Abstract
Fatigue is a common symptom in patients with liver disease and has a significant impact on the health-related quality of life (HR-QoL). Its pathogenesis is poorly understood and is considered multifactorial. The liver is central in the pathogenesis of fatigue because it uniquely regulates much of the production, storage, and release of substrate for energy generation. Also, the liver “cross-talks” with the key organs that are responsible for this symptom complex—gut, skeletal muscle, and brain. Fatigue can have both peripheral (i.e., neuromuscular) and central (i.e., resulting from changes in neurotransmission within the brain) components. The treatment strategies for the management of fatigue are behavioral changes and pharmacotherapy, along with dietetic intervention and exercise. However, there is no consensus on management strategies for fatigue in patients with liver disease. This article gives an overview of fatigue as a concept, its pathophysiology, measures to evaluate fatigue in patients with liver disease, the impact of fatigue on chronic liver disease, assessment of fatigue in an appropriate clinical setting, and various interventions to manage fatigue.
Keywords: chronic liver disease, central fatigue, peripheral fatigue, health-related quality of life [HR-QoL], patient-related outcomes [PRO]
Abbreviations: ACG, anterior cingulate gyrus; ADL, activities of daily living; BBB, blood-brain barrier; BNST, bed nucleus of stria terminalis; CEC, cerebral endothelial cell; CFS, chronic fatigue syndrome; CPET, cardio-pulmonary exercise testing; CRH, corticotropin release hormone; DA, dopamine; FAS, fatigue assessment scale; FIS, fatigue impact scale; FSS, fatigue severity scale; HGS, hand-grip strength; HPA, hypothalamus-pituitary-adrenal; HR-QoL, health-related quality of life; IADL, instrumental activities of daily living; iNOS, inducible nitric oxide synthase; ME, meningoencephalitis; NAFLD, nonalcoholic fatty liver disease; NM, neuromuscular; PGE2, prostaglandins; PRO, patient-reported outcomes; PROMIS-F, patient-reported outcome measure information system for fatigue; PSC, primary sclerosing cholangitis; SAMe, S-adenosyl-methionine; SN, substantia nigra; SPPB, short-physical performance battery; ME, meningo-encephalomyelitis; NO, nitric oxide; VAS-F, visual analog scalefatigue; vmPFC, ventromedial prefrontal cortex; VTA, ventral tegmental area; 6MWD, 6 min walk distance
Fatigue may be defined as any transient exercise-induced decrease in muscular force or power output, with or without task failure. Force production depends on the contraction of the muscle—the actin-myosin cross-bridge formation.1 The muscle contractility may be hindered by a number of factors, which could culminate in diminished power output. These processes may be at or beyond the neuromuscular [NM] junction, in which case the fatigue is termed as “peripheral fatigue.” When these processes are proximal to the NM junction, the fatigue is labeled as “central fatigue.”
The actin-myosin cross-bridge function is hampered by a number of factors, mostly metabolic. To enumerate a few, buildup of H+, NH4+ or iP in the muscle, alteration of Na+ -K+ pump function, impaired regulation of Ca2+ within the myocyte, buildup of K+ in the transverse-tubular system, exercise-induced muscle damage, and glycogen depletion.2
Power decrements during exercise depend on the type of exercise, as well as its duration. Typically, peripheral fatigue is witnessed in short duration [<30min] high-intensity exercises or exercises involving a single limb or exercising in a fixed position [e.g., cycling, rowing].
Central fatigue stems from a decrease in neural drive to the exercising muscles. Similar to peripheral fatigue, an array of physiological disruptions contribute to the development of central fatigue, including hypoglycemia, fluctuations in circulating neurotransmitter concentrations, arterial hypoxemia, increases in core temperature, and reductions in cortical excitability. Central fatigue is more common in long-duration exercise (>60 min) or when locomotion causes a large quantum of muscular stress (e.g. marathon running).
