Ethnic Study of Atherosclerosis; NASH, nonalcoholic steatohepatitis; N/A, not applicable.
Data presented as mean ± standard deviation.
Data presented as median (IQR).
Data presented as n (%).
In the whole cohort, there were a total of 79 (53%) DSE performed, out of which 1 (1%) was positive for ischemia, 62 (79%) were negative, and 16 (20%) indeterminate (all negative for ischemia). A total of 27 (18%) SPECT were performed, with 6 (22%) being positive for ischemia, 20 (74%) negative, and 1 (4%) indeterminate (negative for ischemia). Fifty (34%) patients underwent LHC, finding luminal stenosis ≥70% in 12 (24%) and ≥50% in 24 (48%; between 50% and 69% in 12). Of the 124 patients who had a CAC, a total of 35 (28%) had an Agatston score ≥400, 17 (14%) had scores between 100 and 399, and 72 (58%) were <100 (Figure 2). Patients with an Agatston ≥400 had less DSE/SPECT (37% vs. 76% and 79%, P < 0.001) but more LHC (83% vs. 53% and 6%, P < 0.001) than those with an Agatston of 100–399 and <100, respectively.
Figure 2 shows patient's distribution as they moved down our CAD evaluation protocol. Out of the 35 patients who had scores >400, LHC was performed in 29 (83%), finding luminal stenosis ≥70% in 8 patients, and stenosis between 50% and 69% in 6 more (stenosis ≥50% in 14). However, out of 13 patients from this group that underwent DSE/SPECT, inducible ischemia was noted only in one. In the 14 patients that underwent LT, 4 of them with stenosis ≥70% (3 previously treated with PCI), there were no posttransplant CAD complications (Table 2). Five patients with stenosis between 50% and 69% underwent LT, and one of them presented with an early ACS (no PCI before LT on the basis of normal fractional flow reserve). One patient had a cardiac arrest during LT with acute right ventricular failure not related to an ACS, and another one developed post-LT cardiomyopathy, both with a previously normal LHC. Among patients showing an Agatston score 100–399 (n = 17), DSE/SPECT were performed in 13 patients, and 2 showed positive results. Nine of the 17 (53%) patients underwent LHC, showing significant luminal stenosis ≥70% in 2 patients (both treated with PCI), and stenosis between 50% and 69% in 4 additional patients (two of these treated with PCI; ≥50% in 6). In the 10 patients that underwent LT, 1 developed an early ACS associated with in-stent restenosis after bare metal stenting to RCA before LT (Table 2). Finally, in patients with an Agatston score <100 (n = 72), DSE/SPECT was performed in 58, and none was positive for ischemia. LHC was performed in 4 patients disclosing coronary stenosis 50%–69% only in one patient, in a small vessel not amenable to PCI (Table 2). Forty-nine within this group underwent LT with no early posttransplant ACS events, including 9 patients with a favorable clinical profile not tested with DSE/SPECT or LHC. There was one case with torsades de pointes associated with prolonged QT interval and not associated to and ischemic event (Agatston of 0, no DSE/SPECT or LHC performed due to favorable clinical profile).
Table 2.
Patients With Coronary Luminal Stenosis and Percutaneous Intervention Performed During Left Heart Catheterization.
