Abstract
Preeclampsia (PE) is associated with maternal and fetal perinatal morbidity and mortality, which brings tremendous suffering and imposes an economic burden worldwide. The failure of uterine spiral artery remodeling may be related to the abnormal function of trophoblasts and lead to the occurrence and progression of PE. Aberrant expression of long non-coding RNAs (lncRNAs) is involved in the failure of uterine spiral artery remodeling. However, the regulation of lncRNA expression in PE is poorly characterized. Here, we reported that hypoxia-induced microRNA (miR)-218 inhibited the expression of lncRNA TUG1 by targeting FOXP1. Further RNA sequencing and mechanism analysis revealed that silencing of TUG1 increased the expression of DNA demethylase TET3 and proliferation-related DUSP family, including DUSP2, DUSP4, and DUSP5, via binding to SUV39H1 in the nucleus. Moreover, TUG1 modulated the DUSP family in vitro through a TET3-mediated epigenetic mechanism. Taken together, our results unmask a new regulatory network mediated by TUG1 as an essential determinant of the pathogenesis of PE, which regulates cell growth and possibly the occurrence and development of other diseases.
Keywords: preeclampsia, TUG1, TET3, FOXP1, miR-218, hypoxia, DUSP family
Graphical abstract
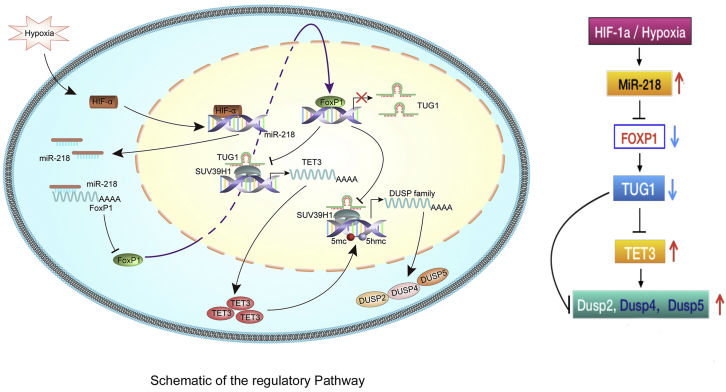
Xu et al. provide novel evidence supporting lncRNA TUG1 as a link between hypoxia and the development of PE. HIF-1α regulated miR-218, which then inhibited FOXP1 expression. FOXP1 activated TUG1, which epigenetically inhibited the expression of TET3 and DUSPs via recruitment of SUV39H1. These findings highlight the importance of TUG1-mediated regulatory network in PE.
Introduction
Preeclampsia (PE) is a pregnancy-specific disorder that originates from the placenta, characterized by new onset of hypertension after 20 weeks of pregnancy.1 It occurs in approximately 5% of pregnant and lying-in women, leading to about 60,000 maternal and fetal deaths worldwide per year.2,3 The only way to cure PE is to induce delivery. Other clinical or experimental strategies are not effective for the treatment of patients with PE.4 The pathologic mechanism of PE (also known as placental dysfunction-mediated syndrome) has not been fully elucidated. The identification of new biomarkers for the prediction, diagnosis, and treatment of PE may improve maternal and infant outcomes.
In the first trimester of pregnancy, extravillous trophoblasts (EVTs) invade maternal decidua, causing spiral artery remodeling (SAR) into highly dilated, low-resistance vessels. SAR ensures high blood flow to the uteroplacental bed.5 However, the function of EVTs in patients with PE is impaired, resulting in arterial stenosis and an increased number of reactive vessels.6 Defective SAR contributes to the development of placental diseases, including hypoxia7 and/or imbalanced placental angiogenesis.8 These pathological changes lead to endothelial dysfunction and the occurrence of PE.9,10 Moreover, aberrant expression of placental genes has been shown to cause trophoblast dysfunction.11 The regulatory roles of these genes in PE remain to be elucidated.
Previous studies of PE focused mainly on the dysregulation of protein-coding genes, which may be used as targets for clinical diagnosis and treatment. However, protein-coding sequences account for less than 2% of the human genome.12 Long non-coding RNAs (lncRNAs) are a group of non-coding RNA transcripts that are more than 200 kb in length. Technological advances enable the investigation of lncRNAs in various human diseases.13,14 LncRNAs are implicated in a variety of physiological and pathological processes,15 such as cell metabolism,16 disease-related development,17,18 cell growth,19 and others. In addition, aberrant lncRNA expression has been reported to positively or negatively regulate the genes related to different human diseases, including PE. Many studies have shown that lncRNAs regulate downstream genes via different mechanisms, including transcriptional, post-transcriptional, and chromatin modifications.20 For instance, lncRNA HOXA11-AS is implicated in the transcriptional process of RND3 by recruiting polycomb repressive complex 2 and lysine-specific histone demethylase 1 and in the post-transcriptional process of HOXA7 by competing for microRNA (miR)-15b-5p, which inhibit trophoblast proliferation and migration in PE.21
LncRNA TUG1 plays an important role in embryonic development and tumorigenesis.22 TUG1 encodes a 7.6-kb-long polyadenylated lncRNA, which is distributed mainly in the nucleus. Abnormal expression of TUG1 has been reported in various diseases, including PE.23,24 Our previous study showed that TUG1 was downregulated in the placental tissues of PE patients compared with a control group. Also, in vitro experiments demonstrated that TUG1 bound to the enhancer of zeste 2 polycomb repressive complex 2 subunit, which recruited H3K27 to inhibit the transcription of RND3, thereby inhibiting trophoblast growth and migration in PE.25 We planned to explore the underlying regulatory mechanism of TUG1 in the pathogenesis of PE.
In this study, we found that hypoxia-induced miR-218 inhibited the expression of FOXP1 at the post-transcriptional level and then inhibited the expression of TUG1. High-throughput sequencing analysis revealed that silencing of TUG1 upregulated the expression of DNA demethylase TET3 and the DUSP family, including DUSP2, DUSP4, and DUSP5, via binding to suppressor of variegation 39 homolog 1 (SUV39H1) in the nucleus. In addition, TUG1 modulated the DUSP family in vitro through a TET3-dependent epigenetic mechanism. Taken together, we proposed a new TUG1-related pathway that regulated cell growth and migration in PE and may also be involved in the occurrence and progression of other diseases.
Results
TUG1 epigenetically regulates the expression of TET3 and the DUSP family by recruiting SUV39H1
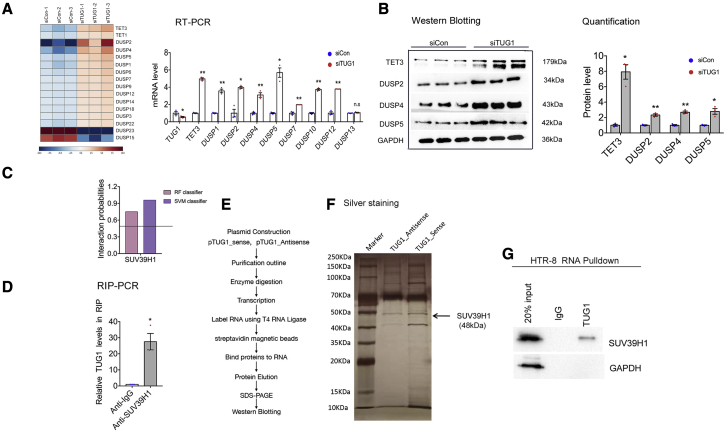
In a previous study we determined that TUG1 can affect trophoblasts’ biological function, including cell growth, migration, and network in vitro, further promoting the progression of preeclampsia.25 TUG1 can modulate trophoblast function by regulating the expression of multiple genes. To investigate the role of the TUG1-associated pathway in PE, we performed RNA sequencing analysis and generated gene expression profiles of HTR-8/SVneo cells transfected with siCon or small interfering RNA (siRNA) against TUG1 (Figure 1A). The most differentially expressed genes were DNA demethylase TET3 and proliferation-related DUSP family members, such as DUSP2, DUSP4, and DUSP5 (Tables S6 and S7). Wu et al.26 demonstrated that TET3 plays a critical role in epigenetic reprogramming of human embryo, and TET3 deficiency in the villus of human embryo might be associated with pregnancy-related diseases. However, the underlying regulatory mechanism of TET3 in PE remains unclear. Here, we transfected HTR-8/SVneo cells with siRNA targeting TUG1 and investigated the effects of TUG1 knockdown on the expression of TET3 and the DUSP family. The results showed that downregulation of TUG1 increased the expression of TET3, DUSP2, DUSP4, and DUSP5 at both mRNA (Figure 1A) and protein (Figure 1B) levels.
Figure 1.
