Abstract
The FGFR3-TACC3 (F3-T3) fusion gene was discovered as an oncogenic molecule in glioblastoma and bladder cancers, and has subsequently been found in many cancer types. Notably, F3-T3 was found to be highly expressed in both untreated and matched recurrence glioblastoma under the concurrent radiotherapy and temozolomide (TMZ) treatment, suggesting that targeting F3-T3 is a valid strategy for treatment. Here, we show that the F3-T3 protein is a client of heat shock protein 90 (HSP90), forming a ternary complex with the cell division cycle 37 (CDC37). Deprivation of HSP90 or CDC37 disrupts the formation of the ternary complex, which destabilizes glycosylated F3-T3, and thereby suppresses F3-T3 oncogenic activity. Gliomas harboring F3-T3 are resistant to TMZ chemotherapy. HSP90 inhibitors sensitized F3-T3 glioma cells to TMZ via the inhibition of F3-T3 activation and potentiated TMZ-induced DNA damage. These results demonstrate that F3-T3 oncogenic function is dependent on the HSP90 chaperone system and suggests a new clinical option for targeting this genetic aberration in cancer.
Keywords: FGFR3-TACC3, glioma, HSP90, CDC37, glycosylation, TMZ resistance
Graphical abstract

Li et al. show that the FGFR3-TACC3 fusion protein is a client of HSP90. Inhibition of HSP90 destabilizes FGFR3-TACC3 glycosylation resulting in the suppression of FGFR3-TACC3 oncogenic activity. Gliomas harboring FGFR3-TACC3 are resistant to temozolomide chemotherapy. HSP90 inhibitors sensitize FGFR3-TACC3 glioma cells to temozolomide and reduced tumor progression in vivo.
Introduction
Fusion genes are chromosomal aberrations in malignancies that can be used as prognostic markers as well as therapeutic targets.1,2 The F3-T3 fusion gene was initially discovered as an oncogenic molecule in glioblastoma and subsequently found in many other cancer types. Based on clinical evidence, F3-T3 was found in glioblastoma patients before and after temozolomide (TMZ) and radiotherapy treatment, suggesting that targeting F3-T3 is a valid strategy for glioblastoma treatment. Here, we report an unrecognized association between F3-T3 and the HSP90/CDC37 chaperone system. The stability and oncogenic function of the F3-T3 fusion protein is highly dependent on HSP90 and co-chaperone CDC37. F3-T3 confers resistance to alkylating agents in glioblastoma (GBM). In combination, HSP90 inhibition sensitizes TMZ treatment response in F3-T3 glioma. In summary, this study elucidates the mechanisms of the HSP90/CDC37 chaperone system involved in F3-T3 oncogenic function and suggests HSP90-targeted therapy as a plausible treatment for F3-T3-containing glioma.
GBM is the most common and aggressive glial-derived malignant primary brain tumor, with a median overall survival of only 14.6 months in patients who received TMZ concurrent with and following postoperative radiotherapy.3,4 Genome-wide molecular characterization studies have identified the genetic alterations and epigenetic profiles associated with different types of gliomas, augmenting the histology-based World Health Organization (WHO) classification to enhance the diagnostic accuracy and guide the use of individualized treatments.5,6
Fusion genes, caused by aberrant genomic rearrangements, are common chromosomal aberrations in many hematopoietic malignancies and have been increasingly identified in solid tumors in recent years.6, 7, 8, 9 Using whole transcriptome sequencing, the F3-T3 gene fusion was discovered as the first recurrent oncogenic fusion event in adult GBM and bladder cancer.10, 11, 12 Subsequent studies also identified F3-T3 fusions in low-grade glioma (LGG) as well as many other cancer types.13, 14, 15, 16, 17 The F3-T3 fusion gene, generated by tandem duplication on 4p16.3, fuses most of the fibroblast growth factor receptor 3 (FGFR3) gene with the coiled-coil domain of transforming acidic coiled-coil containing protein 3 (TACC3) and produces a tumor-specific molecule with powerful tumor-initiating activities.10,11,18,19 F3-T3 is reported to play critical roles in at least two major hallmarks of cancer-genomic instability and mitochondrial metabolism, which establishes this fusion as a key genetic alteration in human cancers.10,19 Notably, F3-T3 was found to be highly expressed in both untreated and matched recurrent GBM, suggesting that F3-T3 gliomas are resistant to concurrent TMZ and radiotherapy treatment.20, 21, 22 Based on the fact that the F3-T3 fusion is indicative of response to FGFR tyrosine kinase inhibitors, a recent Phase II study (NCT01975701) applied molecular therapy to demonstrate F3-T3 as a targetable mutation in GBM;23 however, a lack of success was attributed to tumor heterogeneity and bypass activation of other oncogenic signaling pathways.
We previously reported that fusion of the FGFR3 and TACC3 genes resulted in the loss of the 3′ UTR of FGFR3, thereby escaping epigenetic regulation by miR-99a and leading to enhanced expression levels of F3-T3 RNA.11 In this study, we explore post-translational regulation imposed on F3-T3 protein. Using immunoprecipitation (IP) and liquid chromatography-mass spectrometry (LC-MS), we identify an association between F3-T3 and HSP90/CDC37. Evidence suggests that cancer cells can take advantage of HSP90/CDC37 chaperone machinery to protect unstable oncoproteins from degradation, thus augmenting malignant transformation.24, 25, 26 In this report, we provide evidence that the stability and oncogenic function of the F3-T3 fusion protein is dependent on the HSP90/CDC37 chaperone system, and that F3-T3 confers resistance to alkylating agents in GBM. In combination, we show that HSP90 inhibition sensitizes the TMZ treatment response in F3-T3 glioma cells. Our results reveal a previously unrecognized mechanism of regulation of the oncogenic F3-T3 fusion protein and suggest HSP90-targeted therapy as a plausible treatment for gliomas with F3-T3.
Results
F3-T3 forms a ternary complex with HSP90 and CDC37
Using an antibody against the hemagglutinin (HA) tag, we performed coIP in human GBM U-251 MG cells stably expressing either HA-F3-T3 or HA-FGFR3 and analyzed the protein complexes by 2D-LC/MS (Figure 1A). As F3-T3 contains the majority of wild-type (WT) FGFR3 fused with the C-terminal of TACC3, we used WT FGFR3 as a control (Figure 1B). A total of 1,022 proteins representing 925 hierarchical clusters were identified; 106 were unique to F3-T3, 172 were unique to WT FGFR3, and 647 were present in both samples. By comparing normalized spectra counts of proteins in the F3-T3 sample to those in the WT FGFR3 condition, we identified the most abundant proteins that were uniquely bound to F3-T3. The top protein was CKAP5 (also known as ch-TOG), which has been reported to bind TACC3 residues 672–688, and served to confirm an effective coIP (Table 1).27,28 We also identified those proteins pulled down by both constructs that showed the greatest relative increase or decrease in the level of association with F3-T3 over WT FGFR3, with the top candidates highlighted in Table 1. For those proteins that showed increased binding ratios to F3-T3 over full-length FGFR3, the molecular chaperone proteins encoded by the genes HSP90AB1, HSP90AA1, and CDC37 emerged as fifth, sixth, and seventh on the top 10 list, showing an approximately 4-fold increase in normalized spectral counts.
Figure 1.
F3-T3 shows a strong interaction with HSP90 and CDC37
(A) Schematic diagram of the methodology used for the identification of proteins that potentially interact with F3-T3. Cell lysates from U-251 MG cells stably expressing HA-F3-T3 and HA-FGFR3 underwent immunoprecipitation (IP) with anti-HA antibody and proteins were analyzed using 2D-LC/MS. (B) Schematic diagram of F3-T3, WT FGFR3, and truncated FGFR3 proteins. (C and D) Lysates from U-251 MG cells stably expressing EV, F3-T3, FGFR3, and tFGFR3 were immunoprecipitated with anti-HSP90 or anti-CDC37 antibodies followed by WB as indicated. The arrow indicates the specific CDC37 band. (E) U-251 MG cells stably expressing HA-EV and HA-F3-T3 were treated for 45 min with 100 nM HSP90 inhibitors (onalespib or 17-AAG), and lysates were immunoprecipitated with anti-HA antibody followed by WB as indicated. (F) U-251 MG cells stably expressing HA-F3-T3 were treated for 36 h with CDC37 siRNAs (#1 or #4), and lysates were immunoprecipitated with anti-HA antibody followed by WB as indicated. The blots of (E) and (F) were stripped and subsequently reprobed with anti-FGFR3 antibody to demonstrate the detection of the same HA-F3-T3 bands by anti-HA antibody. See Figure S7 for full images by anti-HA and anti-FGFR3 WB blots.
Table 1.
