Abstract
End-stage liver disease is characterized by massive hepatocyte death resulting in clinical decompensation and organ failures. Clinical consequences in cirrhosis are the results of the loss of functional hepatocytes and excessive scarring. The only curative therapy in advanced cirrhosis is orthotropic liver transplantation, but the clinical demand outweighs the availability of acceptable donor organs. Moreover, this also necessitates lifelong immunosuppression and carries associated risks. The liver has a huge capability for regeneration. Self-replication of quiescent differentiated hepatocytes and cholangiocytes occurs in patients with acute liver injury. Due to limited hepatocyte self-renewal capacity in advanced cirrhosis, great interest has therefore been shown in characterizing the possible role of hepatic progenitor cells and bone marrow-derived stem cells to therapeutically aid this process. Transplantation of cells from various sources that can be properly differentiated into functional liver cells or use of growth factors for ex-vivo expansion of progenitor cells is needed at utmost priority. Multiple researches over the last two decades have aided researchers in refining proliferation, differentiation, and storage techniques and understand the functionality of these cells for use in clinical practice. However, these cell-based therapies are still experimental and have to be used in trial settings.
Keywords: liver regeneration, acute liver failure, chronic liver diseases, GCSF, hepatocyte transplant
Abbreviations: Ang2, angiopoietin 2; BM, Bone marrow; BM-MNCs, bone marrow mononuclear cells; BMSC, bone marrow stem cells; DAMPs, Damage associated molecular patterns; EPCs, endothelial progenitor cells; ESRP2, epithelial splicing regulatory protein 2; HGF, hepatocyte growth factor; Hh, Hedgehog; HPC, Hepatocyte progenitor cells; [Hnf4a], Hepatocyte Nuclear Factor 4 Alpha; HSCs, hematopoietic stem cells; HybHP, hybrid periportal hepatocytes; [Mfsd2a], Major Facilitator Superfamily Domain containing 2A; MMP, matrix metalloprotease; MSCs, mesenchymal stromal cells; OLT, Orthotropic liver transplantation; PAMPs, Pathogen associated molecular patterns; SAH, severe alcoholic hepatitis; SDF1, stromal-derived factor 1; Terthigh, high Telomerase reverse transcriptase; TNFSF12, tumor necrosis factor ligand superfamily member 12
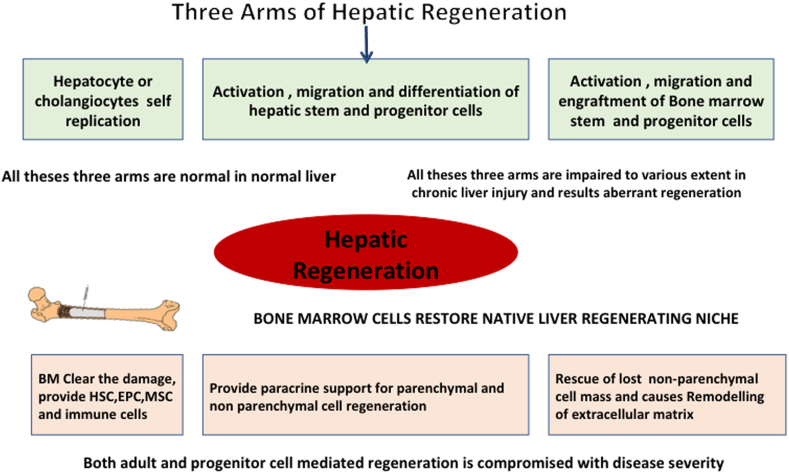
Liver has remarkable regeneration capacity. Systemic inflammation, hepatocyte death, and fibrosis with decreased matrix remodeling are the hallmark of liver cirrhosis. Progression of these changes is associated with impaired liver regeneration and risk of dysfunction and failure of organ systems. Effective and practical alternate approaches to liver transplantation are needed. The challenges to regeneration in patients with liver cirrhosis are different from those with acute hepatic injury. In cirrhosis, there is the massive deposition of extracellular matrix (ECM) and tissue scarring, which results in cellular or functional loss of regenerative niche, architectural distortions, vascular reorganization, depletion of parenchymal cells, and persistent inflammatory response, this would result in failure of engraftment and regeneration of transplanted cells. The discovery of molecular pathways in hepatic regeneration during the last two decades has opened up new vistas in the treatment of liver cirrhosis and given rise to new optimism. Cellular debris, to begin with, should be cleared and inflammation subsided. Hepatocyte progenitor cells (HPC) and bone marrow stem cells (BMSC) play an important role (Figure 1). In this paper, we review the mechanisms of liver regeneration and current therapeutic approaches for enhancing liver regenerative capacities in patients with liver cirrhosis.
Figure 1.
Three arms of hepatic regeneration in relation to chronic liver disease.
Cellular composition of liver
The understanding of the mechanisms of liver injury and the cell types involved in hepatic regeneration requires a thorough knowledge of normal liver cellular composition and architecture. The liver lobule is the basic unit of the liver. It contains cords of hepatocytes and supporting cells, including liver sinusoidal endothelial cells (LSEC), Kupffer cells (KC), cholangiocytes, hepatic stellate cells (HSC), and many other immune cells.1 The unique arrangement of hepatocytes and supporting cells in zones is essential for the wide range of functions performed by the liver. In addition, the liver also contains hepatic stems that are potentially capable of self-renewal.2 Three main types of stem cells in liver are (i) Sox9+ cells in the portal area that express both hepatic and bile duct cells lineage markers and are referred to as hybrid cells.3 These cells are primarily involved in hepatocyte regeneration after chronic liver injury. (ii) Portal area also contains hepatic progenitor cells (HPCs contain oval-shaped nuclei and sparse cytoplasm) that are capable of differentiating in hepatocytes or cholangiocytes whenever the capability of hepatocytes to sufficiently self-renew is compromised in response to injury.4 (iii) Axin2+ cells around the central vein endothelium.5 Various study have shown the distinct cell population in liver with hepatic stem cell-like properties, but whether they are facultative or liver has liver stem cells still remains controversial.6
Normal liver tissue turnover
The average life span of adult hepatocytes varies from 6 to 9 months. For explaining hepatocyte turnover, two competing hypotheses have been presented. As per the “streaming liver” hypothesis, young hepatocytes or cholangiocytes originate from hepatic stem/progenitor cells present in the portal zone.7 Young hepatocytes then subsequently migrate and mature towards the central vein.7 Based on some evidence against the streaming liver hypothesis, the “self-replicating model” was proposed.8 This model suggests that the majority of liver tissue maintenance is accomplished through hepatocytes and cholangiocyte cell division. Currently, there is debate about the role of specialized HPC in proliferation and differentiation into the hepatocytes in hepatic lobules, or every hepatocyte has the capability of repopulating the liver according to the local microenvironment.9 More recently, the pericentral diploid hepatocytes produced by endothelial cells are recognized, which have extensive proliferative capacity yielding mature hepatocytes in response to Wnt signals.5 However, a follow-up study has found that Axin2+ hepatocytes contribute little to a normal hepatocyte turnover and are limited to pericentral hepatocytes.10 Wnt-responsive hepatocytes expressing Lgr4 or Lgr5 have shown limited cell division resulting in limited hepatocyte turnover during homeostasis.11 Lin et al have recognized the specific hepatocytes with high Telomerase reverse transcriptase (Terthigh). These cells are seen scattered throughout the liver lobule without zonal dominance and are clonally capable of repopulating the liver for around a year.12 This study poses a different perspective that multiple populations of regenerating hepatocytes could maintain liver homeostasis without any distinct zonal dominance. However, there is still debate whether there is a differential proliferation of hepatocytes subsets during homeostasis. Some evidence suggests that while Major Facilitator Superfamily Domain containing 2A [Mfsd2a]− expressing periportal hepatocytes are markedly decrease in number during homeostasis,13 whereas Lgr5+ expressing pericentral hepatocytes persist for long term.11
Regeneration in normal liver