TUG1 inhibits the expression of TET3 and the DUSP family
(A) Mean-centered, hierarchical clustering of transcripts altered in siCon-treated cells and siRNA-TUG1-treated trophoblasts (left panel). HTR-8/SVneo trophoblasts were transfected with siCon or siTUG1. Quantitative real-time PCR was used to measure the mRNA expression of genes at 48 h post-transfection (right panel). (B) Protein expression was detected using western blot at 48 h after transfection. (C) Bioinformatics analysis predicted the interaction possibility of TUG1. A probability of >0.5 was considered positive. The RPISeq prediction was based on support vector machine or random forest. (D) RIP assay was performed, followed by quantitative real-time PCR analysis of co-precipitated RNA. (E) Schematic outline of the purification of TUG1-associated ribonucleoproteins and protein identification. (F) Silver staining gel with a protein molecular weight marker (in kilodaltons) labeled on the left. Black arrows indicate the protein bands of SUV39H1. (G) In vitro-transcribed pull-down assay revealed that TUG1 recruited SUV39H1 protein in HTR-8/SVneo cells, but not GAPDH (negative control). All experiments were repeated at least three times.
We further explored the mechanisms underlying TUG1-mediated regulation in trophoblasts. We have previously reported that approximately 70% of TUG1 is located in the nuclei of trophoblasts,25 indicating that TUG1 might play a key role in transcriptional regulation. Bioinformatics analysis (http://pridb.gdcb.iastate.edu/RPISeq/references.php) was then performed to predict potential RNA-binding proteins. As shown in Figure 1C, TUG1 may interact with a panel of chromatin modifier SUV39H1 (H3K9me3) in trophoblasts. To examine the potential interaction between TUG1 and SUV39H1, we performed RNA immunoprecipitation (RIP) coupled with qPCR and found substantial enrichment of SUV39H1 in HTR-8/SVneo cells (Figure 1D). Next, we constructed TUG1-sense/TUG1-antisense plasmids and performed an RNA pull-down assay. The purified ribonucleoproteins were separated using SDS-PAGE followed by silver staining (Figures 1E and 1F). Western blot analysis of affinity-purified ribonucleoproteins confirmed the interaction of SUV39H1 with TUG1-sense but not with IgG (Figure 1G). Collectively, it can be concluded that TUG1 interacts with SUV39H1 in human trophoblasts.
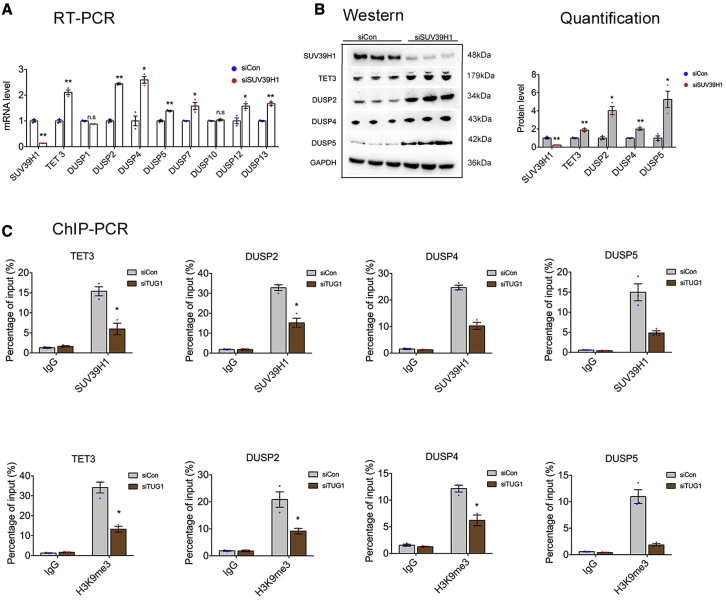
As an RNA-binding protein, SUV39H1 inactivates gene transcription by promoting histone modification, namely, H3K9me3.27 We further explored the correlation between TUG1 and SUV39H1. Silencing of SUV39H1 by siRNAs significantly upregulated TET3, DUSP2, DUSP4, and DUSP5 at both mRNA and protein levels (Figures 2A and 2B). Hence, we speculated that TUG1 might repress the expression of target genes via recruitment of SUV39H1 to the promoter region and induce H3K9me3, thereby altering the phenotype of trophoblasts in PE. Next, we performed chromatin immunoprecipitation (ChIP) coupled with qPCR. Anti-SUV39H1 and anti-H3K9 antibodies were used to precipitate protein-DNA complexes from HTR-8/SVneo cells transfected with siTUG1 or control siRNAs (siCon). As shown in Figure 2C, knockdown of TUG1 suppressed the binding of SUV39H1 to target genes and decreased the H3K9me3 level. These results reveal that SUV39H1 can interact with the promoters of target genes, leading to H3K9 trimethylation at the promoter region.
Figure 2.
TUG1 epigenetically regulates TET3 and the DUSP family by recruiting SUV39H1
(A and B) HTR-8/SVneo trophoblasts were transfected with siCon or siSUV39H1 for 48 h (n = 3). The silencing of SUV39H1 promoted the expression of downstream genes at both mRNA (A) and protein (B) levels. (C) HTR-8/SVneo cells were transfected with siTUG1 or siCon. ChIP coupled with qPCR was performed at 48 h after transfection. The mean relative enrichment of SUV39H1 over input after normalization against IgG (negative control) is shown (n = 3). ChIP coupled with qPCR showed that SUV39H1 and H3K9me3 were enriched in the promoter regions of TET3, DUSP2, DUSP4, and DUSP5, and the enrichment was decreased after silencing of TUG1. Error bars indicate mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01.
TET3 epigenetically regulates expression of the DUSP family
TET3 is a DNA demethylase that oxidizes 5-methylcytosine to 5-hydroxymethylcytosine, which is then converted to unmethylated cytosine.28,29 The enzymatic activity of TET3 is mediated by co-factors (e.g., α-ketoglutarate) generated through the tricarboxylic acid cycle and vitamin C, and by post-translational modification.30 The role of TET3 in cancer and development has been identified. Although altered TET3 expression has been observed in preeclamptic placenta, little is known of its function in PE.31
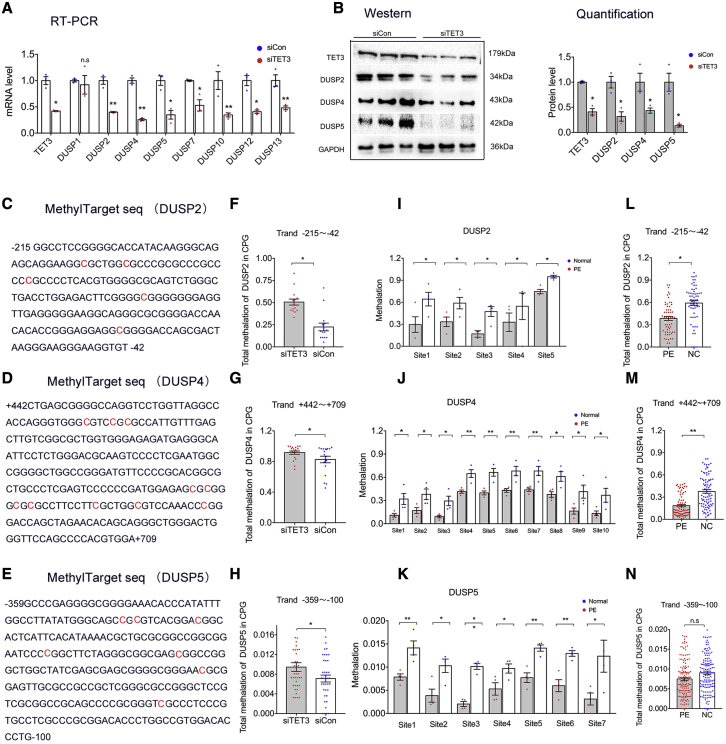
As a DNA-binding protein, TET3 activates gene transcription by promoting DNA demethylation.29 We first investigated whether aberrant expression of TET3 would affect the expression of the DUSP family. Intriguingly, TET3 knockdown markedly decreased the cellular expression of DUSP2, DUSP4, and DUSP5 (Figures 3A and 3B). Next, we examined whether TET3 knockdown altered promoter methylation in the DUSP2, DUSP4, and DUSP5 genes. The genome-wide single-nucleotide resolution of DNA methylation (GEO: GSE117190)32 in human uterine cells with TET3 deficiency showed increased methylation of the DUSP2, DUSP4, and DUSP5 promoters (Data S6).
Figure 3.