Preferential U251 glioma F3-T3 protein interactome (compared to wild-type FGFR3) measured by coIP coupled to mass spectrometry
| Top binding | Gene ID | F3-T3 NTSa | FGFR3 NTSa | F3-T3/FGFR3 | Identified protein | Accession number |
|---|---|---|---|---|---|---|
| Target proteins | ||||||
| FGFR3 | 576 | 407 | 1.4 | fibroblast growth factor receptor 3 isoform 1 precursor | NP_000133.1 | |
| TACC3 | 547 | 0 | – | transforming acidic coiled-coil-containing protein 3 | NP_006333.1 | |
| F3-T3 only | ||||||
| CKAP5 | 160 | 0 | – | cytoskeleton-associated protein 5 isoform a | NP_001008938.1 | |
| MX2 | 49 | 0 | – | interferon-induced GTP-binding protein Mx2 | NP_002454.1 | |
| ZNF318 | 16 | 0 | – | zinc finger protein 318 | NP_055160.2 | |
| ENAH | 16 | 0 | – | protein enabled homolog isoform X9 | XP_016857237.1 | |
| VCP | 16 | 0 | – | transitional ER ATPase isoform 2 | NP_001341856.1 | |
| CAV1 | 16 | 0 | – | caveolin-1 isoform alpha | NP_001744.2 | |
| F3-T3 preferred | ||||||
| HELZ2 | 33 | 3 | 12.3 | helicase with zinc finger domain 2 isoform X2 | XP_024307775.1 | |
| HERC5 | 29 | 5 | 6.4 | E3 ISG15–protein ligase HERC5 | NP_057407.2 | |
| ACIN1 | 17 | 3 | 6.2 | apoptotic chromatin condensation inducer 1, isoform 2 | NP_001158286.1 | |
| PRDX1 | 19 | 4 | 5.2 | Cluster of peroxiredoxin-1 | NP_001189360.1 | |
| HSP90AB1 | 93 | 20 | 4.6 | heat shock protein HSP 90-beta isoform a | NP_001258899.1 | |
| HSP90AA1 | 62 | 15 | 4.3 | heat shock protein HSP 90-alpha isoform 1 | NP_001017963.2 | |
| CDC37 | 54 | 14 | 3.9 | Cluster of HSP90 co-chaperone CDC37 | NP_008996.1 | |
| UTP18 | 59 | 15 | 3.8 | U3 small nucleolar RNA-associated protein 18 homolog | NP_057085.2 | |
| F3-T3 < WT FGFR3 | ||||||
| FAU | 7 | 38 | 0.2 | ubiquitin-like protein fubi and ribosomal protein S30 precursor | NP_001988.1 | |
| TRIOBP | 3 | 19 | 0.2 | TRIO and F-actin-binding protein isoform 6 | NP_001034230.1 | |
| PTDSS1 | 7 | 24 | 0.3 | phosphatidylserine synthase 1 isoform 1 | NP_055569.1 | |
| FXR2 | 10 | 36 | 0.3 | fragile X mental retardation syndrome-related protein 2 | NP_004851.2 | |
| VCAN | 6 | 18 | 0.3 | versican core protein isoform 3 precursor | NP_001157569.1 | |
Experimental schematic design in Figure 1A. Boldface type identifies the proteins investigated for this study.
Normalized total spectra counts.
We performed coIP experiments with western blotting (WB) to validate the result of the proteomic analysis. To resolve the contribution of the shortened FGFR3 segment in F3-T3 protein interactions, we generated truncated (t) FGFR3 (amino acids [aa] 1–759) by cleaving the WT FGFR3 (aa 1–806) at the same exon 18 breakpoint (Figure 1B; Table S2). U-251 MG cells stably expressing F3-T3, WT FGFR3, and tFGFR3 were subjected to coIP using antibodies against HSP90 or CDC37 and were probed for FGFR3 (N-terminal specific detecting all forms) by WB (Figures 1C and 1D). Both the WT FGFR3 and aa 1–759 tFGFR3 appear as 2 bands approximately 10 kDa apart that correspond to the larger fully N-glycosylated protein and the smaller, partially glycosylated form.11,29 Consistent with our MS results, F3-T3 exhibited greater interaction with HSP90 and CDC37 compared to WT FGFR3 (Figures 1C and 1D). Similar to F3-T3, tFGFR3 showed greater interaction with HSP90 than the WT FGFR3, suggesting that the tFGFR3 in F3-T3 may be key to HSP90 recruitment (Figure 1C). CoIP for HSP90 co-chaperone CDC37, however, showed a weak interaction with both the WT and tFGFR3, suggesting that the TACC3 segment (aa 647–838) in F3-T3 may facilitate this interaction (Figure 1D).
To clarify the binding interactions among the three proteins, we performed targeted reduction in protein levels with inhibitors of HSP90, or with small interfering RNA (siRNA) to CDC37, followed by coIP (HA) of F3-T3 to detect binding partners by WB. A short 45-min treatment with 100 nM HSP90 inhibitors onalespib or 17-AAG (17-N-allylamino-17-demethoxygeldanamycin) attenuated the binding between F3-T3 and HSP90 (Figure 1E). HSP90 deprivation strongly reduced the interaction between F3-T3 and CDC37 (Figure 1E). To silence the expression of CDC37, we transfected 4 different CDC37 siRNAs into the F3-T3 overexpression glioma cells for comparison (Figure S1A) and selected 2 with efficient knockdown for these studies. Following 36 h of treatment, we verified that the levels of F3-T3 (HA and FGFR3 probes) remained stable, while the knockdown of CDC37 reduced the coIP detection of CDC37 protein bound to F3-T3 (Figure 1F). Notably, this CDC37 knockdown also attenuated the F3-T3/HSP90 interaction (Figure 1F). These data suggest that F3-T3 forms a ternary complex with HSP90 and CDC37, and the deprivation of either HSP90 or CDC37 are sufficient to disrupt the formation of the F3-T3/HSP90/CDC37 ternary complex.
HSP90/CDC37 chaperone system mediates F3-T3 maturation and stability
In our experiments, treatment for 24 h with onalespib or 17-AAG diminished the glycosylated (mature, 145 kDa) form of F3-T3 protein levels in a concentration-dependent manner, while the partially glycosylated form (135 kDa) appeared unchanged or showed an increase at higher concentrations of the HSP90 inhibitors (Figure 2A and S1D). Over the initial 6 h of treatment, F3-T3 mRNA expression levels remained largely unchanged by onalespib treatment (Figures S1B and S1C), indicating that F3-T3 undergoes post-translational modulation during HSP90 inhibition. Partially glycosylated F3-T3 strongly interacted with HSP90 and CDC37 (Figures 1C and 1D) and was also efficiently dissociated from HSP90 by the inhibitor (Figure 1E). Importantly, the activation of F3-T3, indicated by the detection of phosphorylated FGFR (pFGFR), was significantly abrogated with the disappearance of the glycosylated form, regardless of the status of the partially glycosylated form, suggesting that the mature form accounts for the activation of F3-T3 (Figure 2A). Following 72 h of CDC37 siRNA treatment, there was a clear reduction in the levels of both the partially glycosylated and mature forms of F3-T3, along with the minimal detection of pFGFR (Figure S1A).
Figure 2.
HSP90 deprivation inhibits F3-T3 maturation and results in ubiquitin-mediated degradation of F3-T3
(A) U-251 MG cells stably expressing F3-T3 were exposed for 24 h to increasing doses of HSP90 inhibitors and immunoblotted with the indicated antibodies. (B and C) U-251 MG cells stably expressing F3-T3 were exposed for 2 h to HSP90 inhibitors, and the surface expression of F3-T3 was analyzed using flow cytometry; (B) representative percent-specific label by treatment, and (C) the mean fluorescence intensity (MFI) by treatment as fold of the isotype control from 3 independent experiments. ∗∗∗∗p < 0.0001 by 1-way ANOVA with Tukey’s post test. (D) U-251 MG cells stably expressing F3-T3 were treated with 200 nM HSP90 inhibitors for 2, 4, or 6 h, and subcellular lysates of membrane and cytoplasm were extracted and immunoblotted with the indicated antibodies. (E) U-251 MG cells stably expressing HA-F3-T3 were exposed for 6 h to 200-nM HSP90 inhibitors, with and without 10 μM MG132, and lysates were immunoprecipitated with anti-HA antibody followed by immunoblot with anti-ubiquitin antibody.
Membrane localization was previously reported to be essential for F3-T3 oncogenic signaling activity.18 We used flow cytometry to detect potential alterations in cell surface expression after 2 h of exposure to HSP90 inhibitors. The approximate 30% cell fraction with surface F3-T3 signal (cells positive for FGFR3-allophycocyanin [APC]) under no drug treatment was reduced to approximately 6%–7% F3-T3 positive following the onalespib or 17-AAG inhibitor exposure (Figure 2B). The mean fluorescence intensity of the membrane F3-T3 signal relative to the isotype control was decreased by approximately 80% in response to HSP90 inhibition by either onalespib or 17-AAG compared to the untreated cells (Figure 2C). Cell lysates were isolated into enriched membrane and cytoplasmic protein fractions and probed for FGFR3. Consistent with the flow cytometry results, the F3-T3 membrane levels detected by FGFR3 were highly reduced after the 2-h treatment with HSP90 inhibitors (Figure 2D). The glycosylated form of F3-T3 in the membrane fraction was strikingly downregulated at all time points; however, the partially glycosylated form showed a gradual enrichment in a time-dependent manner within the membrane fraction, indicating that partially glycosylated F3-T3 still participated in membrane trafficking. The cytoplasmic fraction showed a similar but highly attenuated pattern of protein expression (Figure 2D).