Liver regeneration is primarily accomplished through the self-replication of hepatocytes or cholangiocytes. The liver can quickly regenerate back to its former size after partial hepatic resection. The mechanism of hepatocyte regeneration varies depending on the degree of liver resection. Hepatocytes are hypertrophied when 30 percent of the liver is resected, and fast division of hepatocytes occurs to replace the hepatocyte mass when 50–70 percent of the liver is resected.14 It is unclear what regulates the hepatocytes for hypertrophy or rapid proliferation depending on different degrees of liver injury. Other cell sources such as Foxl1+,15 MIC1-1C3+,16 or CK19+17 cells have been shown to yield hepatocytes during a liver injury, but their exact role is still debatable. Some studies have reported transdifferentiation of CK19+ biliary epithelial cells into new hepatocytes and vice-versa.15,17 Few studies have shown liver regeneration to be primarily carried out by hepatocytes as opposed to other cell types.18, 19, 20 Hepatocyte damage in one zone causes a compensatory proliferative response in noninjured hepatocytes in other zones that attribute to the remarkable regenerating potential of hepatocytes in response to injury. In support of this, Pu et al had shown that when the pericentral hepatocytes are injured by CCl4, Mfsd2a+ periportal hepatocytes develop and extend in the liver.13 Periportal hepatocytes expressing Sox9 and/or Hepatocyte Nuclear Factor 4 Alpha [Hnf4a] may multiply and replace hepatocytes when pericentral hepatocytes are chronically damaged.13 Conversely, damage to periportal hepatocytes stimulates compensatory proliferation of pericentral and mid-lobular hepatocytes.20
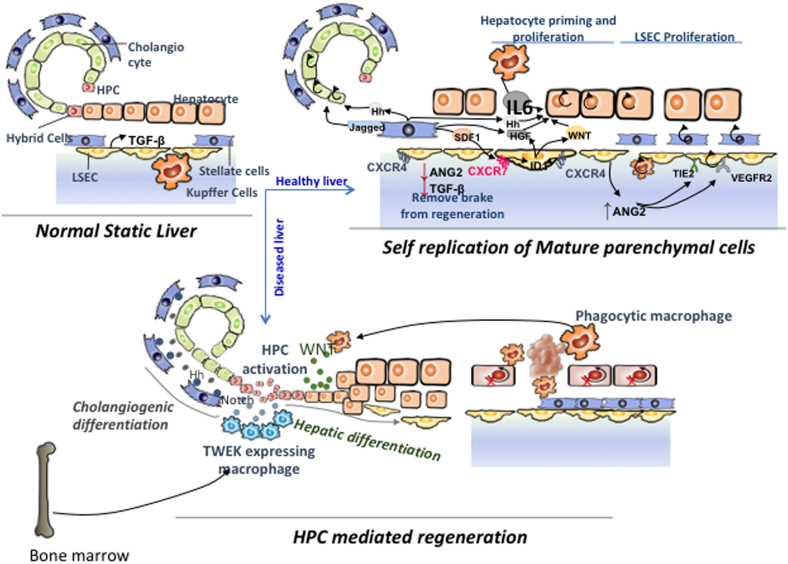
Kupffer cells are the primary hepatic macrophages in the liver that detect damage. Hepatocytes remain in a quiescent state (G0 phase) under physiological conditions, and they respond to TGF generated by LSEC by inhibiting their proliferation. KC gets activated in response to liver injury, which causes HSC and LSEC to become activated as well. Activated KC also produces IL-6 and TNF, which drive G0 hepatocytes to undergo a G0/G1 transition, making them more susceptible to future mutagenic signals.21,22 Angiopoietin 2-mediated TGF synthesis by LSEC decreases in the early stages, releasing the brake on primed hepatocytes. Further, activated HSC generates stromal derived factor 1 (SDF1), hepatocyte growth factor (HGF), Hedgehog (Hh), and Notch ligands.23 Hh and Notch ligands aid in the proliferation of cholangiocytes. LSEC having SDF1 receptors CXCR4 and CXCR7 activated produce the hepatocyte mitogens HGF and Wnt2 through ID1. Primed hepatocytes begin to rapidly multiply in response to HGF and Wnt2. Moreover, they also produce growth factors such as PDGF, VEGF, FGF1, FGF2, and SCF that aid in the regeneration of nonparenchymal cells.23 Hepatocytes proliferation is followed by KC and cholangiocytes proliferation. LSEC regains Ang2 expression later in the process.
Impaired hepatocyte regeneration in chronic liver injury and cirrhosis
Unlike after partial hepatectomy and acute liver injury where liver architecture is intact, in cirrhosis, there are marked changes in liver architecture with fibrosis. There is an increase in the fraction of senescent hepatocytes (with cell arrest at G1/S transition) and telomere shortening.24 In the rodent model, chronic ethanol exposure with partial hepatectomy significantly impair hepatocyte replication.25 Similarly, patients with alcoholic hepatitis showed a marked decrease in Ki67+ hepatocytes and an increase in HPC expansion,26 which also correlate with treatment nonresponse.27 AH patients also showed a significant reduction in cytokines and growth factors associated with liver regeneration26 and had upregulation of cell cycle inhibition. Even in NAFLD, triglycerides in hepatocytes are linked to defects in liver volumetry, suggesting regeneration impairment.28 Underlying cause of poor hepatocyte proliferation or replicative senescence of hepatocyte in cirrhosis is not clearly defined. Exacerbation of cytokine production,29 deficiency in the EGFR pathway,30 and oxidative stress30, 31, 32 also contribute to poor hepatocyte proliferation in NAFLD-related cirrhosis.
In normal hepatocytes, mitochondrial oxidative phosphorylation is the primary source of energy. With a progressive decline in mitochondrial function in cirrhosis, there is a transformation in the energy source in cirrhotic hepatocytes from oxidative phosphorylation to glycolysis.33 In advanced cirrhosis, downregulation of HNF4α (regulates the expression of glucokinase) leads to failure in maintaining glycolysis.33
Hepatic regeneration in chronic liver disease
Gut-derived endotoxins (Pathogen-associated molecular patterns, PAMPs) and DAMPs (Damage-associated molecular patterns) from direct hepatocyte injury activates KC to release IL 6 and TNF. But due to enhanced HSC mediated fibrosis that inhibits hepatocyte self-replication, the regenerative effects of TNF and IL-6 are disrupted in cirrhosis.34 Unlike patients with acute liver injury, hepatocyte self-replication (increased hepatocyte Ki67 expression) is limited. In mild Chronic Injury, hybrid periportal hepatocytes (HybHP) proliferate to regenerate the liver. By contrast, in advanced cirrhosis, both hepatocyte and HybHP are senescent, and there is a ductular proliferation in an attempt to restore liver mass.
A typical ductular reaction appears in the periportal region and is made up of HPCs, inflammatory cells, endothelial cells, and mesenchymal cells.35 HPCs are diverse, with cells having biliary or hepatoblast or stem cell markers.36 In vitro, these cells differentiate into hepatocytes and biliary cells and form hepatocyte buds.37 However, not all studies have confirmed the role of HPCs in regeneration in human liver cirrhosis.38,39 Type and extent of liver injury decide the fate of HPCs. The differentiation into intermediate hepatocytes suggests that HPCs are committed to hepatocyte lineage. This is mediated by Wnt, Notch, and fibroblast growth factor pathways.40,41 During cell death, activated macrophages promotes hepatocyte differentiation through the Wnt pathway and by inhibiting the Notch pathway.40 Hedgehog ligands also recruit macrophages that modulate HPC differentiation into hepatocytes.41 The tumor necrosis factor ligand superfamily member 12 (TNFSF12 or TWEAK) and TNFRSF12A pathway stimulated by T cells and KC is also involved in the commitment of HPCs to hepatocyte lineage in cirrhosis42,43 (Figure 2). Myofibroblasts mediated Notch ligand expression in response to chronic biliary injury induces the HPCs to differentiate into cholangiocytes. Hepatic nonparenchymal cells such as activated HSC might also actively participate in repopulating the liver.44 Activation of HPCs is also linked with an excessive fibrogenic response in liver cirrhosis.37,45,46 Studies in NASH patients have shown a positive correlation between fibrosis stage and ductular reaction.47
Figure 2.
Cellular and molecular mechanisms of hepatic regeneration in chronic liver disease.
Spontaneous recruitment of BMSC in response to liver injury in cirrhosis is limited. Exogenous G-CSF supplementation promotes the recruitment of BMSC and MSCs in the diseased liver and potentiates the regenerative response. A repeated injury also perturbs the endothelial regenerative angiocrine support. BMSC stimulates LSECs for tube formation and angiogenesis.49,50 G-CSF also mobilizes functional neutrophils to the liver. All these processes augment regeneration. On the other hand, by expressing monocyte chemoattractant protein 1 and platelet-derived growth factor, HPCs attract activated HSCs and promote fibrosis.
