Abstract
Affective dysregulation (AD) among persons with schizophrenia spectrum disorders, involving the tendency to exhibit sensitivity to minor stress and negative affective states, is an important diagnostic feature and relates to poorer functional and clinical outcomes. Studies of persons with elevated risk for psychosis demonstrate similar AD to those with schizophrenia, and literature suggest a potential influence of AD in the transition from psychosis-like symptoms (PLEs) to disorder. Cross-sectional investigations to date have supported the link between AD and psychosis, and longitudinal studies have mostly yielded mixed findings without demonstration of potential causal relationships between AD and psychosis. This study examined the concurrent and predictive relationships between AD and PLE in a community sample of youth (n = 630) with attention to distinct facets of AD as a latent construct, including low resiliency, low reactive control, and negative emotionality, using structural equation to estimate a longitudinal cross-lagged and autoregressive model across 3 study waves from 15 to 24 years of age. As hypothesized, AD in the mid-teen years predicted subsequent PLE 3 years later. In addition, we found that increasing PLE in the end of the teen years related to a subsequent increase in AD in the early 20s. A cross-sectional relationship between AD and PLE in the mid-teen years was also supported. Findings overall describe important relationships between AD and PLE that appear to vary with developmental stage, implicating various factors to inform approaches for identifying youth who may be at risk for subsequent PLE or other mental health conditions.
Keywords: affective dysregulation, negative affect, psychosis, structural equation modeling, psychosis-like symptoms
Introduction
The tendency of persons with schizophrenia spectrum disorders (schizophrenia and schizoaffective disorders) to exhibit sensitivity to minor stress, leading to negative affective states, has been increasingly recognized as an important clinical feature of the disorder.1–3 This vulnerability to affective dysregulation (AD) leads to negative affect in response to external stressors, and negative affects also arises in situations not typically seen as stressful. Although “negative” as a descriptor of affect in schizophrenia since the late-20th century has referred to the absence of emotion (eg, anhedonia and flat affect),4,5 original descriptions of schizophrenia in the early 20th century note that affects may be expressed with great energy and include dysphoric mood, anxiety, and stress sensitivity.6 Literature in recent decades has reinforced that flat affect does not mean the absence of emotion.7 Patients with schizophrenia spectrum disorders exhibit more negative affect in the course of their daily lives as shown by experience sampling studies,8 and laboratory studies show a bias to appraise neutral stimuli as more negative.9 Furthermore, negative affect predicts poorer functional outcomes, greater hospitalizations, reduced quality of life, increased need for mental health treatment, and suicide among persons with schizophrenia spectrum disorders, even after controlling for traditional negative symptoms, neurocognition, and positive symptoms.10–14 Given the tendency for poor outcomes in persons with schizophrenia spectrum disorders who experience AD, interest has grown in the role of AD in the development of psychosis.
Studies of persons with an elevated risk for psychosis have demonstrated similar AD as observed in individuals with schizophrenia.12 Walker and colleagues15 studied home videos of children who later developed schizophrenia and observed that negative affect and minimal expressions of joy were greater among children who developed schizophrenia in comparison to their siblings who did not. These findings, along with more recent work, have suggested that AD, typically measured as negative affect, could influence the transition from premorbid to prodromal phases.16–24 Examinations of such relationships have occurred with different measures of negative affect and among various study samples (eg, general population, clinical high risk, diagnostic25). Findings support the cross-sectional relationships between psychosis symptoms and negative affect as a construct,14,25–30 with some studies more specifically measuring anxiety or depressive symptoms,31–34 stress sensitivity,35 and emotion regulation/dysregulation.36,37 Though these cross-sectional data support the link between AD and psychosis, they do not answer the question as to whether AD causes psychosis, or psychosis causes AD, or some combination of both.
Longitudinal studies are one approach to ascertain the influences of AD on the development of psychosis, although results to date have been mixed. In a relapse prevention trial, poor cognition and depressed mood predicted subsequent development of paranoia using structural equation modeling, yet there was no evidence for the reverse influence.38 On the other hand, depression and psychosis were shown to relate concurrently but not across time among a subclinical sample of help-seeking adolescents and young adults (ages 15–24) for nonpsychotic disorders.32 Similarly, significant cross-sectional relationships have been demonstrated between negative affect and positive symptoms among adults with schizophrenia spectrum disorders who were involuntarily hospitalized, but no significant longitudinal effects were found in one year follow-up.39 In Clinical High Risk (CHR) samples, depressive symptoms/diagnoses have been shown to relate to worse functioning and decreased likelihood of remission from CHR status, yet depression is not found to influence the transition to psychosis over time.40,41 Overall, most, but not all, studies have failed to demonstrate a causal relationship between AD and subsequent psychosis in patient samples.
