Abstract
An in vitro cell culture model was used to investigate the long-term effects of azithromycin, rifampin, and the combination of azithromycin and rifampin on Chlamydia trachomatis infection. Although standard in vitro susceptibility testing indicated efficient inhibition by azithromycin, prolonged treatment did not reveal a clear elimination of chlamydia from host cells. Chlamydia were temporarily arrested in a persistent state, characterized by culture-negative, but viable, metabolically active chlamydia, as demonstrated by the presence of short-lived rRNA transcripts. Additionally, azithromycin induced generation of aberrant inclusions and an altered steady-state level of chlamydial antigens, with the predominance of Hsp60 protein compared to the level of the major outer membrane protein. Treatment with azithromycin finally resulted in suppression of rRNA synthesis. Chlamydial lipopolysaccharide and processed, functional rRNA were detectable throughout the entire incubation period. These in vitro data show a good correlation to those from some recent clinical investigations that have reported on the persistence of chlamydia, despite appropriate antibiotic treatment with azithromycin. Rifampin was highly active by in vitro susceptibility testing, but prolonged exposure to rifampin alone for up to 20 days resulted in the emergence of resistance. No development of resistance to rifampin was observed when chlamydia-infected cells were incubated with a combination of azithromycin and rifampin. This combination was shown to be more efficient than azithromycin alone, in that suppression of rRNA synthesis occurred earlier. Thus, such a combination may prove more useful than azithromycin alone.
Infections caused by the obligate intracellular bacterium Chlamydia trachomatis are among the most prevalent causes of ocular and urogenital diseases worldwide. Clinical manifestations of acute infections related to C. trachomatis serovars A to C or serovars D to K are trachoma or cervicitis and urethritis, respectively. These infections can progress to persistent infections, which may initiate a pathogenic process that leads to chronic diseases including blindness or pelvic inflammatory disease, ectopic pregnancy, tubal factor infertility, and chlamydia-induced arthritis, including Reiter's syndrome.
Standard therapy for acute urogenital tract infections is a 7-day course of doxycycline or a single dose of azithomycin. Both regimens have been shown to result in satisfactory cure rates in clinical trials (20, 21, 32, 34, 40, 43, 49).
Relapsing chlamydial infections are, however, a common problem, even though patients are often treated appropriately (6, 24, 56). Usually, recurrent infections are supposed to be a consequence of reinfection. Most of the clinical trials that have addressed relapsing chlamydial infections did not distinguish between reinfection and relapse and thus did not define the role of persistence. There are, however, recent reports of recurrent infections after appropriate antibiotic treatment which appeared to be a result of the persistence of chlamydia (15, 25, 38).
This observation presents an apparent contradiction to results of determination of the MIC and the minimum bactericidal concentration (MBC), which clearly indicated successful suppression of chlamydial growth by clinically used antibiotics. The experimental setting involved with this kind of in vitro testing is, however, not truly reflective of the situation in vivo for chlamydial infection. In natural infections, chlamydia are usually exposed to antimicrobials long after an infection has been well established. In contrast, the conventional in vitro systems used for susceptibility testing represent a quite different condition, in that antibiotics are added usually 48 h after the infectious agent is added or are sometimes added simultaneously with the infectious agent. Recently, we could demonstrate that ciprofloxacin and ofloxacin not only failed to eradicate chlamydia from host cells but induced a persistent infection, although both antibiotics are efficient in susceptibility testing (16). Using this in vitro model, we investigated the efficacies of azithromycin, rifampin, and the combination of azithromycin and rifampin for the elimination of chlamydia from epithelial cells.
MATERIALS AND METHODS
Cells.
Cells of the HEp-2 cells line, a human laryngeal epidermoid cell line, were maintained at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 10% fetal calf serum (Seromed, Berlin, Germany), 1% l-glutamine, and 100 μg of gentamicin (Seromed) per ml.
Growth, purification, and titration of chlamydia.
C. trachomatis serovar K/UW-31/Cx (obtained from the Washington Research Foundation, Seattle) was cultured in HEp-2 cells, as described recently (28). Briefly, at 48 h postinfection the chlamydia were harvested, purified on a discontinuous Renografin gradient (Schering, Berlin, Germany) (10), resuspended in SPG buffer (0.01 M sodium phosphate [pH 7.2], 0.25 M sucrose, 5 mM l-glutamic acid), and stored at −80°C. The infectivity of the chlamydia was expressed as the number of inclusion-forming units (IFU) per milliliter.
Determination of MICs and MBCs.
Determination of the MICs and the MBCs was performed as described recently (16). The MIC was defined as the lowest drug concentration required to inhibit development of chlamydial inclusions after 48 h of incubation. The MBC was defined as the lowest concentration of antibiotic required to suppress generation of infectious chlamydia, as measured by the development of inclusions after passage to fresh HEp-2 cell monolayers. Inclusions were visualized by staining with fluorescein isothiocyanate-conjugated antibody directed against major outer membrane proteins (MOMPs Behring, Schwalbach, Germany).
Infection and treatment with antibiotics.
Azithromycin (Pfizer, Karlsruhe, Germany) and rifampin (Sigma, Deisenhofen, Germany) were supplied as powders and were solubilized according to the manufacturers' instructions. Infection and antibiotic treatment were performed as described previously (16). Briefly, antibiotic-free cultured HEp-2 cells were inoculated with C. trachomatis elementary bodies EBs; multiplicity of infection [MOI], 0.05). Incubation with 0.5 or 1.0 μg of azithromycin per ml, 0.015 μg of rifampin per ml, or the combination of 0.5 μg of azithromycin per ml and 0.015 μg of rifampin per ml was started 2 days after infection.
Immunofluorescence assays.
Visualization of inclusions was done by staining with Hsp60-specific antibody GP 57-19 (kindly provided by R. P. Morrison, Hamilton, Mont.) (16). Fluorescence microscopy was performed with an epifluorescence microscope (Leitz, Wetzlar; Germany). The number of inclusions was counted and was expressed as the number of inclusion bodies per 105 cells.
Detection of infectious chlamydia.
Chlamydial infectivity was determined by titration of cell lysates on confluent HEp-2 cell monolayers (47). After 48 h of incubation, inclusions were visualized by an immunoperoxidase assay (39). The number of inclusions was expressed as the number of IFU/105 cells.
SDS-PAGE and immunoblotting.