TET3-dependent epigenetic regulation of the DUSP family
(A and B) mRNA and protein expression of genes of interest in HTR-8/SVneo cells transfected siTET3 or siCon was measured using quantitative real-time PCR and western blot, respectively. Total RNA and protein were isolated at 48 h after transfection (n = 3). (C–E) Sequences of critical transcription regulatory regions of DUSP2, DUSP4, and DUSP5. The number indicates the position of the nucleotide relative to the transcription initiation site. Differentially methylated cytosine residues are highlighted in red. HTR-8/SVneo cells were transfected with siTET3 or siCon. MethylTarget sequencing analysis was performed at 48 h post-transfection. The methylation levels of the CpG sites in the DUSP2, DUSP4, and DUSP5 genes are shown (F–H) (n = 3). Genomic DNA was extracted from human placental tissues (n = 4 per group) and analyzed using MethylTarget sequencing. The promoter methylation levels of the CpG sites in the DUSP2, DUSP4, and DUSP5 genes are shown (I–K). The total promoter methylation levels of the CpG sites in the DUSP2, DUSP4, and DUSP5 genes are presented (L–N). Error bars indicate mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01.
To determine whether TET3 knockdown in HTR-8/SVneo cells would increase methylation of the DUSP2, DUSP4, and DUSP5 promoters, we performed targeted bisulfite sequencing on the basis of the next-generation sequencing platform to validate the methylation status. As shown in Figures 3C–3K, the methylation level of the CpGs in the DUSP2, DUSP4, and DUSP5 promoters was significantly increased. Pyrosequencing was used to detect promoter demethylation of these genes (Data S5). In addition, the placental tissues of healthy pregnant women and PE patients were collected, and targeted bisulfite sequencing was performed to characterize the methylation status of the CpG sites in the DUSP2, DUSP4, and DUSP5 promoters. As shown in Figures 3L–3N, the methylation level of DUSP2 and DUSP4 promoters in preeclamptic placenta was significantly increased compared with controls. These data suggest that TET3 positively regulates the expression of DUSP2, DUSP4, and DUSP5 in EVTs, likely in part by increasing demethylation at specific CpG sites in the promoter regions of these genes.
Hypoxia-induced miR-218 acts as a FOXP1 sponge and inhibits its expression
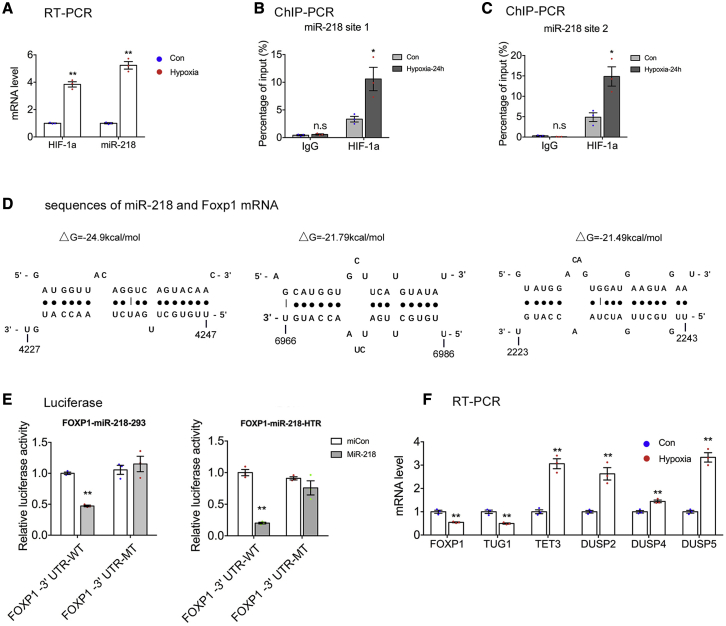
Insufficient uteroplacental oxygenation is implicated in the molecular events that lead to the development of PE.7,33 Fang et al.34 found that hypoxia increased the expression of miRNA-218 by promoting its binding to transcription factor HIF-1α, resulting in the inhibition of trophoblast proliferation and invasion. Here, we investigated whether hypoxia regulated the expression of miR-218. The exposure of HTR-8/SVneo cells to 0.5% O2 for 24 h significantly increased the mRNA expression of HIF-1α and miR-218 (Figure 4A). Then, ChIP was performed to determine whether hypoxia regulated miR-218 expression by promoting the binding of HIF-1α to the promoter of miR-218. As shown in Figures 4B and 4C, transcription factor HIF-1α bound to the promoter region of miR-218 to stimulate its expression.
Figure 4.
Hypoxia-induced miR-218 inhibits the expression of FOXP1
(A) HTR-8/SVneo cells were cultured under hypoxic conditions (0.5% oxygen) for 24 h. Quantitative real-time PCR was used to detect the mRNA expressions of HIF-1α and miR-218. (B and C) ChIP assay showed that HIF-1α was enriched at two sites of the promoter region of miR-218, and the enrichment was increased in HTR-8/SVneo cells exposed to 0.5% oxygen for 24 h. (D) Predicted base-pairing interaction between FOXP1 mRNA and miR-218. (E) Luciferase reporter assay was used to detect the interaction between miR-218 and FOXP1 in 293 and HTR-8/SVneo cells. Relative luciferase activity was normalized to Renilla luciferase activity. (F) Quantitative real-time PCR of genes of interest in HTR-8/SVneo cells exposed to 0.5% oxygen for 24 h (n = 3). Error bars indicate mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01.
Bioinformatics analysis (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/) predicted three binding sites of miR-218 in the 3′ UTR of FOXP1 (Figure 4D). The binding between miR-218 and FOXP1 might lead to FOXP1 mRNA degradation and/or translational repression. To validate this concept, we cultured HTR-8/SVneo cells under 0.5% O2 for 24 h. The mRNA expression of FOXP1 was significantly decreased compared with controls (Figure 4F). Then, 293 and HTR-8/SVneo cells were co-transfected with the 3′ UTR of FOXP1 (or the mutant 3′ UTR of FOXP1) and miR-218. The relative luciferase activity of the reporters containing the 3′ UTR of FOXP1 was significantly reduced in the groups transfected with miR-218 (Figure 4E). In contrast, the luciferase activity of the reporters containing mutant 3′ UTR of FOXP1 had no effect on cells transfected with miR-218 (Figure 4E). After 24 h exposure to 0.5% O2, the mRNA expression of FOXP1 and TUG1 in HTR-8/SVneo cells was significantly decreased, while that of TET3, DUSP2, DUSP4, and DUSP5 was increased (Figure 4F). The above data imply that miR-218 binds to the 3′ UTR of FOXP1 and inhibits its expression.
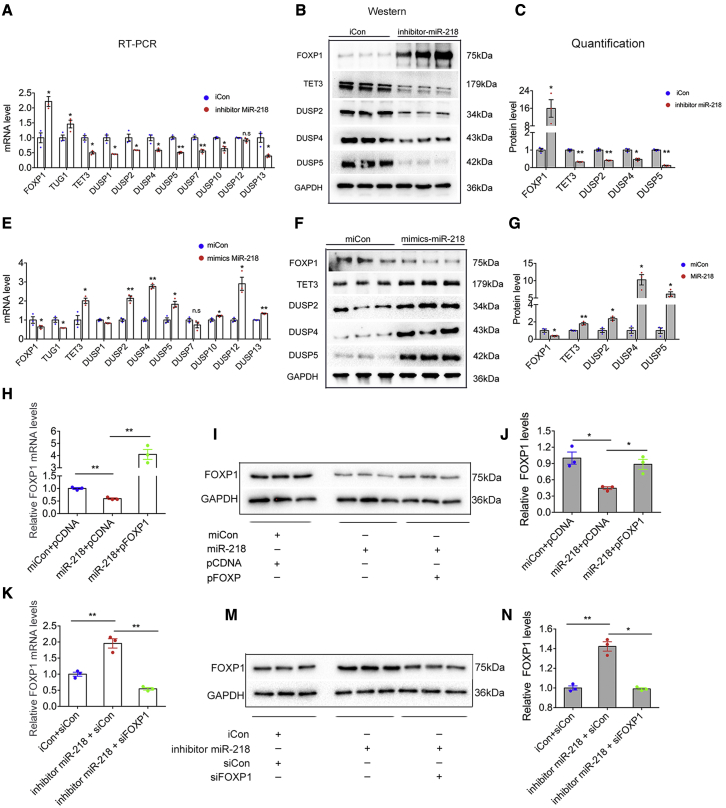
The effects of miR-218 knockdown by inhibitors on the expression of FOXP1, TUG1, TET3, and the DUSP family in HTR-8/SVneo cells were explored. Downregulation of miR-218 increased the expression of FOXP1 and TUG1 and decreased expression of TET3, DUSP2, DUSP4, and DUSP5 at both mRNA (Figure 5A) and protein (Figures 5B and 5C) levels, while overexpression of miR-218 by miR-218 mimics significantly upregulated FOXP1 and TUG1 and downregulated TET3, DUSP2, DUSP4, and DUSP5 at both mRNA and protein levels (Figures 5E–5G). These findings suggest that miR-218 negatively regulates the expression of FOXP1 and downregulates TUG1, TET3, DUSP2, DUSP4, and DUSP5 in HTR-8/SVneo cells.