In alcohol-associated hepatitis, HPCs are not capable of producing mature hepatocytes, and expansion of HPC positively correlated with liver disease severity and short-term mortality.51,52 These findings support the notion that HPCs have a lesser repopulating capability than hepatocytes. We have earlier demonstrated that despite very high HPC expansion in ACLF patients, they do not contribute to the patient’s outcome, and only hepatocyte replication is associated with spontaneous recovery.53 Recently, Hyun et al54 have shown that Inflammatory cytokines generated by heavy ethanol ingestion inhibits epithelial splicing regulatory protein 2 (ESRP2) and thereby limit the transition of adult hepatocyte to progenitor-like cells. This suggests that increased HPC response in cirrhosis is not due to activation and conversion of hepatic stem cells to hepatocytes; rather, it is dedifferentiation of the remaining hepatocytes toward the progenitor cells in response to a change in the inflammatory environment of the liver that further aggravates the inflammation and fibrosis.
Bone marrow stem cell niche in advanced cirrhosis
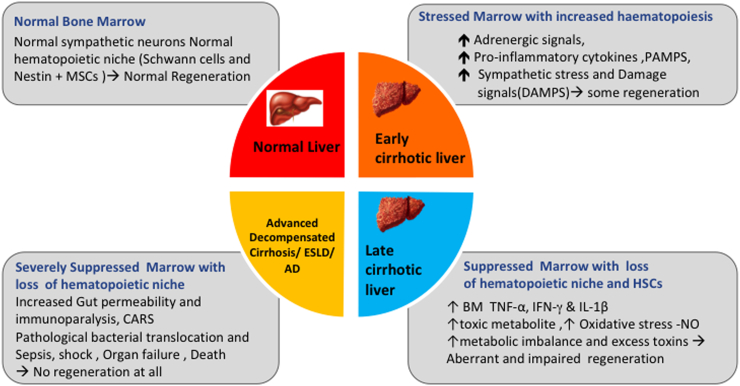
Intact BM is critical for hepatic regeneration in cirrhosis. HSCs increase in the early stages of cirrhosis and decrease with the severity of cirrhosis, regardless of cause.55 The periarteriolar niche made up of Nestin MSCs, sympathetic nerves and related Schwann cells keeps HSCs in a state of quiescence. But in cirrhosis, Nestin MSCs are lost due to the degeneration of the niche.55 Moreover, the growth factors required for HSCs differentiation in the niche are also reduced. Bone marrow nerve injury is known to impair hematopoietic regeneration.56 Alcohol-associated cirrhosis may have reduced bone marrow hematopoiesis secondary to bone marrow suppression secondary to prolonged and excess alcohol use.57 Persistent accumulation of proinammatory cytokines (TNF-a, IFN-Y, and IL-1b) and oxidative stress lead to a decline in BM-HSC pool55 (Figure 3).
Figure 3.
Bone marrow niche in relation to hepatic regeneration and severity of chronic liver disease.
Therapy for hepatic regeneration in cirrhosis
In cirrhosis, massive deposition of extracellular matrix and tissue scarring and consequent cellular or functional loss of regenerative niche, architectural distortions, vascular reorganization, persistent inflammatory response, and depletion of parenchymal cells results in failure of engraftment and regeneration of transplanted cells. Multiple researches over the last two decades have enabled researchers to understand the functionality of different sources for use in clinical practice.
Methods of regenerative therapy (Table 1)
Table 1.
Summary of Clinical Trials on Cell Therapy in Patients With Liver Cirrhosis.
| Authors and journal | N | Type of study | Indication | Type of Cell therapy | Dose and route | Outcome |
|---|---|---|---|---|---|---|
| Hepatocyte cell transplantation | ||||||
| Mito M et al, Cell Transplantation.126 1993 |
10 | Observational, Hepatocyte transplantation in man. |
Liver cirrhosis | Hepatocytes | Intraportal | Upto 11 month survival in one patient |
| Skvorak et al.127 Mol Ther. 2009 | Mice study | Open level experimental | Maple Syrup Urine Disease | Hepatocytes | Direct into liver | |
| Kobayashi et al. Cell Transplant. 2000 | Mice study | Experimental open level | Chronic Liver Failure | Hepatocytes | Spleen | |
| Trials of unsorted Bone Marrow–Derived Mononuclear Cell Transplant in Liver Disease | ||||||
| Saito et al, Stem Cell Dev,128 2011 | 5:Treatment 5:Controls |
RCT | Alcoholic Cirrhosis | BM-MNC | Single dose, peripheral vein | Improved CTP scores and INR, higher serum albumin, and total protein |
| Lyra et al, Eur Jou of Gastr Hepatol,77 2010 | 15: Treatment 15: Controls | RCT | Decompensated cirrhosis on waiting list for LT | BM-MNC | Single dose, hepatic artery | Improved serum albumin and CP score up to 90days |
| Spahr et al,129 PLoS One 2013 | 28: Treatment 30:Controls |
RCT | Decompensated cirrhosis, mean MELD score-19 | BM-MNC + GC-CSF | Single dose, hepatic artery | No significant differences between study groups |
| Trials of Sorted Hematopoietic Stem Cell Transplant in Liver Disease | ||||||
| Gordan et al,81 Stem Cell 2006 | 5 | Phase 1 open Uncontrolled trial |
Decompensated cirrhosis (Ethanol-4, HCV-1) | CD34+ | Single dose, portal vein or hepatic artery | Serum albumin and T Bil improved |
| Spahr L et al,130 Hepatology. 2008 | 11: control 13: treated | RCT | Alcoholic cirrhosis | CD 34+ | 10 μg/kg/day Subcutaneous G-CSF for 5 days. | Effective CD34+ cells mobilization; increased Hepatocyte Growth Factors |
| Levicar et al,82 Cell Prolif 2008 | 5 | Uncontrolled trial | Cirrhosis | CD34+ | Single dose, hepatic artery | Improved T Bil and CP up to 12 months, no short- and long-term side effects |
| Trials of G-CSF–Mobilized Hematopoietic Stem Cell Transplant in Patients with Liver Disease | ||||||
| Han Y et al. Cyto-therapy,131 2008 | 20: control 20: treated | Phase 2 open RCT | Decompensated cirrhosis | PBMCs from G- CSF mobilized PB | Single dose, hepatic artery Vs. peripheral vein for 4 days for HSC mobilization | GC-SF plus PBMNC group had better liver test results up to 6 month follow up, no major adverse effects |
| Shasthry SM et al,86 Hepatology. 2019 | 14:Treatment 14:Placebo |
RCT | Steroid Non responsive Severe Alcoholic Hepatitis | G-CSF | Multiple doses Subcutaneous |
Decrease in MELD, and Maddrey’s discriminant function, Infections and decreased 90-day mortality in the G-CSF arm |
| Kedarisetty CK et al.88 Gastro 2015 | 29:Treatment 26:Placebo |
Double blinded RCT | Decompensated cirrhosis | G-CSF+ Darbopoietin α | Multiple doses, Subcutaneous 4 weeks |
Improved CTP, MELD and survival at 12 month. Decreased sepsis |
| Newsome PN et al.90 Lancet Gastroenterol Hepatol. 2018 | 27: standard care 26: G-CSF 28: G-CSF plus stem-cell infusion |
Multicentre, open-label, randomized, controlled phase 2 trial | Compensated liver cirrhosis and MELD scores of 11–15·5 | G-CSF alone or G-CSF plus stem-cell infusion | G-CSF (lenograstim) at 15 μg/kg bodyweight daily for 5 consecutive days. | No significant changes in MELD score More ascites and encephalopathy in G-CSF group. |
| Philips CA.91 J Clin Exp Hepatol. 2019 | 56: GCSF, per-protocol analysis 24: Matched historical controls |
Retrospective study | Decompensated cirrhosis | G-CSF (5 μg/kg daily 5 days and every 3rd day thereafter until day 26) | Multiple doses Peripheral vein |
Compared to a matched HC group, patients receiving GCSF had higher mortality (75% vs 46%, P = 0.04) at one year. |
| De A.89 Clin Gastroenterol Hepatol. 2020 | 50: standard care 50: G-CSF |
Open-label trial | Decompensated cirrhosis | 5 days of G-CSF every 3 months | Multiple doses Peripheral vein |
GCSF- Significantly more CD34+ cells on day 6 than on day 0 (P < 0.001) Significant reductions in Child-Turcotte-Pugh and model for end-stage liver disease scores, increased ascites control, fewer infections and hospitalizations, lower liver stiffness measurements, and increased quality of life |
| Trials of G-CSF–Mobilized Hematopoietic Stem Cell Transplant in Patients with ACLF | ||||||
| Garg et al.118 Gastro 2012 | 23: Treatment 24:Placebo |
Double blinded RCT | ACLF (APASL) | G-CSF | Multiple doses Peripheral vein |
Improved MELD score, better patient survival Less sepsis, HRS and HE |
| Duan XZ87 et al. WJG 2013 | 27:Treatment 28:Placebo |