A critical confound in these studies is that patients with psychosis diagnoses and those in CHR cohorts are help-seeking individuals, typically presenting with some level of distress. Alternatively, population studies avoid this confound and multiple surveys across diverse societies have shown that psychosis experiences, or psychosis-like experiences (PLEs) are prevalent among 5% of the adult general population42 and 8%–17% of the child and adolescent population.43–45 Research suggests a persistent subclinical psychosis pathway to clinical disorders,43,46,47 and meta-analytic data show PLEs are associated with a 3-times greater risk of mental disorder and 4-times greater risk for psychotic disorder among non-help seeking children and adolescents in a community sample.45 Furthermore, increased prevalence of negative affect (manifested as depression, anxiety, behavioral disorders) has been reported in participants with PLEs.45 In a general population sample of 7000 adults, those who experienced hallucinations with negative emotional states were found to have a greater likelihood of developing delusions 3 years later.27 Among a community sample of adolescents and young adults across 8.6 years, bi-directional associations were observed between psychosis and AD (measured as high and low mood fluctuations).37 Taken together, community samples provide a unique window into the role of AD and psychosis (or PLEs). However, few studies to date have investigated AD prior to the development of PLEs in youth using longitudinal structural equation modeling methods, a theory-driven analytic approach allowing for investigations of complex and causal relationships among latent and observed variables.48
The current paper sought to leverage a longitudinal study in a community sample of youth to address the causal relationship between AD and psychosis, and specifically the occurrence of the former leading to subsequent development of PLE. A cross-lagged panel design was used to evaluate a model across 3 study waves from 15 to 23 years of age to examine cross-sectional associations as well as predictive, cross-lagged effects. In contrast to prior work, we used a latent AD construct, comprised of three measures from the CQS Measure,49 derived from temperament and personality taxonomies developed by Block & Block50 and used in prior investigations of emotional functioning51,52: (1) resiliency, (2) reactive control, and (3) negative emotionality. Resiliency describes psychological flexibility to focus and shift behaviors in response to varying environmental demands, with low levels of resiliency leading to difficulty initiating or inhibiting behaviors. Reactive control is characterized by impulsive responding and behavioral disinhibition.53 Negative emotionality describes deficits in emotion regulation, including depressed mood, symptoms of anxiety, and irritable anger. We predicted, as suggested by prior studies, that AD at one time point will relate to subsequent AD across time, and similarly, PLE will relate to subsequent PLE over time. Critically, our principal hypothesis was that AD will precede subsequent PLE over time.
Methods
Participants
The analytic sample included 630 individuals (67.5% male; 95.9% white) from the Michigan Longitudinal Study (MLS). MLS is an ongoing, prospective study comprised of community-recruited families with parental alcohol use disorder (AUD; 82% of the study sample) and a contrast sample of families without AUD. Additional details on study design and data collection protocols can be found elsewhere.54 Parental AUD diagnoses were determined by meeting criteria during a clinical interview using the Diagnostic Interview Schedule—Version 4.55 The study cohort was also weighted toward having externalizing disorders along with AUD, as the families were recruited by identifying fathers presenting in the court system for driving under the influence. In the present study, participants were from 3 data collection waves to capture an age range of adolescence to young adulthood: T5 (15–17 years of age; N = 522, M = 16.08, SD = .960), T6 (18–20 years of age; N = 565, M = 19.11, SD = 1.044), and T7 (21–23 years of age; N = 630, M = 22.16, SD = 1.149).
All study materials and procedures were approved by the University of Michigan Medical School Institutional Review Board. All participants who were over the age of 18 provided written informed consent. Participants under the age of 18 provided assent and their parent gave written informed consent for their child’s involvement in the study.
Measurement
Affective Dysregulation (AD).
Emotional functioning was measured with 3 subscales of the California Q-Sort (CQS), with the child version (California Child Q-Sort) used at T5 and the adult version used at T6 and T7 (Revised Adult CQS).51,56,57 Subscales include resiliency, reactive control, and negative emotionality. The CQS is a standardized, clinician-administered evaluation of temperament (child version) or personality (adult version) and behavioral functioning. For more detailed description of subscales, see supplementary materials.
Psychosis-Like Experiences (PLE).
The Youth Self Report (YSR)58 and Adult Self Report (ASR)59 were used to assess PLE symptoms, including: (1) paranoia, (2) hallucinations, and (3) bizarre thinking and behaviors at T5 and T6 through T7, respectively. Response options were 0 = “not at all true”, 1 = “somewhat true”, or 2 = “very true”. Response options were subsequently recoded as binary variables for each of the 5 questions to capture the presence or lack of presence of each psychosis experience. Lastly, a single psychosis variable was computed at each time point (T5, T6, T7) to represent a count of the 5 psychosis questions. As a result, scores for the psychosis variable range from 0 to 5, with 5 meaning all symptom questions were endorsed.