HSP90 inhibition was reported to generally disrupt HSP90 chaperoning activity and consequently direct the unstable HSP90 clients to the proteasomal degradation machinery through ubiquitination by E3 ligases.30,31 To determine whether F3-T3 undergoes polyubiquitination upon HSP90 inhibition, HA-F3-T3 expressing cells were treated for 6 h with HSP90 inhibitors, plus or minus MG132 proteasome inhibitor, and then immunoprecipitated for F3-T3 via HA and probed for ubiquitin by WB. At this 6-h time point, the ubiquitin signal was below detection without MG132; however, proteasome inhibition revealed a stronger accumulation of F3-T3-associated ubiquitin signal in samples treated with the HSP90 inhibitors compared to the untreated control (Figure 2E). Accordingly, probing with FGFR3 showed retention of the partially glycosylated F3-T3 levels, the absence of the mature F3-T3 in samples without MG132, and strong accumulation of partially glycosylated F3-T3 in samples protected from degradation by MG132. Further examination of early time points shows the ubiquitination of F3-T3 by 0.5–1 h after initiating HSP90 inhibition, and a clear reduction in F3-T3 levels after 2 h (Figure S1F). It was reported that HSP90 inhibitor-induced client depletion could be overcome by treatment with proteasomal inhibitors.32,33 We observed disproportionate degradation of the mature form of F3-T3 over the partially glycosylated form after inhibiting HSP90 chaperone activity for 24 h both in the presence or absence of MG132 proteasome inhibitor (Figure 2E). We suggest that, as a strong HSP90 client, newly synthesized nascent F3-T3 was not able to be fully glycosylated or was not able to maintain its full glycosylated stability during the deprivation of HSP90 chaperone activity.
F3-T3 is a strong HSP90 client
To better understand the relative dependence of F3-T3 stability on HSP90 function, we compared the outcome of 200 nM HSP90 inhibitor treatments on the levels of HSP90 clients ErbB2, Akt, EGFR, and FGFR3. The fully glycosylated form of F3-T3 was destabilized most rapidly—after 2 h of treatment—whereas this dose of HSP90 inhibitors appeared most effective for the reduction of WT FGFR3 and ErbB2 after 4 h, and insufficient to alter Akt and EGFR up to 6 h after treatment (Figures 3A and 3B). Using 100 nM onalespib or 17-AAG treatment over 6 h in the presence of the protein synthesis inhibitor cycloheximide (CHX), the levels of F3-T3, relative to 0 h, were significantly reduced by the inhibition of HSP90 interaction compared to the DMSO controls (Figures 3D and 3F), while the HSP90 inhibitors had no impact on the relative depletion of WT FGFR3 under the same conditions (Figures 3C and 3E). These studies show that the active, fully glycosylated form of F3-T3 is highly unstable in the absence of HSP90 interaction, and to a greater degree than some other known strong clients, such as FGFR3. This is consistent with our IP results, in which HSP90 and especially CDC37 showed a stronger interaction with F3-T3 compared to WT FGFR3.
Figure 3.
F3-T3 is a strong HSP90 client
(A and B) Time course of 200-nM HSP90 inhibitor treatments in U-251 MG cells stably expressing F3-T3 or FGFR3. Whole-cell lysates were harvested and immunoblotted with the indicated antibodies. (C–F) Upon treatment with DMSO vehicle (Vec) or 100 nM onalespib (C and D) or with (E and F) DMSO Vec or 100 nM 17-AAG, U-251 MG cells stably expressing FGFR3 or F3-T3 were treated with 50 μg/mL CHX, harvested at the indicated times, and lysates were immunoblotted with anti-FGFR3 (a representative WB is shown). WB band densities were quantified, normalized to β-actin, and the mean percent density levels relative to 0 h ± SEMs from 3 independent experiments are plotted. p values were calculated using a paired, 2-tailed Student’s t test.
Targeting the HSP90-CDC37 system inhibits F3-T3 mediated signaling and oncogenic potency
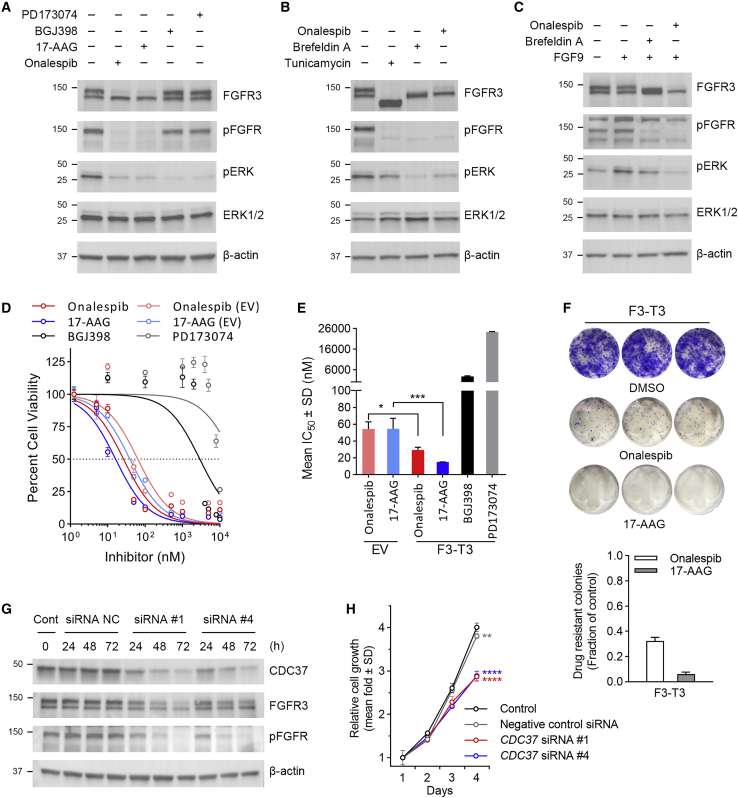
For many HSP90 client proteins involved in signal transduction pathways, association with the chaperone machinery maintains their metastable state that allows activation by ligand binding and phosphorylation.25 To evaluate HSP90 disassociation on F3-T3 signaling activity, we started by comparing 6-h treatment with HSP90 inhibitors (onalespib and 17-AAG) with FGFR inhibitors (BGJ398 or PD173074) on F3-T3 cells. Using equimolar (200 nM) treatments, we observed that HSP90 inhibitors more effectively reduced phosphorylated F3-T3 detection (Figure 4A). Because we have shown that the glycosylated form of F3-T3 is degraded when treating with HSP90 inhibitors, we introduced the N-linked glycosylation inhibitors tunicamycin and brefeldin A to compare the blockade of F3-T3 glycosylation on downstream activity.34,35 Using the same 6-h treatment paradigm, 200 nM onalespib or 5 μg/mL (17.8 μM) brefeldin A resulted in 135-kDa bands representing the partially glycosylated form of F3-T3, while 10 μg/mL (approximately 12 μM) tunicamycin completely shifted the F3-T3 protein to 120 kDa, representing the non-glycosylated form of F3-T3 (Figure 4B). F3-T3 phosphorylation was highly attenuated by all three inhibitors, whereas onalespib and brefeldin A were most effective in the reduction of downstream phosphorylated (p) ERK 1/2 signal transduction. Fibroblast growth factor 9 (FGF9), known as a glia-activating factor, belongs to the FGF subfamily that has the unique properties of activation of the IIIb splice variant of FGFR3.36 To investigate the role of F3-T3 glycosylation status in ligand/receptor interaction, a combination treatment of FGF9 and onalespib or brefeldin A was applied. The FGF9-induced activation of F3-T3 and downstream pERK 1/2 signal transduction were highly reduced by the inhibition of fully glycosylated F3-T3, particularly through onalespib (Figure 4C).
Figure 4.
Targeting the HSP90-cdc37 system inhibits F3-T3 mediated signaling and oncogenic potency
(A) U-251 MG cells stably expressing F3-T3 were exposed for 6 h to 200 nM of HSP90 inhibitors (onalespib and 17-AAG) or FGFR inhibitors (BGJ398 and PD173074) and lysates were immunoblotted with the indicated antibodies. (B) U-251 MG cells stably expressing F3-T3 were exposed for 6 h to 200 nM onalespib, 5 μg/mL brefeldin A (BFA) or 10 μg/mL of tunicamycin and lysates were immunoblotted with the indicated antibodies. (C) U-251 MG cells stably expressing F3-T3 were cultured in the absence or presence of FGF9 with or without 200 nM onalespib or 5 μg/mL BFA for 6 h and lysates were immunoblotted with the indicated antibodies. (D) U-251 MG cells stably expressing EV or F3-T3 were exposed to increasing concentrations of onalespib, 17-AAG, BGJ398, PD173074, or Vec control (DMSO) for 72 h, after which relative cell numbers were estimated using the CCK8 assay. A representative plot from 1 of 3 experiments is shown; means ± SEMs. (E) Mean IC50 of onalespib, 17-AAG, BGJ398, and PD173074 in U-251 MG cells stably expressing EV or F3-T3 from 3 independent experiments as illustrated in (D) and analyzed by 1-way ANOVA with Tukey’s post-test; ∗p < 0.05, ∗∗∗p < 0.001 (selectively indicating matched EV to F3-T3 treatment outcomes). (F) U-251 MG cells stably expressing F3-T3 were treated with DMSO or HSP90 inhibitors (200 nM) for 24 h, after which the drug was washed out and colony formation was visualized after 8 days. (G) U-251 MG cells stably expressing F3-T3 were cultured in the absence or presence of human CDC37 siRNA non-targeting control (NC), siRNA #1, or siRNA #4 for the designated hours, and lysates were immunoblotted with the indicated antibodies. (H) U-251 MG cells stably expressing F3-T3 were cultured in the absence or presence of CDC37 siRNA NC, siRNA #1, or siRNA #4 and relative cell numbers were monitored daily over 4 days using the CCK8 assay, ∗p < 0.01 to control and ∗∗∗p < 0.0001 to control and to NC siRNA on day 4 by 1-way ANOVA with Tukey’s post-test.
We then treated F3-T3 cell lines with increasing concentrations of HSP90 inhibitors or FGFR inhibitors over 72 h and evaluated cell growth. In contrast to FGFR inhibitors, we observed the potent suppression of cell growth by HSP90 inhibitors (Figures 4D and S2A) and a significant reduction in the HSP90 inhibitor half-maximal inhibitory concentrations (IC50) values in cells with F3-T3 compared to empty vector (EV) control cells (Figures 4E and S2B). A clonogenic assay also demonstrated that acute, 24-h exposure of cells to HSP90 inhibitors dramatically inhibited the capacity of the F3-T3 glioma cell lines to form colonies observed with crystal violet staining 8 days later (Figure 4F).