The protein contents of the harvested cells were determined by a micro-Bradford protein assay (Bio-Rad, Munich, Germany) with bovine serum albumin as the standard. Samples of 50 μg of total protein were solubilized by boiling in Laemmli sample buffer and were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12 or 15% acrylamide) (31). The separated proteins were transferred electrophoretically to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). After blocking of the blots in nonfat dried milk powder in phosphate-buffered saline or Roti-Block (Roth, Karlsruhe, Germany), the blots were probed with either anti-Hsp60 (GP 57-5), anti-MOMP (LV-21), or anti-lipopolysaccharide (anti-LPS; S 25-23; kindly provided by H. Brade, Borstel, Germany) antibodies. Antibody bound to chlamydial antigens was detected with alkaline phosphatase-conjugated rabbit or goat anti-mouse immunoglobulin G (Dianova, Hamburg, Germany) and subsequent staining with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (Sigma).
RT-PCR analysis.
Infected or uninfected cells were harvested by centrifugation, washed twice with Hanks balanced salt solution, snap-frozen in liquid N2, and stored at −80°C until use. Total RNA was extracted with an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Prior to reverse transcription (RT) reactions, RNA was treated with RNase-free DNase I (Life Technologies, Gibco-BRL, Karlsruhe, Germany) or RQ1 DNase (Promega, Mannheim, Germany). For RT, 1 μg of RNA was incubated with 200 U of Moloney murine leukemia virus reverse transcriptase or Superscript II RNase H− (Life Technologies, Gibco-BRL) and 100 pmol of primer DS by using the buffers and conditions specified by the manufacturer.
Amplification of unprocessed 16S rRNA transcripts was performed as described recently (17). Demonstration of functional rRNA was done by RT-PCR with downstream primer DS and upstream primer US2b (5′-TTCAGATTGAACGCTGGCGGCGTGGATG-3′), whose sequence is specific for the coding region of the rRNA operon. PCR was carried out in a total volume of 100 μl with 0.3 μM primers and buffers, as described above (17). The reaction mixtures were subjected to an initial denaturation step at 95°C for 5 min and 35 cycles of amplification, performed in a Perkin-Elmer 9600 thermocycler as follows: 1 min of denaturation at 95°C, 1 min of annealing at 60°C, and 1 min of primer extension at 72°C with a final extension at 72°C for 10 min. The PCR products were visualized by electrophoresis on agarose gel stained with ethidium bromide.
RESULTS
Determination of MICs and MBCs.
The MICs, which were the lowest concentrations required to inhibit development of chlamydial inclusions, were 0.25 μg/ml for azithromycin and 0.0075 μg/ml for rifampin. The MBCs, which were defined as the lowest concentrations required to prevent formation of chlamydial inclusions after passage, were 0.5 μg/ml for azithromycin and 0.01 μg/ml for rifampin.
Effect of azithromycin on growth of C. trachomatis.
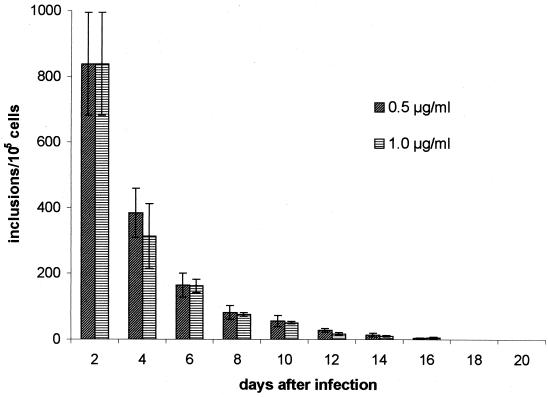
Treatment was started after chlamydial infection had been established, i.e., 2 days after inoculation. The influences of two different concentrations of azithromycin, 0.5 and 1.0 μg/ml, on chlamydial growth were assessed by determination of the numbers of infectious chlamydia and the presence of inclusions. Table 1 shows the effect of the drug on the yield of infectious chlamydia. Both concentrations had equivalent activities in inhibiting productive growth. A significant decrease in infectivity was observed after addition of the drug, resulting in a loss of infectivity after 8 and 6 days with treatment with 0.5 and 1.0 μg of azithromycin per ml, respectively. Although infectious chlamydia could not be recovered on days 8 and 10 after treatment with the two concentrations, respectively, chlamydial inclusions were present for significantly longer periods. At 2 days after infection, inclusions had developed in about 0.8% of host cells, and the numbers of inclusions continuously decreased by treatment with azithromycin (Fig. 1). Typical inclusions could be found only until day 8. Quantification of inclusions was, however, difficult during the later period of culture due to the presence of small atypical inclusions. Single smaller inclusions were detectable for 14 days.
TABLE 1.
Effects of 0.5 and 1.0 μg of azithromycin per ml on infectious chlamydial progeny in HEp-2 cellsa
| Day after infection | Mean no. of IFU/105 cells
|
|
|---|---|---|
| 0.5 μg/ml | 1.0 μg/ml | |
| 2 | 67,672 ± 9,599 | 67,672 ± 9,599 |
| 4 | 24,307 ± 12,441 | 12,871 ± 2,372 |
| 6 | 344 ± 202 | 241 ± 156 |
| 8 | 8 | 0 |
| 10 | 0 | 0 |
HEp-2 cells were infected at a MOI of 0.05. Incubation with azithromycin was started 2 days postinfection. Culture of cells without antibiotics resulted in complete destruction of the cell monolayer in 6 days. The data are the mean numbers of IFU per 105 cells determined in five and four experiments with concentrations of 0.5 and 1.0 μg/ml, respectively.
FIG. 1.
Effect of 0.5 and 1.0 μg of azithromycin per ml on chlamydial inclusions in HEp-2 cells. HEp-2 cells were inoculated at an MOI of 0.05. Incubation with azithromycin was started 2 days after infection. Cultures without azithromycin were run in parallel as a control, with complete destruction of the cell monolayer in 6 days. The figure does not include the numbers of atypical inclusions. Data presented are the means ± standard deviations (error bars) of six and four experiments with concentrations of 0.5 and 1.0 μg/ml, respectively.
Chlamydial viability during treatment with azithromycin.