Quantitative Modeling and Analysis
Data were analyzed in SPSS28 and Mplus8.60 Univariate distributions, bivariate correlations, and missing data were examined among all variables. Confirmatory Factor Analysis (CFA) was conducted to specify and test the fit of the Q-Sort resiliency, reactive control, and negative emotionality subscale factor loadings for a latent AD variable to be used in the measurement model at each time point. Next, series of linear mixed-effect models were performed to independently examine PLE and the 3 indicators of the latent AD variable across the 3 time points (T5–T7): negative emotionality, (reverse) resilience, and (reverse) reactive control.
Longitudinal panel modeling61 was used to test the proposed model using a robust (Huber–White) maximum likelihood algorithm to manage non-normality and variance heterogeneity. Full Information Maximum Likelihood estimators were used to handle missing data62 and model fit was evaluated using both global (chi square, Comparative Fit Index [CFI], Tucker–Lewis Index [TLI], Standardized Root Mean Square Residual [SRMR], Root Mean Square of Approximation [RMSEA]) and focused (standardized residuals and modification indices) fit indices. Acceptable fit was determined by a minimum cutoff of 0.95 for CFI and TLI, a maximum cutoff of 0.06 for RMSEA, and a maximum cutoff of 0.08 for SRMR.63 Preliminary modeling included investigations of the auto-regressive AD and PLE relationships modeled separately over time, auto-regressive relationships together over time without crossed-lagged paths, and the proposed longitudinal panel model with sex included as a covariate.
The final longitudinal panel model included auto-regressive and crossed-lagged effects over 3 points of time (T5, T6, and T7) such that: (1) PLE scores were regressed on earlier PLE scores (ie, T7 on T6 and T6 on T5), (2) AD scores were regressed on earlier AD scores, (3) PLE scores were regressed on earlier AD scores, and (4) AD scores were regressed on earlier PLE scores. In addition, both PLE and AD were concurrently correlated, and sex was included as a covariate in each time point due to the imbalance in participant sex given the study originally recruited male children. Given the complexity of the models, if adequate model fit was not achieved initially, modification indices and theoretical relationships between variables were used to guide determination of residual correlations to achieve adequate model fit.
Our primary model used only sex as a covariate, but to explore other contributing factors, such as substance use, we also tested 4 supplementary models including other additional covariates in the past year of assessment conducted as sensitivity analyses (sex and number of binge drinking days; sex and volume of alcohol consumed; sex and number of days marijuana used; and sex and a composite score of substance use representing alcohol, marijuana, and tobacco use).64 Substance use was measured using the Drinking and Drug History Questionnaire.65
Results
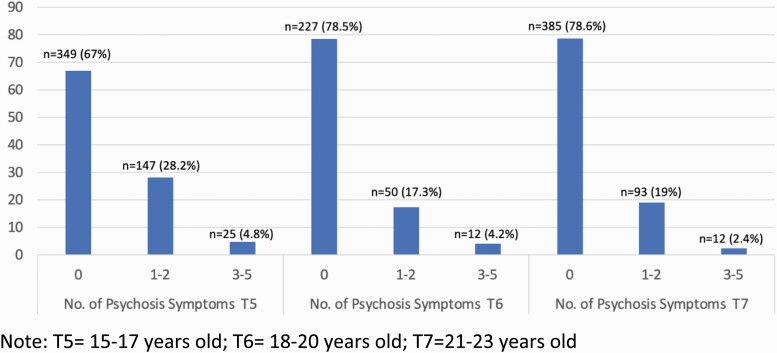
General means and standard deviations for all the study variables are presented in table 1. For the PLE variable, 33% (n = 172) of participants endorsed at least 1 PLE item at T5, 21.5% (n = 62) at T6 and 21.4% (n = 105) at T7 (see figure 1). A series of linear mixed effect models confirmed a significant decrease in PLE (b = cc.101, SE = .02, P < .001) and in the 3 indicators of the latent AD variable across the 3 waves of data: negative emotionality (b = –.336, SE = .03, P < .001), low resilience (b = –.101, SE = .03, P < .001; higher scores mean less resilience), and low reactive control (b = –.094, SE = .02, P < .001; higher scores mean less reactive control). No participants at T6 or T7 met criteria for schizophrenia. Substance use characteristics, of focus in sensitivity analyses, along with diagnostic detail of the sample are illustrated in supplementary materials.
Table 1.
