Abstract
Background
This is a placebo-controlled multi-national trial of roluperidone, a compound with antagonist properties for 5-HT2A, sigma2, and α1A-adrenergic receptors, targeting negative symptoms in patients with schizophrenia. This trial follows a previous trial that demonstrated roluperidone superiority over placebo in a similar patient population.
Methods
Roluperidone 32 mg/day, roluperidone 64 mg/day, or placebo was administered for 12 weeks to 513 patients with schizophrenia with moderate to severe negative symptoms. The primary endpoint was the PANSS-derived Negative Symptom Factor Score (NSFS) and the key secondary endpoint was Personal and Social Performance scale (PSP) total score.
Results
NSFS scores were lower (improved) for roluperidone 64 mg compared to placebo and marginally missing statistical significance for the intent-to-treat (ITT) analysis data set (P ≤ .064), but reached nominal significance (P ≤ .044) for the modified-ITT (m-ITT) data set. Changes in PSP total score were statistically significantly better on roluperidone 64 mg compared to placebo for both ITT and m-ITT (P ≤ .021 and P ≤ .017, respectively).
Conclusions
Results of this trial confirm the potential of roluperidone as a treatment of negative symptoms and improving everyday functioning in patients with schizophrenia. Study registration: Eudra-CT: 2017-003333-29; NCT03397134.
Keywords: schizophrenia, negative symptoms, treatment
Introduction
Schizophrenia is characterized by recurrent positive symptoms and persistent negative symptoms. The introduction of Diagnostic and Statistical Manual (DSM) classification focused on positive psychotic symptoms, such as delusions and hallucinations, which can be more reliably observed, quantified, and communicated than negative symptoms. The availability of antipsychotic drugs, which interfere with Dopamine (DA) neurotransmission and ameliorate positive symptoms and agitation, further shifted attention towards positive symptoms. Yet, for most schizophrenia patients, negative symptoms emerge during the prodromal phase of the illness and for many patients persist for the entire life-span leading to poor quality of life, disabilities, and societal burden of care.1, 2
Negative symptoms are classified as intrinsic to schizophrenia, or primary negative symptoms, such as alogia, avolition (poor motivation), asociality, and apathy and secondary negative symptoms which are mostly induced by antipsychotic drugs.3–5 Paradoxically, antipsychotic drugs both improve and worsen negative symptoms. On one hand, by ameliorating hallucinations, delusions and agitation antipsychotics facilitate social interactions. On the other hand, by interfering with DA neurotransmission and the brain reward circuits ,6 poor motivation, a core component of negative symptoms is aggravated 7 and induce extrapyramidal adverse effects which are often indistinguishable from primary negative symptoms.
Studies on the treatment of negative symptoms reported that amisulpride, a drug mostly acting as a D2/D3 antagonist, seems to have advantages over haloperidol,8 just as cariprazine, a D2/D3 antagonist has advantages over risperidone.9 However, the advantages are relatively small, still under debate,10 and appear to be related to better tolerability rather than a direct and specific effect on primary negative symptoms. A thorough meta-analysis did not find significant advantages for any medication in the treatment of negative symptoms,11 and to date, there are no medications approved for the treatment of negative symptoms by the FDA.
The realization that negative symptoms constitute a major unmet need has stimulated researchers in academia and in the pharmaceutical industry to search for a solution beyond interference with DA neurotransmission. Attempts to target N-methyl-D-aspartic acid (NMDA)/glutamate neurotransmission, via glycine re-uptake inhibition and other strategies were initially encouraging, but the results were not replicated in a subsequent larger trial.11 Pharmacological manipulations of the trace amine-associated receptor 1 (TAAR1), or PDE10A inhibition have not yet produced conclusive results.12, 13 Therefore, developing well-tolerated drugs, with innovative mechanisms of action (MoA), which can ameliorate negative symptoms, remains a priority.
Roluperidone is a novel cyclic amide derivative with antagonistic properties for serotoninergic 5-HT2A, sigma2 and α 1A-adrenergic receptors, and to a lesser extent, α 1B-adrenergic receptors. Roluperidone has no affinity for DA, cholinergic, or histaminergic receptors (data on file). Although roluperidone has no affinities for pre- or postsynaptic DA receptors, dopaminergic neurotransmission might be modulated by both the 5-HT2A and sigma2 antagonisms.14, 15 Antagonism at the sigma2 receptors might also modulate glutamatergic pathways,16 and affect calcium neuronal modulation.17 Taken together, it could be hypothesized that sigma2 receptors are involved in counteracting dysregulations in key DA and glutamate neurotransmitter pathways. It should be noted that several antipsychotic drugs such as haloperidol possess sigma binding activities,18 but the role of sigma receptors in affecting schizophrenia symptoms has not been fully elucidated. Finally, the α 1A-adrenergic antagonistic activity might contribute to improve synaptic efficacy and plasticity, thus facilitating learning and memory functions.19
A previous placebo-controlled, 12-week trial, which enrolled 244 patients with stable positive symptoms of schizophrenia, reported that roluperidone was superior to placebo in decreasing the negative symptoms as measured by the pentagonal model structure negative symptoms scores of the Positive and Negative Syndrome Scale (PANSS) for both the 32 mg and 64 mg dose (P ≤ .024, ES = 0.45, and ≤ .004, ES = 0.57).20 Significant improvements were also found on the Personal and Social Performance (PSP) total score for the 64 mg dose (P ≤ .003, ES = 0.59).21 The aim of this large, multi-site trial presented here was to confirm the results of the previous trial using similar methodology. ClinicalTrials.gov Identifier: NCT03397134.
Methods
Between December 2017 and February 2020, 513 patients 18–55 years of age, received roluperidone or placebo at 61 sites in Europe, Israel, and the USA. Patients were recruited from outpatient clinics, supervised residential facilities, and psychiatric hospital wards. The trial protocol was approved by Institutional Review Boards, local ethics committees, and national regulatory bodies and all participants signed an informed consent form.