Several in vitro studies have demonstrated that an abrogation or deviation from the typical developmental cycle could be induced by gamma interferon (IFN-γ) penicillin, ciprofloxacin, ofloxacin, or depletion of essential amino acids with this deviation resulting in a culture-negative but viable state for the chlamydia (2, 13, 16, 29, 35). Hence, the failure to detect infectious chlamydia does not necessarily exclude the presence of viable bacteria. Therefore, we used an RT-PCR analysis that targets unprocessed 16S rRNA transcripts and that provides a sensitive method for the identification of viable chlamydia. Such transcripts are detectable only in viable, metabolically active organisms and are processed to functional rRNA rapidly (16); thus, their presence indicates the viability of the chlamydia infecting host cells.
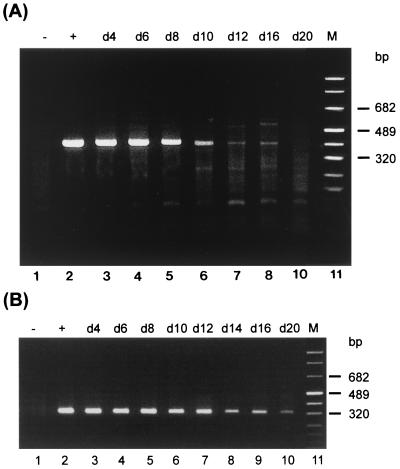
RT-PCR assay demonstrated unprocessed transcripts in cells treated with 0.5 μg of azithromycin per ml for 16 days (Fig. 2A). Incubation with the higher concentration of 1.0 μg/ml resulted in a slightly better inhibition since unprocessed rRNA was present only for 12 days (data not shown).
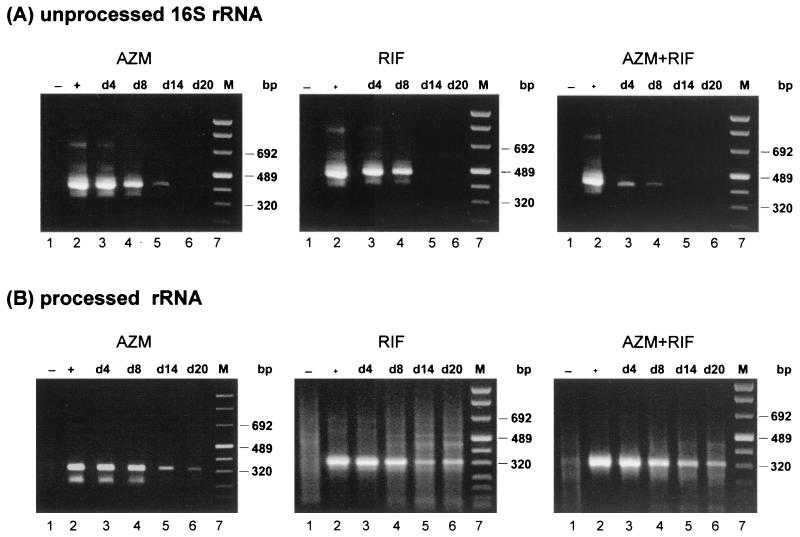
FIG. 2.
RT-PCR analysis of HEp-2 cells treated with azithromycin. Cells were infected at an MOI of 0.05 and were treated with 0.5 μg of azithromycin per ml starting 2 days after infection. Detection of unprocessed 16S rRNA transcripts (A) and processed, functional rRNA (B) was performed as described in Materials and Methods. Lanes: 1, uninfected cells; 2, control cells at 2 days postinfection; 3 to 10, chlamydia-infected cells treated with azithromycin; 11, marker (M).
RT-PCR analysis with upstream primer US2b, whose sequence is specific for the coding region of the rRNA operon, offers a method for the detection of unprocessed and functional, processed 16S rRNA. Samples which were shown to be negative for unprocessed 16S rRNA transcripts were analyzed to detect processed, functional rRNA. Although de novo synthesis of rRNA was inhibited during the later stage of incubation with antibiotic, functional rRNA was detectable throughout the entire period (Fig. 2B). The same result was obtained with the higher drug concentration of 1.0 μg/ml (data not shown).
Analysis of chlamydial antigens during treatment with azithromycin.
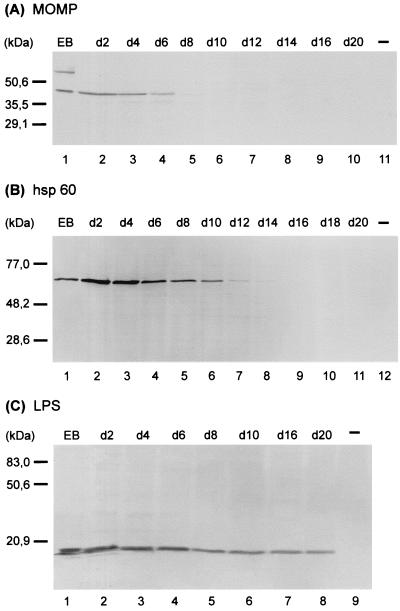
Antigen analyses were done to investigate the effect of azithromycin on key chlamydial antigens. The antigens assessed were MOMP, Hsp60, and LPS. Hsp60 and LPS have been implicated in the elicitation of strong immunopathogenic reactions (22, 36, 37). MOMP, a major structural constituent of the chlamydial outer envelope, is thought to play a role in protective immunity (9, 14, 50, 58). In vitro persistent infection induced by IFN-γ, iron depletion, or ciprofloxacin is characterized by an obvious imbalance of chlamydial antigens, with near normal levels of Hsp60 and significantly down-regulated levels of MOMP (2, 16, 44). According to the ability of azithromycin to inhibit bacterial protein synthesis, synthesis of MOMP and Hsp60 was suppressed within the culture period. Development of atypical inclusions upon azithromycin treatment was associated to some extent with decreasing levels of both chlamydial antigens. No MOMP could be demonstrated in HEp-2 cells by immunoblotting for 8 days after infection, whereas Hsp60 was detectable in HEp-2 cells for up to 14 days after infection (Fig. 3). Chlamydial LPS was present throughout the entire incubation period, although the amount was decreased (Fig. 3C).
FIG. 3.