It has been observed that in certain clinical conditions, the diminished willingness to exert effort may occur not just during ongoing activity but also at rest. The regions of the brain involved in computing the value of effort options, and hence, making effort-based decisions are anterior cingulate gyrus [ACG}, bilateral anterior insula, and ventromedial prefrontal cortex [vmPFC]. It has been proposed that feeling of fatigue may arise from inconsistencies between beliefs about the consequences of actions and actual sensory inputs and motor outputs “performance error, metacognitive theory.” It has been suggested that brain networks that process the proprioceptive and exteroceptive signals from muscles and interoceptive signals from the internal state of the body and visceral organs [temperature, pH, lactate, glycemia, altitude etc.] could be critical for generating feelings of effort and fatigue.3
The concept that fatigue could be a disease entity in itself and not just a symptom of pathological/clinical conditions gained traction after acceptance of ME/CFS [meningo-encephalomyelitis/chronic fatigue syndrome] as a chronic complex systemic disease. ME/CFS has distinct diagnostic criteria [Institute of Medicine criteria, iom.nationalacademies.org/reports/2015/me-cfs aspx], case definition, and biological abnormalities. These are post-exertional malaise, unrefreshing sleep, cognitive impairment, and orthostatic intolerance.4
Fatigue as a construct
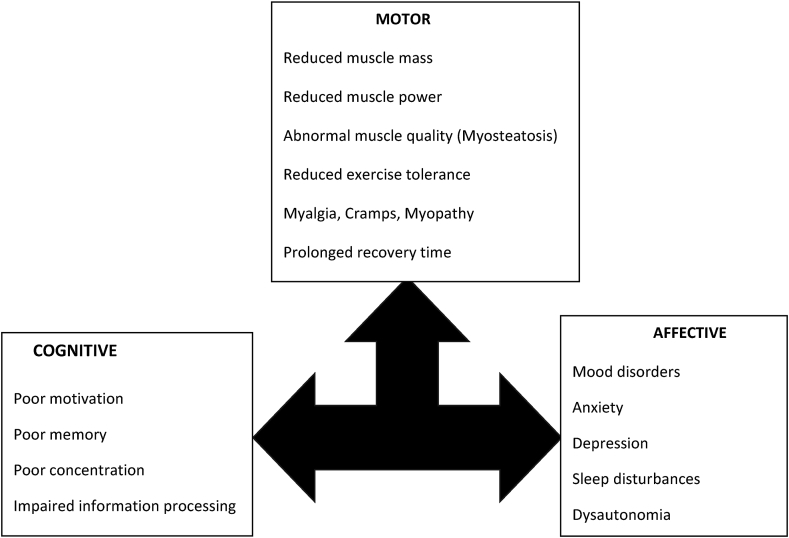
Fatigue is an abnormal perceptual state arising from abnormal effort perception. This perceptual state is experienced by all humans, albeit transiently. It has three components—motor, cognitive and affective [Figure 1]. When it is nontransient or starts to affect the quality of life of an individual, it becomes pathological. Chronic fatigue is difficult to investigate due to: (a) lack of precise definition, (b) confusing terminology [mental-fatigue, physical fatigue], (c) absence of an obvious trigger in many instances, (d) subjective nature with no reliable, objective, measurable behavior surrogate, (e) mistaken notion that it is a secondary symptom [although there is no objective proof of the same], and (f) significant overlap with other clinical symptoms like anxiety, apathy, depressive symptoms. In many disease states, one uses the diagnostic criteria for pathological fatigue given by the International Classification of Diseases 10th edition for cancer-related fatigue [Table 1].5
Figure 1.
Fatigue as a construct can be described along three axes—motor, cognitive, affective. The performance and integrity of the muscles and the neuromuscular junction is the measurable part and has received the maximum attention in recent years. The cognitive and affective domains are similar to other disease states in which fatigue is a prominent symptom. These domains are potential targets for pharmacotherapy, although large clinical trials using specific agents have not been performed in patients with chronic liver disease. Patients with advanced liver disease may have hepatic encephalopathy, which makes interpretation of cognitive and affective changes difficult.
Table 1.
Diagnosis of Pathological Fatigue.
| Necessary [all must be present] |
| Significant fatigue |
| Diminished energy |
| Increased need to rest disproportionate to level of activity |
| Anciliary [at least five of these symptoms must be present] |
| Experience of limb heaviness of generalized weakness |
| Diminished concentration or attention |
| Decreased motivation or interest to engage in usual activities |
| Insomnia or hypersomnia |
| Experience sleep as unrefreshing or nonrestorative |
| Perceived need to struggle to overcome inactivity |
| Marked emotional reactivity to feeling fatigued |
| Perceived problems with short term memory |
| Postexertional malaise for several hours |
Modified from Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment by A. Fabi. Annals of Oncology, Volume 31, Issue 6, 2020.