Sample Characteristics
| Variables | T5 (15–17 years old) |
T6 (18–20 years old) |
T7 (21–23 years old) |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Age (M, SD) | 522 | 16.08, .960 | 529 | 19.11, 1.04 | 630 | 22.16, 1.15 |
| Psychosis symptoms (M, SD) | 521 | .55, .934 | 289 | .37, .869 | 490 | .32, .695 |
| Affective dysregulationa(M, SD) | ||||||
| Resiliency subscale | 516 | 4.03, 1.05 | 565 | 4.25, 1.14 | 528 | 3.84, 1.14 |
| Reactive control subscale | 516 | 5.04, 1.04 | 565 | 5.00, 1.03 | 528 | 4.88, 0.89 |
| Negative emotionality subscale | 516 | 4.45, 1.00 | 556 | 4.38, 0.97 | 518 | 3.79, 1.32 |
Note: Participant data ranges for distinct variables at each time point.
aHigher scores on all 3 subscales of the AD construct indicate greater dysregulation.
Figure 1.
Number and percent of participants who endorsed psychosis symptoms at each time Point. T5 = 15–17 years old; T6 = 18–20 years old; T7 = 21–23 years old.
The CFA model for the latent AD variable at all 3 time points demonstrated good fit (χ 2 = 46.20, df = 15, P < .05; CFI = 0.97, TLI = 0.93; RMSEA = 0.05, SRMR = 0.05). In each time point, all AD indicator factors (resiliency, reactive control, and negative emotionality) loaded significantly onto their respective latent constructs (P < .05), with loadings ranging from 0.32 (reactive control) to .98 (resiliency) at T5, 0.23 (reactive control) to 0.93 (resiliency) at T6, and 0.40 (reactive control) to 0.73 (resiliency) at T7.
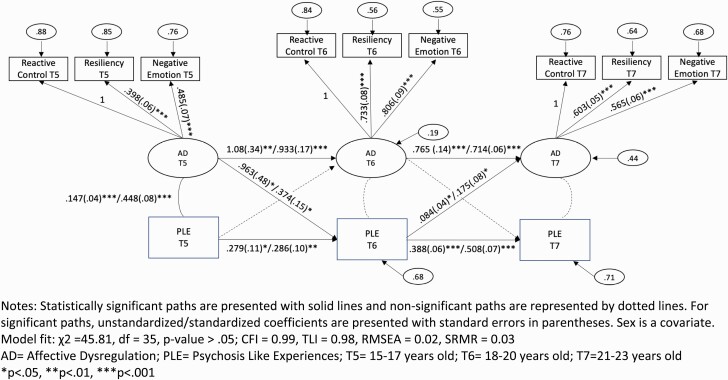
Good model fit was achieved by all preliminary longitudinal panel models and only the results of the final model with sex as a covariate is reported here (figure 2). The panel model achieved good fit per global (χ 2 = 45.81, df = 35, P > .05; CFI = 0.99, TLI = 0.98, RMSEA = 0.02, SRMR = 0.03) and focused fit indices (standardized residuals < |2| and modification indices < |4|). AD and PLE were significantly concurrently correlated at T5 (unstandardized r = .15, SE = .04, P < .001; standardized r = .45, SE = .08, P < .001).
Figure 2.
Cross-lagged and autoregressive AD and PLE model findings. Statistically significant paths are presented with solid lines and nonsignificant paths are represented by dotted lines. For significant paths, unstandardized/standardized coefficients are presented with SEs in parentheses. Sex is a covariate. Model fit: χ 2 = 45.81, df = 35, P > .05; CFI = 0.99, TLI = 0.98, RMSEA = 0.02, SRMR = 0.03 AD = affective dysregulation; PLE = psychosis-like experiences; T5 = 15–17 years old; T6 = 18–20 years old; T7 = 21–23 years old *P < .05, **P < .01, ***P < .001.
Autoregressive paths revealed that both AD and PLE were independently related over time. Crossed-lagged paths between AD and PLE indicated 2 predictive relationships over time. In line with our principal hypothesis, AD at T5 related to PLE at T6 (b = .96, SE = .48, P < .05; b = .37, SE = .15, P < .05). In addition, PLE at T6 related to AD at T7 (b = .08, SE = .04, P < .05; b = .18, SE = .08, P < .05).
Model fit varied in adequacy across all 4 models including additional covariates conducted as sensitivity analyses (supplementary materials). When the number of binge drinking days variable was included as an additional covariate, acceptable model fit was achieved, and results were essentially identical to the sex-only covariate model. When the days of marijuana use variable was included as an additional covariate, acceptable model fit was achieved, and the cross-lagged path from T5 AD to T6 PLE did not emerge (b = .22, SE = .15, P > .05). Similarly, the T5 AD to T6 PLE cross-lagged path did not emerge in the other 2 models (alcohol volume and composite substance use as additional covariates), but local fit indices indicated points of stress and poor overall model fit.