To explore the impact of CDC37 on F3-T3 activation, we first treated F3-T3 U251 MG cells with CDC37 siRNAs (#1, #4), comparing treatment times of 24, 48, and 72 h. Incremental reduction of the CDC37 protein with a longer treatment time was verified by WB, and both the fully glycosylated and the actively phosphorylated F3-T3 showed a similar reduction in a time-dependent manner (Figure 4G). Treatment for 4 days with negative control (NC) siRNA showed a small 5% reduction in the relative growth of F3-T3 cells, while CDC37 siRNAs further reduced cell growth by an additional 25% compared to untreated controls (Figure 4H).
F3-T3 confers resistance to TMZ-induced DNA damage
Recently, FGFR pathway signaling was shown to be involved in DNA repair and to confer therapeutic resistance in human cancers.37,38 We analyzed The Cancer Genome Atlas (TCGA) and GSE16011 GBM cohort for the impact of FGFR1, FGFR2, FGFR3, and FGFR4 expression. Only the patients with high FGFR3 expression consistently showed significantly poorer survival rates across both cohorts (Figures 5A and S3A–S3F). Higher FGFR3 expression was also associated with lower survival in LGG patients from the TCGA (Figure S4C). Furthermore, high FGFR3 expression in GBM and LGG patients treated with alkylating therapy was associated with lower overall survival (Figures 5B and S4D).
Figure 5.
F3-T3 confers resistance to alkylating chemotherapy in glioma
(A) Kaplan-Meier overall survival curves in GBM patients based on the upper and lower 30% quantiles of FGFR3 expression (TCGA and GSE16011). (B) TCGA GBM patients separated by FGFR3 expression and alkylating treatment modality. Non-treated and treated patients were analyzed, respectively, with Kaplan-Meier curves by upper and lower 30% quantiles of FGFR3 expression. p values were calculated by log rank test. (C) U-251 MG cells stably expressing EV, FGFR3, F3-T3, or F3-T3 K508R were treated with increasing concentrations of TMZ. After 5 days, relative viability was measured using the CCK8 assay. Means ± SEMs. p values compare best-fit IC50 estimates (all to EV or F3-T3 to FGFR3) by a normalized log (inhibitor) model. (D) U-251 MG cells stably expressing F3-T3 were exposed to either indicated concentrations of TMZ or relative concentrations of DMSO for 7 days, and lysates were immunoblotted with the indicated antibodies. (E) GSEA plots show the upregulation of hallmark DNA repair genes in human astrocytes expressing F3-T3 treated with Vec (F3-T3, n = 5 replicates) compared to human astrocytes expressing F3-T3 treated with the FGFR inhibitor PD173074 for 12 h (F3-T3 PD, n = 5 replicates), F3-T3 K508M treated with Vec (F3-T3 KD, n = 3 replicates) and empty vector treated with Vec (n = 3 replicates). p value = 1E−10 (F3-T3 vs F3-T3 PD; F3-T3 vs F3-T3 KD; F3-T3 vs Vec). NES, normalized enrichment score. (F) U-251 MG cells stably expressing EV, F3-T3, or F3-T3 K508R were treated with DMSO or TMZ and assayed for colony formation after 8 days. The data show mean value relative to Vec ±SD (n = 3), ∗∗p < 0.01 by 1-way ANOVA with Tukey’s post test. (G) U-251 MG cells stably expressing EV, F3-T3, or F3-T3 K508 were treated with 50 μM TMZ for 24 h and compared to 0 h lysates immunoblotted with indicated antibodies. (H) Nuclear pH2AX foci immunofluorescence (DAPI counterstain) in U-251 MG cells stably expressing EV, F3-T3, or F3-T3 K508R after 48 h exposure to DMSO or 50 μM TMZ. Scale bar, 25 μm. Mean fraction of positive nuclei ±SD analyzed by 2-way ANOVA with Tukey’s post test; ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
The F3-T3 fusion protein includes most of the FGFR3 protein with its major phosphorylation sites (Figure S4A). We treated F3-T3 glioma cell lines with TMZ, an alkylating agent that is used for frontline chemotherapy for GBM.39 We observed a statistically significant TMZ-induced reduction in cell viability, with cells expressing FGFR3 or F3-T3 requiring ∼2- to 4-fold higher TMZ concentrations to achieve the mean IC50 values observed in the EV and F3-T3 K508R (kinase-dead F3-T3) groups (Figure 5C). The 95% confidence intervals of the IC50 measurements for the different treatment groups were calculated: EVIC50 = 10.9–20.9 μM; FGFR3IC50 = 22.1–41.9 μM; F3-T3IC50 = 44.9–88.5 μM; and F3-T3 K508RIC50 = 12.9–21.2 μM. We also confirmed significant TMZ growth resistance in LN-229 glioma cells comparing EVIC50 = 21.0–54.3 μM to F3-T3IC50 = 66.4–163.4 μM (Figure S2C).
With increasing concentrations of TMZ, we observed the stimulation of the F3(Y647/648)-T3 activation loop (Figure S4A), visualized by WB using an antibody against phosphorylated-FGFR(Y653/654) (Figure 5D). Similarly, the expression level of F3-T3 (FGFR3 probe) appeared strikingly elevated in response to increasing concentrations of TMZ (Figure 5D). While appearing elevated in response to TMZ, the pERK band intensity did not appear to differ across concentrations (Figure 5D). Using a clonogenic assay, we showed that after 8 days of treatment with 25 μM TMZ compared to DMSO controls, the fraction of drug-resistant colonies was significantly elevated in glioma cells expressing F3-T3 (Figures 5F and S2D) or WT FGFR3 (Figure S4F) compared to EV controls. Furthermore, U251 MG cells expressing F3-T3 K508R remained sensitive to TMZ, with a significantly lower fraction of colonies than F3-T3, similar to the EV control (Figure 5F).
We conducted a gene set enrichment analysis (GSEA) on gene expression from human astrocytes transduced with a lentivirus expressing F3-T3 (F3-T3) and treated with vehicle (Vec; DMSO), F3-T3 transduced cells treated with the FGFR inhibitor PD173074 for 12 h (F3-T3 PD), F3-T3 K508M (F3-T3 KD, F3-T3 kinase-dead form) transduced cells treated with Vec, and EV transduced cells treated with Vec.19 The genes involved in DNA repair were significantly enriched and positively associated with F3-T3 activation (Figures 5E and S4E). We examined levels of the DNA damage marker pH2AX (H2AX phosphorylated on serine 139) in U-251 MG cells expressing EV, F3-T3, and F3-T3 K508R. Comparing 24-h TMZ treatment to 0-h control lysates, the pH2AX band intensity increased in cells expressing EV or F3-T3 K508R, whereas levels appeared unchanged in the F3-T3 cells with hyperphosphorylation of pFGFR (Figure 5G). We used pH2AX immunofluorescence labeling to quantify damage, setting ≥25 foci per nucleus as a positive threshold. After 48 h treatment with 100 μM TMZ, cells expressing EV, F3-T3, and F3-T3 K508R all showed significant pH2AX DNA damage compared to DMSO controls; however, the fraction of pH2AX-positive nuclei in U-251 MG cells expressing F3-T3 was significantly lower compared to cells expressing EV or F3-T3 K508R (Figures 5H and S5A).
Targeting F3-T3 glioma through combined TMZ and HSP90 inhibition
We performed drug combination testing experiments in LN-229 and U-251 cells with F3-T3 (Figures 6A and S5B) and examined the joint effect with CalcuSyn software 2.0 (Biosoft) that calculates a combinatorial index (CI): synergism (CI < 1) and antagonism (CI > 1). Heatmap matrices (Figures 6A and S5B) illustrating the percentages of cell death relative to the untreated cells suggested the combinatorial drug benefit. The CI values at various effective dose (ED) combinations were calculated, showing a synergic inhibition of F3-T3 tumor cell growth by the combination of onalespib and TMZ (Table S1). Consistently, a clonogenic assay demonstrated that the combination of with TMZ significantly inhibited the formation of TMZ-resistant colonies in F3-T3 glioma cell lines (Figure 6B). We next examined levels of the DNA damage marker pH2AX by WB in U-251 MG cells expressing F3-T3 after 24 h of treatment (+/−) TMZ and HSP90 inhibitors. The increased intensity of the pH2AX level was evident in the combinatorial treatment of TMZ with onalespib or with 17-AAG, while both the TMZ-induced activation of F3-T3 (pFGFR) and the levels of mature F3-T3 (FGFR3) appeared attenuated by the HSP90 inhibitors (Figure 6C). These results were recapitulated by the assessment of pH2AX in situ showing that foci were significantly induced in U251 F3-T3 cells treated with the TMZ and HSP90 inhibitor combination treatments compared to TMZ alone, while neither of the HSP90 inhibitors had any effect on pH2AX foci levels alone (Figures 6D and S5C).
Figure 6.