| CAC (Agatston) | Etiology | MELD/MELD-Na | Cardiac Stress Test | No. Affected Vessels | Main Affected Vessel | Maximum Obstruction | Intervention (stent type) | Post-LT Outcome |
|---|---|---|---|---|---|---|---|---|
| Significant stenosis (≥70%) | ||||||||
| 842 | EtOH | 16/27 | (−) DSE | 1 | PDA | 70% | No PCI | No ACS |
| 1809 | HCV | 9/12 | (+) SPECT | 3 | RPL | 70% | POBA | No ACS |
| 1561 | HBV | 9/9 | N/A | 3 | RCA | 80% | BMS | No ACS |
| 2024 | Crypto | 26/29 | N/A | 2 | RCA | 90% | BMS | No ACS |
| 699 | EtOH | 16/21 | (I) DSE | 1 | OM | 90% | BMS | No LT |
| 512 | NASH | 13/13 | N/A | 1 | LAD | 70% | DES | No LT |
| 1752 | EtOH | 16/18 | N/A | 3 | OM | 90% | POBA | No LT |
| 479 | Crypto | 31/33 | N/A | 1 | LCx | 70% | BMS | No LT |
| 347 | NASH | 16/19 | (+) SPECT | 3 | RCA | 95% | BMS | NSTEMI |
| 351 | HCV | 14/20 | N/A | 1 | LAD | 80% | BMS | No LT |
| Intermediate stenosis (50%–69%) | ||||||||
| 1193 | ALD | 29/34 | (−) DSE | 3 | LCx | 60% | No PCI | No ACS |
| 811 | NASH | 21/25 | N/A | 1 | LAD | 50% | No PCI | NSTEMI |
| 472 | NASH | 8/14 | N/A | 1 | RCA | 50% | No PCI | No ACS |
| 1193 | ALD | 10/15 | (I) DSE | 1 | RCA | 50% | No PCI | No ACS |
| 884 | HCV | 14/15 | (−) DSE | 3 | LAD | 60% | No PCI | No LT |
| 1123 | HBV | 7/6 | N/A | 4 | LAD | 60% | DES | No ACS |
| 315 | HCV | 9/10 | N/A | 1 | LAD | 60% | BMS | No ACS |
| 220 | HCV | 13/12 | N/A | 1 | RCA | 50% | No PCI | No LT |
| 310 | HCV | 14/14 | (−) DSE | 1 | OM | 60% | No PCI | No LT |
| 388 | Crypto | 10/10 | N/A | 1 | LAD | 60% | BMS | No LT |
| 68 | NASH | 20/26 | (I) DSE | 1 | RI | 50% | No PCI | No ACS |
CAC, coronary calcium score; DSE, dobutamine stress echocardiogram; DES, drug-eluting stent; I, indeterminate cardiac stress test; LCx, left circumflex; OM, obtuse marginal; PDA, posterior descending artery; POBA, plain old balloon angioplasty; RCA, right coronary artery; RI, ramus intermedius; RPL, right posterolateral; SPECT, single-photon emission computed tomography.
ROC curve analysis identified an Agatston score of 346 as the best cutoff point to identify significant coronary luminal stenosis (CAD ≥ 70) in our population. Usefulness of various CAD risk stratification and testing strategies was compared against CAC with an Agatston score of 346, as shown in Table 3. All 148 patients were considered for this analysis. CAC was the only test significantly associated with CAD ≥ 70 with a very high negative predictive value, whereas DSE/SPECT combined were not more useful than accounting for the presence of 3 or more classic CAD risk factors or high ASCVD risk. Optimizing CAC to include classic cardiovascular risk factors (high MESA score) negatively affected its predictive usefulness.
Table 3.
Operational Characteristics of Various Strategies to Detect Luminal Stenosis ≥70%.
| Variable | Sensitivity | Specificity | PPV | NPV | P |
|---|---|---|---|---|---|
| Troponin I | 22% | 94% | 50% | 82% | 0.14 |
| ≥2 CV Risk Factors | 92% | 14% | 26% | 83% | 0.63 |
| ≥3 CV Risk Factors | 33% | 57% | 20% | 72% | 0.54 |
| ASCVD High Risk | 83% | 32% | 30% | 85% | 0.29 |
| DSE/SPECT | 50% | 80% | 43% | 84% | 0.14 |
| MESA High Risk | 100% | 10% | 28% | 100% | 0.29 |
| CAC >346 | 100% | 31% | 31% | 100% | 0.04 |
ASCVD, atherosclerosis cardiovascular disease risk; CAC, coronary calcium score; CV, cardiovascular; DSE, dobutamine stress echocardiogram; MESA, Multi-Ethnic Study of Atherosclerosis; NPV, negative predictive value; SPECT, single-photon emission computed tomography; PPV, positive predictive value.