Eligibility Criteria
To be eligible, patients had to meet DSM-5 criteria for schizophrenia confirmed by Mini International Neuropsychiatric Interview, diagnosed with schizophrenia for at least one year, be symptomatically stable by history, and manifest negative symptoms for ≥ 6 months prior to entering the trial. Patients had to be either outpatients, or inpatients admitted for social reasons and not for symptomatic worsening. Patient must have had a score of > 20 on the PANSS negative symptoms subscale (N-1 Blunted affect, N-2 Emotional withdrawal, N-3 Poor rapport, N-4 Passive/apathetic social withdrawal, N-5 Difficulty in abstract thinking, N-6 Lack of spontaneity & flow of conversation, N-7 Stereotyped thinking) with no change between screening and baseline of more than 3 points. There was no severity limit on the total positive symptoms score, but patients had to have scores ≤ 4 on PANSS items related to agitation (P4 Excitement, P6 Suspiciousness/persecution, P7 Hostility, G8 Uncooperativeness, and G14 Poor impulse control). Patients were excluded if they had a Calgary Depression Scale for Schizophrenia (CDSS) total score > 6, at least a moderate degree of akathisia based on the Barnes Akathisia Rating Scale (BARS), or a BMI ≥ 35 kg/m2. Patients were also excluded if they had a personal or familial history of long QT syndrome, a QTc (Fridericia-corrected) > 430 ms for males and > 450 ms for females, or if they were poor or intermediate metabolizers for P450 CYP2D6, as determined by genotyping. Patients with an Axis I diagnosis of another mental disorder, significant risk of suicide, a positive urine test for illicit drugs, history of substance abuse, or unstable medical disorders were also excluded.
Study Design
Eligible patients had their depot antipsychotic medications discontinued for at least a treatment cycle (1 to 3 months depending on the drug formulation) and all their psychotropic drugs were discontinued at least 2 days prior to randomization and throughout the study duration hence, patients received roluperidone monotherapy throughout the study. Patients were assigned to roluperidone 32 mg/day, 64 mg/day, or placebo in a 1:1:1 ratio for a 12-week study duration. Patients were randomized based on a computer-generated randomization schedule prepared before the study. The randomization was balanced by using randomly permuted blocks and were stratified by region (United States, all other countries). Investigators, patients, and the sponsor were blinded to assignment at all times during the study. After randomization, patients had to be hospitalized for at least 36 hours and then could remain hospitalized or be discharged at the discretion of the investigator. No psychotropic medications were allowed during the duration of the trial except for rescue medications given for insomnia or agitation in doses allowed by the local regulations (oral lorazepam, zolpidem). Anticholinergic medications were discontinued at baseline in all patients but were allowed during the trial to treat emergent EPS. Patients were evaluated in person at screening, baseline, and Weeks 1, 2, 3, 4, 8, and 12 (end of double-blind study). Patients who completed the 12-week double-blind trial could continue to receive the same dose of roluperidone or, if receiving placebo, be switched at random to either dose of roluperidone for 40 additional weeks in an open-label extension. The roluperidone dose was blinded through the extension phase; data are not presented here).
Outcome Measures
Primary Outcome Measure.
Primary outcome measure was the change from baseline to week 12 on the NSFS (N1 to N4, N6, G7, and G16) of the PANSS .22, 23
Key Secondary Outcome Measure.
Key secondary outcome measure was the PSP total score ,24 a scale that assesses socially useful activities, personal and social relationships, self-care, and disturbing and aggressive behaviors.
Secondary Outcome Measures.
Secondary outcome measures were the Clinical Global Impression—Severity Scale (CGI-S), Clinical Global Impression—Improvement Scale (CGI-I), other PANSS-derived subscales and domains, assessment of cognitive function measured by verbal fluency test, and of depressive symptoms measured by CDSS.
Safety and Tolerability.
Safety and tolerability were evaluated by monitoring the frequency, severity, and timing of adverse events, clinical laboratory test results, ECG, vital signs, body weight, abnormal involuntary movement scale (AIMS), BARS, Simpson-Angus Scale (SAS), and Sheehan Suicidality Tracking Scale.
Sample Size and Statistical Analysis
The sample size for this study was based on the assumption of a treatment difference of 3 points in the mean change from baseline to Week 12 in NSFS between any roluperidone dose and placebo. A standard deviation of 6.5 in the change in NSFS score from baseline was hypothesized. Assuming an equal allocation to placebo and each of the 2 roluperidone doses, 167 patients in each treatment group, adjusted for 40% noncompleters, were required to detect the treatment difference with a power of 90% at an overall 2-sided significance level of .05 (P-value)
Statistical Methods
The primary efficacy endpoint was the change in the NSFS from baseline to Week 12 (the 12-week double-blind treatment phase). This endpoint was analyzed using MMRM with fixed effects for treatment group (roluperidone 64 mg, roluperidone 32 mg, and placebo), region (USA, rest of the world), visit, and treatment-by-visit interaction, a random effect for patient within treatment group, and baseline NSFS as covariate. An unstructured covariance matrix was used to model the covariance of within-patient scores. The Kenward-Roger approximation 25 was used to estimate denominator degrees of freedom. These analyses were performed based on all post-Baseline scores using only the observed cases without imputation of missing values. Comparison against placebo was performed with the roluperidone 64 mg and 32 mg doses.
The truncated Hochberg method for adjustment of multiplicity to control the type I error rate at the 2-sided 0.050 alpha level within the family of primary and the key secondary hypotheses was utilized and was chosen based on the phase 2b results.
Responder analysis based on pre-defined changes from baseline in NSFS, PANSS total score, and PSP total score was performed, and the treatment groups were compared using logistic regression model with treatment and Baseline values as covariate.
All analyses were performed using the safety and ITT sets. Before the database was locked and unblinded, a data quality review with the help of AI revealed that one site reported data that were behaviorally and physiologically implausible. Throughout the 12 weeks of the trial, the 17 patients recruited at the site had none or negligible variations in terms of symptoms severity as reflected by CGI or blood pressure measurement, no reports of adverse effects, and no dropout from the trial. Therefore, efficacy analyses were repeated both as ITT and as modified ITT (m-ITT) set which excluded from the ITT set the 17 patients from the site.