Hsp90 inhibitor chemosensitizes F3-T3 glioma cells to TMZ
(A) Combination drug matrix for estimates of interactions using CalcuSyn software. LN-229 cells stably expressing F3-T3 were treated (+/−) onalespib and TMZ as shown and relative CCK8 viability was measured after 72 h. Percentages are the means from 3 independent experiments and indicate cell death relative to the untreated controls. (B) U-251 MG cells or LN-229 cells stably expressing F3-T3 treated with onalespib or 17-AAG in combination with DMSO or TMZ and assayed for colony formation after 8 days. Mean colonies (treatment/DMSO ±SD (n = 3); ∗∗∗p < 0.001 each drug alone to each combination by 1-way ANOVA with Tukey’s post-test. (C) U-251 MG cells stably expressing F3-T3 were treated with either 200 nM onalespib or 17-AAG combined with DMSO or 50 μM TMZ for 24 h, and lysates were immunoblotted with the indicated antibodies. (D) Nuclear pH2AX foci immunofluorescence (DAPI counterstain) in U-251 MG cells stably expressing F3-T3 in response to 24-h exposure with 200 nM onalespib or 17-AAG combined with DMSO or 50 μM TMZ. Scale bar, 25 μm. Mean fraction of positive nuclei ±SD analyzed by 2-way ANOVA with Tukey’s post-test; ∗∗∗∗p < 0.0001.
We next examined the potential therapeutic effects of onalespib and TMZ on mice bearing intracranial tumors. Intracranial U-251 MG tumors harboring F3-T3 were established (injection of 2 × 105 cells). TMZ treatment was given for 3 consecutive days and administered onalespib only twice, initially 1 day before TMZ start and then again 7 days later (Figure 7A). We also repeated the intracranial model in a different condition (Figure S6B). The combination treatments of onalespib and TMZ significantly improved mouse survival. The improvement was in dramatic contrast to the onalespib or TMZ single-agent treatment groups (Figures 7B and S6C). Bioluminescence imaging showed that the combination treatment exerted a potent antitumor effect on tumor growth (Figures 7C, 7D, S6D, and S6E).
Figure 7.
Targeting F3-T3 glioma through combined TMZ and Hsp90 inhibition
(A) Schematic representation of the preclinical study design to assess the combination effects of onalespib and TMZ on U251 MG F3-T3 glioma xenograft growth and mouse survival in vivo. Four treatment groups consisted of n = 10 mice (5 males/5 females): Vec, TMZ, onalespib, or combination. Fourteen days after tumor engraftment, mice were treated with 5 mg/kg TMZ on days 15–17 (or Vec control), and/or with 30 mg/kg onalespib on days 14 and 21 (or Vec control). (B) Survival of glioma-bearing mice was tracked after intracranial implantation. ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 by log rank (Mantel-Cox) test. (C) Relative xenograft bioluminescence quantification results from 14–63 days after implantation. Mean fold of 14-day level ±SEM; the black bar indicates the treatment window over days 14–21; (a) p < 0.05, (b) p < 0.01 by 2-way ANOVA with Tukey’s post-test (points compare to Vec control on days 49, 56, and 63; lines compare between TMZ or onalespib and combination on days 56 and 63). (D) IVIS bioluminescence images (photons/s/cm2/sr) of mice bearing xenografts derived from F3-T3-luciferase-coexpressing U-251 MG cells. (E) Proposed model for the post-translational regulation by the HSP90/CDC37 chaperone system on F3-T3 protein. F3-T3 achieves mature status by association with the HSP90/CDC37 chaperone system. Subsequent chaperone interaction is necessary to maintain the fully glycosylated F3-T3 status enabling oncogenic function. Hsp90 inhibitor blockade of this interaction leads to the ubiquitin-mediated degradation of the mature F3-T3 form, as well as suspension of F3-T3 maturation, resulting in the inactivation of F3-T3.
Discussion
Chromosomal translocations that generate in-frame oncogenic gene fusions provide numerous potential targets for targeted cancer therapies.19 Recently, efforts have been made seeking strategies for targeting the F3-T3 fusion gene.19,40,41 Our previous study demonstrated mechanisms for F3-T3 upregulation at the gene level through tandem duplication and loss of the miR-99a control at the 3′UTR of FGFR3 upon fusion with TACC3.11 In this study, we characterize a regulatory mechanism of F3-T3 fusion protein through the HSP90/CDC37 chaperone system. We provide evidence that glycosylated F3-T3, or the mature form of F3-T3, is stabilized in tumor cells by association to HSP90/CDC37. Furthermore, the maintenance of glycosylation through this interaction mediates an FGFR-driven pathway by F3-T3 that promotes TMZ-resistant tumor growth (Figure 7E). Using a preclinical mouse model, we demonstrate that combination therapy using TMZ and an HSP90 inhibitor sensitizes the F3-T3 glioma tumor cells to the alkylating agent (Figure S6A) and significantly improves survival.
The FGFR family consists of four member proteins that control key cell behaviors, including proliferation, survival, and migration. As such, signaling of FGFRs is susceptible to hijacking by cancer cells and has been shown to play oncogenic roles in many cancers.42 As a molecular chaperone, HSP90 interacts with numerous substrate proteins that are called clients.43 Among the FGFRs, FGFR3 was shown to be a strong HSP90 client.44 In this study, we found that F3-T3 strongly associated with HSP90, showing an even greater HSP90 chaperoning interaction compared to FGFR3. Because CDC37 was recognized as a highly specialized co-chaperone adaptor for kinases,43 we also investigated the role of HSP90 co-chaperone CDC37 in the F3-T3 chaperoning system. Our data showed a stronger interaction between CDC37 and F3-T3 compared to WT FGFR3. We demonstrated that F3-T3 forms a ternary complex with HSP90/CDC37, and the disassociation of either HSP90 or CDC37 results in the disruption of this ternary complex.
It is reported that HSP90 tends to associate with intrinsically unstable clients instead of binding specific sequence motifs, and clients adopt a conformation that HSP90 and CDC37 can recognize.43 The F3-T3 fusion protein contains most of the FGFR3 sequence fused to the C terminus of TACC3. One scenario is that this fusion results in intrinsic instability in the protein conformation of F3-T3, thus making it a better client for HSP90. Another scenario is that loss of the C-terminal tip of FGFR3 in the F3-T3 fusion is sufficient to generate instability that is more recognizable by HSP90. In our results, both tFGFR3 (the protein sequence is the same as the FGFR segment within F3-T3) and F3-T3 showed a stronger interaction with HSP90 compared to WT FGFR3, suggesting that the truncated FGFR segment in F3-T3 plays a larger role in changes for HSP90 association. Numerous HSP90 client kinases have been reported to be associated with this chaperone primarily as a function of their instability rather than any salient structural features, or for protection from degradation, and with CDC37 primarily participating by conferring kinase recognition.43 For CDC37 interaction, we did not detect a significant difference between tFGFR3 and WT FGFR3. However, F3-T3 showed the strongest interaction with CDC37, suggesting that the TACC3 sequence in F3-T3 influences the association with CDC37. Because the TACC3 domain has been shown to mediate dimerization of the fusion protein,45 CDC37 binding may be enhanced in the dimerized configuration.
We also provided evidence that disruption of HSP90/CDC37 chaperoning resulted in a significant degradation of the glycosylated form of F3-T3 and the inhibition of F3-T3 maturation. The process of HSP90/CDC37 chaperoning requires ATPase activity. The inhibition of the ATPase activity of HSP90 prevents the reloading of clients onto the chaperone, thus redirecting the clients to degradation or aggregation.43 Intriguingly, our data suggest that the fate of F3-T3 varies depending on the glycosylation status following inhibitor-induced dissociation from HSP90. Glycosylated (mature) F3-T3 underwent a significant proteasome-dependent degradation, whereas partially glycosylated F3-T3 was not efficiently degraded. Similarly, it has been shown in the literature that some strong clients were not degraded, even though they were efficiently dissociated from HSP90 by the inhibitor.43 Among these client proteins, the receptor tyrosine kinase AXL and FGFR2 fusion proteins (FFs) also showed different fates upon HSP90 inhibition depending on glycosylation status.32,33 Interestingly, the HSP90 dissociation-induced degradation of glycosylated AXL and FGFR2 fusion proteins was attenuated by proteasomal inhibitors.32,33 In contrast, degradation of glycosylated F3-T3 after HSP90 dissociation was not blocked by proteasome inhibitors, suggesting that glycosylated F3-T3 is particularly “addicted” to the HSP90-mediated protein stabilization mechanism.
This dependence on HSP90 provides a potential window for therapeutic intervention. Our in vitro and preclinical mouse model experiments support this notion, that F3-T3 activation was blocked by HSP90 dissociation as strongly as that by the N-linked glycosylation inhibitor or the FGFR inhibitor. As set forth, the F3-T3 fusion gene was discovered as an oncogenic molecule in many cancer types. We suggest that the association between HSP90 and F3-T3 is not limited to glioblastoma and that targeting of HSP90 can be also applied to other cancer types harboring F3-T3. Emerging evidence indicates that the FGFR pathway promotes DNA repair in response to chemotherapy in cancer.37,38 Consistently, we showed that high expression of FGFR3 was associated with a worse response to alkylating chemotherapy in GBM. F3-T3 was found to be highly expressed in both the primary and matched recurrence GBM.20, 21, 22 Our GSEA analysis reveals that F3-T3-associated genes are enriched in DNA repair. Thus, F3-T3 is likely to promote DNA repair in response to TMZ via the FGFR signaling pathway.