Figure 5.
MiR-218 regulates the expression of FOXP1 at the post-transcriptional level
(A–C) Quantitative real-time PCR and western blot analysis of genes of interest in HTR-8/SVneo cells transfected with iCon or miR-218 inhibitor for 48 h (n = 3). (E–G) Quantitative real-time PCR and western blot analysis of genes of interest in HTR-8/SVneo cells transfected with miCon or mimics miR-218 for 48 h (n = 3). (H–J) HTR-8/SVneo cells were transfected with miCon + pCDNA, miR-218 + pCDNA, or miR-218 + pFOXP1 for 48 h. Total RNA and protein were extracted and analyzed using quantitative real-time PCR (left panel) and western blot (right panels), respectively (n = 3). (K–N) HTR-8/SVneo cells were transfected with iCon + siCon, inhibitor miR-218 + siCon, or inhibitor miR-218 + siFOXP1 for 48 h. Total RNA and protein were isolated and analyzed using quantitative real-time PCR (left panel) and western blot (right panels), respectively (n = 3). Data are from three independent experiments. Error bars indicate mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01.
Subsequently, a rescue experiment was performed to determine whether the regulation was mediated by miR-218. As expected, the transfection of cells overexpressing miR-218 with pFOXP1 increased the expression of FOXP1 (Figures 5G and 5H). However, knockdown of FOXP1 in cells transfected with miR-218 inhibitors eliminated the effects of miR-218 deficiency on the upregulation of FOXP1 (Figures 5K–5N). These findings indicate that miR-218 regulates the expression of FOXP1 in vitro at the post-transcriptional level.
Transcription factor FOXP1 activates TUG1 and inhibits the expression of TET3 and the DUSP family
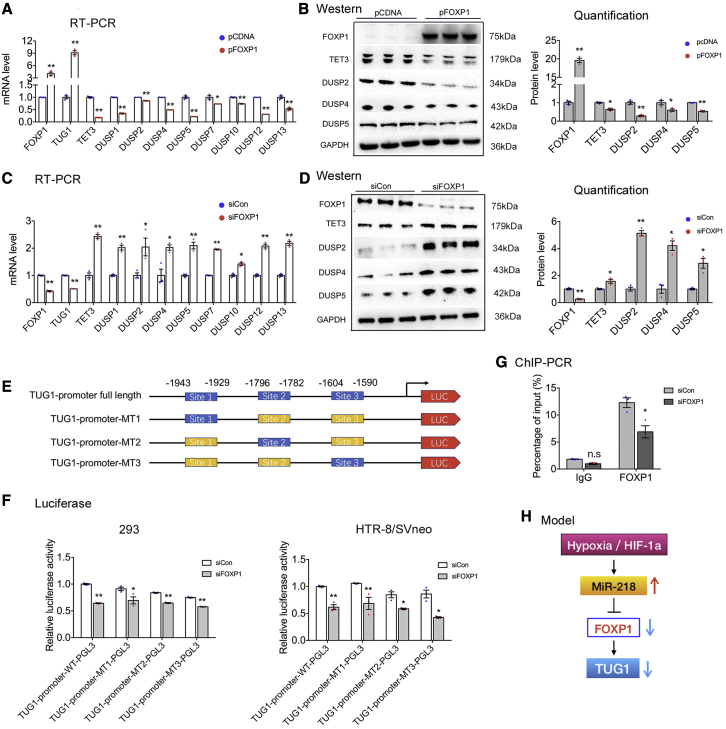
The expression of most human lncRNAs is mediated by various transcription factors. The JASPAR database (http://jaspar2014.genereg.net) was used to identify potential factors that may activate or inactivate the transcription of TUG1. As shown in Figure 6E, there were several FOXP1-binding sites in the promoter region of TUG1, implying that TUG1 might be regulated by FOXP1 in trophoblasts. Then, we transfected HTR-8/SVneo cells with designated plasmids and siRNAs to either upregulate or downregulate FOXP1. The level of TUG1 was significantly increased in cells overexpressing FOXP1, and the expression of TET3, DUSP2, DUSP4, and DUSP5 was also greatly increased (Figures 6A and 6B). An opposite trend was observed in cells with insufficient FOXP1 expression (Figures 6C and 6D).
Figure 6.
Transcription factor FOXP1 activates the transcription of TUG1 and inhibits the expression of TET3 and the DUSP family.
(A and B) Quantitative real-time PCR and western blot were performed to assess the expression of target genes in HTR-8/SVneo cells transfected FOXP1 plasmid (pFOXP1) or empty vector (pCDNA) for 48 h (n = 3). (C and D) Quantitative real-time PCR and western blot analysis of target genes in HTR-8/SVneo cells transfected with siCon or siTET3 for 48 h (n = 3). The putative binding sites of FOXP1 in the TUG1 promoter were predicted by JASPAR. The potential binding sites were highlighted in blue (E). Luciferase reporter assay (F) was used to determine the interaction between FOXP1 and TUG1. The luciferase activity was normalized to Renilla luciferase activity. (G) HTR-8/SVneo cells were transfected with siFOXP1 or siCon for 48 h. ChIP coupled with qPCR was then performed. The mean relative enrichment of FOXP1 over input after normalization against IgG (negative control) is shown (n = 3). (H) Upstream regulation of TUG1. Error bars indicate mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01.
Next, we performed luciferase reporter assay (Figure 6F) and ChIP assay (Figure 6G) to examine whether TUG1 regulation was mediated by FOXP1. As expected, FOXP1 knockdown significantly reduced the binding of FOXP1 to the TUG1 promoter, which was consistent with the aforementioned data showing the physical interaction between FOXP1 and the TUG1 promoter. These findings indicated that FOXP1 bound to the promoter region of the TUG1 gene to stimulate its expression. Taken together, these results support the statement that the hypoxia-miR-218/FOXP1/TUG1 axis regulates the function of trophoblasts in a cell-autonomous manner (Figure 6H).
Expression of TUG1, TET3, and the DUSP family was significantly changed in rats with L-NAME-induced PE and human preeclamptic placenta
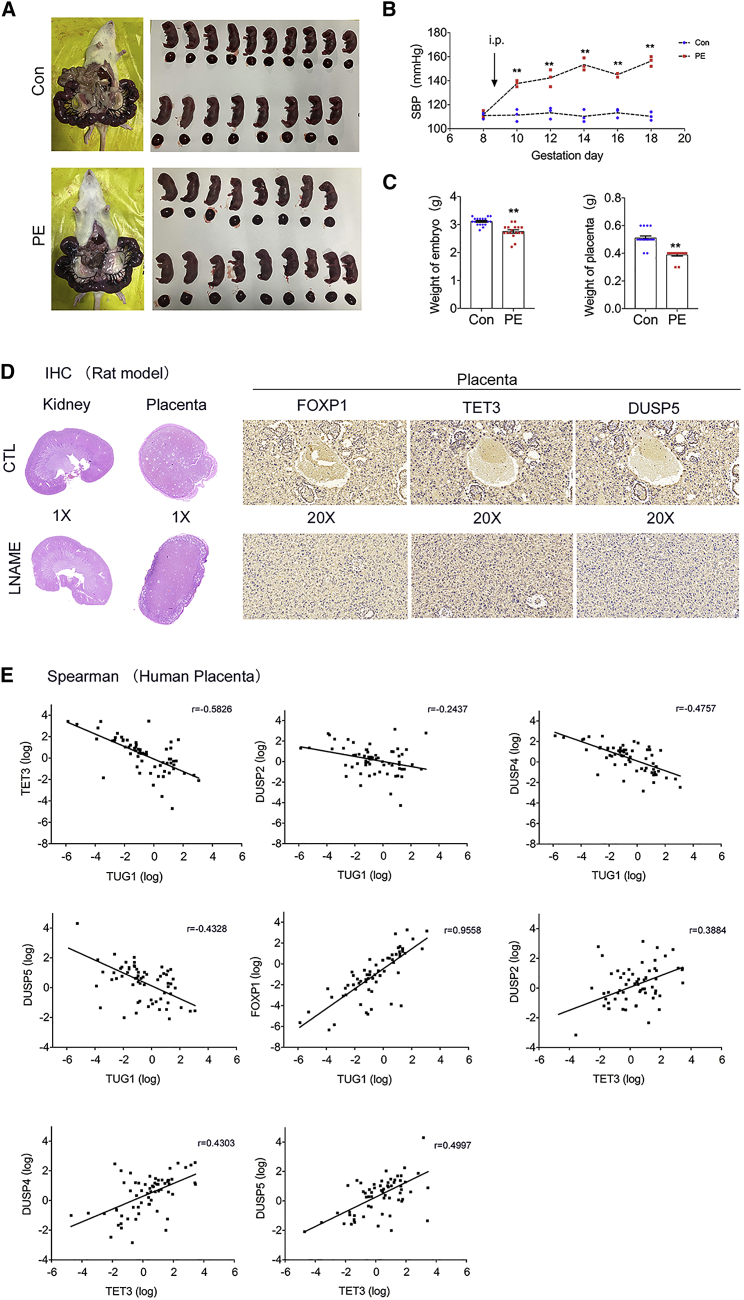
We further measured the expression of TUG1, TET3, and the DUSP family in rats with L-NAME-induced PE (Figure 7A). The mean systolic blood pressure (SBP) of the control group on gestational day (GD) 8 was 110.77 ± 4.12 mm Hg, and that of pregnant rats awaiting L-NAME treatment was 146.67 ± 6.94 mm Hg (p < 0.05). After L-NAME treatment, the mean SBP of rats with L-NAME-induced PE was increased on GD 8 and maintained until GD 18.5. The mean SBP of the PE group was significantly increased compared with the controls (Figure 7B). The average weights of the fetuses and placental tissues of the PE group were also significantly lower than those of healthy controls (Figure 7C). Immunohistochemistry analysis showed that the protein expression of TET3, DUSP2, and DUSP5 in the placental tissues of PE rats was increased, while FOXP1 was downregulated (Figure 7D; Data S7). The results of the animal study were in line with those observed in HTR-8/SVneo cells.