Discussion
The current paper goes beyond prior research of relationships between AD and PLE by applying a SEM cross-lagged panel design in longitudinal investigations of AD and PLE, with attention to distinct facets of AD as a latent construct, including low resiliency, low reactive control, and negative emotionality. In contrast to prior studies in help-seeking clinical samples, the current study utilized longitudinal data in a community sample of youth to examine the presence of AD prior to the development of PLE.43,46,47 Our principal hypothesis was supported, with AD relating to subsequent PLE over time. Specifically, more AD in the mid-teen years (T5) related to more PLE 3 years later (T6). In addition, we found that increasing PLE at this phase, the end of the teen years (T6), related to a subsequent increase in AD in the early 20s (T7). Consistent with prior literature, our findings also supported the cross-sectional relationship between AD and PLE in the mid-teen years (T5).14,25–30 Findings overall describe important relationships between AD and PLE, although these relationships appear to vary with developmental stage, implicating multiple factors, and considerations discussed below.
When our participants were in their mid-teen years (average age of 16 years old), the experience of PLE and AD correlated, as expected. If one is having thoughts of suspiciousness, mistrust, and/or unusual perceptual experiences, experiencing negative affect is an expected reaction to those unusual experiences.1–3 It is also possible that the affective disturbances reflect the same underlying process giving rise to the PLE, but we cannot eliminate the possibility of this confound in a cross-sectional analysis. However, the finding that more AD, which also includes the trait-like phenomena of low resilience and poor reactive control, links to more PLE three years later is suggestive of a causal relationship—direct or indirect during this developmental stage. This is consistent with postulations that aberrant stimulus processing, particularly of salient stimuli, underlies the development of psychosis1,42,66 and could also lead to impaired detection and regulation of emotional states, as well as over-reactivity to minor stresses, manifested as AD.
Another notable aspect of our findings is that these relationships changed with the developmental stage of our cohort. In the second 2 waves of assessments (T6–T7), obtained at the end of the participants’ teen years and beginning of their 20s, we no longer observed a cross-sectional relationship between AD and PLE. Furthermore, AD in late teens did not directly translate into PLE in their early 20s. Interestingly, PLE in late teens showed a direct influence on AD in the early 20s. We noted that in our sample, PLEs and AD indicators significantly declined over time. While PLE means and some of the AD indicators (reactive control and negative emotionality) consistently decreased at each time point, one AD indicator (resiliency) slightly decreased in late teens (T6) and subsequently improved in early 20s (T7). While it was not possible to tease apart the individual contributions of each of these 3 measures to the latent variable for AD, it is possible to speculate that emotional maturation occurring between adolescence and early adulthood altered the relationship of AD with PLE. The changing nature of the relationship between PLE and AD with development is an interesting topic for future research.
As we reviewed above, PLEs place individuals at risk for the development of future psychosis,1,42,66 a finding that has important clinical implications. With an analytic sample of 630, we would expect ~6 would meet criteria for schizophrenia, assuming a population lifetime prevalence of 1%. None met diagnostic criteria in our sample, which is likely attributable to MLS excluding individuals with a first-degree relative with a psychotic disorder, thus lowering the genetic risk of the participants in this cohort. However, at a mean age of 22 in the last timepoint of assessment (T3), 2.3% of our sample had a diagnosis of bipolar disorder, which is close to the expected incidence. As about 50% of patients with bipolar disorder have psychosis, it is possible that the PLEs recorded in our sample reflected a bipolar diathesis. Although the vast majority of adolescents and young adults who experience AD and/or PLE will not go on to develop a psychotic disorder, other diagnosable psychiatric conditions that affect functioning remain likely including bipolar disorder.67,68
The potential role of substance abuse in this cohort requires consideration, as significant numbers of participants met criteria for alcohol and marijuana abuse (13.3% and 6.9%, respectively, at T7). When we included binge drinking days in the model, the relationships described above were essentially unchanged. With the alcohol volume and composite measure models, fit indices ranged from poor to less acceptable, so it was not possible to evaluate whether our reported relationships held when co-varying for these factors. Model fit was slightly more acceptable when days of marijuana use were entered into the model, and then the relationship between early AD and subsequent PLE disappeared. As marijuana use has been associated with PLE,69,70 it is possible that variance in PLE was absorbed by the marijuana covariate, reducing the variance in PLE that could be explained by AD. Strictly speaking, we cannot rule out the possibility that marijuana use was a third variable causing both PLE and AD, although the other relationships between AD and PLE (cross-sectional at T5, and the cross-lagged relationship of PLE to AD from T6 to T7) were preserved with marijuana usage in the model, suggesting that AD and PLE were not explained away as secondary to marijuana use. AD, PLE, and marijuana use may have more complex relationships that merit continued and future investigations.