Discussion
Accurate cardiovascular assessment and risk estimation is a crucial step in the LT evaluation process. However, to date, there is no agreement on the best strategy to evaluate CAD risk in LT candidates. Patients with ESLD experience a unique change in hemodynamic parameters and express different cardiovascular responses when compared to the general population. Some relevant changes include an increased cardiac output, marked splanchnic and peripheral vasodilation, activation of the renin-angiotensin-aldosterone system, increased sympathetic tone and vasopressin levels, and diminished free-water excretion resulting in fluid overload.17, 18, 19, 20, 21, 22, 23 Moreover, in an attempt to reduce the risk for variceal bleeding, many patients with ESLD are placed on beta-blockers, further compromising hemodynamic homeostasis. Therefore, it is not surprising for cardiac stress tests to underperform in this population. In the case of DSE, patients might not reach target heart rate or rate-pressure product to support appropriate stress induction, and as for SPECT, pre-existing coronary vasodilation might preclude blood flow gradient generation across areas including a dominant stenosis. Sarcopenia and frailty are also prevalent in this population (20%–50%),24 thus limiting mobility and the possibility of obtaining exercise-induced cardiac stress testing. By following strict criteria, we found a significant number of indeterminate DSE/SPECT (20% and 4%, respectively) in our population, significantly affecting their predictive usefulness. These figures are within the spectrum of what has been described for patients with ESLD in the literature.3,25,26
To complicate things even further, age of LT recipients and obesity prevalence are rising, as well as the prevalence of diabetes mellitus and nonalcoholic fatty liver disease (NAFLD), thus negatively impacting the cardiovascular risk profile. With improvement in the management of LT, patient mortality associated to cardiovascular complications now poses the greatest risk in the posttransplant period, especially in patients with pre-existing CAD.27,28 Although CAD prevalence in LT candidates varies depending on the test used as well as the population, a large study assessing prevalence of CAD in LT recipients from the United States Organ Procurement and Transplantation Network database including 17,482 patients found a CAD prevalence of 7.4% in NAFLD, 2.9% in alcoholic liver disease, and 2.7% in hepatitis C.29 Similarly, in 420 subjects with alcohol- or NAFLD-related ESLD, significant CAD (≥70%) was present in 2% of patients with alcoholic liver disease and 13% of the NAFLD group.30 Another study including 161 LT candidates without a previous history of CAD evaluated with LHC found moderate to severe CAD (≥50% luminal stenosis) in 24%, and the most common associated risk factors were male gender, age >50 years, diabetes, and hypertension.31 Remarkably, our results showed a higher prevalence of moderate to severe CAD of 48% with 24% having severe disease (≥70%). This higher LHC yield is the result of the selection bias imposed by our CAC-based algorithm to stratify CAD risk and need for LHC, as 83% of patients with an Agatston score >400% underwent LHC, compared to 53% when the score was 399–100 or 6% when it was <100 (the latter representing 58% of population). It was also notable that our studied population had a high prevalence of ASCVD risk factors, including smoking, high ASCVD score, and obesity in approximately half or our patients.
With an increasing ASCVD risk among the LT population, there is an urgent need to have a more accurate CAD evaluation as part of the LT evaluation process, what takes further relevance among populations with a higher adiposity in whom finding an adequate imaging window affects cardiac stress testing validity. CAC allows ASCVD risk assessment, and it is advantageous to LT candidates through its noninvasiveness, lack of use of radio-contrast, minimal radiation exposure, conserved accuracy in patients with poor exercise capacity and increased adiposity; the method actually benefits from the use of beta-blockers which are commonly prescribed in patients with ESLD.32 Our protocol increased our confidence to pursue transplantation in many patients having an Agatston <100, thus disregarding the presence of multiple ASCVD risk factors and avoiding LHC and its potential complications. In fact, we were able to proceed with LT in selected patients with an Agatston <100 without further CAD evaluation (DSE or SPECT). After the 30-day post-LT follow-up, we did not observe a single ACS episode in the group of patients with an Agatston score <100. In support of our findings, a CAD evaluation approach including the use of CAC was recently recommended as part of a consensus document.33 Although coronary computed tomography angiography can identify both calcified and noncalcified coronary plaque4 and is as reliable as LHC for the diagnosis of CAD,34 it requires sophisticated cardiac imaging expertise, and it is more expensive than CAC. Also, it cannot be used among the growing number of ESLD patients with acute or chronic kidney injury because of the need for iodinated contrast. The applicability of coronary computed tomography angiography for evaluation of CAD in LT candidates is limited; therefore, further research is required to standardize its use and to determine its prognostic role along with CAC.35,36
When considering severe CAD (luminal stenosis ≥70%) as an endpoint in the whole cohort, CAC outperformed other strategies commonly used to assess CAD, including cardiac stress testing. Although DSE and SPECT are commonly used for cardiac evaluation of LT candidates, they have shown suboptimal to poor predictive usefulness. When used to predict cardiovascular events after LT, DSE is reported to miss up to 25% of patients with CAD,23 and more recently, it was shown to lack prognostic value.37 When compared in a cross-sectional fashion against LHC, the sensitivity and specificity to detect significant CAD was reported as 41% and 47%, respectively.38 With a similar design, SPECT has shown sensitivities between 35% and 62% and specificities between 82% and 88%, which were no different from the predictive usefulness of accounting for traditional ASCVD risk factors.39 Our results also failed to show a role for DSE/SPECT in predicting significant CAD. Neither elevated troponin I, a recently described ASCVD predictor in LT recipients,40 nor patients with a high ASCVD risk score were associated with significant CAD per LHC. However, with an Agatston score ≥346, CAC showed a sensitivity and negative predictive value of 100%, thus providing clinically useful information: A result between 0 and 345 rules out significant CAD. These results mirror findings from prior studies, where a high correlation between CAC and obstructive CAD was found, along with high negative predictive values.32,41 Moreover, CAC has also shown to predict posttransplant ASCVD complications in those with CAC > 400 (8% vs. 2%).10,11,42 Thus, pretransplant CAC serves a prognostic role after LT, and in agreement with recommendations from the American Heart Association,43 we now prescribe statins to all patients with a CAC > 100 in the early posttransplant period as part of their primary ASCVD prophylaxis, irrespective of other clinical risk factors. Surprisingly, the MESA score did not improve the predictive usefulness of CAC.