Results
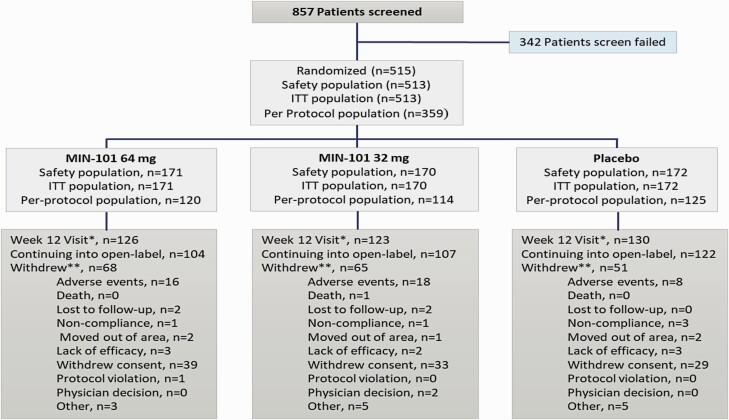
A total of 857 patients were screened, 515 patients were randomized, and 513 patients received at least one dose of roluperidone or placebo. Of the 513 patients, 172 received placebo, 170 received the 32 mg roluperidone dose, and 171 received the 64 mg roluperidone dose. A total of 379/513 patients completed 12-week of double-blind treatment: 76% in the placebo group, 72% in the 32 mg group, and 74% in the 64 mg group (figure 1). The most frequent reason for study discontinuation in all treatment groups was withdrawal of consent. All demographics and disease characteristics were comparable for the 3 groups (tables 1 and 2). Before the wash-out period required prior to randomization, 434 (84.6%) patients received oral antipsychotics, 32 (6.2%) patients depot antipsychotics, and 47 (9.2%) patients received no antipsychotic medication. The most frequently used concomitant medication administered during the 12-week double-blind portion of the trial were benzodiazepines, in 19 patients (11%) in the placebo group, 31 patients (18%) in the 32 mg roluperidone group, and 31 patients (18%) in the 64 mg roluperidone group.
Fig. 1.
Patients distribution.
Table 1.
Demographic data
| Placebo (N = 172) |
Roluperidone | Overall (N = 513) |
|||
|---|---|---|---|---|---|
| 32 mg (N = 170) | 64 mg (N = 171) | Total (N = 341) | |||
| Age at informed consent (years) | |||||
| Mean (SD) | 41 (8.7) | 41 (9.4) | 41 (9.3) | 41 (9.3) | 41 (9.1) |
| Median | 41 | 42 | 42 | 42 | 42 |
| Min, max | 18, 55 | 18, 55 | 18, 55 | 18, 55 | 18, 55 |
| Sex, n (%) | |||||
| Male | 106 (62%) | 106 (62%) | 103 (60%) | 209 (61%) | 315 (61%) |
| Female | 66 (38%) | 64 (38%) | 68 (40%) | 132 (39%) | 198 (39%) |
| Race, n (%) | |||||
| American Indian or Alaska Native | 0 | 1 (<1%) | 0 | 1 (<1%) | 1 (<1%) |
| Asian | 0 | 1 (<1%) | 1 (<1%) | 2 (<1%) | 2 (<1%) |
| Black or African American | 20 (12%) | 19 (11%) | 18 (11%) | 37 (11%) | 57 (11%) |
| NativeHawaiian orother PacificIslander | 0 | 2 (1%) | 1 (<1%) | 3 (<1%) | 3 (<1%) |
| White | 152 (88%) | 147 (86%) | 151 (88%) | 298 (87%) | 450 (88%) |
| Other | 0 | 0 | 0 | 0 | 0 |
| BMI (kg/m2) | |||||
| N | 172 | 170 | 170 | 340 | 512 |
| Mean (SD) | 25.8 (4.14) | 25.6 (4.31) | 25.7 (4.04) | 25.6 (4.17) | 25.7 (4.16) |
| Median | 24.7 | 25.2 | 25.5 | 25.4 | 25.1 |
| Min, max | 18.6, 34.8 | 16.8, 34.7 | 17.6, 34.7 | 16.8, 34.7 | 16.8, 34.8 |
| USA | 27 (16%) | 27 (16%) | 27 (16%) | 54 (16%) | 81 (16%) |
| Rest of World | 145 (84%) | 143 (84%) | 144 (84%) | 287 (84%) | 432 (84%) |
Table 2.
Baseline Characteristics
| Assessments at Baseline | Placebo (N = 172) |
Roluperidone | Overall (N = 513) |
||
|---|---|---|---|---|---|
| 32 mg (N = 170) | 64 mg (N = 171) | Total (N = 341) | |||
| PANSS | |||||
| NSFS | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 24 (3.0) | 25 (3.4) | 25 (3.2) | 25 (3.3) | 25 (3.2) |
| Median | 24 | 25 | 26 | 25 | 25 |
| Min, max | 17, 32 | 16, 39 | 19, 36 | 16, 39 | 16, 39 |
| Total Score | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 77 (9.9) | 80 (11.8) | 79 (10.5) | 79 (11.1) | 79 (10.8) |
| Median | 77 | 80 | 79 | 80 | 79 |
| Min, max | 56, 113 | 53, 117 | 56, 109 | 53, 117 | 53, 117 |
| Positive Subscore | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 14 (3.6) | 15 (4.1) | 14 (4.0) | 15 (4.0) | 14 (3.9) |
| Median | 14 | 15 | 14 | 15 | 14 |
| Min, max | 7, 23 | 7, 25 | 7, 27 | 7, 27 | 7, 27 |
| Negative Subscore | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 26 (3.3) | 27 (3.6) | 27 (3.4) | 27 (3.5) | 27 (3.4) |
| Median | 26 | 27 | 27 | 27 | 27 |
| Min, max | 21, 40 | 21, 43 | 21, 39 | 21, 43 | 21, 43 |
| PSP Scale Total Score | |||||
| N | 171 | 170 | 171 | 341 | 512 |