Immunoblot analysis of azithromycin-treated HEp-2 cells with anti-MOMP (A), anti-hsp60 (B), and anti-LPS (C) antibodies. HEp-2 cells were infected at an MOI of 0.05 and were treated with 0.5 μg of azithromycin per ml starting 2 days after infection. Lanes: 1, C. trachomatis serovar K EBs; 11 (A), 12 (B) and 9 (C), uninfected (−) cells; 2 to 10 (A), 11 (B), and 8 (C), chlamydia-infected cells treated with 0.5 μg of azithromycin per ml on the indicated day (d).
Treatment with a higher concentration of azithromycin (1.0 μg/ml) resulted in slightly more efficient suppression, as shown by the lack of detection of both Hsp60 and MOMP 2 days earlier than the time of a lack of protein detection observed for the lower concentration of 0.5 μg/ml (data not shown).
Effects of rifampin and the combination of rifampin and azithromycin on chlamydial infection.
Since rifampin has been shown to be very active against C. trachomatis in a number of in vitro cell culture studies (4, 5, 8, 11, 12, 23, 45, 57), we decided to test rifampin alone and the combination of rifampin and azithromycin to gain a better inhibition in our cell culture model. Infection of treated cells was monitored by detection of infectious chlamydia, inclusions, and unprocessed 16S rRNA transcripts at days 4, 8, 14, and 20 after infection. Table 2 shows the effects of the antimicrobial drugs on infectious progeny. All three regimens suppressed productive growth. Few infectious bacteria were detectable at day 8 when cells had been treated with azithromycin or rifampin alone. With the combination of both antibiotics, no infectious chlamydia were detected 8 days after infection.
TABLE 2.
Effects of azithromycin, rifampin, and the combination of azithromycin and rifampin on chlamydial infectivitya
| Day postinfection | Infectivity (% IFU/105 cells) after treatment with:
|
||
|---|---|---|---|
| AZM (0.5 μg/ml) | RIF (0.015 μg/ml) | AZM (0.5 μg/ml) + RIF (0.015 μg/ml) | |
| 2 | 100 | 100 | 100 |
| 4 | 22.8 ± 3.2 | 12.9 ± 2.2 | 23.9 ± 3.3 |
| 8 | 0.009 ± 0.006 | 0.003 ± 0.001 | 0 |
| 14 | 0 | 0 | 0 |
Treatment with 0.5 μg of azithromycin (AZM) per ml or 0.015 μg of rifampin (RIF) per ml, in both, was started 2 days after infection. Culture of cells without antibiotics resulted in complete destruction of the cell monolayer in 6 days. The data are the means ± standard deviations of four experiments.
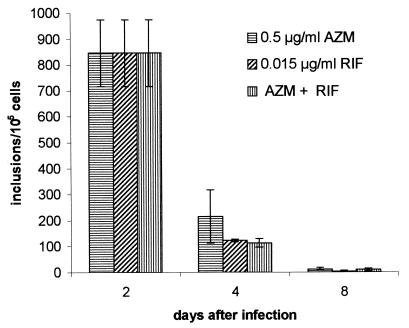
Antibiotic treatment of infected cells had to be done substantially longer to eliminate chlamydial inclusions from host cells. The number of inclusions was significantly reduced at day 4 for about 74 to 85% of the cells (Fig. 4). At day 8 only single inclusions that were significantly smaller than typical ones found in untreated cells were present. Host cells were found to be free of typical inclusions at 14 days postinfection for all three treatment regimens. As already shown for azithromycin, generation of atypical small inclusions during treatment occurred, and this was observed at days 4, 8, and 14.
FIG. 4.
Effects of azithromycin (AEM), rifampin (RIF), and azithromycin plus rifampin on chlamydial inclusions. Untreated control cells were completely destroyed in 6 days. Antibiotic incubation was started 2 days postinfection. Host cells were found to be free of typical inclusions at day 14 for all three treatment regimens. Data are presented as the means ± standard deviations of four (azithromycin) and six (rifampin and azithromycin plus rifampin) experiments.
Azithromycin, rifampin, and the combination of both drugs were all sufficient to suppress de novo synthesis of rRNA (Fig. 5A). Unprocessed transcripts are detectable in cells treated with azithromycin alone on days 4, 8, and 14. Rifampin and the combination of azithromycin and rifampin were shown to be more effective, as demonstrated by the presence of primary rRNA only on days 4 and 8. Nevertheless, use of primer US2b, whose sequence is specific for the coding region of the 16S rRNA gene, in another assay demonstrated functional rRNA throughout the entire period of incubation with azithromycin, rifampin, or azithromycin plus rifampin (Fig. 5B).
FIG. 5.
RT-PCR analyses of cells treated with azithromycin (AZM), rifampin (RIF), or azithromycin plus rifampin. Cells were infected at an MOI of 0.05 and were treated with 0.5 μg of azithromycin per ml or 0.015 μg of rifampin per ml, or both, starting 2 days after infection. Detection of unprocessed 16S rRNA transcripts (A) and processed, functional rRNA (B) was performed as described in Materials and Methods. Lanes: 1, uninfected cells; 2, control cells at 2 days postinfection; 3 to 6, chlamydia-infected cells treated with antibiotic on the indicated day (d); 7, marker (M).
Development of rifampin-resistant chlamydia.
Although incubation with rifampin alone was generally active in the inhibition of chlamydial growth and suppression of rRNA synthesis, in some cases recurrent infection with the appearance of typical inclusions and the recovery of infectious chlamydia occurred.
To verify the development of resistant strains, the MICs of rifampin for these chlamydial isolates were determined. The original chlamydia were tested in parallel as control organisms with known susceptibility to rifampin. The results of in vitro susceptibility testing are summarized in Table 3. The data clearly show the emergence of resistance for all isolates tested, with MICs ranging from 4 to 256 μg/ml, whereas the MIC for the original stock of chlamydia was 0.0075 μg/ml. This phenomenon was observed in two independent experiments after incubation with rifampin alone for at least 12 days in 3 of 50 wells (6%) and 2 of 18 wells (11%), respectively. Simultaneous incubation of chlamydia-infected cells with azithromycin and rifampin prevented the emergence of resistance to rifampin.
TABLE 3.
Development of resistant chlamydia during treatment with rifampina
| Expt no. | Day of harvest postinfection | MIC (μg/ml) |
|---|---|---|
| Original chlamydia | 0.0075 | |
| 1 | 14 | 4 |
| 1 | 16 | 64 |
| 1 | 20 | 256 |
| 2 | 14 | 4 |
| 2 | 20 | 4 |
Emergence of resistance was observed in 3 of 50 wells (6%) for experiment 1 and 2 of 18 wells (11%) for experiment 2.