RCT | HBV related ACLF | G-CSF | Multiple doses Peripheral vein |
Increased CD34 (+) cell mobilization, improved the liver function and survival rate. |
| Singh V119 et al Am Jour Gastroenterol 2014 | 23:Treatment 23:Placebo |
Open RCT | Severe Alcoholic hepatitis | G-CSF | Multiple doses Peripheral vein |
Increased CD34 (+) cell mobilization, CTP, MELD, mDF score, and survival rate. |
| Engelmann C et al.92 J Hepatol 2021 | 88: Treatment 88: SMT |
Open-label, Plase 2 RCT | ACLF defined by EASL-CLIF criteria | G-CSF (5 μg/kg daily 5 days and every 3rd day thereafter until day 26) | Multiple doses Peripheral vein |
No improvement in overall and transplant free 90 and 360 day survival. No prevention of infection. |
| Trials of Mesenchymal Stem Cell Transplant in Liver Disease | ||||||
| Peng et al,97 Hepatology 2011 | 53: Treatment 105: Controls |
Phase 2, open, RCT | HBV Related Cirrhosis-Decompensated | BM-MSC | Single dose infusion, hepatic artery | No mortality benefit. Decreased Bilirubin, improved INR and MELD score. No complications |
| Amin MA et al, Clinical Transplantation.120 2013 | 20 | Open level, Uncontrolled trial, for safety | Post HCV child C liver cirrhosis | bone marrow derived mesenchymal stem cells | Intrasplenic injection | Decreased Bilirubin, AST, ALT, PT; improved Albumin, and INR |
| Mohamadnejad et al.96 Liv Int 2013 | 15:Treatment 12: Placebo |
RCT | Decompensated cirrhosis MELD >15 | BM-MSC | Single dose, peripheral vein | No differences between the groups |
| Liang J et al, International Journal of Rheumatic Diseases. 2017 | 26 | Open level, uncontrolled | Cirrhosis related to Autoimmune liver diseases | Allogeneic MSCs | Peripheral vein | improved MELD and liver function, without any side effect |
| El-Ansary et al.121 Stem cell rev 2012 | 15:Treatment, and 10:Controls | Phase 2, open, Uncontrolled trial | HCV-related cirrhosis and MELD score >12 | BM-MSC | Single dose, Peripheral vein | Decreased Bilirubin, improved INR, albumin, and MELD score |
| Trials of Mesenchymal Stem Cell Transplant in Patients with ACLF | ||||||
| Shi M et al. Stem Cells Transl Med,122 2012 | 24:Treatment 19: Placebo |
open-labeled and controlled | HBV related ACLF | UC-MSC | three times at 4-week intervals, Peripheral vein | Increase 90 day survival, reduced the MELD scores; increased serum albumin, and platelet counts |
| Li YH et al. Stem Cell Rev Rep,123 2016 | 11:PE + MSC 34:Only PE |
Prospective study, open-labeled | HBV related ACLF | UC-MSC | Single doses, Peripheral vein | Improves the hepatic function and survival |
| Lin BL et al124 Hepatology 2017 | 56:Treatment 54:Placebo |
open-label, RCT | HBV related ACLF | Allogeneic BM-MSC |
weekly for 4 weeks, Peripheral vein | Improved survival and liver function tests, Decrease incidence of Sepsis and multiorgan failure |
| Macrophage therapy | ||||||
| Thomas JA et al,104 Hepatology. 2011 | Experiential mice study | Mice study | Liver Cirrhosis | Macrophages | Single dose treatment | Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. |
| Bird TG et al, Proc Natl Acad Sci U S A.125 2013 | Experimental mice study | Mice study | Liver cirrhosis | Macrophage | Single dose treatment, intravascular | Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. |
Hepatocyte Replacement
This can be achieved by two predominant methods, one to completely transplant liver in the setting of decompensated cirrhosis and second cell therapy with an aim to replace senescent hepatocytes with healthy hepatocytes or its progenitor as a definitive treatment or a bridge to liver transplantation.
Orthotropic Liver Transplantation (OLT)
Liver transplant is a well-established treatment modality in advanced cirrhosis, and with improved surgical technique and organ preservation methods over time, the rate of graft failure and mortality has substantially decreased. The main issues are the limited availability of suitable organs and the high procedure cost. With respect to OLT, cell therapy is still in the experimental stage.
Hepatocyte Cell Transplantation
Hepatocytes have unique proliferative and regenerative potential. Hepatocyte cell transplantation has been studied in UGT1 enzyme deficiency (Crigler-Najjar syndrome) and low-density lipoprotein receptor deficiency (familial hypercholesterolemia). However, studies in cirrhosis are limited to small case series with variable results.58, 59, 60, 61, 62
The use of hepatocyte transplantation in practice has limitations (1) lack of suitable hepatocytes due to organ shortages, poor cell survival, and difficulties in isolation, characterization, and failure of long-term cryopreservation of cells.63 The cell numbers decrease after thawing, and the freezing process can cause loss of metabolic function and downregulation of adhesion proteins (integrin-b1 and E-cadherin).64 Optimizing cryopreservation and thawing techniques and the use of apoptosis inhibitors65 and N-acetylcysteine66,67 can improve cell quality and viability. (2) Difficulties in delivery or transfusion of the isolated hepatocytes into the liver sinusoids. The cells can be injected either through the portal vein, peripheral vein, intrasplenic or intraperitoneal route. Multiple risks are involved in cirrhosis in view of coagulopathy, portal hypertension mediated shear forces causing transplanted cell destruction, and inadvertent arterial injections into the hepatic or splenic artery leading to embolic process.68 (3) Poor engraftment of transplanted cells in the liver.
Hepatocyte Progenitor Cells (HPC)
HPCs have a high proliferative ability to differentiate into mature hepatocytes and cholangiocytes. Lgr5 in mice recognizes cells that have an HPC trait. These Lgr5+ cells may be developed into high-clonogenic-capacity organoids.69 EpCAM+/NCAM+ progenitor cells in the fetal liver can expand and differentiate into hepatocytes.70 Study of human fetal HPC transplant into patients with cirrhosis has shown clinical improvement.71 In cirrhosis, intrasplenic injection of fetal hepatocytes has shown to improve MELD score72. In the future, well-designed and adequately powered studies to demonstrate safety and efficacy while overcoming technical issues are needed.
Regenerative Niche Correction
Stem cells can be totipotent, pluripotent, multipotent, or unipotent. Stem cells are ideal for liver regeneration in cirrhosis due to their ability to divide, proliferate and differentiate into other cell types. Stem cells also provide a favorable milieu for hepatocytes cell growth. Main methodologies for stem cell-based therapies include (1) direct injection of cells, (2) in vitro differentiation to hepatocyte-like cells, and then transplantation, or (3) Ex vivo mobilization of stem cells into the regenerative niche.
Bone Marrow Stem Cell Therapy
Bone marrow (BM) is a common source of three different pluripotent cell types; hematopoietic stem cells (HSCs), mesenchymal stromal cells (MSCs), and endothelial progenitor cells (EPCs). In clinical trials, autologous BMSC transplantation had shown improved quality of life without complications.73 BMSC role in liver regeneration has been studied in a number of studies in recent years. In comparison to hepatocytes, HSCs and MSCs can be collected from the BM of living donors, lowering the chance of graft rejection.74 In recent years, the role of BMSC in liver regeneration has been explored in various trials. BM also contains macrophages that produce matrix metalloprotease (MMP) that are antifibrotic.