| Mean (SD) | 53 (11.0) | 53 (12.2) | 53 (10.5) | 53 (11.4) | 53 (11.2) |
| Median | 50 | 51 | 50 | 50 | 50 |
| Min, max | 21, 80 | 20, 100 | 15, 75 | 15, 100 | 15, 100 |
| CGI-S Score | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 4 (0.6) | 4 (0.6) | 4 (0.6) | 4 (0.6) | 4 (0.6) |
| Median | 4 | 4 | 4 | 4 | 4 |
| Min, max | 2, 6 | 3, 6 | 3, 6 | 3, 6 | 2, 6 |
| Verbal Fluency Test (words per min) | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 25 (9.3) | 23 (10.1) | 23 (9.7) | 23 (9.9) | 24 (9.7) |
| Median | 24 | 23 | 22 | 22 | 23 |
| Min, max | 4, 51 | 0, 47 | 0, 51 | 0, 51 | 0, 51 |
| CDSS Score | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 1 (1.5) | 1 (1.5) | 1 (1.5) | 1 (1.5) | 1 (1.5) |
| Median | 0 | 0 | 0 | 0 | 0 |
| Min, max | 0, 6 | 0, 6 | 0, 6 | 0, 6 | 0, 6 |
| AIMS Composite Score | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 0 (1.0) | 0 (1.4) | 0 (1.0) | 0 (1.2) | 0 (1.2) |
| Median | 0 | 0 | 0 | 0 | 0 |
| Min, max | 0, 11 | 0,12 | 0,10 | 0,12 | 0,12 |
| BARS Total Score | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 0 (0.4) | 0 (0.4) | 0 (0.4) | 0 (0.4) | 0 (0.4) |
| Median | 0 | 0 | 0 | 0 | 0 |
| Min, max | 0, 2 | 0, 2 | 0, 3 | 0, 3 | 0, 3 |
| S-AS | |||||
| N | 172 | 170 | 171 | 341 | 513 |
| Mean (SD) | 0 (1.0) | 1 (2.2) | 1 (1.5) | 1 (1.9) | 1 (1.7) |
| Median | 0 | 0 | 0 | 0 | 0 |
| Min, max | 0, 7 | 0, 15 | 0, 10 | 0, 15 | 0, 15 |
| STS Total Score | |||||
| N | 171 | 170 | 171 | 341 | 512 |
| Mean (SD) | 0 (0.1) | 0 (1.5) | 0 (0.3) | 0 (1.1) | 0 (0.9) |
| Median | 0 | 0 | 0 | 0 | 0 |
| Min, max | 0, 1 | 0, 20 | 0, 3 | 0, 20 | 0, 20 |
Note: AIMS, Abnormal Involuntary Movement Scale; BARS, Barnes Akathisia Rating Scale; CDSS, Calgary Depression Scale for Schizophrenia; CGI-S, Clinical Global Impression of Severity; Max, maximum; Min, minimum; NSFS, Marder Negative Symptoms Factor Score; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance; SAS, Simpson-Angus Scale; SD, standard deviation; STS, Sheehan Suicidality Tracking Scale.
Efficacy
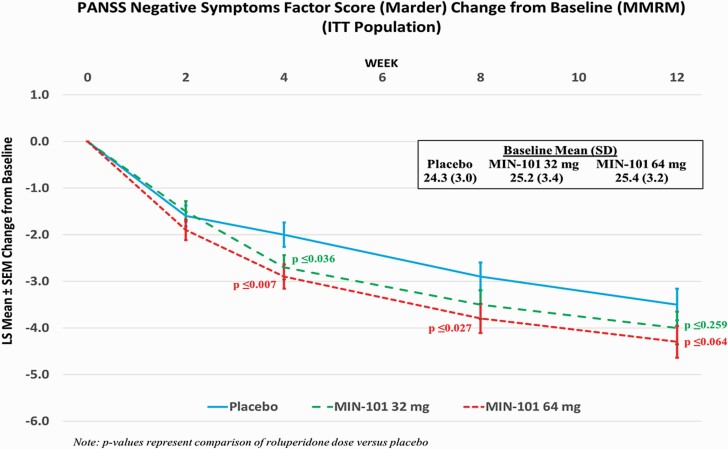
Table 3 presents summary of findings for primary, key secondary, and other secondary and exploratory endpoints. The analysis of the change from baseline to Week 12 in NSFS for the ITT population, showed a treatment difference versus placebo of –0.52 (95% CI: –1.42, 0.38) for the 32 mg roluperidone group and –0.85 (95% CI: –1.75, 0.05) for the 64 mg roluperidone group (P ≤ .260 and P ≤ .064, respectively; figure 2). The analysis of the change from Baseline to Week 12 in NSFS for the m-ITT population for the 64 mg roluperidone group demonstrated nominally statistically significant superiority compared to placebo (difference versus placebo: –0.96 [95% CI: –1.89, –0.03], P ≤ .044; ES = 0.26; table 3). Statistically, significantly more patients had a 20% reduction from baseline at Week 12 in the 64 mg roluperidone dose in both NSFS (39% versus 23% on placebo; P ≤ .006) and PANSS total score (20% versus 9% on placebo; P ≤ .021).
Table 3.
Summary of Select Efficacy Endpoints At Week 12
| Change from Baseline (LS Means (SEM)) |
P-value | Effect Sizea | |||||
|---|---|---|---|---|---|---|---|
| MIN-101 | MIN-101 versus Placebo | MIN-101 versus Placebo | |||||
| Placebo | 32 mg | 64 mg | 32 mg | 64 mg | 32 mg | 64 mg | |
| Primary Objective | |||||||
| Marder’s NSFS (ITT) | –3.5 (0.34) | –4.0 (0.35) | –4.3 (0.34) | .259 | .064 | 0.13 | 0.21 |
| Marder’s NSFS (mITT) | –3.5 (0.35) | –4.0 (0.35) | –4.5 (0.35) | .286 | .044 | 0.13 | 0.26 |
| Key Secondary Objectives | |||||||
| PSP Total Score (ITT) | 3.9 (0.73) | 4.5 (0.75) | 6.1 (0.73) | .542 | .021 | 0.07 | 0.27 |
| PSP Total Score (mITT) | 3.8 (0.75) | 4.4 (0.77) | 6.2 (0.77) | .551 | .017 | 0.07 | 0.29 |