Pathophysiology of fatigue
The pathogenesis of fatigue is poorly understood and is complex and multifactorial. Fatigue constitutes a part of nonspecific symptom complexes such as malaise, lethargy, anorexia, listlessness, loss of social interest, and inability to concentrate, which are commonly associated with a number of disease states. These symptoms have been termed as “sickness behaviors” and are felt to be secondary to the disease process. These symptoms do not have objective clinical definitions or quantitative measures. At times, these terms are used interchangeably. As an example, malaise is commonly defined as an indefinite feeling of debility or lack of health, often accompanying the onset of illness. However, it is well known that malaise may persist even after the disappearance of the trigger that precipitated it in the first place. Any conceptual framework that is postulated to explain the genesis of fatigue as a symptom in patients with CLD needs to include the following features: (a) peripheral signaling pathways must exist that link the diseased liver to the brain, and that activation of these pathways, in turn, leads to and (b) changes in neurotransmission within the brain that gives rise to changes in behavior, culminating in the clinical expression of fatigue. It is apparent that communication pathways between the liver and the brain could result in changes in central neural activity leading to the development of subsequent sickness behaviors and associated symptoms, including fatigue.
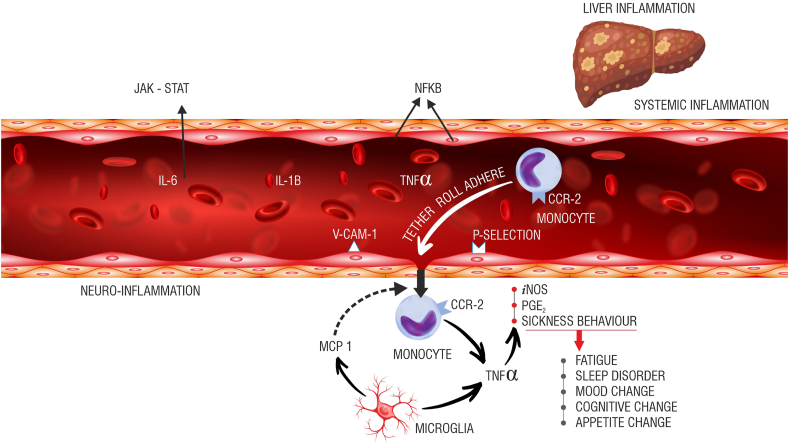
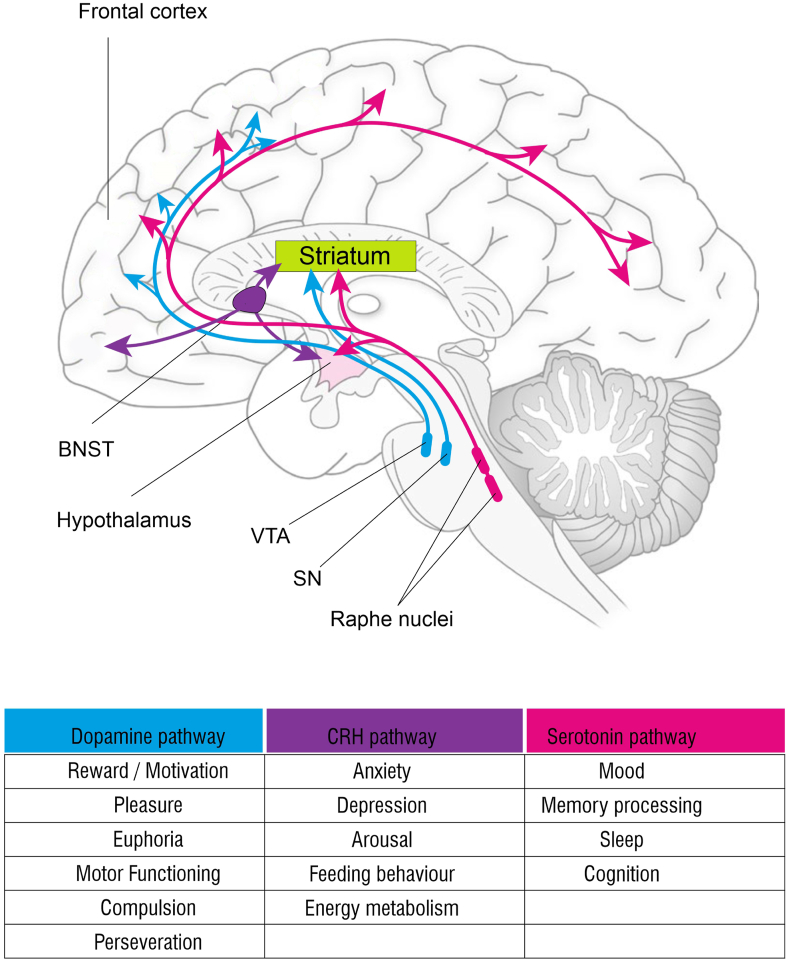
The role of inflammation in the genesis of this symptom is highlighted in Figure 2. The source of inflammation may be the liver itself [chronic viral hepatitis, alcohol use-related liver disease, NAFLD/NASH, etc.] or the portal hypertension that is a common accompaniment to cirrhosis [gut edema, increased gut permeability, altered gut-microbiome, etc.].This results in increased levels of circulating cytokines [TNF-alfa, Il-1 beta, IFN-gamma, Il-6] as highlighted in [Figure 2].6 The key central pathways that modulate fatigue are highlighted in Figure 3. The altered neurotransmission mediates both the physical component of fatigue [directional and activational aspects of movement/locomotion], as well as affective/cognitive components [motivation, reward, anxiety, depression].7, 8, 9 Experimentally, a small amount of lipo-polysaccharide injected into control subjects [which mimics the scenario in patients with cirrhosis, who have elevated LPS levels in certain situations] leads to characteristic changes on PET scan and functional MRI in the areas of the brain mentioned above—ACG, insula, and vmPFC. Therefore, it is not difficult to imagine that fatigue is a common symptom across etiologies of CLD.