Unsorted BM-derived mononuclear cell (BM-MNC) transplant
Several trials and small studies have shown that autologous BM-MNCs transplantation is both safe and effective.75,76 A pilot study by Lyra et al showed that infusion of autologous BM-MNC through hepatic artery causes liver function improvement in patients with cirrhosis.77 A recent meta-analysis of 15 studies has shown the effectiveness of autologous BMSC therapy for liver improvement and coagulation in patients with liver cirrhosis. The therapeutic effect was generated at 2–4 weeks after transplantation. The effect lasted for 24 weeks but no more than 48 weeks. The greatest benefit to patients was observed with a 4 × 108 autologous BMSC transplant via the hepatic artery.78
Sorted hemopoietic stem cell transplant
CD34 is a cellular marker of HSCs. CD34+ HSC promotes repopulation of cells by fusion with hepatocytes forming hybrid cells, which helps in liver regeneration. In mouse models, purified BMSC infusion has shown improved regeneration in cirrhosis with a reduction in liver fibrosis.79 Small case series have shown improvement in liver functions with HSC therapy, whereas larger randomized controlled trials showed mixed benefits.80 A study by Gordon et al has shown improvement in serum bilirubin and albumin after CD 34+ cells injection via a portal vein or hepatic artery and with no complications.81 Levicar et al in a similar study showed that the effect lasts for about 12 months.82
G-CSF mobilized HSC therapy
G-CSF stimulates the BM to release neutrophils and CD34+ HSC into the circulation. CD133+ cells are a subset of CD34+ cells that can differentiate more easily.83 G-CSF acts as a chemoattractant and a mitogen for oval cells in vitro. G-CSF therapy results in a significant increase in oval cell reaction and liver repair.84 However, G-CSF may sometimes activate molecular pathways that can be associated with fibrosis progression.83
G-CSF is mainly studied for treatment for severe alcoholic hepatitis (SAH) and ACLF. A recent meta-analysis by Marot et al showed that as compared to controls, G-CSF treatment is related with a 70% reduction in mortality after 3 months in SAH patients but shows a beneficial role only in Asian studies.85 Shasthry et al used GCSF therapy in steroid nonresponsive SAH patients (12 doses of 300 mcg GCSF over 28 days) vs. placebo. There was no mortality benefit at day 28, whereas at day 90, there was a significant reduction in MELD, infection rates, and lower mortality with GCSF therapy.86 A randomized study in hepatitis B-related ACLF patients have shown that G-CSF therapy improved liver function and survival.87 In a randomized trial by Kedarisetty CK and colleagues, combination of G-CSF and darbopoietinα in decompensated cirrhosis was associated with survival benefit with decreased sepsis and reduction in liver severity scores as compared to placebo.88 In another randomized trial by Arke De et al, administration of multiple cycles of G-CSF increases the numbers of hematopoietic stem cells and survival of patients with decompensated cirrhosis.89
Not all studies on G-CSF in cirrhosis have shown promising results. In a multicentre, open-label, randomized, controlled trial by Newsome and colleagues in patients with compensated liver cirrhosis and MELD scores of 11·0–15·591. Treatment with subcutaneous G-CSF (lenograstim) 15 μg/kg for 5 days, or treatment with G-CSF for 5 days followed by leukapheresis and intravenous infusion of three doses of CD133-positive hemopoietic stem cells (0·2 × 10⁶ cells per kg per infusion) did not improve liver dysfunction or fibrosis and was associated with increased frequency of adverse events such as ascites. In fact, survival was shorter than what was expected in the natural history of the disease after G-CSF use in patients with decompensated cirrhosis.91 The study by Engelmann et al (GRAFT study) failed to show a significant beneficial effect of G-CSF in treating patients with acute-on-chronic liver failure.92 The use of G-CSF neither improved 3- and 12-month transplant-free survival nor lead to improvement in MELD score or reduction in the incidence of new infections. This was independent of the nature of the precipitating event, the severity of ACLF, or the type of organ failure.
Mesenchymal stem cell therapy
MSCs are multipotent fibroblast-like cells that are mostly generated from the bone marrow but can also be obtained from the umbilical cord and adipose tissue. Phase I/II studies on MSCs based therapies in liver cirrhosis had shown promising results. MSCs reduce inflammation, fibrosis and increase liver regeneration response better and rapidly than HSCs.93,94 MSCs increase MMP expression and phagocytosis, promoting the regenerative process.95
An RCT of peripheral administration of autologous MSCs showed minimal benefit in cirrhosis.96 The subsequent RCT in cirrhotics patients using HSC given via portal vein followed by peripheral BM-MSCs infusion 1 week later showed improvement in liver functions.97 In another study, MSCs given through the hepatic artery (two infusions of 50 million BM-MSCs) in alcoholic cirrhosis showed improvements in CTP score without any benefit in MELD score.98 At present, large human studies on MSC are hindered by ethical and safety concerns, lack of molecular data, and immunological mismatch.
A recent pooled analysis, including 39 studies, have concluded that MSC-based therapy is relatively safe and improves liver function during the first 6 months after administration.99 A single injection administration via the hepatic artery and MSCs derived from bone marrow are optimal in terms of improving liver function. However, the long-term efficacy of MSC therapy remains unknown.
Endothelial progenitor cell (EPC) therapy
EPCs could repair endothelial injury of hepatic sinusoids, reduce fibrosis and stimulate liver regeneration.100 EPC also has immunomodulatory effects for better homing and expansion to injured organs. EPCs were shown to be antifibrotic and capable of inducing liver regeneration in rat models of liver fibrosis by Nakamura et al.101 Kaur and colleagues showed increased levels of EPC in cirrhosis, and these cells stimulated angiogenesis in vitro.50
Macrophage Therapy
Monocyte-derived macrophages have a dual role. They recruit immune cells to the injury site and activate HSC, which promotes liver fibrosis.102 They also initiate progenitor-mediated liver regeneration and hepatocytes differentiation. Despite significant chemotactic and paracrine actions, repeated injected macrophages are required as they last in the liver for a brief time. In cirrhosis, macrophages trigger the ductular response via Tweak/FN14 signaling.103 In liver disease, BM-derived macrophages are given via venous or intrasplenic injections. Thomas et al showed reduced liver fibrosis after 4 weeks intraportal infusion of BM-derived macrophage on murine model.104
Embryonal Stem Cell (ESC) Therapy
Differentiation of cultured ESC toward hepatocyte-like cells involves the administration of several growth factors and cytokines in a sequential manner (fibroblast growth factor 2/4, bone morphogenetic protein 2/4, and activin A).105 As hepatocyte isolation is difficult, Asialoglycoprotein receptor ASGPR1 (hepatocyte-specific cell surface marker) expression-based sorting is used to yield hepatocytes.106 Despite the promising studies, there are ample ethical issues for the use of human ESCs in practice.107
iPSC Therapy (Induced Pluripotent Stem Cells (iPSCs))
iPSCs are induced pluripotent cells that are reprogrammed from adult cells using pluripotency factors (Activin A and Wnt3a) and maturation factors (hepatocyte growth factor and oncostatin-M) to form hepatocyte-like cells.108 Unlike ESC, they do not require embryonic material, and since they are autologous, they need no immunosuppression. Nowadays, for the production of functionally efficient iPSCs, excisable viral transfection, microRNA transfection, and mRNA transfection techniques are being used.109,110
Liver Support Devices and Their Role in Improving Liver Regeneration
Extracorporeal liver support devices are primarily aimed at detoxification of the liver and thereby promote the microenvironment to facilitate regeneration.111 They can be classified as (1) artificial liver support systems e.g. molecular adsorbent recirculating system (MARS), single-pass albumin dialysis (SPAD), and fractionated plasma separation and adsorption system (FPSA, Prometheus). (2) bioartificial liver support system e.g. Extracorporeal liver assist device (ELAS), Bioartificial liver assist system (BLSS), and Radial flow bioreactor (RFB). (3) Hybrid system e.g. Hybrid artificial liver assist system (TECA) and Molecular extracorporeal liver support system (MELS).111,112
Liver assist devices might be useful in a subgroup of patients with cirrhosis presenting with acute decompensation and ACLF, either as a bridge to transplant or regeneration.113,114 Albumin dialysis helps by improvement in the cardiovascular system (by increasing systemic vascular resistance index and mean arterial pressure), cerebral function (by decreasing hepatic encephalopathy and intracranial pressure), renal function (by increase in urine output and decrease in creatinine), liver function (increase in indocyanine green plasma disappearance rate, and improves others parameter) and improves the quality of life (by decreasing pruritus).115 Extremely high costs, complexity, and shortage of suitable large prospective trials have curtailed the routine use of such systems to date.