Figure 7.
Altered expression of TUG1, TET3, and the DUSP family in rats with L-NAME-induced PE and human preeclamptic placenta
(A) L-NAME or normal saline was intraperitoneally injected into pregnant rats. The embryos and placental tissues were collected, counted, and weighted. (B) Changes in SBP in the two groups. ∗∗p < 0.001 compared with SBP on GD 8 before intraperitoneal injection of L-NAME or normal saline. (C) The average weights of the embryos and placental tissues of the control and L-NAME groups were measured. (D) Four images on the left show representative photomicrographs of hematoxylin-eosin-stained kidney and placenta sections of saline- or L-NAME-treated rats on GD 18.5. Six images on the right show immunohistochemistry staining of placental tissues using antibodies against FOXP1, TET3, and DUSP5. Magnification, 20×; scale bar, 100 μm. (E) Spearman’s correlation analysis revealed negative correlations between the expression of TUG1 and TET3 and between TUG1 and its target genes (DUSP2, DUSP4, and DUSP5). Positive correlations between mRNA expression of TUG1 and FOXP1 and between TET3 and DUSP2, DUSP4, and DUSP5 were found in 64 paired preeclamptic and control placental tissues. Error bars indicate mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01.
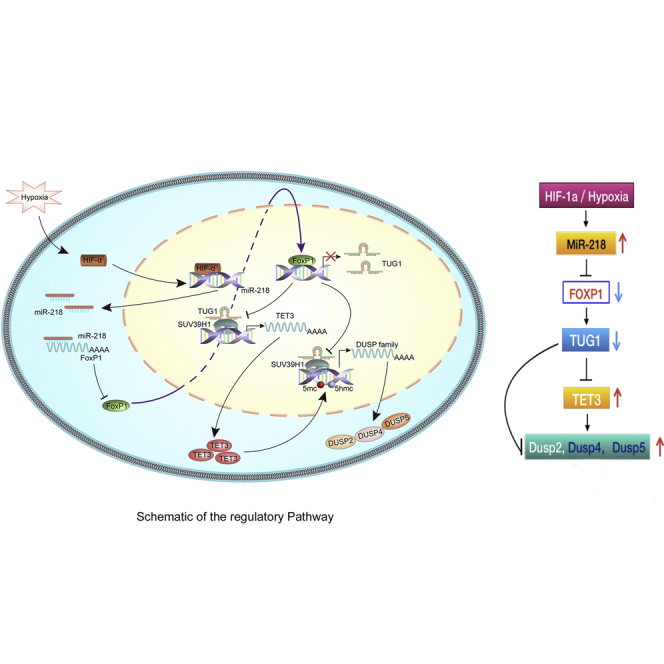
In addition, we examined the correlations of the mRNA levels of these genes in human preeclamptic placenta. The mRNA levels of TUG1 and TET3, TUG1 and DUSP2, TUG1 and DUSP4, and TUG1 and DUSP5 were significantly and negatively correlated (Figure 7E). A significant positive correlation between the levels of FOXP1 and TUG1 was also observed (Figure 7E). We further analyzed the mRNA expression of TET3 and its target genes, DUSP2, DUSP4, and DUSP5, in preeclamptic placenta and matched normal placental tissues. As shown in Figure 7E, the expression of TET3 was positively correlated with the levels of all target genes, confirming the in vitro results that TUG1 knockdown in trophoblasts increased the expression of TET3, DUSP2, DUSP4, and DUSP5, and regulation by TUG1 was at the epigenetic level. Collectively, these data implicate FOXP1-mediated regulation of TUG1 in the pathogenesis of PE. The potential regulatory pathway is shown in Figure 8. Whether TUG1 would regulate other genes or pathways in placental trophoblasts warrants further investigation.
Figure 8.
Schematic of the regulatory pathway
Discussion
PE is a common obstetric complication among pregnant women that may lead to maternal and fetal morbidity and mortality.35 Current treatments for PE are not satisfactory and the only way to prevent the progression of PE is to induce delivery.36 Hence, it is of significant importance to comprehensively understand the pathogenic mechanisms of PE and to identify new prognostic factors. Increasing evidence has shown the involvement of lncRNAs in cell development and human diseases.37,38 LncRNAs are also involved in human normal development, cell differentiation, RNA decay, genomic imprinting, chromatin modification, and regulation of sponge-like miRNAs.39,40 Our previous study demonstrated that TUG1 was markedly downregulated in the placental tissues of PE patients.25 Moreover, TUG1 might result in SAR impairment in PE. Thus, we proposed that TUG1 might play an essential role in the progression of PE. Here, we investigated the regulatory mechanisms of TUG1 in the development of PE.
The TET proteins are a group of DNA demethylases that oxidize 5-methylcytosine to 5-hydroxymethylcytosine.41 The DUSP family is characterized by highly conserved amino acid sequences, while different DUSP members may have different functions.42 The DUSP family is implicated in a variety of biological processes. DUSP9 maintains the stem cell-like characteristics of cancer cells and is related to the development of breast cancer.43 Importantly, the biological functions of lncRNAs depend largely on their subcellular localization.44 We have shown that most TUG1 is located in the nuclei of trophoblasts, indicating its potential function. In this study, RNA sequencing analysis, gene expression profiles, and in vitro cell function experiments showed that TET3 and the DUSP family were negatively regulated by TUG1. Moreover, the expression of TET3 and the DUSP genes were significantly correlated in PE. One of the pathogenesis of preeclampsia has been confirmed to be related to abnormal changes in methylation.45,46 Thus, we suspect that the occurrence of preeclampsia may be related to TET3-DUSPs in some way. The genome-wide single-nucleotide resolution of DNA methylation, pyrosequencing analysis, and bisulfite sequencing indicated that TET3 regulated the expression of the DUSP genes partially by increasing demethylation at specific CpGs in their promoter regions. These results were confirmed in the rat model of L-NAME-induced PE. Our resulting data demonstrated that TET3 positively regulates the expression of DUSP2, DUSP4, and DUSP5 in EVTs, likely in part by increasing demethylation at specific CpG sites in the promoter regions of these genes. We believe that the molecular and functional interaction between TET3 and the DUSPs characterized in this report represents a critical mechanism in the impairment of spiral artery remodeling in PE.
Epigenetic regulation plays a crucial role in various diseases, including PE.47 Approximately 70% of TUG1 is located in the nuclei of trophoblasts, suggesting that TUG1 may play an essential role in epigenetic regulation. To further investigate how TUG1 regulated TET3 and the DUSP family, bioinformatics analysis and RIP coupled with qPCR were performed. Our results showed a binding preference of TUG1 to SUV39H1 in human trophoblasts. As previously reported, genes recruit the H3K9MTases SUV39H1 to the genome, resulting in the enrichment of H3K9me2/me3 and therefore promoting the development of many cancers.48 However, little is known about the roles of SUV39H1 and SUV39H1-associated H3K9me3 modification in PE. In the present study, silencing of SUV39H1 upregulated both TET3 and the DUSP family. In addition, TUG1 knockdown suppressed the binding of SUV39H1 and decreased the H3K9me3 level. The above findings indicate that TUG1 might regulate the progression of PE via mediating the expression of TET3 and the DUSP family by recruiting SUV39H1.