As the frontline therapeutic strategy of GBM, concomitant treatment with radiotherapy plus TMZ result in a clinically meaningful and statistically significant survival benefit with minimal additional toxicity.46 However, the prognosis of GBM is still poor, with a median survival time of 14.6 months following diagnosis. The first-in-human study with oral HSP90 inhibitor TAS-116 showed promising antitumor efficacy in patients with advanced solid tumors.47,48 Multiple inhibitors of HSP90 are being clinically evaluated in combination with other anticancer agents.49 To improve the therapeutic effect of TMZ on GBM with the F3-T3 gene fusion, we propose to evaluate combination therapy with TMZ and HSP90 inhibitors. Several studies did not detect a synergetic effect with the combined therapy of TMZ and HSP90 inhibitor in glioma,50,51 but these studies did not consider the F3-T3 status. Here, we demonstrated that F3-T3 is addicted more to the HSP90/CDC37 chaperone system than other known clients and that HSP90 inhibition decreased F3-T3 activity to sensitize glioma cells to TMZ treatment. The inhibition of other known oncogenic clients may provide additional suppression of F3-T3-fueled tumor growth.
In summary, this study elucidates mechanisms of the HSP90/CDC37 chaperone system involved in F3-T3 oncogenic function and provides laboratory justification and motivation for possible therapeutic interventions in clinical trials that specifically target this genetic event in tumors harboring the F3-T3 fusion gene.
Materials and methods
Plasmids, cloning, and siRNAs
HA-tagged F3-T3 in pcDNA3.1 expression plasmid was made from GBM-13 patient samples, as previously described.11 FGFR3 and HA-FGFR3 constructs were purchased from Genscript. The constructs of F3-T3, F3-T3 K508R, HA-F3-T3, FGFR3, HA-FGFR3, and truncated FGFR3 were inserted into the pcDNA3.1 expression plasmid (Invitrogen). HA was tagged at the C-terminal of the proteins. The aa sequences of plasmids are listed in Table S2 and the generation of F3-T3 K508R was described in Table S3. siRNAs targeting human CDC37 (#1) J-003231-09, (#2) J-003231-10, (#3) J-003231-11, (#4) J-003231-12, and non-targeting control (NC) D-001810-01 were purchased from Dharmacon and were transfected into cells using Lipofectamine RNAiMAX (Thermo Fisher Scientific).
Cell culture and cell line generation
The human GBM U-251 MG cell line was obtained from the European Collection of Authenticated Cell Cultures (ECACC) through Millipore-Sigma and the human GBM LN-229 cell line was obtained from the American Type Culture Collection (ATCC). For the generation of stably transfected cell lines, 3 × 105 U-251 MG and LN-229 cells were transfected with 2.5 μg HA-F3-T3, F3-T3 K508R, WT HA-FGFR3, or truncated FGFR3 plasmids, respectively, via Lipofectamine 3000 (Invitrogen) following the manufacturer’s instructions. Stably transfected cells were selected by antibiotic resistance to 0.5 mg/mL G418 (Invitrogen) for a minimum of 2 weeks, after which relative overexpression of the constructs was confirmed by immunoblot analysis using a mouse monoclonal antibody probing for FGFR3 amino acids 25–124 (sc-13121, Santa Cruz), which is common to all 3 constructs.
Identification of F3-T3 binding partners by MS
CoIP analysis of HA tag-captured protein complexes was performed in cell line U-251 MG stably engineered to express HA-FGFR3 or HA-F3-T3. Cells were incubated in Pierce IP Lysis Buffer plus Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific) for 10 min on ice, and soluble protein supernatants were collected after centrifugation at 14,000 × g for 15 min at 4°C. IP with anti-HA antibody-conjugated magnetic beads (11,846; Cell Signaling Technology) was performed overnight at 4°C with rotation, followed by 3 washes with cold PBS. The final wash was removed, the beads were frozen at −80°C, and shipped on dry ice to the Mass Spectrometry Core Lab at the Whitehead Institute for Biomedical Research (Massachusetts Institute of Technology [MIT], Cambridge, MA). Digestion protocol eluates were analyzed using 2-dimensional (2D) liquid chromatography (8 fractions) followed by MS-MS using 60-min gradients per fraction (2D-LC/MS). Processed data were provided in a Scaffold 4 proteome analysis file. Normalized total spectrum counts from the samples were obtained in Scaffold 4 using a 95% minimum peptide threshold and a 99% minimum protein threshold with a 3-peptide minimum. Fold changes in normalized spectral counts between the HA-F3-T3 and the HA-FGFR3 control group were used to determine the top proteins that showed unique, increased, or decreased associations with the F3-T3 fusion protein construct. The result of the MS is provided in Table S4.
Immunoblot and IP
Immunoblot was performed as previously described.11 The following primary antibodies were used: FGFR3 (sc-13121), HSP90 (sc-13119), CDC37 (sc-17758), HA probe (sc-7392), ubiquitin (sc-7392), and β-actin (sc-47778) from Santa Cruz Biotechnology; phospho-(tyr653/654)-FGFR (3471), CDC37 (4793), Na/K-ATPase (3010), EGFR (4267), Akt (4691), HER2/ErbB2 (2242), Erk1/2 (2242), and phospho-Erk1/2 (9101) from Cell Signaling Technology; and pH2AX (05–636, clone JBW301) from Millipore-Sigma. The quantification of WB band densities was provided in Table S6.
For IP, cells under treatments were lysed with Pierce IP Lysis Buffer (Thermo Fisher Scientific) plus Halt PI-PhI for 10 min on ice. Supernatants were collected after centrifuging at 14,000 x g for 15 min at 4°C. IP was performed by incubating cellular lysate supernatants with either isotype control antibody- or anti-HA antibody-conjugated magnetic beads (Cell Signaling Technology, 8726 and 11846), or with Santa Cruz mouse monoclonal anti-HSP90 (sc-13119) or CDC37 (sc-17758) antibodies followed by protein A agarose (Santa Cruz, sc-2001) or protein G plus agarose (Santa Cruz, sc-2002). IP incubations were performed by gentle rotation overnight at 4°C, after which the immune complexes were washed three times in cold PBS, suspended in 3 parts IP lysis buffer:1 part 4× sample buffer (Bio-Rad), boiled at 95°C for 5 min and analyzed by SDS-PAGE and immunoblot.
Cell viability and clonogenic assays
A CCK8 assay (Dojindo) was performed to measure relative cell viability. Cells were seeded at 1,000 cells in 100-μL medium per well in 96-well plates in quintuplicate, allowed to attach overnight, and then exposed to the indicated treatments. At 72 or 120 h after cell plating, 10 μL CCK8 reagent was added per well and incubated for 2 h. Relative cell viability was measured as relative 96-well absorbance levels measured at 450 nm using an Epoch Microplate Reader and Gen5 2.0 software (Biotek). CalcuSyn software (Biosoft) was used to determine the CI in drug combination studies. GraphPad Prism 9.0.2 was used to generate drug treatment best-fit lines and IC50 values using log (inhibitor) compared to normalized response analysis (Hill slope fixed at −1).
For the clonogenic assay, cells were seeded in 6-well plates at 2,000 cells/well in triplicate, allowed to attach overnight, and then exposed to the indicated treatments. Cells were incubated for 8 days to allow the formation of colonies and then were gently washed twice with ice-cold PBS and fixed with cold 100% methanol for 10 min on ice. Colonies were stained with 0.5% Crystal Violet solution in 25% methanol for 10 min, washed with water several times, and then air dried. Colony numbers were counted, and the surviving fraction was calculated as the ratio of the plating efficiency of the treated cells to that of drug diluent controls.
Flow cytometry
Cells were harvested after the indicated treatments and times, washed, adjusted in cell suspension to a concentration of 5 × 106 cells/mL in ice-cold PBS plus 3% fetal bovine serum (FBS). The cells were incubated with APC-conjugated anti-human FGFR3 (136334, R&D) or mouse immunoglobulin 1 (IgG1) control (11711, R&D) primary antibody for 45 min at 4°C in the dark. Cells were washed 3×, centrifuged at 300 × g for 5 min, and resuspended in 500 μL ice-cold PBS plus 3% FBS for immediate analysis on a BD Accuri C6 Plus Flow Cytometer.
Subcellular fractionation
Enriched membrane proteins were obtained using the Mem-PER Plus Membrane Protein Extraction Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, approximately 3 × 106 cells were harvested by scraping, centrifuged at 300 × g, and resuspended twice in cell wash solution. The final pellet was suspended in 0.45 mL permeabilization buffer, vortexed briefly, and incubated for 10 min at 4°C with constant mixing. After a 15-min centrifugation at 16,000 × g, the supernatant containing cytosolic proteins was carefully transferred to a new tube, and 0.3 mL solubilization buffer was added to the pellet and incubated at 4°C for 30 min. After centrifuging at 16,000 × g for 15 min at 4°C, the supernatant containing solubilized membrane and membrane-associated proteins was transferred to a new tube, aliquoted, and stored at −80°C for future use.
Gene expression analysis
Cells-to-cDNA TM II, reverse transcription without RNA isolation kit (Thermo Fisher Scientific) was used to synthesize cDNA directly from treated cells following the instructions provided by the manufacturer. The sequences of primers used for the amplification of the fusion gene (FGFR3-TACC3) and 18S rRNA cDNAs were as follows:
- FGFR3-TACC3 forward: 5′-CCGTGACGTCCACCGACGTAAAGGCGACACAGG-3′
- Reverse: 5′-CCTTCTGCTTCTGAACTTCCTCCATGGCCTGG-3′.
- 18S rRNA forward: 5′-GCTTAATTTGACTCAACACGGGA-3′
- Reverse: 5′-AGCTATCAATCTGTCAATCCTGTC-3′.