Several limitations are important to note. First, because the sample was collected for enriched AUD risk, these participants may represent a proximally “clinical” sample that is not fully representative of a general population-based community sample. However, it remains a sample that was not determined by clinical help-seeking. Second, though the selection of covariates in all models were informed by prior research and available data, we acknowledge there are additional factors that could conceivably relate to AD and PLE (eg, other drug use, childhood trauma, antisocial personality characteristics), requiring additional future investigations. Third, participants most often identified as male and white, therefore the sample is not representative of youth and young adults across the United States and the globe, and the results may not generalize to diverse populations. Lastly, PLE assessment relied on self-report which is inherently vulnerable to subjectivity and recall bias (though is often the primary way of evaluating experiences that are not outwardly observable like suspiciousness or perceptual abnormalities).
In sum, these findings describe important relationships between AD and PLE, appearing to vary with developmental age and implicating various factors. There may be benefits in counseling families of youth identified with high levels of AD through routine interactions with parents, school professionals, or general medical practitioners on the risk for subsequent PLE or other mental health conditions. Future research is needed to elucidate the complex longitudinal relationships between AD and PLE within clinical and CHR samples of adolescents, involving investigations of potential mechanisms at-play (eg, substance use, family history of psychotic disorder, trauma, etc.) in the risk for a subsequent clinical disorder.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Lindsay A Bornheimer, University of Michigan, School of Social Work, Ann Arbor, MI, USA; Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Meghan E Martz, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Takakuni Suzuki, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA; Department of Psychology, University of Michigan, Ann Arbor, MI, USA.
Ivy F Tso, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA; Department of Psychology, University of Michigan, Ann Arbor, MI, USA.
Cynthia Z Burton, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Juliann Li Verdugo, University of Michigan, School of Social Work, Ann Arbor, MI, USA.
Tyler Grove, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Mary M Heitzeg, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Stephan F Taylor, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA; Department of Psychology, University of Michigan, Ann Arbor, MI, USA.
Funding
This work was supported by the National Institute of Mental Health (L30MH127715: Suzuki; R01MH118634-01: Taylor; K23MH108823:Tso; R01MH122491: Tso) National Institute on Alcohol Abuse and Alcoholism (R01AA007065: Heitzeg; R01AA025790: Heitzeg; K01AA027558: Martz), National Alliance on Mental Illness (Suzuki), and National Center for Advancing Translational Sciences (UL1TR002240: Suzuki; KL2TR00224: Suzuki).
References
- 1. Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27(4):409–424. [DOI] [PubMed] [Google Scholar]
- 2. Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: towards the development of new models of investigation. Clin Psychol Rev. 2007;27(3):307–317. [DOI] [PubMed] [Google Scholar]
- 3. Taylor M, Jauhar S. Are we getting any better at staying better? The long view on relapse and recovery in first episode nonaffective psychosis and schizophrenia. Ther Adv Psychopharm 2019;9:2045125319870033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psych 1982;39(7):784–788. [DOI] [PubMed] [Google Scholar]
- 5. Carpenter WT, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiat 1988;145(5):578–583. [DOI] [PubMed] [Google Scholar]
- 6. Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York, NY: International University Press; 1911. [Google Scholar]
- 7. Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia Bull 2008;34(5):819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho H, Gonzalez R, Lavaysse LM, Pence S, Fulford D, Gard DE. Do people with schizophrenia experience more negative emotion and less positive emotion in their daily lives? A meta-analysis of experience sampling studies. Schizophr Res. 2017;183:49–55. [DOI] [PubMed] [Google Scholar]
- 9. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophrenia Bull 2010;36(1):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tso IF, Grove TB, Taylor SF. Self-assessment of psychological stress in schizophrenia: preliminary evidence of reliability and validity. Psychiat Res 2012;195(1-2):39–44. [DOI] [PubMed] [Google Scholar]