Figure 2.
Potential inflammatory pathways implicated in the causation of fatigue. The vagal afferents [not shown here] innervating the liver can respond to cytokines like Il-1b, TNF-a, and Il-6. These afferents project to different parts of the brain. The cytokines may directly interact with the cerebral endothelial cells [CECs] and generate secondary signals creating a milieu of neuro-inflammation. Nitric oxide [NO] through inducible nitric oxide synthase [iNOS] and prostaglandins [PGE2] are generated as a result of this interaction. The cytokines can directly access the brain through parts of the brain, which lacks the blood-brain barrier [BBB]. It is not a coincidence that the brain’s resident immune cells [microglia] have a greater density around these areas. A complex interaction between transmigrating activated circulating monocytes [chemokine receptor-2, CCR-2 positive] and activated microglia [monocyte chemoattractant protein-1, MCP-1 secreting] amplifies the neuro-inflammation to produce sickness behaviors, including fatigue.
Figure 3.
Central pathways mediating fatigue. The key neurotransmitters are serotonin [5HT], dopamine [DA], corticotropin release hormone [CRH], norepinephrine, and endocannabinoids. The most important amongst these [5HT, DA, and CRH] are color coded. The neurotransmitters may be produced locally in the brain or gut derived [e.g., 5HT]. The same neurotransmitter may have a contrasting effect depending on its projections. The receptor subtypes [not shown here] of these neurotransmitters are also clinically relevant. For example, SSRIs [selective serotonin receptor uptake inhibitors] act on 5HT1A receptors, and ondansetron acts on 5HT3 receptors, yet both have been used clinically to treat fatigue and depression. The important projection pathways are DA: Substantia nigra [SN] and ventral tegmental area [VTA] projecting to the striatum, basal ganglia, and frontal cortex. 5HT: Raphe nucleus [brainstem] projecting to the striatum, basal ganglia, and frontal cortex. CRH: hypothalamus, bed nucleus of stria terminalis [BNST], and frontal cortex projecting to the striatum and basal ganglia and regulating DA transmission. CRH pathways are important in states of inflammation. The hypothalamus-pituitary-adrenal [HPA] axis has an important role to play in this situation. High cortisol inhibits CRH release, leading to diminished motivation and fatigue.