The expression of human lncRNAs is mediated by various transcription factors. FOXP1 is an evolutionally conserved transcription factor that binds to highly conserved regions of the DNA and is required for the proper development of pulmonary, neural, intestinal, cardiovascular, and lymphoid tissues.49 Here, the JASPAR database was used to identify the potential upstream targets of TUG1 in PE. The results showed that there were several binding sites of FOXP1 in the promoter region of TUG1. Also, FOXP1 knockdown significantly decreased the expression of TUG1 but increased the expression of TET3 and the DUSP family. Furthermore, luciferase reporter assay and ChIP assay indicated that FOXP1 bound to the promoter region of TUG1 to stimulate its expression.
The upregulation of hypoxia-inducible transcription factors and hypoxia-related genes in the placenta suggests that hypoxia is essential for the pathogenesis of PE.33 HIF-1α, a marker of cellular oxygen deprivation, is highly expressed in proliferative trophoblasts and in the placenta of PE patients.50 A previous study reported that miR-218 promoted trophoblast invasion, induced EVT-to-enEVT differentiation, and accelerated SAR.51 In this study, we identified HIF-1α as an interactor of miR-218. The binding preference of these hypoxia-inducible factors under hypoxic conditions was confirmed by ChIP assay.
Recently, considerable attention has been focused on the sponge effect of miRNA.52 miRNAs regulate gene expression by directly binding mRNAs and subsequently suppressing mRNA translation or inducing mRNA degradation.53 Here, we predicted the binding sites of miR-218 in the 3′ UTR of the FOXP1 mRNA, indicating that miR-218 might repress the translation of FOXP1 mRNA and/or induce the degradation of FOXP1 mRNA. Moreover, the expression of FOXP1 was significantly decreased under hypoxic conditions. Further RT-PCR and luciferase reporter assay confirmed the binding of miR-218 to FOXP1. Our data implied that miR-218 negatively regulated the expression of FOXP1 and decreased the levels of TUG1, TET3, DUSP2, DUSP4, and DUSP5 in trophoblasts, indicating that hypoxia-induced HIF-1α mediated the expression of FOXP1 and its downstream genes through a miR-218-dependent mechanism.
Finally, we investigated the correlations of the expression of these genes in human placenta. The level of TUG1 was significantly and negatively correlated with the expression of TET3, DUSP2, DUSP4, and DUSP5. A significant positive correlation between the expression of TUG1 and FOXP1 was also observed. Furthermore, the TET3 expression was positively correlated with the levels of DUSP2, DUSP4, and DUSP5.
In conclusion, this study provides novel evidence supporting lncRNA TUG1 as a link between hypoxia and the development of PE. Our results suggest that hypoxia-induced-HIF-1α regulated miR-218, which then inhibited the expression of the transcription factor FOXP1. Moreover, FOXP1 activated TUG1, which epigenetically inhibited the expression of TET3 and the DUSP family via recruitment of SUV39H1. These findings highlight the importance of TUG1-mediated regulatory network in PE and provide new insights into the potential therapeutic targets for PE.
Materials and methods
Establishment of animal model with L-NAME
Pregnant Sprague-Dawley (SD) rats (8 weeks old) were obtained from the Experimental Animal Research Institute of Nanjing Medical University and housed in a controlled environment (temperature 21 ± 3°C, 12 h/12 h light/dark cycle). All rats had free access to water and standard food before experiments. This study approved by the Animal Care and Use Committee of Nanjing Medical University in accordance with the National Institutes of Health Guidelines for Use and Care of Animals.
Pregnant SD rats were randomly separated into two groups, an L-NAME group and a control group. Rats in the L-NAME group received intraperitoneal injections of L-NAME (125 mg/kg body weight) on gestational days 9.5, 10.5, 11.5, 12.5, 13.5, 14.5, and 15.5. Rats in the control group were administered phosphate-buffered saline (1 mL/kg body weight) following the same procedure.
Blood pressure (BP) was measured on GDs 8.5, 10.5, 12.5, 14.5, and 16.5 using a programmed electro-sphygmomanometer (BP-98A; Softron, Tokyo, Japan) according to the tail-cuff method. Blood was obtained from the orbital sinus of each rat. The urine samples were also collected. On GD 16.5, rats were euthanized. Placentas and fetuses were dried and weighed.
Cell culture and transfections of cell lines
We selected cell lines that were related to pregnancy. HTR-8/SVneo cells were generously furnished by Prof. Charies Graham (Queen’s University, Kingston, ON, Canada). The 293T cell lines were purchased from the Institute of the Chinese Academy of Sciences (Shanghai, China). HTR-8 and 293T cells were maintained in RPMI 1640 medium (Gibco, Nanjing, China) supplemented with 10% fetal bovine serum (FBS) (Gibco, BRL, Invitrogen, Carlsbad, CA) and antibiotics (100 μg/mL streptomycin and 100 U/mL penicillin G). All cell lines were cultured in humidified air at 37°C and 5% CO2. For hypoxia treatment, cells were exposed to 0.5% O2 in a hypoxic incubator (BioSpherix, Redfield, NY) for the indicated time.
For transfection of suspended HTR-8/SVneo cells, a volume of 1–2 μL Lipofectamine 3000 transfection reagent was mixed with 25 μL OPTI-MEM. Meanwhile, 20 pmol siRNAs were mixed with 25 μL OPTI-MEM. After 5 min, the mixture containing siRNAs and Lipofectamine 3000 reagent was mixed and placed at room temperature for 5–10 min. Then, 50 μL transfection solution was added to re-suspend cells (6 × 104 cells per well in 24-well plates). After 10 min incubation at room temperature, 450 μL fresh culture medium was added to suspended cells, which were then transferred to the culture plate.
For on-plate transfection of primary trophoblasts, cells were seeded in six-well plates (6 × 104 per well). After 6 h, on-plated transfection was performed. A volume of 240 μL OPTI-MEM was incubated with 10 μL siRNAs (100 pmol) for 5 min. Meanwhile, 240 μL OPTI-MEM was mixed with 10 μL Lipofectamine 3000 reagent and placed at room temperature for 5 min. Then, the mixture containing siRNAs and Lipofectamine 3000 reagent was mixed and placed at room temperature for 20 min. Subsequently, 500 μL total solution was added to each well of the six-well plate. After 6 h of transfection, transfected solution was replaced by fresh culture medium. HTR-8/SVneo and 293 cells were seeded in six-well plates and transfected with 40 nM miR-218 inhibitor, miR-218 mimic, or corresponding controls (RiboBio, Guangzhou, China) for 48 h. Cells were also plated in six-well plates and transfected with 3 μg plasmid (vector, pcDNA3.1 + FOXP1, and pcDNA3.1 + TET3).
Placenta samples collection
Placental tissue samples were obtained from 64 PE patients and 64 paired healthy controls who underwent cesarean section at the First Affiliated Hospital of Nanjing Medical University from 2017 to 2019. All placental tissues were washed with sterile saline and stored in liquid nitrogen. The clinicopathological characteristics of healthy pregnant women and PE patients are summarized in Table S1 (published by Zhang et al.54). The study protocol was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University. All subjects provided written informed consent.
Quantitative real-time PCR
This experiment was performed as described by Geng et al.55 with minor modifications. Briefly, total RNA was extracted from placental tissues or cultured cells using PureLink RNA Mini Kit (catalog no. 12183018A; Ambion). Total RNA (0.8 μg) was reverse-transcribed to cDNA in a reaction volume of 10 μL using PrimeScript RT Reagent Kit (catalog no. RR037A; Takara, Tokyo, Japan). Quantitative real-time PCR was performed using SYBR Premix Ex Taq (Takara, Dalian, China) and the ABI 7500 system. The expressions of target genes was normalized to those of housekeeping genes ACTB and GAPDH. The sequences of PCR primers for rat and human tissues were summarized in Data S1.
Transcriptome sequencing analysis
Transcriptome sequencing analysis was performed as described in our previous study.25
RNA immunoprecipitation assay (RIP)
RIP was performed as previously described.25
Chromatin immunoprecipitation assay (ChIP)
ChIP was performed as previously described by Xu et al.56
Western blotting
Protein expression was measured using western blot as previously described.56 Protein samples (5–10 μL) were loaded onto a 10% SDS gel and analyzed using western blot. GAPDH was used as an internal control. The antibodies used in this experiment and the conditions of western blot are shown in Table S2. The intensity of protein bands was quantified using Quantity One software (Bio-Rad, Hercules, CA). The uncropped western blot images are shown in Data S2.
Luciferase reporter assay
As previously described by Zhang et al.,57 the amplified cDNA fragments of the TUG1 and FOXP1 promoters were subcloned into the downstream of the luciferase gene in the pGL3 plasmid. The mutant plasmids (i.e., pGL3-FOXP1-3′-UTR-MUT and pGL3-TUG1-MUT) were obtained using Platinum Pfx DNA Polymerase. The luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). In brief, HTR-8/SVneo cells (1 × 105 per well) were plated in 24-well plates for 36 h. At 48 h post-transfection, cells were lysed and collected. The relative luciferase activity was normalized to Renilla luciferase activity. The binding sites of miR-218 in the 3′-UTR of FOXP1 are shown in Table S3 and Data S3.