DISCUSSION
Although regimens for the treatment of acute chlamydial urogenital infections are well established, there are recent reports of the persistence of chlamydia, despite appropriate antibiotic treatment with doxycycline or azithromycin (15, 25). These observations are in contrast to those from conventional in vitro susceptibility testing, which clearly indicated good activity. An experimental setting of this kind, however, is not truly reflective of the situation in vivo for chlamydial infection, since determination of the MIC is done by addition of the antibiotics soon after infection of the cell culture or sometimes simultaneously with infection of the cell culture. Some studies have shown an increase in the MIC when the antibiotic is added up to 24 h after inoculation (41, 42). The main disadvantage of these models is the short incubation period of at most 72 h, which is not sufficient to investigate whether the drugs are capable to eradicate chlamydia from the host cells. Therefore, we established a cell culture system by consideration of this point, in that we used a longer incubation period of 20 days (16). Using this model, we could demonstrate that in vitro treatment with ciprofloxacin and ofloxacin induced a state of chlamydial persistence, although determination of the MIC and the MBC clearly indicated successful suppression of chlamydial growth.
In the present study we could show by conventional susceptibility testing that azithromycin has good activity, as demonstrated by an MIC of 0.25 μg/ml and an MBC of 0.5 μg/ml. These results are within the ranges reported by others (1, 7, 33, 46, 48, 52, 53, 54). Extended treatment of an established chlamydial infection with azithromycin did not reveal a clear elimination of the chlamydia. The course of infection in HEp-2 cells treated with azithromycin was characterized by three distinct stages. Infectious chlamydia and typical inclusions were found during the first stage. Further incubation with azithromycin, however, suppressed generation of infectious bacteria, resulting in a culture-negative state after 8 or 10 days. Synthesis of rRNA could be detected for substantially longer times than infectious organisms could. That means that chlamydia are temporarily arrested in a nonproductive, but viable state. Inclusion morphology was affected by azithromycin, as shown by the development of aberrant, small inclusions whose numbers decreased during further treatment. Additionally, an imbalance between chlamydial Hsp60 and MOMP, with a predominance of Hsp60, was noted during this stage of culture. It appeared that azithromycin treatment resulted in a temporary persistence, which shows some striking similarities to in vitro models, as persistence was induced by exposure to IFN-γ, ciprofloxacin, or ofloxacin or depletion of essential nutrients (2, 13, 16). Chlamydia that had restricted growth but that were also viable longer than infectious organisms were also detected. An altered antigen profile was additionally shown for cells whose growth was arrested by exposure to IFN-γ, ciprofloxacin, and ofloxacin.
Finally, exposure of infected cells to azithromycin was successful in suppressing de novo synthesis of rRNA and the simultaneous disappearance of Hsp60. Chlamydial components such as processed rRNA and LPS, however, persisted in host cells throughout the culture period, even after rRNA synthesis was inhibited.
There may be two explanations for these in vitro results. First, suppression of rRNA synthesis corresponds to a bactericidal effect of azithromycin. The chlamydial components that are present would represent remnants of degraded organisms. These macromolecules may, however, have consequences for the sequelae of chlamydial infections. Some cell wall constituents of bacteria such as LPS are known to hinder the accessibility of cell wall-degrading enzymes (19). It has been reported that C. trachomatis envelopes persist in human polymorphonuclear leukocytes (59). Another in vitro study done by Wyrick and coworkers (55) showed, that following azithromycin exposure of infected epithelial cells, residual chlamydial envelopes can persist in inclusions for up to 4 weeks, although metabolically active reticulate bodies are effectively destroyed. Although chlamydia may be killed, the presence of chlamydial LPS could provide a source for sustained inflammation. Additionally, Wyrick et al. (55) demonstrated that chemotaxis of polymorphonuclear leukocytes is stimulated by epithelial cells containing residual envelopes (55). The role for chlamydial LPS in elicitation of the proinflammatory response was confirmed by Ingalls et al. (22). Purified LPS was shown to induce tumor necrosis factor alpha production from whole blood ex vivo.
The second explanation for the results of the present study is that suppression of rRNA synthesis does not necessarily mean that chlamydia are killed. We cannot entirely exclude the possibility that intact chlamydia which exhibit some kind of metabolic activity are present. Recently, the failure of azithromycin to suppress the growth of Chlamydia pneumoniae in vitro was reported in two studies (18, 30).
Previous clinical trials revealed high cure rates after treatment of acute, urogenital chlamydial infections with azithromycin (20, 21, 32, 34, 40, 41, 49). Surprisingly, the in vitro inhibitory effect of azithromycin developed relatively slowly compared to the time to the development of the inhibitory effect in the in vivo study, and prolonged incubation was not successful in complete eradication of chlamydial antigens. Thus, in vitro data demonstrate an apparent contrast to clinical observations. A critical point that must be kept in mind for these clinical studies is the relatively short follow-up period of 4 weeks. Kjær et al. (27) screened patients with urogenital C. trachomatis infection for recurrent infections after antibiotic treatment. Although the study did not distinguish between reinfection and relapse after antibiotic treatment, the incidence of recurrent infection was 29% during 24 weeks of follow-up after patients had tested negative for C. trachomatis at some point during the first 4 to 8 weeks after treatment. These data strongly suggest that retesting after more than 4 weeks after treatment, substantially longer than was usually done in the studies mentioned above, revealed good clinical results for azithromycin.
Two recent studies have demonstrated an important role for the persistence of chlamydia, despite appropriate antibiotic therapy with azithromycin or doxycycline. Katz et al. (25) presented the results of an epidemiologic study in which they evaluated factors affecting chlamydial persistence or recurrence after treatment with a single dose of azithromycin. They reported a 10% rate of “recurrence” of chlamydial infection 1 month after treatment for male and female adolescents reporting no sexual activity during this period. At 3 months, the recurrence rate was 13%. All patients in this group were found to be culture negative at 1 month. Similar recurrence rates were found for adolescents who used condoms during both time periods. A more detailed study in terms of persistent chlamydial infections was published by Dean et al. (15). They identified 552 women with three or more recurrent cervical infections over a period of >2 years among 11,212 culture-positive women attending a sexually transmitted disease clinic. Of these 552 women, 130 (24%) had recurrences caused by the same serovar. For further genotyping, 45 isolates from seven women with 3 to 10 repeated infections over 2 to 5 years were selected. As determined by omp1 genotyping, four women had identical genotypes at each recurrence and one women was persistently infected with a unique genotype, genotype Ja. Many intervening culture-negative samples were positive when tested by ligase chain reaction. These data strongly suggest that cervical C. trachomatis infections may persist over many years.