Limitations of cell-based therapies
It is unknown how a short course of therapy can have mortality benefit at three months. The homing of CD34 cells, particularly to the liver, after a peripheral mobilization, is unexplained. Some studies have documented the adverse effects related to treatment, such as new-onset ascites and portal vein thrombosis after hepatic artery infusion.116 None of the proposed regenerative therapies have long-term effects. The effect wanes over time.117 It is difficult to identify leucocytosis due to immune paresis in ACLF when G-CSF is used.
future perspective
Regenerating capacity of the normal liver is well known and this had revolutionized the concept of living donor liver transplant, where donor liver and recipient liver both get regenerated, but the same requires expertise, motivation of the donor, and other legalities. In the presence of organ shortage, high cost, and the need for life-long immunosuppression after liver transplant, various newer approaches of regenerative medicine can be helpful in the regeneration of the native liver. In patients with cirrhosis, the variations in intracellular matrix composition, paracrine effects from nonparenchymal mesenchymal cells, cytokines, and growth factors produced by inflammatory cells and bone marrow-derived stem and progenitor cells are the key elements involved in the supporting role in regeneration. Several studies conducted and still ongoing efforts are in place, which can provide mechanisms and processes to reverse the fibrosis or cirrhosis process, but all in experimental phases. G-CSF therapy seems to be the more efficient in certain group of patients with liver diseases, particularly those without organ failure and bacterial infection, but it is still a double-edged sword and intact BM is critical for hepatic regeneration in cirrhosis. Hope in positivity is the key, with constant efforts going in place despite the conflicting results.
Author contribution
AJ – Writing manuscript, critical analysis; RKJ – Compilation of data; AK – Writing manuscript.
Author disclosures
None.
Credit authorship contribution statement
Ankur Jindal: Conceptualization, Methodology, Writing – original draft, editing; Rakesh K. Jagdish: Writing – original draft, Figures, Tables; Anupam Kumar: Writing – original draft, Figure.
Conflicts of interest
The authors have none to declare.
Funding
None.
References
- 1.Krishna M. Microscopic anatomy of the liver. Clin Liver Dis. 2013;2(suppl 1):S4–S7. doi: 10.1002/cld.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrera M.B., Fonsato V., Bruno S., et al. Human liver stem cells improve liver injury in a model of fulminant liver failure. Hepatology. 2013;57:311–319. doi: 10.1002/hep.25986. [DOI] [PubMed] [Google Scholar]
- 3.Font-Burgada J., Shalapour S., Ramaswamy S., et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Canc Res. 1956;16:142–148. [PubMed] [Google Scholar]
- 5.Wang B., Zhao L., Fish M., Logan C.Y., Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pek N.M.Q., Liu K.J., Nichane M., Ang L.T. Controversies surrounding the origin of hepatocytes in adult livers and the in vitro generation or propagation of hepatocytes. Cell Mol Gastroenterol Hepatol. 2021;11:273–290. doi: 10.1016/j.jcmgh.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zajicek G., Oren R., Weinreb M., Jr. The streaming liver. Liver. 1985;5:293–300. doi: 10.1111/j.1600-0676.1985.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 8.Michalopoulos G.K. Advances in liver regeneration. Expet Rev Gastroenterol Hepatol. 2014;8:897–907. doi: 10.1586/17474124.2014.934358. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Chen L., Zern M.A., et al. The diversity and plasticity of adult hepatic progenitor cells and their niche. Liver Int. 2017;37:1260–1271. doi: 10.1111/liv.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun T., Pikiolek M., Orsini V., et al. Axin2+ pericentral hepatocytes have limited contributions to liver homeostasis and regeneration. Cell Stem Cell. 2020;26 doi: 10.1016/j.stem.2019.10.011. 97–107.e106. [DOI] [PubMed] [Google Scholar]
- 11.Ang C.H., Hsu S.H., Guo F., et al. Lgr5 +pericentral hepatocytes are self-maintained in normal liver regeneration and susceptible to hepato-carcinogenesis. Proc Natl Acad Sci USA. 2019;116:19530–19540. doi: 10.1073/pnas.1908099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin S., Nascimento E.M., Gajera C.R., et al. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. 2018;556:244–248. doi: 10.1038/s41586-018-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pu W., Zhang H., Huang X., et al. Mfsd2a+ hepatocytes repopulate the liver during injury and regeneration. Nat Commun. 2016;7:13369. doi: 10.1038/ncomms13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyaoka Y., Ebato K., Kato H., Arakawa S., Shimizu S., Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;CB 22:1166–1175. doi: 10.1016/j.cub.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Shin S., Upadhyay N., Greenbaum L.E., Kaestner K.H. Ablation of Foxl1-cre-labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. YGAST. 2015;148 doi: 10.1053/j.gastro.2014.09.039. 192–202.e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarlow B.D., Pelz C., Naugler W.E., et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raven A., Lu W.-Y., Man T.Y., et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanger K., Knigin D., Zong Y., et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Huang X.Z., He L., et al. Genetic tracing of hepatocytes in liver homeostasis, injury, and regeneration. J Biol Chem. 2017;292:8594–8604. doi: 10.1074/jbc.M117.782029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen F., Jimenez R.J., Sharma K., et al. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell. 2020;26 doi: 10.1016/j.stem.2019.11.001. 27-33.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijer C., Wiezer M.J., Diehl A.M., et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- 22.Cressman D.E., Greenbaum L.E., DeAngelis R.A., et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 23.Forbes S.J., Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 24.Aravinthan A., Scarpini C., Tachtatzis P., et al. Alexander Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J Hepatol. 2013;58:549–556. doi: 10.1016/j.jhep.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Dippold R.P., Vadigepalli R., Gonye G.E., Hoek J.B. Chronic ethanol feeding enhances miR-21 induction during liver regeneration while inhibiting proliferation in rats. Am J Physiol Gastrointest Liver Physiol. 2012;303:G733–G743. doi: 10.1152/ajpgi.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubuquoy L., Louvet A., Lassailly G., et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015;64:1949–1960. doi: 10.1136/gutjnl-2014-308410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanthier N., Rubbia-Brandt L., Lin-Marq N., et al. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J Hepatol. 2015;63:609–621. doi: 10.1016/j.jhep.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Truant S., Bouras A.F., Petrovai G., et al. Volumetric gain of the liver after major hepatectomy in obese patients: a case-matched study in 84 patients. Ann Surg. 2013;258:696–702. doi: 10.1097/SLA.0b013e3182a61a22. [DOI] [PubMed] [Google Scholar]
- 29.Leclercq L.A., Field J., Farrell G.C. Leptin-specific mechanisms for impaired liver regeneration in ob/ob mice after toxic injury. Gastroenterology. 2003;124:1451–1464. doi: 10.1016/s0016-5085(03)00270-1. [DOI] [PubMed] [Google Scholar]
- 30.de l'Hortet C., Zerrad-Saadi A., Prip-Buus C., et al. GH administration rescues fatty liver regeneration impairment by restoring GH/EGFR pathway deficiency. Endocrinology. 2014;155:2545–2554. doi: 10.1210/en.2014-1010. [DOI] [PubMed] [Google Scholar]
- 31.Gentric G., Maillet V., Paradis V., et al. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J Clin Invest. 2015;125:981–992. doi: 10.1172/JCI73957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itoh T. Stem/progenitor cells in liver regeneration. Hepatology. 2016;64:663–668. doi: 10.1002/hep.28661. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa T., Bellance N., Damm A., et al. A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J Hepatol. 2014;60:1203–1211. doi: 10.1016/j.jhep.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 35.Gouw A.S., Clouston A.D., Theise N.D. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 36.Schaub J.R., Malato Y., Gormond C., Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuramitsu K., Sverdlov D.Y., Liu S.B., et al. Popov Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol. 2013;183:182–194. doi: 10.1016/j.ajpath.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur S., Siddiqui H., Bhat M.H. Hepatic progenitor cells in action: liver regeneration or fibrosis? Am J Pathol. 2015;185:2342–2350. doi: 10.1016/j.ajpath.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Reid L.M. Paradoxes in studies of liver regeneration: relevance of the parable of the blind men and the elephant. Hepatology. 2015;62:330–333. doi: 10.1002/hep.27917. [DOI] [PubMed] [Google Scholar]