Fatigue in chronic liver diseases
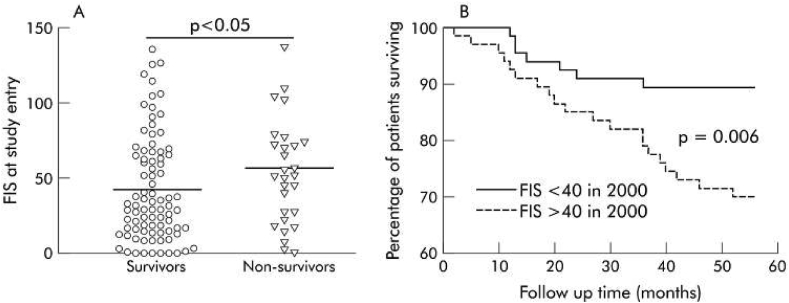
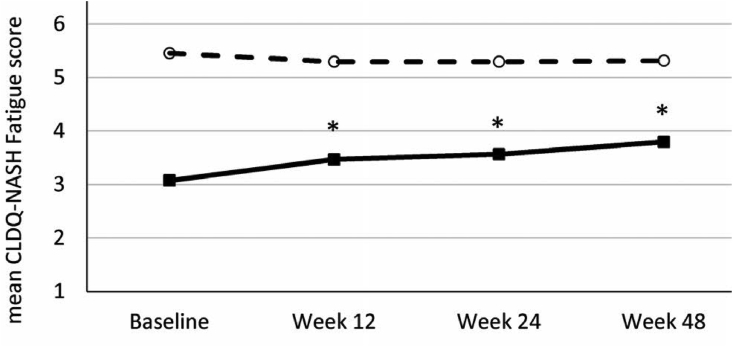
The prevalence of this symptom in the general population is ∼5% and in community practice ∼20%.10 Traditionally, fatigue has been described in patients with cholestatic liver diseases like primary biliary cholangitis [PBC] and primary sclerosing cholangitis [PSC]. In these disorders, the prevalence of fatigue might be seen in up to 50% of cases, with fatigue being a debilitating symptom in a quarter of cases.11,12 The early work on pathophysiologic pathways elucidating fatigue was described in the mouse model of cholestatic liver disease. So much so, fatigue was described as having a bearing on the survival of patients with PBC in one study performed in the United Kingdom about 15 years ago [Figure 4].13 In more recent times, fatigue has been shown to be a cardinal feature of many chronic liver diseases, including alcohol use related liver disease, chronic hepatitis C [with or without treatment, and with or without having attained sustained virologic response] drug-induced liver disease, nonalcoholic fatty liver disease [NAFLD], to name a few. Recent research has focused on health-related quality of life [HR-QoL] measurements, and this has facilitated the assessment of “sickness behavior” to a large extent. Patient-reported outcomes [PRO] have centered around the study of orphan symptoms like fatigue, pruritus, sleep disturbances, mood disorders, anxiety and depression, work performance, sickness absenteeism, etc. The commonly used assessment tools include: fatigue assessment scale [FAS], fatigue impact scale [FIS], fatigue severity scale [FSS], SF-36 questionnaire, patient-reported outcome measure information system for fatigue [PROMIS-F], visual analog scale-fatigue [VAS-F].14 As might be expected, the symptom of fatigue varies over a period of time. This is true for chronic liver diseases as also for a number of other conditions in which fatigue is a prominent symptom. In a recent evaluation of a NASH cohort, it was seen that the patients with cirrhosis [F4 fibrosis] had a higher incidence of fatigue than those with advanced fibrosis [F3 fibrosis]. However, on follow up, even the patients who were treated with placebo reported improvement in fatigue, suggesting that the symptom may be transient [Figure 5].15.
Figure 4.
Four years follow up on a geographically defined cohort of patients with PBC, [n = 136, follow up from 2000 to 2004]. A. Mean fatigue impact scale [FIS] was 36 in patients who survived [n = 108] and 55 in those who died [n = 28]. B. Patients with FIS >40/160 at enrolment had a lower survival to follow up than those whose FIS was < 40.13
Figure 5.