Although it is not possible to state whether PCI before LT can actually improve cardiovascular outcomes, particularly given existing literature in the non-ESLD population,44,45 we have not had any post-LT mortality since our CAC protocol started in 2014, and it made us become more aggressive with post-LT revascularization (data not shown). Moreover, our CAC-based protocol permitted us to limit the number of LHC performed as part of the LT evaluation process. Had we used stratification solely on the basis of the number of cardiovascular risk factors or ASCVD scores, we would have performed LHC in approximately half of our population, whereas within the cohort of patients having undergone CAC, we were able to limit LHC to 34% (42 out of 124). As such, the high rate of false positives still results in less unnecessary LHC when compared to risk stratification based on traditional ASCVD risk factors or scoring. Therefore, CAC might prove to be a strategy helping to decrease the number of unnecessary LHC and acquisition costs associated to LT evaluation; however, confirmatory studies are needed.
Our study had a few limitations. We could not obtain a CAC in all eligible patients, and although there were some violations to the protocol, these mostly worked in our advantage by providing us with additional data to analyze (i.e., DSE or SPECT in patients with a CAC ≥400). Not all patients underwent LT, yet, given that our primary aim was a cross-sectional comparison with LHC, we believe our results still provide a valid picture of LT evaluation in patients with ESLD. Given the design of our study, the number of patients with SPECT or DSE who underwent LHC was small, precluding a separate analysis per cardiac stress test type. Finally, although the number of patients undergoing both LHC and CAC was not large, it is the largest of its kind, and we provided a novel stratification approach to use CAC in a more rational way by considering it in conjunction with traditional risk factors. We acknowledge that larger studies are needed to confirm our findings before unequivocally recommending CAC in daily clinical practice.
In conclusion, among various cardiac evaluation strategies, CAC was the most useful single predictor of CAD in asymptomatic patients undergoing LT evaluation. Along with a structured cardiac evaluation based on ASCVD risk factors, the negative predictive value from a low CAC can reassure the transplant team about the lack of CAD, which is remarkably important for severely decompensated ESLD patients in whom DSE/SPEC lack accuracy and LHC can result in severe adverse events. Finally, CAC scores can help better triage LHC, thus avoiding unnecessary catheterizations and reducing transplant evaluation costs.
Credit authorship contribution statement
S.B. and B.L.-M. contributed to original draft preparation, visualization, and submission. M.A.-S., F.H., K.A., M.K., Z.A., and D.B.-C. contributed to investigation. M.G.-S.-de-S. and A.D.-R. contributed to supervision, project administration, reviewing, and funding acquisition.
Conflicts of interest
The authors have none to declare.
Funding
This work is partially supported by the University of Arkansas for Medical Sciences College of Medicine Clinician Scientist Program, Little Rock, Arkansas, USA.
Footnotes
This work was presented at the American Physician Scientists Association in April 2017, in Chicago, IL, USA.