- 40.Boulter L., Govaere O., Bird T.G., et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon H., Song K., Han C., et al. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease. Hepatology. 2016;63:1155–1169. doi: 10.1002/hep.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar D., Rooge S., Kumar A., Sarin S.K. Alternatively activated M2 macrophages promotes hepatocyte differentiation in hepatic progenitor cell mediated liver regeneration in acute on chronic liver failure patients [abstract 423] Hepatology. 2014;60(suppl 1):102A. [Google Scholar]
- 43.Karaca G., Swiderska-Syn M., Xie G., et al. TWEAK/Fn14 signaling is required for liver regeneration after partial hepatectomy in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0083987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilchrist E.S., Plevris J.N. Bone marrow-derived stem cells in liver repair: 10 years down the line. Liver Transplant. 2010;16:118–129. doi: 10.1002/lt.21965. [DOI] [PubMed] [Google Scholar]
- 45.Richardson M.M., Jonsson J.R., Powell E.E., et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Williams M.J., Clouston A.D., Forbes S.J. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 47.Gomez D., Malik H.J., Bonney G.K., et al. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 49.Uda Y., Hirano T., Fujimoto J. Angiogenesis is crucial for liver regeneration after partial hepatectomy. Surgery. 2013;153:70–77. doi: 10.1016/j.surg.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 50.Kaur S., Tripathi D., Sakhuja P., Sarin S.K. Increased number and function of endothelial progenitor cells stimulate angiogenesis by resident liver sinusoidal endothelial cells (SECs) in cirrhosis through paracrine factors. J Hepatol. 2012;57:1193–1198. doi: 10.1016/j.jhep.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 51.Sancho-Bru P., Altamirano J., Rodrigo-Torres D., et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012;55:1931–1941. doi: 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- 52.Cai X., Zhai J., Kaplan D.E., et al. Wu Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular- cholangiocarcinoma. Hepatology. 2012;56:1804–1816. doi: 10.1002/hep.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shubham S., Kumar D., Rooge S., et al. Cellular and functional loss of liver endothelial cells correlates with poor hepatocyte regeneration in acute-on-chronic liver failure. Hepatol Int. 2019;13:777–787. doi: 10.1007/s12072-019-09983-y. [DOI] [PubMed] [Google Scholar]
- 54.Hyun J., Sun Z., Ahmadi A.R., et al. Epithelial splicing regulatory protein 2-mediated alternative splicing reprograms hepatocytes in alcoholic hepatitis. J Clin Invest. 2020;130:2129–2145. doi: 10.1172/JCI132691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bihari C., Anand L., Rooge S., et al. Bone marrow stem cells and its niche components are adversely affected in advanced cirrhosis of liver. Hepatology. 2016;64:1273–1288. doi: 10.1002/hep.28754. [DOI] [PubMed] [Google Scholar]
- 56.Lucas D., Scheiermann C., Chow A., et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. 2013;19:695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latvala J., Parkkila S., Niemelä O. Excess alcohol consumption is common in patients with cytopenia: studies in blood and bone marrow cells. Alcohol Clin Exp Res. 2004;28:619–624. doi: 10.1097/01.alc.0000122766.54544.3b. [DOI] [PubMed] [Google Scholar]
- 58.Sokal E.M. Treating inborn errors of liver metabolism with stem cells: current clinical development. J Inherit Metab Dis. 2014;37:535–539. doi: 10.1007/s10545-014-9691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vacanti J.P., Kulig K.M. Liver cell therapy and tissue engineering for transplantation. Semin Pediatr Surg. 2014;23:150–155. doi: 10.1053/j.sempedsurg.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Strom S.C., Fisher R.A., Thompson M.T., et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 61.Fox I.J., Chowdhury J.R., Kaufman S.S., et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 62.Hansel M.C., Gramignoli R., Skvorak K.J., et al. The history and use of human hepatocytes for the treatment of liver diseases: the first 100 patients. Curr Protoc Toxicol. 2014;62:14 12 11–14 12 23. doi: 10.1002/0471140856.tx1412s62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terry C., Dhawan A., Mitry R.R., Hughes R.D. Cryopreservation of isolated human hepatocytes for transplantation: state of the art. Cryobiology. 2006;53:149–159. doi: 10.1016/j.cryobiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Terry C., Hughes R.D., Mitry R.R., Lehec S.C., Dhawan A. Cryopreservation induced nonattachment of human hepatocytes: role of adhesion molecules. Cell Transplant. 2007;16:639–647. doi: 10.3727/000000007783465000. [DOI] [PubMed] [Google Scholar]
- 65.Terry C., Dhawan A., Mitry R.R., Lehec S.C., Hughes R.D. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transplant. 2010;16:229–237. doi: 10.1002/lt.21983. [DOI] [PubMed] [Google Scholar]
- 66.Fujita R., Chelly M., Achilles A. The effect of antioxidants and a caspase inhibitor on cryopreserved rat hepatocytes cell. Transplantation. 2005;14:391–396. doi: 10.3727/000000005783982981. [DOI] [PubMed] [Google Scholar]
- 67.Bartlett David C., Hodson James, Bhogal Ricky H., Youster Janine, Newsome Phil N. Combined use of N-acetylcysteine and Liberase improves the viability and metabolic function of human hepatocytes isolated from human liver. Cytotherapy. 2014;16:800–809. doi: 10.1016/j.jcyt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi N., Ito M., Nakamura J., et al. Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology. 2000;31:851–857. doi: 10.1053/he.2000.5636. [DOI] [PubMed] [Google Scholar]
- 69.Komori J., Boone L., DeWard A., Hoppo T., Lagasse E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat Biotechnol. 2012;30:976–983. doi: 10.1038/nbt.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huch M., Dorrell C., Boj S.F., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fomin M.E., Beyer A.I., Muench M.O. Human fetal liver cultures support multiple cell lineages that can engraft immunodeficient mice. Open Biol. 2017;7:170108. doi: 10.1098/rsob.170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khan A.A., Shaik M.V., Parveen N., et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19:409–418. doi: 10.3727/096368910X498241. [DOI] [PubMed] [Google Scholar]
- 73.Zekri A.R.N., Salama H., Medhat E., et al. The impact of repeated autologous infusion of haematopoietic stem cells in patients with liver insufficiency. Stem Cell Res Ther. 2015;6:118. doi: 10.1186/s13287-015-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Austin T.W., Lagasse E. Hepatic regeneration from hematopoietic stem cells. Mech Dev. 2003;120:131–135. doi: 10.1016/s0925-4773(02)00339-8. [DOI] [PubMed] [Google Scholar]
- 75.Terai S., Ishikawa T., Omori K., et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cell. 2006;24:2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 76.Park C.H., Bae S.H., Kim H.Y., et al. A pilot study of autologous CD34-depleted bone marrow mononuclear cell transplantation via the hepatic artery in five patients with liver failure. Cytotherapy. 2013;15:1571–1579. doi: 10.1016/j.jcyt.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 77.Lyra A.C., Soares M.B., da Silva L.F., et al. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22:33–42. doi: 10.1097/MEG.0b013e32832eb69a. [DOI] [PubMed] [Google Scholar]
- 78.Wu C.X., Wang D., Cai Y., Luo A.R., Sun H. Effect of autologous bone marrow stem cell therapy in patients with liver cirrhosis: a meta-analysis. J Clin Transl Hepatol. 2019;7:238–248. doi: 10.14218/JCTH.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vassilopoulos G., Wang P.R., Russell D.W. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 80.Moore J.K., Stutchfield B.M., Forbes S.J. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Aliment Pharmacol Ther. 2014;39:673–685. doi: 10.1111/apt.12645. [DOI] [PubMed] [Google Scholar]
- 81.Gordon M.Y., Levicar N., Pai M., et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cell. 2006;24:1822–1830. doi: 10.1634/stemcells.2005-0629. [DOI] [PubMed] [Google Scholar]
- 82.Levicar N., Pai M., Habib N.A., et al. Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell Prolif. 2008;41(suppl 1):115–125. doi: 10.1111/j.1365-2184.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Philips C.A., Augustine P., Ahamed R., et al. Role of granulocyte colony-stimulating factor therapy in cirrhosis. J Clin Transl Hepatol. 2019;7:371–383. doi: 10.14218/JCTH.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piscaglia A.C., Shupe T.D., Oh S.H., Gasbarrini A., Petersen B.E. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology. 2007;133:619–631. doi: 10.1053/j.gastro.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marot A., Singal A.K., Moreno C., Deltenre P. Granulocyte colony-stimulating factor for alcoholic hepatitis: a systematic review and meta-analysis of randomised controlled trials. JHEP Rep. 2020;2:100139. doi: 10.1016/j.jhepr.2020.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shasthry S.M., Sharma M.K., Shasthry V., Pande A., Sarin S.K. Efficacy of granulocyte colony-stimulating factor in the management of steroid-nonresponsive severe alcoholic hepatitis: a double-blind randomized controlled trial. Hepatology. 2019;70:802–811. doi: 10.1002/hep.30516. [DOI] [PubMed] [Google Scholar]