| Secondary and Exploratory | |||||||
| Clinical Global Impression of Severity | –0.3 (0.06) | –0.4 (0.06) | –0.5 (0.06) | .221 | .073 | 0.12 | 0.24 |
| PANSS Constructs | |||||||
| Total Score | –5.5 (0.84) | –7.1 (0.87) | –7.4 (0.85) | .168 | .098 | 0.17 | 0.20 |
| Negative Symptoms Subscore | –3.8 (0.35) | –4.2 (0.35) | –4.7 (0.35) | .392 | .046 | 0.10 | 0.23 |
| Positive Symptoms Subscore | –0.2(0.25) | –0.3 (0.26) | –0.4 (0.25) | .783 | .478 | 0.04 | 0.07 |
| General Psychopathology Subscore | –1.7 (0.45) | –2.8 (0.47) | –2.3 (0.46) | .092 | .380 | 0.22 | 0.12 |
| Marder’s Positive Symptoms Factor Score | –1.0 (2.70) | –1.5 (2.65) | –1.6 (2.86) | .190 | .039 | 0.14 | 0.24 |
| Marder’s Anxiety/Depression Factor Score | –0.5 (0.20) | –0.6 (0.20) | –0.7 (0.20) | .622 | .448 | 0.05 | 0.09 |
| Marder’s Disorganized Thought Factor Score | –1.2 (0.24) | –1.6 (0.25) | –1.4 (0.25) | .279 | .514 | 0.15 | 0.07 |
| Marder’s Uncontrolled Hostility/Excitement Factor Score | 0.2 (0.18) | 0.3 (0.18) | 0.5 (0.18) | .684 | .153 | –0.05 | –0.15 |
| NSFS Emotional Experience Score | –1.3 (0.16) | –1.5 (0.16) | –1.8 (0.16) | .401 | .020 | 0.11 | 0.28 |
| NSFS Emotional Expression Score | –2.3 (0.22) | –2.6 (0.22) | –2.6 (0.22) | .352 | .349 | 0.17 | 0.17 |
| PSP Domains | |||||||
| Self-Care | –0.3 (0.06) | –0.4 (0.06) | –0.3 (0.06) | .261 | .819 | 0.15 | 0.04 |
| Socially Useful Activities | –0.3 (0.05) | –0.3 (0.06) | –0.4 (0.05) | .865 | .047 | 0.02 | 0.18 |
| Personal and Social Relationships | –0.3 (0.06) | –0.4 (0.06) | –0.3 (0.06) | .076 | .501 | 0.15 | 0.00 |
| Disturbing and Aggressive Behaviors | 0.0 (0.05) | 0.0 (0.05) | 0.0 (0.05) | .961 | .186 | 0.00 | –0.07 |
| Clinical Global Impression of Improvementb | 3.3 (0.08) | 3.4 (0.08) | 3.3 (0.08) | .683 | .746 | –0.12 | 0.02 |
| Calgary Depression Scale for Schizophrenia | 0.1 (0.13) | –0.2 (0.13) | –0.1 (0.13) | .093 | .139 | 0.21 | 0.14 |
| Total Verbal Fluencyc | 2.4 (0.64) | 3.9 (0.66) | 1.7 (0.63) | .067 | .327 | 0.21 | –0.10 |
| Responder Analysis d | |||||||
| Number of Patients with 20% Reduction in NSFS at Week 12 | 30/128 (23%) | 32/116 (28%) | 48/122 (39%) | .418 | .006 | 0.05 | 0.17 |
| Number of Patients with 20% Reduction in PANSS Total Score at Week 12 | 12/128 (9%) | 20/116 (17%) | 24/122 (20%) | .061 | .021 | 0.12 | 0.15 |
| Number of Patients with 7-Point Improvement in PSP Total at Week 12 |
37/128 (29%) | 37/116 (32%) | 50/122 (41%) | .589 | .032 | 0.03 | 0.13 |
Note: NSFS, Negative symptoms factor score; PANSS, Positive and negative syndrome scale; PSP, Personal and social performance.
aBased on Cohen’s d.
bObserved data analyzed using MMRM with baseline CGI-S score as covariate.
cBased on sum of 3 trials.
dEffect size is based on Cohen’s W.
Fig. 2.
PANSS Negative Symptoms Factor Score (Marder) change from baseline (MMRM) ITT population.
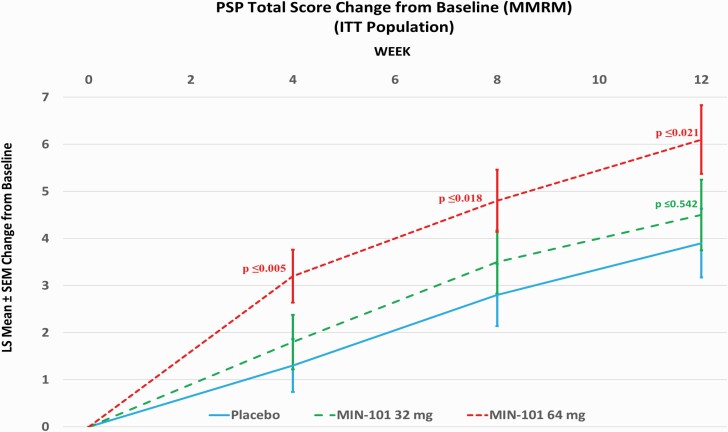
There was an increase (improvement) in the PSP total score at Week 12 in all 3 treatment groups, with the greatest improvement in the 64 mg roluperidone group (for ITT population: LSM difference versus placebo: 2.20; 95% CI: 0.33, 4.06; P ≤ .021; figure 3; for m-ITT population: LSM difference versus placebo: 2.40; 95% CI: 0.44, 4.35; P ≤ .017). Statistically, significantly more patients had a 7-point response in the PSP total score at Week 12 in the 64 mg roluperidone dose (41% versus 29% on placebo; P ≤ .032). Roluperidone 64 mg group was nominally statistically significantly superior to placebo on the derived PANSS emotional experience score (N2 + N4 + G16)26 and for the N2 +N4 reflective of avolition.
Fig. 3.
Personal Social Performance (PSP) total score change from baseline (MMRM) ITT population.