- 11. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophrenia Bull 2006;32(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophrenia Bull 2008;34(5):888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grove TB, Yao B, Mueller SA, et al. A Bayesian model comparison approach to test the specificity of visual integration impairment in schizophrenia or psychosis. Psych Res 2018;265:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crutchfield NR. The relationship of work engagement, work-life balance, and occupational commitment on the decisions of agricultural educators to remain in the teaching profession [dissertation]. Texas A&M University; 2010. [Google Scholar]
- 15. lker E, Grimes KE, Davis DM, Smith AJ. Childhood precursors of schizophrenia: facial expressions of emotion. Am J Psychiat 1993;150:1654–1660. [DOI] [PubMed] [Google Scholar]
- 16. Phillips LJ, Francey SM, Edwards J, McMurray N. Strategies used by psychotic individuals to cope with life stress and symptoms of illness: a systematic review. Anxiety Stress Copin 2009;22(4):371–410. [DOI] [PubMed] [Google Scholar]
- 17. Bryson G, Bell M, Lysaker P. Affect recognition in schizophrenia: a function of global impairment or a specific cognitive deficit. Psych Res 1997;71(2):105–113. [DOI] [PubMed] [Google Scholar]
- 18. Sellers R, Gaweda L, Wells A, Morrison AP. The role of unhelpful metacognitive beliefs in psychosis: relationships with positive symptoms and negative affect. Psych Res 2016;246:401–406. [DOI] [PubMed] [Google Scholar]
- 19. Debbané M, Van der Linden M, Gex‐Fabry M, Eliez S. Cognitive and emotional associations to positive schizotypy during adolescence. J Child Psychol Psyc 2009;50(3):326–334. [DOI] [PubMed] [Google Scholar]
- 20. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psych Psych Epid 2002;37(3):97–104. [DOI] [PubMed] [Google Scholar]
- 21. Freeman D, Garety PA. Connecting neurosis and psychosis: the direct influence of emotion on delusions and hallucinations. Behav Res Ther. 2003;41(8):923–947. [DOI] [PubMed] [Google Scholar]
- 22. Paulik G, Badcock JC, Maybery MT. The multifactorial structure of the predisposition to hallucinate and associations with anxiety, depression and stress. Pers. Individ. Dif. 2006;41:1067–1076. [Google Scholar]
- 23. Malla AK, Norman RM. Prodromal symptoms in schizophrenia. Brit J Psych 1994;164(4):487–493. [DOI] [PubMed] [Google Scholar]
- 24. Zisook S, McAdams LA, Kuck J, et al. Depressive symptoms in schizophrenia. Am J Psychiat 1999;156(11):1736–1743. [DOI] [PubMed] [Google Scholar]
- 25. Stainsby LM, Lovell GP. Proneness to hallucinations and delusions in a non-clinical sample: exploring associations with metacognition and negative affect. Aust J Psychol 2014;66(1):1–7. [Google Scholar]
- 26. Calderon-Mediavilla M, Vila-Badia R, Dolz M, et al. Depressive symptoms and their relationship with negative and other psychotic symptoms in early onset psychosis. Eur Child Adoles Psy 2021;30(9):1383–1390. [DOI] [PubMed] [Google Scholar]
- 27. Hanssen M, Bak M, Bijl R, Vollebergh W, Van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. Brit J Clin Psych 2005;44(2):181–191. [DOI] [PubMed] [Google Scholar]
- 28. Jeppesen P, Clemmensen L, Munkholm A, et al. Psychotic experiences co-occur with sleep problems, negative affect and mental disorders in preadolescence. J Child Psychol Psyc 2015;56(5):558–565. [DOI] [PubMed] [Google Scholar]
- 29. Lincoln TM, Hartmann M, Köther U, Moritz S. Do people with psychosis have specific difficulties regulating emotions? Clin Psychol Psychot 2015;22(6):637–46. [DOI] [PubMed] [Google Scholar]
- 30. van der Steen Y, Gimpel-Drees J, Lataster T, et al. Clinical high risk for psychosis: the association between momentary stress, affective and psychotic symptoms. Acta Psychiat Scand 2017;136(1):63–73. [DOI] [PubMed] [Google Scholar]
- 31. Unterrassner L, Wyss TA, Wotruba D, Ajdacic-Gross V, Haker H, Rössler W. Psychotic-like experiences at the healthy end of the psychosis continuum. Front Psychol. 2017;8:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wigman JT, van Winkel R, Raaijmakers QA, et al. Evidence for a persistent, environment-dependent and deteriorating subtype of subclinical psychotic experiences: a 6-year longitudinal general population study. Psychol Med. 2011;41(11):2317–2329. [DOI] [PubMed] [Google Scholar]
- 33. Van Os J, Verdoux H, Maurice-Tison S, et al. Self-reported psychosis-like symptoms and the continuum of psychosis. Soci Psych Psych Epid 1999;34(9):459–463. [DOI] [PubMed] [Google Scholar]
- 34. Owens DC, Miller P, Lawrie SM, Johnstone EC. Pathogenesis of schizophrenia: a psychopathological perspective. Brit J Psychiat 2005;186(5):386–393. [DOI] [PubMed] [Google Scholar]