Immunofluorescent labeling of pH2AX foci
U-251 MG and LN-229 cells expressing EV, F3-T3, F3-T3 K508R, and WT FGFR3 were seeded and grown overnight in LabTek Chamber Slides (Thermo Fisher Scientific). Cells were treated with 100 μM TMZ, 200 nM onalespib or 17-AAG, or DMSO Vec. After 24 h, cells were fixed with 4% formaldehyde, washed with PBS, permeabilized with 0.5% Triton X-100 (10 min), blocked in 5% normal donkey serum (20 min), and then probed with antibodies detecting pH2AX (Millipore) followed by Alexa Fluor 594-conjugated secondary antibody. Slides were mounted with SlowFade Diamond mountant with DAPI (Thermo Fisher Scientific) and analyzed using an Eclipse Ts2R microscope (Nikon). To quantify foci, a threshold of ≥25 foci/nuclei was set to score cells as positive, and the fraction of positive cells/nuclei was calculated for each treatment condition.
Intracranial xenograft glioma models
All of the animal studies were performed in accordance with Wake Forest School of Medicine institutional animal care and use committee (IACUC)-approved protocols. For each survival study, forty 6- to 12-week-old athymic mice (Nu/Nu, 20 male and 20 female, Charles River Laboratories) were implanted in the right frontal lobe (medial-lateral [ML] 2 mm, anterior-posterior [AP] 0 mm, dorsal-ventral [DV] 2.75 mm) with 0.2×106 or 0.5×106 U-251 MG cells expressing F3-T3. Two weeks after implantation, mice were randomized using tumor size (in vivo imaging system [IVIS] bioluminescence estimates) across 4 treatment cohorts (N = 10: 5 males, 5 females) consisting of Vec, TMZ, onalespib, or TMZ and onalespib in combination. TMZ (5 mg/mL in 20% DMSO) was administered by oral gavage and onalespib (6 mg/mL in 15% SelleckChem Captisol-S4592 in water) by tail vein injection given on the days and in the concentrations indicated in the Figures 7A and S6B) schematic overview. Animals were weighed every 1–2 days and tumor growth was monitored by IVIS bioluminescence measurements made up to 49 days following cell implantation. Animals were humanely euthanized at survival endpoints when declining health and well-being were observed.
Public data
TCGA glioma samples
TCGA molecular data (gene expression) and clinical characteristics (survival time, censored status, and treatment history) for GBM and LGG patients were accessed through the University of California, Santa Cruz (UCSC) Xena platform.52 Among the TCGA GBM samples, there were 9 F3-T3 fusion samples: TCGA-06-6388, TCGA-06-6390, TCGA-19-5958, TCGA-12-0820, TCGA-12-0826, TCGA-27-1835, TCGA-28-2506, TCGA-74-6578, and TCGA-76-4925.19 The clinicopathological information of TCGA glioma samples is provided in Table S5.
GSE16011 glioma samples
Data (n = 276) were collected from the Erasmus University Medical Center tumor archive (1989–2005).53 The raw gene expression data and clinical information were obtained through the GEO database. The clinicopathological information of GSE16011 glioma samples is provided in Table S5.
E-MTAB-6037 astrocyte samples
Comparative analysis of gene expression was obtained from human astrocytes transduced with a lentivirus expressing F3-T3 treated with DMSO (n = 5), F3-T3 treated with the PD173074 for 12 h (n = 5), F3-T3 K508M treated with DMSO (n = 3), and EV treated with DMSO (n = 3).19 Ranked lists for GSEA were respectively obtained from three independent comparisons: F3-T3 versus F3-T3 PD; F3-T3 versus F3-T3 KD; F3-T3 versus Vec. GSEA was performed using the ClusterProfiler R package.54 Transcriptomic microarray gene expression data were obtained in ArrayExpress with accession number E-MTAB-6037.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 9.0.2 software as indicated in the text and included the following: ANOVA (1-way and 2-way), 2-tailed Student’s t test (unpaired and paired), normalized log (response) inhibition for IC50 estimates, and log rank (survival) test, as indicated. The survival R package (http://cran.r-project.org/web/packages/survival/index.html) was used for the survival analysis. Kaplan-Meier curves with p values from the log rank test were used for presenting the results of survival analysis.
Study approval
The animal study was approved by the institutional review board of the Wake Forest School of Medicine.
Acknowledgments
We thank Denise Herpai, Puja Sharma, and Yue Huang (Debinski lab) for sharing their expertise on performing orthotopic xenograft surgery of glioma and IVIS tumor imaging for our mouse model studies. This work was supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award no. R01CA183153 to WZ, and by the NCI Cancer Center Support Grant to the Wake Forest Baptist Comprehensive Cancer Center (P30CA012197). W.Z. is supported by the Hanes and Willis Professorship in Cancer and a fellowship from the National Foundation for Cancer Research. X.J.Y. and T.L. are supported by the National Natural Science Foundation of China (no. 81872063). T.L. is also supported by the National Natural Science Foundation of China (no. 82102951).
Author contributions
Conceptualization, T.L., W.Z., F.M.-G., and P.-C.C. Data curation, Q.S. and X.W. Formal analysis, T.L., M.E.F., S.V.N., Q.S., and X.W. Funding acquisition, W.Z. Investigation, T.L., F.M.-G., M.E.F., and E.A.B. Methodology, T.L., F.M.-G., M.E.F., E.A.B., P.-C.C., B.C.P.K., and W.D. Project administration, W.Z. and X.Y. Resources, W.Z., M.E.F., and E.A.B. Supervision, W.Z. and X.Y. Validation, T.L., W.Z., and M.E.F. Visualization, T.L. and M.E.F. Writing – original draft, T.L., M.E.F., and W.Z. Writing – review & editing, F.M.-G., Q.S., P.-C.C., B.C.P.K., F.F.L., G.L., W.D., and X.Y.
Declaration of interests
W.Z. reports receiving fees from Astellas US and Astellas Pharma US outside of the submitted work. The other authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.02.009.
Contributor Information
Xuejun Yang, Email: ydenny@126.com.
Wei Zhang, Email: wezhang@wakehealth.edu.
Supplemental information
References
- 1.Schram A.M., Chang M.T., Jonsson P., Drilon A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 2017;14:735–748. doi: 10.1038/nrclinonc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw A.T., Hsu P.P., Awad M.M., Engelman J.A. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat. Rev. Cancer. 2013;13:772–787. doi: 10.1038/nrc3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom Q.T., Gittleman H., Truitt G., Boscia A., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20:iv1–iv86. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koshy M., Villano J.L., Dolecek T.A., Howard A., Mahmood U., Chmura S.J., Weichselbaum R.R., McCarthy B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012;107:207–212. doi: 10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan C.W., Verhaak R.G., McKenna A., Campos B., Noushmehr H., Salama S.R., Zheng S., Chakravarty D., Sanborn J.Z., Berman S.H., et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reifenberger G., Wirsching H.G., Knobbe-Thomsen C.B., Weller M. Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017;14:434–452. doi: 10.1038/nrclinonc.2016.204. [DOI] [PubMed] [Google Scholar]
- 7.Mitelman F., Johansson B., Mertens F. The impact of translocations and gene fusions on cancer causation. Nat. Rev. Cancer. 2007;7:233–245. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 8.Brien G.L., Stegmaier K., Armstrong S.A. Targeting chromatin complexes in fusion protein-driven malignancies. Nat. Rev. Cancer. 2019;19:255–269. doi: 10.1038/s41568-019-0132-x. [DOI] [PubMed] [Google Scholar]
- 9.Mertens F., Johansson B., Fioretos T., Mitelman F. The emerging complexity of gene fusions in cancer. Nat. Rev. Cancer. 2015;15:371–381. doi: 10.1038/nrc3947. [DOI] [PubMed] [Google Scholar]
- 10.Singh D., Chan J.M., Zoppoli P., Niola F., Sullivan R., Castano A., Liu E.M., Reichel J., Porrati P., Pellegatta S., et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker B.C., Annala M.J., Cogdell D.E., Granberg K.J., Sun Y., Ji P., Li X., Gumin J., Zheng H., Hu L., et al. The tumorigenic FGFR3-TACC3 gene fusion escapes miR-99a regulation in glioblastoma. J. Clin. Invest. 2013;123:855–865. doi: 10.1172/JCI67144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams S.V., Hurst C.D., Knowles M.A. Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 2013;22:795–803. doi: 10.1093/hmg/dds486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research N., Brat D.J., Verhaak R.G., Aldape K.D., Yung W.K., Salama S.R., Cooper L.A., Rheinbay E., Miller C.R., Vitucci M., et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granberg K.J., Annala M., Lehtinen B., Kesseli J., Haapasalo J., Ruusuvuori P., Yli-Harja O., Visakorpi T., Haapasalo H., Nykter M., Zhang W. Strong FGFR3 staining is a marker for FGFR3 fusions in diffuse gliomas. Neuro Oncol. 2017;19:1206–1216. doi: 10.1093/neuonc/nox028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majewski I.J., Mittempergher L., Davidson N.M., Bosma A., Willems S.M., Horlings H.M., de Rink I., Greger L., Hooijer G.K., Peters D., et al. Identification of recurrent FGFR3 fusion genes in lung cancer through kinome-centred RNA sequencing. J. Pathol. 2013;230:270–276. doi: 10.1002/path.4209. [DOI] [PubMed] [Google Scholar]