Analysis adjusted for CDSS, or SAS baseline scores did not change the study results suggesting that the observed improvement with roluperidone was independent of the depression or drug-induced extrapyramidal syndrome scores
Safety and Tolerability
Treatment-emergent adverse events (TEAE) were experienced by 33% of patients who received placebo, 42% of patients who received roluperidone 32 mg, and 37% of patients who received roluperidone 64 mg. The most commonly reported TEAEs were insomnia (10% Roluperidone, 10% placebo), worsening of schizophrenia (8.0% roluperidone, 3.0% placebo), headache (5.0% roluperidone, 5.0% placebo), anxiety (4.0% roluperidone, 2.0% placebo), and agitation (3.0% roluperidone, 2.0% placebo). The remainder of reported TEAEs occurred in < 3% of the patients. The majority of TEAEs reported in all treatment groups were mild to moderate in severity with severe TEAEs reported in 17 patients (3%) overall with similar incidence in all treatment groups. There were 25 (5%) patients with serious adverse events (SAEs), 5 (3%) in the placebo group, and 20 (6%) in the roluperidone groups. There were 2 deaths during the trial in the roluperidone 32 mg dose, one patient committed suicide and another died from gastrointestinal bleeding 6 days after he withdrew consent and discontinued the trial. Neither death was considered by the investigator related to treatment. Overall, 5% of patients in the placebo group, 11% of patients in the 32 mg roluperidone group, and 9% of patients in the 64 mg roluperidone group reported TEAEs that led to the study drug being discontinued. Relapse, defined as worsening of schizophrenia symptoms was the most frequent reason for discontinuation from the trial with the highest incidence in the roluperidone 32 mg dose group (11%) and the lowest incidence in the placebo group (5%). Four patients in the roluperidone treatment groups were discontinued due to a cardiac disorder or ECG abnormality (roluperidone 32 mg: left ventricular dysfunction and T wave inversion [both events in the same patient]); roluperidone 64 mg: electrocardiogram QT prolongation (3 patients), and none in the placebo group.
There was no notable change from baseline in weight (placebo = –0.2 ± 2.97 kg; 32 mg = 0.0 ± 3.96 kg; 64 mg = +0.1 ± 2.87 kg) or waist circumference (placebo = –1.0 ± 9.17 cm; 32 mg = –0.9 ± 7.76 cm; 64 mg = +0.8 ± 9.53 cm) in any of the 3 groups. There were no differences in the prolactin plasma variations between the 3 groups. There were no changes in vital signs, routine laboratory values, and extrapyramidal symptom ratings measured by AIMS, BARS, and SAS scores. There were no changes in suicidality expressed as S-STS scores.
Discussion
In this 12-week randomized, double-blind, placebo-controlled trial of symptomatically stable schizophrenia patients with moderate to severe negative symptoms, roluperidone 64 mg dose was associated with improvements in several indices of negative symptoms and social functioning. Additionally, improvement was also manifested on both experience and expression constructs of negative symptoms27 probably driven by roluperidone’s observed effect on motivation (avolition)7. These results are consistent with the results of a similarly designed roluperidone trial,20 which had as the primary endpoint a similar construct of PANSS negative symptoms and as secondary endpoint the Brief Negative Symptom Scale (BNSS), a measure specifically designed to assess negative symptoms of schizophrenia. Different from the previous trial in which statistical significance for the primary endpoint was reached for both 64 mg/mg and 32 mg/day20 this trial did not reach statistical significance on the primary endpoint. Roluperidone was well-tolerated with no adverse events that could have unmasked the drug/placebo assignment.
There are several possible reasons why two trials with almost identical patients, study designs, and comparable results may differ in terms of statistical significance. First, larger trials, which involve more study sites (61 sites in this study versus 36 in the previous study), might have increased data variability hence, reduce the likelihood to achieve statistical significance between active treatment and placebo arm.28 Some of the data variability can be mitigated by thorough rater training to reduce observed and reported subjective interpretations of symptoms severity.29 Second, a first, positive successful trial, may raise patients’ and investigators’ expectations of benefit hence, increasing the placebo effect during a second, confirmatory trial. Hence when designing a trial that follows a positive trial, the challenge is to include a sample sufficiently large to overcome the placebo effect generated by expectations yet, limit the variability associated with increasing numbers of sites. Ideally, a few well-trained and supervised sites should address this challenge.
Designs of trials targeting negative symptoms pose several challenges: (a) whether to administer the investigational drug as monotherapy or as an add-on to an antipsychotic drug; (b) what endpoint to use for the assessment of symptoms that may be sensitive to treatment effects, and (c) identifying the patient population most likely to benefit from the intervention. All 3 are challenging tasks since the presumed interaction between the pathophysiology of negative symptoms and the mechanism of action of the pharmacological intervention in question are far from elucidated.
Monotherapy vs. Add-on
Because of the prevailing practice to treat most or all schizophrenia patients continuously with antipsychotic drugs to reduce risk for exacerbation, most trials targeting specific aspects of schizophrenia such as negative symptoms or cognitive impairment employ an add-on design. However, this design introduces a potential confounder that might obscure the effect of the experimental compound. Antipsychotic drugs on one hand indirectly improve negative symptoms by reducing preoccupation with delusions and hallucinations thus enhancing social interactions, but on the other hand, interfere with dopamine-driven motivation circuits hence aggravate avolition which is a major component of the schizophrenia intrinsic negative symptoms. Therefore, by using an add-on design it is difficult to disentangle between the direct effect of the experimental drug on negative symptoms and the pseudo-effect derived from the treatment with antipsychotics that blockade the D2 receptors. To overcome this limitation it has recently been suggested that trials targeting negative symptoms should use monotherapy and placebo-controlled designs,23 which is the option taken in this trial and the previous trial.20 Furthermore, data are accumulating showing that over a third of patients do not experience exacerbation during one year of placebo administration and some might even benefit from antipsychotic drug reduction or discontinuation.30–33
Assessment Instruments
There is currently no agreement on what constitutes the best instrument to assess negative symptoms. Assessments based on raters’ observations and collateral reports have their inherent limitations29, 34 while passive digital phenotyping instruments are still under development.35 For the current trial, the NSFS and the PSP were selected as primary and key secondary endpoints as informed by the previous trial data where they appear to capture the effect of roluperidone.
Patient Population
In designing the inclusion criteria for this trial the investigator intended to target a patient population that on one hand is sufficiently large to make the results clinically relevant and on the other hand to restrict it to patients who can maintain symptoms stability without continuous maintenance treatment with antipsychotic drugs. Historically, trials targeting negative symptoms have included patients who have relatively severe negative symptoms but do not exceed a pre-established threshold on positive symptoms. However, by defining overly stringent inclusion criteria a large proportion of schizophrenia patients would be excluded from trials, since many of the patients have substantial—albeit stable—, positive symptoms, making the results less clinically relevant, and less generalizable.36 Therefore, the trial presented here did not require an upper limit on the subscore of positive symptoms. On the other hand, we tried to select a group of patients who can maintain symptoms stability without maintenance treatment with antipsychotics. These are patients who for at least 6 months immediately prior to the trial: (1) manifested moderate to severe negative symptoms, 2) manifested no substantial variation in psychotic symptoms, 3) have a low level of symptoms related to agitation, poor impulse control, hostility, suspiciousness, and uncooperativeness, 4) were not actively using illegal drugs 5) have not manifested behaviors which put the patient or those around them at risk. This patient group which constitutes over 60% of the outpatient schizophrenia population37 has already been described in the literature.38, 39, 40 Interestingly the drop-out rate from the trial due to exacerbation of positive symptoms was very low during the 12 weeks double-blind part of the trial as well as during 9 months of open-label trial (data not shown) consistent with the hypothesis that this patient population can maintain symptoms stability on Roluperidone monotherapy.