References
- 1.Kristensen S.D., Knuuti J. New ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J. 2014;35:2344–2345. doi: 10.1093/eurheartj/ehu285. [DOI] [PubMed] [Google Scholar]
- 2.Martin P., DiMartini A., Feng S., Brown R., Jr., Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American association for the study of liver diseases and the American society of transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 3.Harinstein M.E., Flaherty J.D., Ansari A.H., et al. Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates. Am J Transplant. 2008;8:1523–1528. doi: 10.1111/j.1600-6143.2008.02276.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi J.M., Kong Y.G., Kang J.W., Kim Y.K. Coronary computed tomography angiography in combination with coronary artery calcium scoring for the preoperative cardiac evaluation of liver transplant recipients. BioMed Res Int. 2017;2017:4081525. doi: 10.1155/2017/4081525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald L.A., Beohar N., Wang N.C., et al. A comparison of arterial closure devices to manual compression in liver transplantation candidates undergoing coronary angiography. J Invasive Cardiol. 2003;15:68–70. [PubMed] [Google Scholar]
- 6.Sharma M., Yong C., Majure D., et al. Safety of cardiac catheterization in patients with end-stage liver disease awaiting liver transplantation. Am J Cardiol. 2009;103:742–746. doi: 10.1016/j.amjcard.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Azarbal B., Poommipanit P., Arbit B., et al. Feasibility and safety of percutaneous coronary intervention in patients with end-stage liver disease referred for liver transplantation. Liver Transplant. 2011;17:809–813. [Google Scholar]
- 8.Assy N., Djibre A., Farah R., Grosovski M., Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254:393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 9.Raval Z., Harinstein M.E., Skaro A.I., et al. 2011. Cardiovascular Risk Assessment of the Liver Transplant Candidate. [Google Scholar]
- 10.Kong Y.G., Ha T.Y., Kang J.W., Hwang S., Lee S.G., Kim Y.K. Incidence and predictors of increased coronary calcium scores in liver transplant recipients. Transplant Proc. 2015;47:1933–1938. doi: 10.1016/j.transproceed.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Kong Y.G., Kang J.W., Kim Y.K., et al. Preoperative coronary calcium score is predictive of early postoperative cardiovascular complications in liver transplant recipients. Br J Anaesth. 2015;114:437–443. doi: 10.1093/bja/aeu384. [DOI] [PubMed] [Google Scholar]
- 12.Rumberger J.A., Sheedy P.F., 3rd, Breen J.F., Schwartz R.S. Coronary calcium, as determined by electron beam computed tomography, and coronary disease on arteriogram. Effect of patient's sex on diagnosis. Circulation. 1995;91:1363–1367. doi: 10.1161/01.cir.91.5.1363. [DOI] [PubMed] [Google Scholar]
- 13.Greenland P., Bonow R.O., Brundage B.H., et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of cardiology foundation clinical expert consensus task force (ACCF/AHA writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the society of atherosclerosis imaging and prevention and the society of cardiovascular computed tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Neves P.O., Andrade J., Monção H. Coronary artery calcium score: current status. Radiol Bras. 2017;50:182–189. doi: 10.1590/0100-3984.2015.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff D.C., Lloyd-Jones D.M., Bennett G., et al. 2013. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. [Google Scholar]
- 16.The Multiethnic Study of Atherosclerosis. ScienceDirect; 2017. [Google Scholar]
- 17.Burra P., Senzolo M., Adam R., et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry) Am J Transplant. 2009;10:138–148. doi: 10.1111/j.1600-6143.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 18.Gaglio P.J., Jr., Gaglio P.J., Sr Complications in patients with alcohol-associated liver disease who undergo liver transplantation. Clin Liver Dis. 2012;16:865–875. doi: 10.1016/j.cld.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Vaillant G.E. A 60-year follow-up of alcoholic men. Addiction. 2003;98:1043–1051. doi: 10.1046/j.1360-0443.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 20.Lucey M.R. Liver transplantation for alcoholic liver disease. Nat Rev Gastroenterol Hepatol. 2014;11:300–307. doi: 10.1038/nrgastro.2013.247. [DOI] [PubMed] [Google Scholar]
- 21.Neuberger J., Adams D., MacMaster P., Maidment A., Speed M. Assessing priorities for allocation of donor liver grafts: survey of public and clinicians. BMJ. 1998;317:172–175. doi: 10.1136/bmj.317.7152.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Hogan B.J., Gonsalkorala E., Heneghan M.A. Evaluation of coronary artery disease in potential liver transplant recipients. Liver Transplant. 2017;23:386–395. [Google Scholar]
- 24.Duarte-Rojo A., Ruiz-Margain A., Montano-Loza A.J., Macias-Rodriguez R., Ferrando A., Kim W.R. Exercise and physical activity for patients with ESLD: improving functional status and sarcopenia while on the transplant waitlist. Liver Transplant. 2018;24:122–139. [Google Scholar]