- 87.Duan X.Z., Liu F.F., Tong J.J., et al. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2013;19:1104–1110. doi: 10.3748/wjg.v19.i7.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kedarisetty C.K., Anand L., Bhardwaj A., et al. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology. 2015;148 doi: 10.1053/j.gastro.2015.02.054. 1362–1370.e7. [DOI] [PubMed] [Google Scholar]
- 89.De A., Kumari S., Singh A., et al. Multiple cycles of granulocyte colony stimulating factor increase survival times of patients with decompensated cirrhosis in a randomized trial. Clin Gastroenterol Hepatol. 2021;19:375–383.e5. doi: 10.1016/j.cgh.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 90.Newsome P.N., Fox R., King A.L., et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:25–36. doi: 10.1016/S2468-1253(17)30326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Philips C.A., Augustine P., Rajesh S., et al. Granulocyte colony-stimulating factor use in decompensated cirrhosis: lack of survival benefit and probable predisposition to hepatocellular carcinoma. J Clin Exp Hepatol. 2019 doi: 10.1016/j.jceh.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engelmann C., Herber A., Franke A., et al. Granulocyte-Colony Stimulating Factor (G-CSF) to treat acute-on-chronic liver failure, a multicenter randomized trial (GRAFT study) J Hepatol. 2021;S0168–8278 doi: 10.1016/j.jhep.2021.07.033. 1963–2. [DOI] [PubMed] [Google Scholar]
- 93.Li Q., Zhou X., Shi Y., et al. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PloS One. 2013;8 doi: 10.1371/journal.pone.0062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alfaifi M., Eom Y.W., Newsome P.N., Baik S.K. Mesenchymal stromal cell therapy for liver diseases. J Hepatol. 2018;68:1272–1285. doi: 10.1016/j.jhep.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 95.Tsuchiya A., Takeuchi S., Watanabe t., et al. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as “conducting cells” for improvement of liver fibrosis and regeneration. Inflamm Regen. 2019;39:18. doi: 10.1186/s41232-019-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mohamadnejad M., Alimoghaddam K., Bagheri M., et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490–1496. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 97.Peng L., Xie D.Y., Lin B.L., et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 98.Suk K.T., Yoon J.H., Kim M.Y., et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology. 2016;64:2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 99.Zhao L., Chen S., Shi X., et al. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res Ther. 2018;9:72. doi: 10.1186/s13287-018-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakamoto M., Nakamura T., Torimura T., et al. Transplantation of endothelial progenitor cells ameliorates vascular dysfunction and portal hypertension in carbon tetra-chloride induced rat liver cirrhotic model. J Gastroenterol Hepatol. 2013;28:168–178. doi: 10.1111/j.1440-1746.2012.07238.x. [DOI] [PubMed] [Google Scholar]
- 101.Nakamura T., Torimura T., Sakamoto M., et al. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology. 2007;133:e101. doi: 10.1053/j.gastro.2007.03.110. [DOI] [PubMed] [Google Scholar]
- 102.Wilhelm A., Shepherd E.L., Amatucci A., et al. Interaction of TWEAK with Fn14 leads to the progression of fibrotic liver disease by directly modulating hepatic stellate cell proliferation. J Pathol. 2016;239:109–121. doi: 10.1002/path.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramachandran P., Dobie R., Wilson-Kanamori J.R., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomas J.A., Pope C., Wojtacha D., et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011 Jun;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 105.Itskovitz-Eldor J., Schuldiner M., Karsenti D., et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88. [PMC free article] [PubMed] [Google Scholar]
- 106.Hay D.C., Fletcher J., Payne C., et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires Activin A and Wnt3a signaling. Proc Natl Acad Sci USA. 2008;105:12301–12306. doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Basma H., Soto-Gutiérrez A., Yannam G.R., et al. Differentiation and transplantation of human embryonic stem cell–derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyoshi N., Ishii H., Nagano H., et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 109.Yu J., Hu K., Smuga, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Warren L., Manos P.D., Ahfeldt T., et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carpentier B., Gautier A., Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58:1690–1702. doi: 10.1136/gut.2008.175380. [DOI] [PubMed] [Google Scholar]
- 112.Struecker B., Raschzok N., Sauer I.M. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11:166–176. doi: 10.1038/nrgastro.2013.204. [DOI] [PubMed] [Google Scholar]
- 113.Bañares R., Nevens F., Larsen F.S., et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153–1162. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 114.Kribben A., Gerken G., Haag S., et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:782–789. doi: 10.1053/j.gastro.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 115.Tsipotis E., Shuja A., Jaber B.L. Albumin dialysis for liver failure: a systematic review. Adv Chron Kidney Dis. 2015;22:382–390. doi: 10.1053/j.ackd.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 116.Huang X.L., Luo L., Luo L.Y., et al. Clinical outcome of autologous hematopoietic stem cell infusion via hepatic artery or portal vein in patients with end-stage liver diseases. Chin Med Sci J. 2014;29:15–22. doi: 10.1016/s1001-9294(14)60018-3. [DOI] [PubMed] [Google Scholar]
- 117.Sharma M., Kulkarni A., Sasikala M., et al. Long-term outcome of autologous hematopoietic stem cell infusion in cirrhosis: waning effect over time. J Clin Transl Hepatol. 2020;8:385–390. doi: 10.14218/JCTH.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garg V., Garg H., Khan A., et al. Granulocyte colony-stimulating factor mobilizes CD34(þ) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142 doi: 10.1053/j.gastro.2011.11.027. 505–512.e1. [DOI] [PubMed] [Google Scholar]
- 119.Singh V., Sharma A.K., Narasimhan R.L., et al. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109:1417–1423. doi: 10.1038/ajg.2014.154. [DOI] [PubMed] [Google Scholar]
- 120.Amin M.A., Sabry D., Rashed L.A., et al. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27:607–612. doi: 10.1111/ctr.12179. [DOI] [PubMed] [Google Scholar]
- 121.El-Ansary M., Mogawer S., Abdel-Aziz I., Abdel-Hamid S. Phase I trial: mesenchymal stem cells transplantation in end stage liver disease. J Am Sci. 2010;6:135–144. [Google Scholar]
- 122.Shi M., Zhang Z., Xu R., et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Y.H., Xu Y., Wu H.M., et al. Umbilical cord-derived mesenchymal stem cell transplantation in hepatitis B virus related acute-on-chronic liver failure treated with plasma exchange and entecavir: a 24-month prospective study. Stem Cell Rev Rep. 2016;12:645–653. doi: 10.1007/s12015-016-9683-3. [DOI] [PubMed] [Google Scholar]
- 124.Lin B.L., Chen J.F., Qiu W.H. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66:209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 125.Bird T.G., Lu W.Y., Boulter L., et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci U S A. 2013;110:6542–6547. doi: 10.1073/pnas.1302168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mito M., Kusano M. Hepatocyte transplantation in man. Cell Transplant. 1993;2:65–74. [PubMed] [Google Scholar]
- 127.Skvorak K.J., Paul H.S., Dorko K., et al. Hepatocyte transplantation improves phenotype and extends survival in a murine model of intermediate Maple syrup urine disease. Mol Ther. 2009;17:1266–1273. doi: 10.1038/mt.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saito T., Okumoto K., Haga H., et al. Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cell Dev. 2011;20:1503–1510. doi: 10.1089/scd.2011.0074. [DOI] [PubMed] [Google Scholar]
- 129.Spahr L., Chalandon Y., Terraz S., et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spahr L., Lambert J.F., Rubbia-Brandt L., et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221–229. doi: 10.1002/hep.22317. [DOI] [PubMed] [Google Scholar]
- 131.Han Y., Yan L., Han G., et al. Controlled trials in hepatitis B virus-related decompensate liver cirrhosis: peripheral blood monocyte transplant versus granulocyte-colony-stimulating factor mobilization therapy. Cytotherapy. 2008;10:390–396. doi: 10.1080/14653240802129901. [DOI] [PubMed] [Google Scholar]