Choice of Adjustment of Multiplicity and of Data Set
Perhaps the most challenging aspect of this trial and the conclusions that can be drawn are related to the pre-specification of the truncated Hochberg method for controlling the Type I error rate at the 2-sided 0.050 alpha level within the family of primary and the key secondary hypotheses. The investigators’ choice was informed by the results of the previous trial 20 where both doses of roluperidone were statistically superior to placebo in using the same multiplicity adjustment. If a more traditional adjustment method such as the step-down approach had been selected, the study results would have shown statistical significance on both the primary endpoint and the key secondary endpoint for the 64 mg roluperidone dose.
The quality of the data was analyzed with the help of logic checks on site, during the rating and, centrally with the help of artificial intelligence. This analysis revealed that at 1/61 participating sites, a site that recruited 17/513 patients reported implausible behavioral (schizophrenia symptoms) and physiological (blood pressure data). All 17 subjects had the same CGI-S score between Baseline and any of the visits during the 12-week study duration as well as extreme clustering of blood pressure without the expected physiologically variability within and between subjects. Of note, throughout the 12-week double-blind study duration, the site reported only 1 TEAE across all 17 subjects which is not impossible, but very unusual in double-blind placebo-controlled schizophrenia studies. The decision to exclude these 17 patients and create the equivalent of an m-ITT analysis set was taken before the database was locked and the treatment codes unblinded. Nevertheless, the results section reports both ITT and m-ITT.
Limitations
Among the limitations of this trial are the short washout period and the possibility that some of the improvement in NSFS might be attributed to the withdrawal of antipsychotics and decrease in secondary negative symptoms. However, the randomization should have mitigated such effects. Also, some of the statistically significant advantages of roluperidone are derived from posthoc analysis. Nevertheless, the advantages of the active drug versus placebo are consistent within-trial and between the two trials.
In summary, the results of this trial are consistent with the results of a previous similar trial suggesting that roluperidone might benefit negative symptoms and social functioning in schizophrenic patients with stable negative and positive symptoms. Supporting the validity of the results of this and the previous trial is the fact that roluperidone is indistinguishable from placebo by subjective or observable adverse effects, hence drug/placebo unmasking is not possible.
Because aspects of negative symptoms manifest in 19 distinct DSM-5 categories41 and in adolescents suspected of prodromal and spectrum schizophrenia,42 future trials targeting negative symptoms or aspects of negative symptoms such as avolition43 across DSM categories or along the research domain criteria principles44 will be conducted.
Acknowledgments
MD, RL, JS authors are employees of Minerva Neurosciences the sponsor of the trial and CS, EL, NN. SW are employees of PPRS the CRO of the study. MW, PH, GS have received consultancy fee from Minerva Neurosciences.
Contributor Information
Michael Davidson, Minerva Neurosciences, Watham, MA, USA; Department Of Psychiatry Nicosia Cyprus, Nicosia University Medical School, Egkomi, Cyprus.
Jay Saoud, Minerva Neurosciences, Watham, MA, USA.
Corinne Staner, PPRS, 4e Av. du Général de Gaulle, Colmar, Grand EST, France.
Nadine Noel, PPRS, 4e Av. du Général de Gaulle, Colmar, Grand EST, France.
Sandra Werner, PPRS, 4e Av. du Général de Gaulle, Colmar, Grand EST, France.
Elisabeth Luthringer, PPRS, 4e Av. du Général de Gaulle, Colmar, Grand EST, France.
David Walling, Collaborative Neuroscience Network, Suite 3, Garden Grove, CA, USA.
Mark Weiser, University of Tel Aviv School of Medicine, Ramat Aviv, Israel.
Philip D Harvey, Department of Psychiatry, University of Miami Miller School of Medicine, Miami, FL, USA.
Gregory P Strauss, Department of Psychology, University of Georgia, Athens, GA, USA.
Remy Luthringer, Minerva Neurosciences, Watham, MA, USA.
References
- 1. Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5:664–677. [DOI] [PubMed] [Google Scholar]
- 2. Robertson BR, Prestia D, Twamley EW, Patterson TL, Bowie CR, Harvey PD. Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schizophr Res. 2014;160:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szczypiński JJ, Gola M. Dopamine dysregulation hypothesis: the common basis for motivational anhedonia in major depressive disorder and schizophrenia? Rev Neurosci. 2018;29:727–744. [DOI] [PubMed] [Google Scholar]
- 4. Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645–692. [DOI] [PubMed] [Google Scholar]
- 5. Kirkpatrick B. Developing concepts in negative symptoms: primary vs secondary and apathy vs expression. J Clin Psychiatry. 2014;75:30–37. [DOI] [PubMed] [Google Scholar]
- 6. Culbreth AJ, Moran EK, Kandala S, Westbrook A, Barch DM. Effort, avolition, and motivational experience in schizophrenia: analysis of behavioral and neuroimaging data with relationships to daily motivational experience. Clin Psychol Sci. 2020;8:555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strauss GP, Zamani Esfahlani F, Sayama H, et al. Network analysis indicates that avolition is the most central domain for the successful treatment of negative symptoms: evidence from the roluperidone randomized clinical trial. Schizophr Bull. 2020;46:964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carriere P, Bonhomme D, Lemperiere T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study (the Amisulpride Study Group. Eur Psychiatry. 2000;15:321–329. [DOI] [PubMed] [Google Scholar]
- 9. Németh B, Molnár A, Akehurst R, et al. Quality-adjusted life year difference in patients with predominant negative symptoms of schizophrenia treated with cariprazine and risperidone. J Comp Eff Res. 2017;6:639–648. [DOI] [PubMed] [Google Scholar]
- 10. Leucht S, Davis JM.Schizophrenia, primary negative symptoms, and soft outcomes in psychiatry. Lancet. 2017;389(10074):1077–1078. doi: 10.1016/S0140-6736(17)30181-2. [DOI] [PubMed] [Google Scholar]