The clear difference between former studies reporting high degrees of efficacy in curing chlamydial infections and those published by Katz et al. (25) and Dean et al. (15) is the significantly longer follow-up in the last two studies. Patients were retested over a period of up to 5 years in the study of Dean et al. (15). It is plausible that a follow-up of 4 weeks is too short to detect persistent chlamydia due to the biologic properties, demonstrated in in vitro systems, mentioned above. Thus, the ambiguous inhibitory effects of azithromycin on in vitro chlamydial infections are not necessarily contradictory to the results of clinical trials that have obtained good results with azithromycin. Another important point reported in the study by Dean et al. (15) was a significant association of C class serovars (serovars H, I, Ia, J, and K) with persistent cervical infection, although it is known that the B class serovars (serovars D, E, and F) are the most prevalent serovars in lower genital tract chlamydial infections. We used serovar K, a C class serovar, for our studies, as well as for most in vitro investigations of chlamydial persistence. One may assume that there are particular biologic properties of class C serovars that favor the induction of persistence. Additional in vitro studies are necessary, however, to evaluate a possible correlation between chlamydial serovars and the rate of persistence.
Rifampin, a potent inhibitor of DNA-dependent RNA polymerase, has been shown to be highly active against C. trachomatis in a number of in vitro studies (3, 8, 11, 12, 23). The rifampin MIC of 0.0075 μg/ml and MBC of 0.01 μg/ml determined in the present study confirmed the high degree of susceptibility of C. trachomatis to rifampin. Long-term treatment of in vitro chlamydial infection, however, resulted in the emergence of resistance. This observation is in agreement with previous data published by Keshishyan et al. (26), Jones et al. (23), Treharne et al. (51), and Zanetti et al. (57). Keshishyan et al. (26) found that resistance to rifampin developed rather easily during passage in chlamydia-infected egg yolk sacs. This observation has since been confirmed in tissue culture systems (23, 51, 57). These data strongly indicate that should not be used rifampin alone for the treatment of chlamydial infections due to the potential favoring of the development of resistance.
The combination of rifampin and azithromycin, however, revealed more encouraging results. Development of resistance was prevented when cells were treated with azithromycin and rifampin. Similar observations were made by Jones et al. (23), who reported that subinhibitory concentrations of erythromycin and oxytetracycline inhibited the development of resistant strains under conditions in which such resistance would otherwise have emerged. Additionally, the combination of rifampin and azithromycin proved to be more efficient than azithromycin alone, in that elimination of typical and aberrant inclusions and suppression of rRNA synthesis occurred earlier.
Although we cannot be sure that this combination treatment is indeed effective in killing chlamydia, as discussed above for azithromycin, such a combination may prove to be more useful than azithromycin alone. Finally, we can assume that such a combination may possibly represent a new treatment strategy.
ACKNOWLEDGMENTS
This study was supported by the German Ministry of Technology (grant 01VM9708/4) and by a research program of the Medical School Hannover (HiLF program).
REFERENCES
- 1.Agacfidan A, Moncada J, Schachter J. In vitro activity of azithromycin (CP-62, 993) against Chlamydia trachomatis and Chlamydia pneumoniae. Antimicrob Agents Chemother. 1993;37:1746–1748. doi: 10.1128/aac.37.9.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon γ-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker Y, Zackay-Rones Z. Rifampicin—a new antitrachoma drug. Nature. 1969;222:851–853. doi: 10.1038/222851a0. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi A, Scieux C, Salmeron C M, Casin I, Perol Y. Rapid determination of MICs of 15 antichlamydial agents by using an enzyme immunoassay (Chlamydiazyme) Antimicrob Agents Chemother. 1988;32:1350–1353. doi: 10.1128/aac.32.9.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackman H J, Yoneda C, Dawson C R, Schachter J. Antibiotic susceptibility of Chlamydia trachomatis. Antimicrob Agents Chemother. 1977;12:673–677. doi: 10.1128/aac.12.6.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blythe J M, Katz B P, Batteiger B E, Ganser J A, Jones R B. Recurrent genitourinary chlamydial infections in sexually active female adolescents. J Pediatr. 1992;121:487–493. doi: 10.1016/s0022-3476(05)81812-8. [DOI] [PubMed] [Google Scholar]
- 7.Børsum T, Dannevig L, Størvold G, Melby K. Chlamydia trachomatis: in vitro susceptibility of genital and ocular isolates to some quinolones, amoxillin and azithromycin. Chemotherapy (Basel) 1990;36:407–415. doi: 10.1159/000238797. [DOI] [PubMed] [Google Scholar]
- 8.Bowie W R, Lee C K, Alexander E R. Prediction of efficacy of antimicrobial agents in treatment of infectious due to Chlamydia trachomatis. J Infect Dis. 1978;138:655–659. doi: 10.1093/infdis/138.5.655. [DOI] [PubMed] [Google Scholar]
- 9.Byrne G I, Stephens R S, Ada G, Caldwell H D, Su H, et al. Workshop on in vitro neutralization of Chlamydia trachomatis: summary of proceedings. J Infect Dis. 1993;168:415–420. doi: 10.1093/infdis/168.2.415. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cevenini R, Landini M P, Donati M, Rumpianesi F. Antimicrobial drug susceptibility of 15 strains of Chlamydia trachomatis recently isolated from cases of nongonococcal urethritis in Italy. J Antimicrob Chemother. 1980;6:285–300. doi: 10.1093/jac/6.2.294. [DOI] [PubMed] [Google Scholar]