- 16.Capelletti M., Dodge M.E., Ercan D., Hammerman P.S., Park S.I., Kim J., Sasaki H., Jablons D.M., Lipson D., Young L., et al. Identification of recurrent FGFR3-TACC3 fusion oncogenes from lung adenocarcinoma. Clin. Cancer Res. 2014;20:6551–6558. doi: 10.1158/1078-0432.CCR-14-1337. [DOI] [PubMed] [Google Scholar]
- 17.Yuan L., Liu Z.H., Lin Z.R., Xu L.H., Zhong Q., Zeng M.S. Recurrent FGFR3-TACC3 fusion gene in nasopharyngeal carcinoma. Cancer Biol. Ther. 2014;15:1613–1621. doi: 10.4161/15384047.2014.961874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson K.N., Meyer A.N., Wang C.G., Donoghue D.J. Oncogenic driver FGFR3-TACC3 is dependent on membrane trafficking and ERK signaling. Oncotarget. 2018;9:34306–34319. doi: 10.18632/oncotarget.26142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frattini V., Pagnotta S.M., Tala A., Fan J.J., Russo M.V., Lee S.B., Garofano L., Zhang J., Shi P., Lewis G., et al. A metabolic function of FGFR3-TACC3 gene fusions in cancer. Nature. 2018;553:222–227. doi: 10.1038/nature25171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Stefano A.L., Fucci A., Frattini V., Labussiere M., Mokhtari K., Zoppoli P., Marie Y., Bruno A., Boisselier B., Giry M., et al. Detection, characterization, and inhibition of FGFR-TACC fusions in IDH wild-type glioma. Clin. Cancer Res. 2015;21:3307–3317. doi: 10.1158/1078-0432.CCR-14-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballester L.Y., Moghadamtousi S.Z., Leeds N.E., Huse J.T., Fuller G.N. Coexisting FGFR3 p.K650T mutation in two FGFR3-TACC3 fusion glioma cases. Acta Neuropathol. Commun. 2019;7:63. doi: 10.1186/s40478-019-0721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Cazzato E., Ladewig E., Frattini V., Rosenbloom D.I., Zairis S., Abate F., Liu Z., Elliott O., Shin Y.J., et al. Clonal evolution of glioblastoma under therapy. Nat. Genet. 2016;48:768–776. doi: 10.1038/ng.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen P.Y., Weller M., Lee E.Q., Alexander B.M., Barnholtz-Sloan J.S., Barthel F.P., Batchelor T.T., Bindra R.S., Chang S.M., Chiocca E.A., et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22:1073–1113. doi: 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trepel J., Mollapour M., Giaccone G., Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitesell L., Lindquist S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 26.Verba K.A., Agard D.A. How Hsp90 and Cdc37 lubricate kinase molecular switches. Trends Biochem. Sci. 2017;42:799–811. doi: 10.1016/j.tibs.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hood F.E., Williams S.J., Burgess S.G., Richards M.W., Roth D., Straube A., Pfuhl M., Bayliss R., Royle S.J. Coordination of adjacent domains mediates TACC3-ch-TOG-clathrin assembly and mitotic spindle binding. J. Cell Biol. 2013;202:463–478. doi: 10.1083/jcb.201211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson K.N., Meyer A.N., Siari A., Campos A.R., Motamedchaboki K., Donoghue D.J. Oncogenic gene fusion FGFR3-TACC3 is regulated by tyrosine phosphorylation. Mol. Cancer Res. 2016;14:458–469. doi: 10.1158/1541-7786.MCR-15-0497. [DOI] [PubMed] [Google Scholar]
- 29.Chen F., Hristova K. The physical basis of FGFR3 response to fgf1 and fgf2. Biochemistry. 2011;50:8576–8582. doi: 10.1021/bi200986f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neckers L., Schulte T.W., Mimnaugh E. Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Invest. New Drugs. 1999;17:361–373. doi: 10.1023/a:1006382320697. [DOI] [PubMed] [Google Scholar]
- 31.Xu W., Yuan X., Xiang Z., Mimnaugh E., Marcu M., Neckers L. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat. Struct. Mol. Biol. 2005;12:120–126. doi: 10.1038/nsmb885. [DOI] [PubMed] [Google Scholar]
- 32.Krishnamoorthy G.P., Guida T., Alfano L., Avilla E., Santoro M., Carlomagno F., Melillo R.M. Molecular mechanism of 17-allylamino-17-demethoxygeldanamycin (17-AAG)-induced AXL receptor tyrosine kinase degradation. J. Biol. Chem. 2013;288:17481–17494. doi: 10.1074/jbc.M112.439422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamberti D., Cristinziano G., Porru M., Leonetti C., Egan J.B., Shi C.X., Buglioni S., Amoreo C.A., Castellani L., Borad M.J., et al. HSP90 inhibition drives degradation of FGFR2 fusion proteins: implications for treatment of cholangiocarcinoma. Hepatology. 2019;69:131–142. doi: 10.1002/hep.30127. [DOI] [PubMed] [Google Scholar]
- 34.Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 35.Choudhary C., Olsen J.V., Brandts C., Cox J., Reddy P.N., Böhmer F.D., Gerke V., Schmidt-Arras D.E., Berdel W.E., Müller-Tidow C., et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol. Cell. 2009;36:326–339. doi: 10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Ornitz D.M., Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma J., Benitez J.A., Li J., Miki S., Ponte de Albuquerque C., Galatro T., Orellana L., Zanca C., Reed R., Boyer A., et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell. 2019;35:504–518.e7. doi: 10.1016/j.ccell.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., Zhou Y., Huang T., Wu F., Pan Y., Dong Y., Wang Y., Chan A.K.Y., Liu L., Kwan J.S.H., et al. FGF18, a prominent player in FGF signaling, promotes gastric tumorigenesis through autocrine manner and is negatively regulated by miR-590-5p. Oncogene. 2019;38:33–46. doi: 10.1038/s41388-018-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munoz J.L., Rodriguez-Cruz V., Greco S.J., Ramkissoon S.H., Ligon K.L., Rameshwar P. Temozolomide resistance in glioblastoma cells occurs partly through epidermal growth factor receptor-mediated induction of connexin 43. Cell Death Dis. 2014;5:e1145. doi: 10.1038/cddis.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y., Jin W., Wang Q., Zhou J., Wang Y., Tan Y., Cui X., Tong F., Yang E., Wang J., Kang C. Precise editing of FGFR3-TACC3 fusion genes with CRISPR-Cas13a in glioblastoma. Mol. Ther. 2021;29:3305–3318. doi: 10.1016/j.ymthe.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Parker Kerrigan B.C., Ledbetter D., Kronowitz M., Phillips L., Gumin J., Hossain A., Yang J., Mendt M., Singh S., Cogdell D., et al. RNAi technology targeting the FGFR3-TACC3 fusion breakpoint: an opportunity for precision medicine. Neurooncol. Adv. 2020;2:vdaa132. doi: 10.1093/noajnl/vdaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner N., Grose R. Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 43.Taipale M., Krykbaeva I., Koeva M., Kayatekin C., Westover K.D., Karras G.I., Lindquist S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laederich M.B., Degnin C.R., Lunstrum G.P., Holden P., Horton W.A. Fibroblast growth factor receptor 3 (FGFR3) is a strong heat shock protein 90 (Hsp90) client: implications for therapeutic manipulation. J. Biol. Chem. 2011;286:19597–19604. doi: 10.1074/jbc.M110.206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarkar S., Ryan E.L., Royle S.J. FGFR3-TACC3 cancer gene fusions cause mitotic defects by removal of endogenous TACC3 from the mitotic spindle. Open Biol. 2017;7:170080. doi: 10.1098/rsob.170080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 47.Shimomura A., Yamamoto N., Kondo S., Fujiwara Y., Suzuki S., Yanagitani N., Horiike A., Kitazono S., Ohyanagi F., Doi T., et al. First-in-Human phase I study of an oral HSP90 inhibitor, TAS-116, in patients with advanced solid tumors. Mol. Cancer Ther. 2019;18:531–540. doi: 10.1158/1535-7163.MCT-18-0831. [DOI] [PubMed] [Google Scholar]
- 48.Yuno A., Lee M.J., Lee S., Tomita Y., Rekhtman D., Moore B., Trepel J.B. Clinical evaluation and biomarker profiling of Hsp90 inhibitors. Methods Mol. Biol. 2018;1709:423–441. doi: 10.1007/978-1-4939-7477-1_29. [DOI] [PubMed] [Google Scholar]
- 49.Butler L.M., Ferraldeschi R., Armstrong H.K., Centenera M.M., Workman P. Maximizing the therapeutic potential of HSP90 inhibitors. Mol. Cancer Res. 2015;13:1445–1451. doi: 10.1158/1541-7786.MCR-15-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohba S., Hirose Y., Yoshida K., Yazaki T., Kawase T. Inhibition of 90-kD heat shock protein potentiates the cytotoxicity of chemotherapeutic agents in human glioma cells. J. Neurosurg. 2010;112:33–42. doi: 10.3171/2009.3.JNS081146. [DOI] [PubMed] [Google Scholar]
- 51.Sauvageot C.M., Weatherbee J.L., Kesari S., Winters S.E., Barnes J., Dellagatta J., Ramakrishna N.R., Stiles C.D., Kung A.L., Kieran M.W., Wen P.Y. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009;11:109–121. doi: 10.1215/15228517-2008-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldman M., Craft B., Hastie M., Repečka K., Kamath A., McDade F., Rogers D., Brooks A.N., Zhu J., Haussler D. The UCSC Xena platform for public and private cancer genomics data visualization and interpretation. Preprint at bioRxiv. 2019:326470. doi: 10.1101/326470. [DOI] [Google Scholar]
- 53.Gravendeel L.A., Kouwenhoven M.C., Gevaert O., de Rooi J.J., Stubbs A.P., Duijm J.E., Daemen A., Bleeker F.E., Bralten L.B., Kloosterhof N.K., et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009;69:9065–9072. doi: 10.1158/0008-5472.CAN-09-2307. [DOI] [PubMed] [Google Scholar]
- 54.Yu G., Wang L.-G., Han Y., He Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.