STELLAR trial cohort. Patients treated with placebo and followed up for 48 weeks showed spontaneous improvement in fatigue scores [Chronic liver disease questionnaire-CLDQ-NASH]. In the CLDQ-NASH, the lower the score [<4], the greater the severity of fatigue. The study highlights the transient nature of the symptom complex in a proportion of patients.15
Clinical assessment of fatigue
As described earlier, fatigue as a construct has two components: measurable, which is muscle power and performance, and intangible, which is the patient’s perceived response to the action/effort. Not just physical effort, a mental “exercise” can also induce fatigue in patients with chronic liver disease as also in a host of other medical conditions that have fatigue as a common feature. In clinical practice, the symptom is not given the importance it deserves. In patients with more advanced liver disease, the “measurable” component of the construct—sarcopenia has gained a lot of attention in recent times.16 It has been proven unequivocally that the outcome of patients with advanced liver disease is not just captured by the liver disease severity scores like the MELD score or the Child-Pugh status. At least in the context of patients evaluated for liver transplantation, the scientific community has started to look at the performance status of the patients, as also tests of frailty. Fatigue is an important component of most tests of frailty, although this term may not be used as such in all the scores. In clinical practice, muscle power and performance are assessed using hand-grip strength [HGS], short-physical performance battery [SPPB], 6 min walk distance [6MWD], and cardio-pulmonary exercise testing [CPET].17 The impact that fatigue may have on a patient’s clinical condition is readily gauged by observing or through patient-reported response, the activities of daily living [ADL], or instrumental activities of daily living [IADL].18
Management of fatigue
General principles of management
There is extensive literature regarding the management of fatigue in patients with chronic fatigue syndrome and cancer-related fatigue.19 With regards to managing fatigue in patients with liver disease, few specific interventions have shown promise. The TrACE model was developed for patients with PBC and has been used in the management of patients with liver disease of other etiologies too. It includes Treating the treatable causes of fatigue, Ameliorating the modifiable symptoms, Coping, and Empathizing20 patients’ thoughts and beliefs also contribute profoundly to central fatigue. Cognitive behaviour therapy works better in chronic fatigue syndrome, and it can be used in liver disease-associated fatigue also.21 The authors use a similar kind of approach called TRACES for patients with advanced fibrosis, cirrhosis, and liver decompensation [Table 2].
Table 2.
General Principles of Management of Fatigue [TRACES].
| Management element | Associated feature |
|---|---|
| Treat/Rectify | Sarcopenia |
| Sepsis | |
| Pruritus | |
| Hypothyroidism | |
| Hypocortisolism | |
| Cardiopulmonary issues | |
| Renal dysfunction | |
| Electrolyte imbalance | |
| Anemia | |
| Glucose control | |
| Mineral deficiency [K+, Mg2+, Ca2+, Zn] | |
| Vitamin deficiency [B1, B12, folate, Vit D] | |
| Nonselective beta-blockers | |
| Sleep disorders | |
| Depression | |
| Avoid | Smoking |
| Alcohol | |
| Hot baths | |
| Working across time-zones/shifts | |
| Packed work schedule | |
| Denial of the problem | |
| Curb | Bed rest |
| Day time naps | |
| Health drinks | |
| Weight gain | |
| Stressful situations | |
| Exercise | Aerobic |
| Resistance | |
| Structured or unstructured—hours on feet | |
| Substrate | Calories |
| Proteins | |
| Nocturnal snack | |
| Ergogenic foods |
Dietetic intervention and exercise are the cornerstones of the management of these patients. The “motor” component of the construct is easier to detect, and requisite therapy can be instituted. Much of this has to do with providing adequate macro and micro-nutrients. The standard nutrition guidelines for cirrhotics to provide 30–35 kCal/kg body weight calories and 1.2–1.5 gm/kg body weight protein along with the provision of macro/micro-nutrients should be ensured In addition, the value of late-evening snacks for these patients cannot be over-emphasized. Exercise, both aerobic [200–240 min/week] and resistance [70–105 min/week], are instituted before patients become de-conditioned. Regular physical activity and graded exercise programs should be a part of every prescription. Exercise and dietary interventions improve insulin sensitivity, reduce oxidative stress and inflammation, mobilize fat from the liver and improve mitochondrial function. Exercise also improves mood, helps in controlling blood pressure, sleep disturbances and regulates glycemia. Exercise has effects on tryptophan clearance, which plays a role in fatigue mediation.11 The effect of nutritional management and exercise institutions has not been systematically studied in patients with chronic liver disease. This might be in part due to such interventions being directed to sick cirrhotic patients, especially those wait-listed for a liver transplant. The measurable components of such interventions are: change in skeletal muscle index at L3 vertebra [sarcopenia], improvement in hand-grip strength [HGS], and frailty scores. It might be tempting to use malnutrition, sarcopenia, frailty, and fatigue interchangeably, although this is not scientifically correct. Fatigue in patients with chronic diseases tends to have a strong cognitive component [see above, fatigue as a concept].