- 12.Cevenini R, Donati M, Sambri V, Rumpianesi F, LaPlaca M. Enzyme-linked immunosorbent assay for “in vitro” detection of sensitivity of Chlamydia trachomatis to antimicrobial drugs. J Antimicrob Chemother. 1987;20:677–684. doi: 10.1093/jac/20.5.677. [DOI] [PubMed] [Google Scholar]
- 13.Coles A M, Reynolds D J, Harper A, Devitt A, Pearce J H. Low-nutrient induction of abnormal chlamydial development: a novel component of chlamydial pathogenesis. FEMS Microbiol Lett. 1993;106:193–200. doi: 10.1111/j.1574-6968.1993.tb05958.x. [DOI] [PubMed] [Google Scholar]
- 14.Cotter T W, Meng Q, Shen Z L, Zhang Y X, Su H, Caldwell H D. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean D, Suchland R J, Stamm W E. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J Infect Dis. 2000;182:909–916. doi: 10.1086/315778. [DOI] [PubMed] [Google Scholar]
- 16.Dreses-Werringloer U, Padubrin I, Jürgens-Saathoff B, Hudon A P, Zeidler H, Köhler L. Persistence of Chlamydia trachomatis is induced by ciprofloxacin and ofloxacin in vitro. Antimicrob Agents Chemother. 2000;44:3288–3297. doi: 10.1128/aac.44.12.3288-3297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerard H C, Whittum-Hudson J A, Hudson A P. Genes required for assembly and function of the protein synthetic system in Chlamydia trachomatis are expressed early in elementary to reticulate body transformation. Mol Gen Genet. 1997;255:637–642. doi: 10.1007/s004380050538. [DOI] [PubMed] [Google Scholar]
- 18.Gieffers J, Füllgraf H, Jahn J, Klinger M, Dalhoff K, Katus H A, Solbach W, Maass M. Chlamydia pneumoniae infection in circulating human monocytes is refractory to antibiotic treatment. Circulation. 2001;103:351–356. doi: 10.1161/01.cir.103.3.351. [DOI] [PubMed] [Google Scholar]
- 19.Ginsburg I, Lahav M. Lysis and biodegradation of microorganisms in infectious sites may involve cooperation between leukocyte, serum factors and bacterial wall autolysins: a working hypothesis. Eur J Clin Microbiol. 1983;2:186–191. doi: 10.1007/BF02029513. [DOI] [PubMed] [Google Scholar]
- 20.Hammerschlag M R, Golden N H, Oh K, Gelling M, Sturdevant M, Brown P R, Aras Z, Neuhoff S, Dumornay W, Roblin P M. Single dose of azithromycin for the treatment of genital chlamydial infections in adolescents. J Pediatr. 1993;122:961–965. doi: 10.1016/s0022-3476(09)90029-4. [DOI] [PubMed] [Google Scholar]
- 21.Hillis S D, Coles F B, Litchfield B, Black C M, Mojica B, Schmitt K, Louis M E. Doxycycline and azithromycin for prevention of chlamydial persistence or recurrence one month after treatment in women. A use-effectiveness study in public health settings. Sex Transm Dis. 1998;25:5–11. doi: 10.1097/00007435-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones R B, Ridgeway G L, Boulding S, Hunley K L. In vitro activity of rifamycins alone and in combination with other antibiotics against Chlamydia trachomatis. Rev Infect Dis. 1983;5(Suppl. 3):556–561. doi: 10.1093/clinids/5.supplement_3.s556. [DOI] [PubMed] [Google Scholar]
- 24.Katz B P, Caine V A, Batteiger B E, Jones R B. A randomized trial to compare 7 and 21 day tetracycline regimens in the prevention of recurrence of infection with Chlamydia trachomatis. Sex Transm Dis. 1991;18:36–40. doi: 10.1097/00007435-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Katz B P, Fortenberry D, Orr D. Factors affecting chlamydial persistence or recurrence one and three months after treatment. In: Stephens R S, Byrne G I, Christiansen G, et al., editors. Chlamydia infections: proceedings of the Ninth International Symposium on Human Chlamydial Infection. Berkeley, Calif: Berkeley University Press; 1998. pp. 35–38. [Google Scholar]
- 26.Keshishyan H, Hanna L, Jawetz E. Emergence of rifampin-resistance in Chlamydia trachomatis. Nature. 1973;244:173–174. doi: 10.1038/244173a0. [DOI] [PubMed] [Google Scholar]
- 27.Kjær H O, Dimcevski G, Hoff G, Oleson F, Østergaard L. Recurrence of urogenital Chlamydia trachomatis infection evaluated by mailed samples obtained at home: 24 weeks' prospective follow up study. Sex Transm Infect. 2000;76:169–172. doi: 10.1136/sti.76.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Köhler L, Nettelnbreker E, Hudson A P, Ott N, Gérard H C, Branigan P J, Schumacher H R, Drommer W, Zeidler H. Ultrastructural and molecular analyses of the persistence of Chlamydia trachomatis (serovar K) in human monocytes. Microb Pathog. 1997;22:133–142. doi: 10.1006/mpat.1996.0103. [DOI] [PubMed] [Google Scholar]
- 29.Kramer M J, Gordon F B. Ultrastructural analysis of the effects of penicillin and chlortetracycline on the development of a genital tract Chlamydia. Infect Immun. 1971;3:333–341. doi: 10.1128/iai.3.2.333-341.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutlin A, Roblin P M, Hammerschlag M R. In vitro activities of azithromycin and ofloxacin against Chlamydia pneumoniae in a continuous-infection model. Antimicrob Agents Chemother. 1999;43:2268–2272. doi: 10.1128/aac.43.9.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Lauharanta J, Saarinen K, Mustonen M T, Happonen H P. Single-dose oral azithromycin versus seven-day doxycycline in the treatment of non-gonococcal urethritis in males. J Antimicrob Chemother. 1993;31(Suppl. E):177–183. doi: 10.1093/jac/31.suppl_e.177. [DOI] [PubMed] [Google Scholar]
- 33.Lefevre J C, Bauriaud R, Gaubert E, Escaffre M C, Lareng M B. In vitro activity of sparfloxacin and other antimicrobial agents against genital pathogens. Chemotherapy (Basel) 1992;38:303–307. doi: 10.1159/000239018. [DOI] [PubMed] [Google Scholar]