- 11. Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia—results from the phase III FlashLyte and DayLyte studies. Biol Psychiatry. 2017;82:8–16. [DOI] [PubMed] [Google Scholar]
- 12. Gomes FV, Grace AA. Beyond dopamine receptor antagonism: new targets for schizophrenia treatment and prevention. Int J Mol Sci . 2021;22:4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menniti FS, Chappie TA, Schmidt CJ. PDE10A inhibitors—clinical failure or window into antipsychotic drug action? Front Neurosci. 2020;14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katz JL, Su T-P, Hiranita T, et al. A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals. 2011;4:880–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lever JR, Miller DK, Green CL, et al. A selective sigma-2 receptor ligand antagonizes cocaine-induced hyperlocomotion in mice. Synapse 2014;68:73–84. [DOI] [PubMed] [Google Scholar]
- 16. Skuza G. Pharmacology of sigma (σ) receptor ligands from a behavioral perspective. Curr Pharm Des. 2012;18:863–874. [DOI] [PubMed] [Google Scholar]
- 17. Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. J Pharmacol Exp Ther. 2000;292:900–911. [PubMed] [Google Scholar]
- 18. Hayashi T, Su T-P. The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr Neuropharmacol. 2005;3:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perez DM. α1-Adrenergic receptors in neurotransmission, synaptic plasticity, and cognition. Front Pharmacol. 2020;11:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;174:1195–1202. [DOI] [PubMed] [Google Scholar]
- 21. Rabinowitz J, Badescu S, Palamarchuk P, et al. Personal and social adjustment effects of roluperidone in patients with schizophrenia and negative symptoms: Results from an exploratory outcome of a randomized placebo-controlled trial. Schizophr Res. 2019;211:103–104. doi: 10.1016/j.schres.2019.07.029. PMID: 31375316. [DOI] [PubMed] [Google Scholar]
- 22. White L, Harvey PD, Opler L, Lindenmayer J. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. Psychopathology 1997;30:263–274. [DOI] [PubMed] [Google Scholar]
- 23. Marder SR, Davidson M, Zaragoza S, et al. Issues and perspectives in designing clinical trials for negative symptoms in schizophrenia: consensus statements. Schizophrenia Bulletin Open 2020;1:sgz001. [Google Scholar]
- 24. Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand. 2007;116:403–418. [DOI] [PubMed] [Google Scholar]
- 25. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997:983–997. [PubMed] [Google Scholar]
- 26. Khan A, Liharska L, Harvey PD, Atkins A, Ulshen D, Keefe RS. Negative symptom dimensions of the positive and negative syndrome scale across geographical regions: implications for social, linguistic, and cultural consistency. Innovat Clin Neurosci. 2017;14:30. [PMC free article] [PubMed] [Google Scholar]
- 27. Harvey PD, Khan A, Keefe RS. Using the positive and negative syndrome scale (PANSS) to define different domains of negative symptoms: prediction of everyday functioning by impairments in emotional expression and emotional experience. Innovat Clin Neurosci. 2017;14:18. [PMC free article] [PubMed] [Google Scholar]
- 28. Leucht S, Chaimani A, Mavridis D, et al. Disconnection of drug-response and placebo-response in acute-phase antipsychotic drug trials on schizophrenia? Meta-regression analysis. Neuropsychopharmacology. 2019;44:1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr Bull. 2011;37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA psychiatry 2013;70:913–920. [DOI] [PubMed] [Google Scholar]
- 31. Luther L, Suor JH, Rosen C, Jobe TH, Faull RN, Harrow M. Clarifying the direction of impact of negative symptoms and neurocognition on prospective work functioning in psychosis: a 20-year longitudinal study. Schizophr Res. 2020;220:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davidson M. The debate regarding maintenance treatment with antipsychotic drugs in schizophrenia. Dialogues Clin Neurosci. 2018;20:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harrow M, Jobe T, Faull R. Does treatment of schizophrenia with antipsychotic medications eliminate or reduce psychosis? A 20-year multi-follow-up study. Psychol Med. 2014;44:3007–3016. [DOI] [PubMed] [Google Scholar]
- 34. Kirkpatrick B, Saoud JB, Strauss GP, et al. The brief negative symptom scale (BNSS): sensitivity to treatment effects. Schizophr Res. 2018;197:269–273. [DOI] [PubMed] [Google Scholar]
- 35. Cohen AS, Schwartz E, Le TP, et al. Digital phenotyping of negative symptoms: the relationship to clinician ratings. Schizophr Bull. 2021;47:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunayevich E, Chen CY, Marder SR, Rabinowitz J. Restrictive symptomatic inclusion criteria create barriers to clinical research in schizophrenia negative symptoms: an analysis of the CATIE dataset. Eur Neuropsychopharmacol. 2014;24:1615–1621. [DOI] [PubMed] [Google Scholar]
- 37. Bobes J, Arango C, Garcia-Garcia M, Rejas J. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry. 2009;70:280–286. [DOI] [PubMed] [Google Scholar]
- 38. McGorry P, Alvarez-Jimenez M, Killackey E. Antipsychotic medication during the critical period following remission from first-episode psychosis: less is more. JAMA Psychiatry 2013;70:898–900. [DOI] [PubMed] [Google Scholar]
- 39. Bogers JPAM, Hambarian G, Michielis M, et al. Risk factors for psychotic relapse after dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia: a systematic review and meta-analysis. Schizophrenia Bull Open. 2020;1:1–12. doi: 10.1093/schizbullopen/sgaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murray RM, Quattrone D, Natesan S, et al. Should psychiatrists be more cautious about the long-term prophylactic use of antipsychotics? Br J Psychiatry. 2016;209:361–365. [DOI] [PubMed] [Google Scholar]
- 41. Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull. 2017;43:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yung AR, Nelson B, McGorry PD, Wood SJ, Lin A. Persistent negative symptoms in individuals at Ultra High Risk for psychosis. Schizophr Res. 2019;206:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strauss GP, Bartolomeo LA, Harvey PD. Avolition as the core negative symptom in schizophrenia: relevance to pharmacological treatment development. NPJ Schizophr. 2021;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cuthbert BN. The role of RDoC in future classification of mental disorders. Dialogues Clin Neurosci. 2020;22:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]