Specific pharmacological therapies targeting central fatigue in patients with liver disease are currently not available. Treatment with serotonin receptor antagonists has shown improvement in fatigue as documented in patients with chronic hepatitis C that were treated with ondansetron, a 5-HT3 receptor antagonist. Nonspecific CNS stimulant such as modafinil [agent used for day-time somnolence] has been tried but found unsuccessful in treating fatigue.
S-adenosylmethionine (a methyl donor) is thought to work through the dopamine pathway and has been shown to mitigate symptoms of depression. S-adenosyl-methionine (SAMe) is a molecule that participates in multiple cellular reactions (transmethylation, trans-sulfuration, and amino-propylation) It is a precursor for the synthesis of glutathione. It is the principal methyl -donor in methyltransferase reactions that modify DNA, RNA, histones, and other proteins. SAMe treatment inhibits TNF-alfa production in numerous preclinical models of liver disease.22 Clinically, the evidence of efficacy is low, although some therapeutic benefit has been reported in uncontrolled studies.23
Phosphodiesterase inhibitors like pentoxifylline have been used for their anti-TNF-alfa action. However, they have not been found to be consistently useful.24 Although liver transplantation can clearly reduce the clinical impact of advanced liver disease, there is little evidence that the fatigue symptom complex is completely ameliorated after this procedure, at least in the early follow-up period.25,26
Future perspectives
Since its first elaborate description by Angelo Mosso in 1891, fatigue continues to be easy to understand the concept of a diminution in muscular force, although not as much an abnormal sensation for perceived effort. Strict criteria as described in the meningoencephalitis chronic fatigue syndrome [ME/CFS] do not exist for patients with chronic liver disease, although this problem is quite prevalent, persistent, and perplexing in this cohort of patients. Biomarkers to define the symptom complex have remained elusive. Hepatic encephalopathy and dysautonomia confound the interpretation of the cognitive and affective domains of this symptom in chronic liver disease patients. The authors have noted in their experience that there is a physical differential in fatigue in patients with chronic liver disease—upper limb <lower limbs, recumbent < upright. This is different from other disease conditions where fatigue is an important feature. The mainstay of therapy has been adequate substrate provision and building good quality muscle mass through physical exercise. Study of bio-energetics and tissue-specific energy substrate assessment [OXPHOS/ETC] hold promise as future research tools.
Credit authorship contribution statement
Dr Krishnagopal Bhandari: Conceptualization,Writing – original draft preparation. Writing- Reviewing and Editing. Dr Dharmesh Kapoor: Conceptualization,Writing – original draft preparation. Writing- Reviewing and Editing.
Conflicts of interest
The authors have none to declare.
Funding
None.