- 34.Martin D H, Mroczkowski T F, Dalu Z A, McCarty J, Jones R B, Hopkins S J, Johnson R B. A controlled trial of a single dose of azithromycin for the treatment of chlamydial urethritis and cervicitis. N Engl J Med. 1992;327:921–925. doi: 10.1056/NEJM199209243271304. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto A, Manire G P. Electron microscopic observation on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970;101:278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison R P, Lyng K, Caldwell H D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989;169:663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison R P, Belland R J, Lyng K, Caldwell H D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989;170:1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munday P E, Thomas B J, Gilroy C B, Gilchrist C, Taylor-Robinson D. Infrequent detection of Chlamydia trachomatis in a longitudinal study of women with treated cervical infection. Genitourin Med. 1995;71:24–26. doi: 10.1136/sti.71.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nettelnbreker E, Zeidler H, Bartels H, Dreses-Werringloer U, Däubener W, Holtmann H, Köhler L. Effect of IFN-γ on persistent infection by Chlamydia trachomatis serovar K in TPA-differentiated U937 cells. J Med Microbiol. 1997;46:1–9. doi: 10.1099/00222615-47-2-141. [DOI] [PubMed] [Google Scholar]
- 40.Nilsen A, Halsos A, Johansen A, Hansen E, Tørud E, Moseng D, Ånestad G, Størvold G. A double blind study of single dose azithromycin and doxycycline in the treatment of chlamydial urethritis in males. Genitourin Med. 1992;68:325–327. doi: 10.1136/sti.68.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Notomi T, Ikeda Y, Nagayama A. Minimum inhibitory and minimal lethal concentration against Chlamydia trachomatis dependent on the time of addition and the duration of the presence of antibiotics. Chemotherapy (Tokyo) 1999;45:242–248. doi: 10.1159/000007192. [DOI] [PubMed] [Google Scholar]
- 42.Nyström-Rosander, Hultén C K, Gustavsson I, Cars O, Engstrand L, Hjelm E. Susceptibility of Chlamydia pneumoniae to azithromycin and doxycycline: methodological aspects on the determination of minimal inhibitory and minimal bactericidal concentrations. Scand J Infect Dis. 1997;29:513–516. doi: 10.3109/00365549709011865. [DOI] [PubMed] [Google Scholar]
- 43.Ossewarde J M, Plantema F H F, Rieffe M, Nawrocki R P, de Vries A, van Loon A M. Efficacy of single-dose azithromycin versus doxycycline in the treatment of cervical infections caused by Chlamydia trachomatis. Eur J Clin Microbiol Infect Dis. 1992;11:693–697. doi: 10.1007/BF01989972. [DOI] [PubMed] [Google Scholar]
- 44.Raulston J E. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect Immun. 1997;65:4539–4547. doi: 10.1128/iai.65.11.4539-4547.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridgeway G L, Owen J M, Oriel J D. The antimicrobial susceptibility of Chlamydia trachomatis in cell culture. Br J Vener Dis. 1978;54:103–106. doi: 10.1136/sti.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scieux C, Bianchi A, Chappey B, Vassias I, Perol Y. In vitro activity of azithromycin against Chlamydia trachomatis. J Antimicrob Chemother. 1990;25(Suppl. A):7–10. doi: 10.1093/jac/25.suppl_a.7. [DOI] [PubMed] [Google Scholar]
- 47.Shemer Y, Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985;48:592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slaney L, Chubb H, Ronald A, Brunham R. In vitro activity of azithromycin, erythromycin, ciprofloxacin and norfloxacin against Neisseria ghonorrhoea, Haemophilus ducreyi and Chlamydia trachomatis. J Antimicrob Chemother. 1990;25(Suppl. A):1–5. doi: 10.1093/jac/25.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 49.Steingrimsson O, Olafsson J H, Thorarinsson H, Ryan R W, Johnson R B, Tilton R C. Azithromycin in the treatment of sexually transmitted disease. J Antimicrob Chemother. 1990;25(Suppl. A):109–114. doi: 10.1093/jac/25.suppl_a.109. [DOI] [PubMed] [Google Scholar]
- 50.Su H, Watkins N G, Zhang Y X, Caldwell H D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treharne J D, Yearsley P J, Ballard R C. In vitro studies of Chlamydia trachomatis susceptibility and resistance to rifampin and rifabutin. Antimicrob Agents Chemother. 1989;33:1394. doi: 10.1128/aac.33.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh M, Kappus E W, Quinn T C. In vitro evaluation of C-62,933, erythromycin, clindamycin, and tetracycline against Chlamydia trachomatis. Antimicrob Agents Chemother. 1987;31:811–812. doi: 10.1128/aac.31.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welsh L, Gaydos C A, Qinn T C. In vitro evaluation of activities of azithromycin, erythromycin, and tetracycline against Chlamydia trachomatis and Chlamydia pneumoniae. Antimicrob Agents Chemother. 1992;36:291–294. doi: 10.1128/aac.36.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyrick P B, Davis C H, Knight S T, Choong J. In vitro activity of azithromycin on Chlamydia trachomatis infected, polarized human endometrial epithelial cells. J Antimicrob Chemother. 1993;31:139–150. doi: 10.1093/jac/31.1.139. [DOI] [PubMed] [Google Scholar]
- 55.Wyrick P B, Knight S T, Paul T R, Rank R G, Barbier C S. Persistent chlamydial envelope antigens in antibiotic-exposed infected cells trigger neutrophil chemotaxix. J Infect Dis. 1999;179:954–966. doi: 10.1086/314676. [DOI] [PubMed] [Google Scholar]
- 56.Xu F, Schillinger J A, Markowitz L E, Sternberg M R, Aubin M R, St Louis M E. Repeat Chlamydia trachomatis infection in women: analysis through a surveillance case registry in Washington State, 1993–1998. Am J Epidemiol. 2000;152:1164–1170. doi: 10.1093/aje/152.12.1164. [DOI] [PubMed] [Google Scholar]
- 57.Zanetti S, Usai D, Nonis A, Fadda G. In vitro activity of 3-azinomethyl-rifamycin (SPA-S-565) against Chlamydia trachomatis. J Antimicrob Chemother. 1996;37:357–359. doi: 10.1093/jac/37.2.357. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y S, Stewart S, Joseph T, Taylor H R, Caldwell H D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987;138:575–581. [PubMed] [Google Scholar]
- 59.Zvillich M, Sarov I. The persistence of Chlamydia trachomatis elementary body cell walls in human polymorphonuclear leucocytes and induction of a chemiluminescent response. J Gen Microbiol. 1989;135:95–104. doi: 10.1099/00221287-135-1-95. [DOI] [PubMed] [Google Scholar]