- 25.Elhendy A., van Domburg R.T., Bax J.J., et al. The functional significance of chronotropic incompetence during dobutamine stress test. Heart. 1999;81:398–403. doi: 10.1136/hrt.81.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krenning B.J., Geleijnse M.L., Poldermans D., Roelandt J.R. Methodological analysis of diagnostic dobutamine stress echocardiography studies. Echocardiography. 2004;21:725–736. doi: 10.1111/j.0742-2822.2004.03161.x. [DOI] [PubMed] [Google Scholar]
- 27.Safadi A., Homsi M., Maskoun W., et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009;120:1189–1194. doi: 10.1161/CIRCULATIONAHA.108.847178. [DOI] [PubMed] [Google Scholar]
- 28.Nicolau-Raducu R., Gitman M., Ganier D., et al. Adverse cardiac events after orthotopic liver transplantation: a cross-sectional study in 389 consecutive patients. Liver Transplant. 2015;21:13–21. [Google Scholar]
- 29.Gologorsky E., Pretto E.A., Jr., Fukazawa K. Coronary artery disease and its risk factors in patients presenting for liver transplantation. J Clin Anesth. 2013;25:618–623. doi: 10.1016/j.jclinane.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Patel S., Kiefer T.L., Ahmed A., et al. Comparison of the frequency of coronary artery disease in alcohol-related versus non-alcohol-related endstage liver disease. Am J Cardiol. 2011;108:1552–1555. doi: 10.1016/j.amjcard.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Carey W.D., Dumot J.A., Pimentel R.R., et al. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation. 1995;59:859–864. [PubMed] [Google Scholar]
- 32.Kemmer N., Case J., Chandna S., Neff G.W. The role of coronary calcium score in the risk assessment of liver transplant candidates. Transplant Proc. 2014;46:230–233. doi: 10.1016/j.transproceed.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 33.VanWagner L.B., Harinstein M.E., Runo J.R., et al. Multidisciplinary approach to cardiac and pulmonary vascular disease risk assessment in liver transplantation: an evaluation of the evidence and consensus recommendations. Am J Transplant. 2018;18:30–42. doi: 10.1111/ajt.14531. official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dewey M., Zimmermann E., Deissenrieder F., et al. Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation. 2009;120:867–875. doi: 10.1161/CIRCULATIONAHA.109.859280. [DOI] [PubMed] [Google Scholar]
- 35.An J., Shim J.H., Kim S.O., et al. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: a registry-based matched case-control study. Circulation. 2014;130:1353–1362. doi: 10.1161/CIRCULATIONAHA.114.009278. [DOI] [PubMed] [Google Scholar]
- 36.Hughes D.L., Rice J.D., Burton J.R., et al. Presence of any degree of coronary artery disease among liver transplant candidates is associated with increased rate of post-transplant major adverse cardiac events. Clin Transplant. 2020;34 [Google Scholar]
- 37.Patel K.K., Young L., Carey W., et al. Preoperative dobutamine stress echocardiography in patients undergoing orthotopic liver transplantation. Clin Cardiol. 2018;41:931–935. doi: 10.1002/clc.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snipelisky D., Levy M., Shapiro B. Utility of dobutamine stress echocardiography as part of the pre-liver transplant evaluation: an evaluation of its efficacy. Clin Cardiol. 2014;37:468–472. doi: 10.1002/clc.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhutani S., Tobis J., Gevorgyan R., et al. Accuracy of stress myocardial perfusion imaging to diagnose coronary artery disease in end stage liver disease patients. Am J Cardiol. 2013;111:1057–1061. doi: 10.1016/j.amjcard.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Fussner L.A., Heimbach J.K., Fan C., et al. Cardiovascular disease after liver transplantation: when, what, and who is at risk. Liver transplantation : official publication of the American association for the study of liver diseases and the. International Liver Transplantation Society. 2015;21:889–896. [Google Scholar]
- 41.Cassagneau P., Jacquier A., Giorgi R., et al. Prognostic value of preoperative coronary computed tomography angiography in patients treated by orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2012;24:558–562. doi: 10.1097/MEG.0b013e3283522df3. [DOI] [PubMed] [Google Scholar]
- 42.McAvoy N.C., Kochar N., McKillop G., Newby D.E., Hayes P.C. Prevalence of coronary artery calcification in patients undergoing assessment for orthotopic liver transplantation. Liver Transplant. 2008;14:1725–1731. [Google Scholar]
- 43.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary. Circulation. 2018; [Google Scholar]
- 44.Poldermans D., Schouten O., Vidakovic R., et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49:1763–1769. doi: 10.1016/j.jacc.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 45.Stone G.W., Maehara A., Lansky A.J., et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]