References
- 1.Huxley A.F., Simmons R.M. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- 2.Debold E.E.P. Recent insights into muscle fatigue at the cross-bridge level. Front Physiol. 2012;3:151. doi: 10.3389/fphys.2012.00151. Published 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan P.S., Chen S.X., Teh W.W., Chib V.S. Neural mechanisms underlying the effects of physical fatigue on effort-based choice. Nat Commun. 2020;11:4026. doi: 10.1038/s41467-020-17855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green C.R., Cowan P., ELK R., o'Neil K.M., Rasmussen A.L. National institute of health pathways to prevention workshop: advancing the research on myalgic encephalomyelitis/chronic fatigue syndrome. Ann Intern Med. 2015;16:860–865. doi: 10.7326/M15-0338. 1629120. [DOI] [PubMed] [Google Scholar]
- 5.Fabi A., Bhargava R., Fatigoni S., et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol. 2020;31 doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 6.D'Mello C., Swain M.G. Liver-brain inflammation axis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G749–G761. doi: 10.1152/ajpgi.00184.2011. [DOI] [PubMed] [Google Scholar]
- 7.Burak K.W., Le T., Swain M.G. Increased sensitivity to the locomotor-activating effects of corticotropin-releasing hormone in cholestatic rats. Gastroenterology. 2002;122:681–688. doi: 10.1053/gast.2002.31878. [DOI] [PubMed] [Google Scholar]
- 8.Crofford L.J., Demitrack M.A. Evidence that abnormalities of central neurohormonal systems are key to understanding fibromyalgia and chronic fatigue syndrome. Rheum Dis Clin N Am. 1996:267–284. doi: 10.1016/s0889-857x(05)70272-6. [DOI] [PubMed] [Google Scholar]
- 9.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatr. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 10.Adams R.D., Victor M., Ropper A.H. In: Principles of Neurology. 6 th. Adams R.D., et al., editors. McGraw-Hill; New York: 1997. Fatigue, asthenia, anxiety and depressive reactions; pp. 497–507a. [Google Scholar]
- 11.Huet P.M., Deslauriers J., Tran A., Faucher C., Charbonneau J. Impact of fatigue on the quality of life in patients with primary biliary cirrhosis. Am J Gastroenterol. 2000;95:760–767. doi: 10.1111/j.1572-0241.2000.01857.x. [DOI] [PubMed] [Google Scholar]
- 12.Cauch-Dudek K., Abbey S., Stewart D.E., Heathcote E.J. Fatigue in primary biliary cirrhosis. Gut. 1998;43:705–710. doi: 10.1136/gut.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones D.E., Bhala N., Burt J., Goldblatt J., Prince M., Newton J.L. Four year follow up of fatigue in a geographically defined primary biliary cirrhosis patient cohort. Gut. 2006;55:536–541. doi: 10.1136/gut.2005.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber L.H., Weinstein A.A., Mehta R., Younossi Z.M. Importance of fatigue and its measurement in chronic liver disease. World J Gastroenterol. 2019;25:3669–3683. doi: 10.3748/wjg.v25.i28.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi Z.M., Wai-Song Wong V., Anstee M.Q., et al. Fatigue and pruritus in patients with advanced fibrosis due to non-alcoholic steato-hepatitis: the impact on patient reported outcomes. Hepatol Comm. 2020;4:1637–1650. doi: 10.1002/hep4.1581. [liver disease] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasarathy S., Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–1244. doi: 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai J.C., Feng S., Terrault N.A., Lizola B., Hayssen H., Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav A., Chang Y.-H., Carpenter S., et al. Relationship between sarcopenia, six-minute walk distance and health-related quality of life in liver transplant candidates. Clin Transpl. 2015;29:134–141. doi: 10.1111/ctr.12493. [DOI] [PubMed] [Google Scholar]
- 19.Hilfiker R., Meichtry A., Eicher M., et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating a indirect-comparisons meta-analysis. Br J Sports Med. 2018;52:651–658. doi: 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swain M.G., Jones D.E.J. Fatigue in chronic liver disease: new insights and therapeutic approaches. Liver Int. 2019;39:6–19. doi: 10.1111/liv.13919. [DOI] [PubMed] [Google Scholar]
- 21.Swain M.G. Fatigue in chronic disease. Clin Sci (Lond). 2000;99:1–8. [PubMed] [Google Scholar]
- 22.Ara A.I., Xia M., Ramani K. S-adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–1666. doi: 10.1002/hep.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noureddin M., Sander-Struckmeier S., Mato J.M. Early treatment efficacy of S-adenosylmethionine in patients with intrahepatic cholestasis: a systematic review. World J Hepatol. 2020;12:46–63. doi: 10.4254/wjh.v12.i2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Wagner Lisa B., Koppe Sean W.P., Brunt Elizabeth M. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277–286. [PubMed] [Google Scholar]
- 25.Carbone M., Bufton S., Monaco A., Griffiths L., Jones D.E., Neuberger J.M. The effect of liver transplantation on fatigue in patients with primary biliary cirrhosis: a prospective study. J Hepatol. 2013;59:490–494. doi: 10.1016/j.jhep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Kalaitzakis Evangelos, Josefsson Axel, Castedal Maria, et al. Factors related to fatigue in patients with cirrhosis before and after liver transplantation. Clin Gastroenterol Hepatol. 2012;10:174–181.e1. doi: 10.1016/j.cgh.2011.07.029. 1016. [DOI] [PubMed] [Google Scholar]