Targeted bisulfite sequencing
MethylTarget sequencing analysis was performed by Shanghai Genesky Biotechnology Company (Shanghai, China). Primers were designed and validated using Methylation Primer software using bisulfite-converted DNA. The primer sets were designed to flank each targeted CpG site in 200 nt regions (Table S4). After PCR amplification using HotStarTaq Polymerase Kit (Takara) and library construction, samples were sequenced using the MiSeq Benchtop Sequencer (Illumina) following the paired-end sequencing protocol. Detailed experimental procedures are shown in Data S4.
RNA pull-down assay
Briefly, RNA transcription in vitro was performed using mMESSAGE mMACHINE T7 Transcription Kit according to the manufacturer’s instructions (catalog no. AM1344; Invitrogen). Then, TUG1 RNAs were labeled with desthiobiotinylation using the Pierce RNA 3′ End Desthiobiotinylation Kit (catalog no. 20164, Magnetic RNA-Protein Pull-Down Kit, Components; Thermo Fisher Scientific). RNA pull-down assays were conducted using the Pierce Magnetic RNA-Protein Pull-Down Kit according to the manufacturer’s instructions (catalog no. 20164, Magnetic RNA-Protein Pull-Down Kit). After elution of lncRNA-interacting proteins, they were subjected to western blotting analysis. Detailed process of RNA pull-down assay are listed in Data S9.
Pyrosequencing
Pyrosequencing was performed to detect protein expression using a PyroMark Q96 instrument (Qiagen) as previously described.58 The bisulfite-converted DNA was amplified using HotStarTaq DNA Polymerase (Qiagen). PCR products were immobilized on streptavidin-Sepharose beads (GE Healthcare, Chicago, IL), washed, denatured, and released into the annealing buffer containing sequencing primer (Table S5). Pyro CpG software (Qiagen) was used to calculate the percentage of methylation.
Statistical analysis
Normally distributed data are shown as mean ± SEM and were compared using two-tailed Student’s t test using GraphPad Prism 7.0 (www.graphpad.com). The number of independent experiments is shown in figure legends. A p value of less than 0.05 was considered to indicate statistical significance (∗p < 0.05 and ∗∗p < 0.01; ns, not statistical significance).
Acknowledgments
This project was supported by the National Natural Science Foundation of China (grant 82001578 to Y.X.; grant 81801472 to Y.Z.), the Project of Natural Science Foundation of Jiangsu Province (grant BK2020107 to Y.X.; grant BK20181080 to Y.Z), the Postdoctoral Fund Project in Jiangsu Province (grant 2020Z087 to Y.X.), the Postdoctoral Fund Project in Nanjing (grant 2021BSH209 to Y.X.), Maternal and child health "young talents" project (grant FRC202151 to Y.X.), and the China Postdoctoral Fund Project (NO. 2020M671394 to Y.X.).
Author contributions
Y.X., Y.Z., and L. Sun designed and performed the experiments, collected and analyzed the data, and wrote the manuscript. Y.X., D.W., B.H., and L. Shu performed the experiments and collected and analyzed the data. N.Y., X.T., and C.W. performed the experiments and collected the data. Y.Z. provided intellectual insights and critical discussion of the project. Y.Y. and L. Shu created a heatmap showing the expression of differential genes after suppression of TUG1. All authors revised the manuscript critically for important intellectual content and read and approved the final version.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.01.043.
Contributor Information
Nana Yang, Email: nanayang210@163.com.
Ziyan Jiang, Email: zyjiangchm@163.com.
Yuanyuan Zhang, Email: zhangyuanyuan5518@jsph.org.cn.
Lizhou Sun, Email: sunlizhou@njmu.edu.cn.
Supplemental information
References
- 1.American College of Obstetricians and Gynecologists Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists' task force on hypertension in pregnancy. Obstet. Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Altman D., Carroli G., Duley L., Farrell B., Moodley J., Neilson J., Smith D., Magpie Trial Collaboration G. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie tTrial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–1890. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 3.Hogberg U. The World Health Report 2005: "make every mother and child count" - including Africans. Scand. J. Public Health. 2005;33:409–411. doi: 10.1080/14034940500217037. [DOI] [PubMed] [Google Scholar]
- 4.Genest D.S., Falcao S., Gutkowska J., Lavoie J.L. Impact of exercise training on preeclampsia: potential preventive mechanisms. Hypertension. 2012;60:1104–1109. doi: 10.1161/HYPERTENSIONAHA.112.194050. [DOI] [PubMed] [Google Scholar]
- 5.Meekins J.W., Pijnenborg R., Hanssens M., McFadyen I.R., van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 6.Pijnenborg R., Vercruysse L., Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Soleymanlou N., Jurisica I., Nevo O., Ietta F., Zhang X., Zamudio S., Post M., Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J. Clin. Endocrinol. Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaiworapongsa T., Chaemsaithong P., Yeo L., Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014;10:466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 11.Sitras V., Paulssen R.H., Gronaas H., Leirvik J., Hanssen T.A., Vartun A., Acharya G. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30:424–433. doi: 10.1016/j.placenta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P., et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles Richard J.L., Eichhorn P.J.A. Platforms for investigating LncRNA functions. SLAS Technol. 2018;23:493–506. doi: 10.1177/2472630318780639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan W., Li M., Zhao K., Liu J., Wu F.X., Pan Y., Wang J. LDAP: a web server for lncRNA-disease association prediction. Bioinformatics. 2017;33:458–460. doi: 10.1093/bioinformatics/btw639. [DOI] [PubMed] [Google Scholar]
- 15.Kopp F., Mendell J.T. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akerman I., Tu Z., Beucher A., Rolando D.M.Y., Sauty-Colace C., Benazra M., Nakic N., Yang J., Wang H., Pasquali L., et al. Human pancreatic beta cell lncRNAs control cell-specific regulatory networks. Cell Metab. 2017;25:400–411. doi: 10.1016/j.cmet.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz S.U., Grote P., Herrmann B.G. Mechanisms of long noncoding RNA function in development and disease. Cell Mol. Life Sci. 2016;73:2491–2509. doi: 10.1007/s00018-016-2174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao K., Xu J., Yang W., You X., Zhong Q., Wang X. The research progress of LncRNA involved in the regulation of inflammatory diseases. Mol. Immunol. 2018;101:182–188. doi: 10.1016/j.molimm.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Ghazal S., McKinnon B., Zhou J., Mueller M., Men Y., Yang L., Mueller M., Flannery C., Huang Y., Taylor H.S. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol. Med. 2015;7:996–1003. doi: 10.15252/emmm.201505245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhade V.S., Pal D., Kanduri C. Long noncoding RNA: genome organization and mechanism of action. Adv. Exp. Med. Biol. 2017;1008:47–74. doi: 10.1007/978-981-10-5203-3_2. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y., Wu D., Liu J., Huang S., Zuo Q., Xia X., Jiang Y., Wang S., Chen Y., Wang T., Sun L. Downregulated lncRNA HOXA11-AS affects trophoblast cell proliferation and migration by regulating RND3 and HOXA7 expression in PE. Mol. Ther. Nucleic Acids. 2018;12:195–206. doi: 10.1016/j.omtn.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin P.C., Huang H.D., Chang C.C., Chang Y.S., Yen J.C., Lee C.C., Chang W.H., Liu T.C., Chang J.G. Long noncoding RNA TUG1 is downregulated in non-small cell lung cancer and can regulate CELF1 on binding to PRC2. BMC Cancer. 2016;16:583. doi: 10.1186/s12885-016-2569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q., Zhang J., Su D.M., Guan L.N., Mu W.H., Yu M., Ma X., Yang R.J. lncRNA TUG1 modulates proliferation, apoptosis, invasion, and angiogenesis via targeting miR-29b in trophoblast cells. Hum. Genomics. 2019;13:50. doi: 10.1186/s40246-019-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Ai Y., Chen M., Liu J., Ren L., Yan X., Feng Y. lncRNA TUG1 promotes endometrial fibrosis and inflammation by sponging miR-590-5p to regulate Fasl in intrauterine adhesions. Int. Immunopharmacol. 2020;86:106703. doi: 10.1016/j.intimp.2020.106703. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y., Ge Z., Zhang E., Zuo Q., Huang S., Yang N., Wu D., Zhang Y., Chen Y., Xu H., et al. The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis. 2017;8:e3104. doi: 10.1038/cddis.2017.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu A.H., Yang D.Y., Liu Y.D., Chen X., Chen X.L., Lu S., Chen S.L. Expression of TET and 5-HmC in trophoblast villi of women with normal pregnancy and with early pregnancy loss. Curr. Med. Sci. 2018;38:505–512. doi: 10.1007/s11596-018-1907-0. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu D., Kimura H., Kotoshiba K., Tachibana M., Shinkai Y. Pericentric H3K9me3 formation by HP1 interaction-defective histone methyltransferase Suv39h1. Cell Struct. Funct. 2016;41:145–152. doi: 10.1247/csf.16013. [DOI] [PubMed] [Google Scholar]
- 28.An J., Rao A., Ko M. TET family dioxygenases and DNA demethylation in stem cells and cancers. Exp. Mol. Med. 2017;49:e323. doi: 10.1038/emm.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen K.D., Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30:733–750. doi: 10.1101/gad.276568.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minor E.A., Court B.L., Young J.I., Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013;288:13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma M., Zhou Q.J., Xiong Y., Li B., Li X.T. Preeclampsia is associated with hypermethylation of IGF-1 promoter mediated by DNMT1. Am. J. Transl. Res. 2018;10:16–39. [PMC free article] [PubMed] [Google Scholar]
- 32.Cao T., Jiang Y., Wang Z., Zhang N., Al-Hendy A., Mamillapalli R., Kallen A.N., Kodaman P., Taylor H.S., Li D., Huang Y. H19 lncRNA identified as a master regulator of genes that drive uterine leiomyomas. Oncogene. 2019;38:5356–5366. doi: 10.1038/s41388-019-0808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S.B., Nakashima A., Huber W.J., Davis S., Banerjee S., Huang Z., Saito S., Sadovsky Y., Sharma S. Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. 2019;10:927. doi: 10.1038/s41419-019-2162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang M., Du H., Han B., Xia G., Shi X., Zhang F., Fu Q., Zhang T. Hypoxia-inducible microRNA-218 inhibits trophoblast invasion by targeting LASP1: implications for preeclampsia development. Int. J. Biochem. Cell Biol. 2017;87:95–103. doi: 10.1016/j.biocel.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Mol B.W.J., Roberts C.T., Thangaratinam S., Magee L.A., de Groot C.J.M., Hofmeyr G.J. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 36.Phipps E.A., Thadhani R., Benzing T., Karumanchi S.A. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019;15:275–289. doi: 10.1038/s41581-019-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 2021;20 doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian X., Zhao J., Yeung P.Y., Zhang Q.C., Kwok C.K. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem. Sci. 2019;44:33–52. doi: 10.1016/j.tibs.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Schertzer M.D., Braceros K.C.A., Starmer J., Cherney R.E., Lee D.M., Salazar G., Justice M., Bischoff S.R., Cowley D.O., Ariel P., et al. lncRNA-induced spread of polycomb controlled by genome architecture, RNA abundance, and CpG island DNA. Mol. Cell. 2019;75:523–537.e10. doi: 10.1016/j.molcel.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brook I., Yocum P. Comparison of the microbiology of group A and non-group A streptococcal tonsillitis. Ann. Otol. Rhinol. Laryngol. 1988;97:243–246. doi: 10.1177/000348948809700306. [DOI] [PubMed] [Google Scholar]
- 41.Tsagaratou A. Deciphering the multifaceted roles of TET proteins in T-cell lineage specification and malignant transformation. Immunol. Rev. 2021;300:22–36. doi: 10.1111/imr.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramkissoon A., Chaney K.E., Milewski D., Williams K.B., Williams R.L., Choi K., Miller A., Kalin T.V., Pressey J.G., Szabo S., et al. Targeted inhibition of the dual specificity phosphatases DUSP1 and DUSP6 suppress MPNST growth via JNK. Clin. Cancer Res. 2019;25:4117–4127. doi: 10.1158/1078-0432.CCR-18-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jimenez T., Barrios A., Tucker A., Collazo J., Arias N., Fazel S., Baker M., Halim M., Huynh T., Singh R., Pervin S. DUSP9-mediated reduction of pERK1/2 supports cancer stem cell-like traits and promotes triple negative breast cancer. Am. J. Cancer Res. 2020;10:3487–3506. [PMC free article] [PubMed] [Google Scholar]
- 44.Guo C.J., Ma X.K., Xing Y.H., Zheng C.C., Xu Y.F., Shan L., Zhang J., Wang S., Wang Y., Carmichael G.G., et al. Distinct processing of lncRNAs contributes to non-conserved functions in stem cells. Cell. 2020;181:621–636.e2. doi: 10.1016/j.cell.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Matsui H., Iriyama T., Sayama S., Inaoka N., Suzuki K., Yoshikawa M., Ichinose M., Sone K., Kumasawa K., Nagamatsu T., et al. Elevated placental histone H3K4 methylation via upregulated histone methyltransferases SETD1A and SMYD3 in preeclampsia and its possible involvement in hypoxia-induced pathophysiological process. Placenta. 2021;115:60–69. doi: 10.1016/j.placenta.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Kazmi N., Sharp G.C., Reese S.E., Vehmeijer F.O., Lahti J., Page C.M., Zhang W., Rifas-Shiman S.L., Rezwan F.I., Simpkin A.J., et al. Hypertensive disorders of pregnancy and DNA methylation in newborns. Hypertension. 2019;74:375–383. doi: 10.1161/HYPERTENSIONAHA.119.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apicella C., Ruano C.S.M., Mehats C., Miralles F., Vaiman D. The role of epigenetics in placental development and the etiology of preeclampsia. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen J.Z., Qiu Z., Wu Q., Finlay D., Garcia G., Sun D., Rantala J., Barshop W., Hope J.L., Gimple R.C., et al. FBXO44 promotes DNA replication-coupled repetitive element silencing in cancer cells. Cell. 2021;184:352–369.e3. doi: 10.1016/j.cell.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhuang T., Liu J., Chen X., Zhang L., Pi J., Sun H., Li L., Bauer R., Wang H., Yu Z., et al. Endothelial Foxp1 suppresses atherosclerosis via modulation of Nlrp3 inflammasome activation. Circ. Res. 2019;125:590–605. doi: 10.1161/CIRCRESAHA.118.314402. [DOI] [PubMed] [Google Scholar]
- 50.Frazier S., McBride M.W., Mulvana H., Graham D. From animal models to patients: the role of placental microRNAs, miR-210, miR-126, and miR-148a/152 in preeclampsia. Clin. Sci. (Lond) 2020;134:1001–1025. doi: 10.1042/CS20200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brkic J., Dunk C., O'Brien J., Fu G., Nadeem L., Wang Y.L., Rosman D., Salem M., Shynlova O., Yougbare I., et al. MicroRNA-218-5p promotes endovascular trophoblast differentiation and spiral artery remodeling. Mol. Ther. 2018;26:2189–2205. doi: 10.1016/j.ymthe.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomson D.W., Dinger M.E. Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 2016;17:272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 53.Kumar V., Kumar V., Chaudhary A.K., Coulter D.W., McGuire T., Mahato R.I. Impact of miRNA-mRNA profiling and their correlation on medulloblastoma tumorigenesis. Mol. Ther. Nucleic Acids. 2018;12:490–503. doi: 10.1016/j.omtn.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S., Zou Y., Tang X., Zhang Y., Yang N., Xu K., Xu Y. Silencing of AFAP1-AS1 lncRNA impairs cell proliferation and migration by epigenetically promoting DUSP5 expression in pre-eclampsia. J. Cell Biochem. 2021;122 doi: 10.1002/jcb.30072. [DOI] [PubMed] [Google Scholar]
- 55.Geng T., Liu Y., Xu Y., Jiang Y., Zhang N., Wang Z., Carmichael G.G., Taylor H.S., Li D., Huang Y. H19 lncRNA promotes skeletal muscle insulin sensitivity in part by targeting AMPK. Diabetes. 2018;67:2183–2198. doi: 10.2337/db18-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Y., Sun X., Zhang R., Cao T., Cai S.Y., Boyer J.L., Zhang X., Li D., Huang Y. A positive feedback loop of TET3 and TGF-beta1 promotes liver fibrosis. Cell Rep. 2020;30:1310–1318.e5. doi: 10.1016/j.celrep.2019.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang E., Han L., Yin D., He X., Hong L., Si X., Qiu M., Xu T., De W., Xu L., et al. H3K27 acetylation activated-long non-coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma. Nucleic Acids Res. 2017;45:3086–3101. doi: 10.1093/nar/gkw1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu Y.C., Jiang Y., Yang M.M., He S.N., Xi X., Xu Y.T., Hu W.S., Luo Q. Hypermethylation of delta-like homolog 1/maternally expressed gene 3 loci in human umbilical veins: insights into offspring vascular dysfunction born after preeclampsia. J. Hypertens. 2019;37:581–589. doi: 10.1097/HJH.0000000000001942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.