- 35. Reininghaus U, Kempton MJ, Valmaggia L, et al. Stress sensitivity, aberrant salience, and threat anticipation in early psychosis: an experience sampling study. Schizophrenia Bull 2016;42(3):712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boldrini T, Buglio GL, Giovanardi G, Lingiardi V, Salcuni S. Defense mechanisms in adolescents at high risk of developing psychosis: an empirical investigation. Res Psychother: Psychopathol Process Outcome 2020;23(1):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Rossum I, Dominguez MD, Lieb R, Wittchen HU, van Os J. Affective dysregulation and reality distortion: a 10-year prospective study of their association and clinical relevance. Schizophrenia Bull 2011;37(3):561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fowler T, Zammit S, Owen MJ, Rasmussen F. A population-based study of shared genetic variation between premorbid IQ and psychosis among male twin pairs and sibling pairs from Sweden. Arch Gen Psych 2012;69(5):460–466. [DOI] [PubMed] [Google Scholar]
- 39. Crutchfield P. Delusion, proper function, and justification. Neuroethics 2020:1–2. [Google Scholar]
- 40. Kline E, Keshavan M. Innovations in first episode psychosis interventions: the case for a “RAISE-Plus” approach. Schizophr Res. 2017;182:2–3. [DOI] [PubMed] [Google Scholar]
- 41. Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophrenia Bull 2014;40(1):120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Linscott RJ, Van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43(6):1133–1149. [DOI] [PubMed] [Google Scholar]
- 43. Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42(9):1857–1863. [DOI] [PubMed] [Google Scholar]
- 44. Maijer K, Begemann MJ, Palmen SJ, Leucht S, Sommer IE. Auditory hallucinations across the lifespan: a systematic review and meta-analysis. Psychol Med. 2018;48(6):879–888. [DOI] [PubMed] [Google Scholar]
- 45. Healy C, Brannigan R, Dooley N, et al. Childhood and adolescent psychotic experiences and risk of mental disorder: a systematic review and meta-analysis. Psychol Med. 2019;49(10):1589–1599. [DOI] [PubMed] [Google Scholar]
- 46. de Gracia Dominguez M, Viechtbauer W, Simons CJ, van Os J, Krabbendam L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol Bull. 2009;135(1):157. [DOI] [PubMed] [Google Scholar]
- 47. Van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophrenia Bull 2008;34(6):1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hancock GR, Mueller RO, eds. Structural equation modeling: A second course. 2nd ed. Charlotte, NC: Information Age Publishing; 2013. [Google Scholar]
- 49. Eisenberg N, Valiente C, Fabes RA, et al. The relations of effortful control and ego control to children’s resiliency and social functioning. Dev Psychol. 2003;39(4):761–776. [DOI] [PubMed] [Google Scholar]
- 50. Block JH, Block J. The role of ego-control and ego-resiliency in the organization of behavior. In: Development of cognition, affect, and social relations. New York, NY: Psychology Press; 2014:49–112. [Google Scholar]
- 51. Heitzeg MM, Cope LM, Martz ME, Hardee JE, Zucker RA. Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev Cogn Neuros-neth 2015;16:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martel MM, Pierce L, Nigg JT, et al. Temperament pathways to childhood disruptive behavior and adolescent substance abuse: testing a cascade model. J Abnorm Child Psych 2009;37(3):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nigg JT. Annual research review: on the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J Child Psychol Psyc 2017;58(4):361–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zucker RA, Ellis DA, Fitzgerald HE, Bingham CR, Sanford K. Other evidence for at least two alcoholisms II: life course variation in antisociality and heterogeneity of alcoholic outcome. Dev Psycohpathol 1996;8(4):831–848. [Google Scholar]
- 55. Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV). Washington University School of Medicine; St. Louis, MO; 2000. [Google Scholar]
- 56. Wong MM, Nigg JT, Zucker RA, et al. Behavioral control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev. 2006;77(4):1016–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eisenberg N, Oettingen G, Gollwitzer PM. Self-regulation: conceptual issues and relations to developmental outcomes in childhood and adolescence. In: Self-Regulation in Adolescence Vol 1. New York, NY: Cambridge University Press; 2015:57–77. [Google Scholar]
- 58. Achenbach TM. Manual for the Youth Behavior Self-Report and 1991 Profile. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- 59. Achenbach TM, Dumenci L, Rescorla LA. Ratings of relations between DSM-IV diagnostic categories and items of the Adult Self-Report (ASR) and Adult Behavior Checklist (ABCL). In Research Center for Children, Youth and Families. 2003. [Google Scholar]
- 60. Muthén LK, Muthén BO. Mplus User’s Guide: Statistical Analysis with Latent Variables. 7th ed. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- 61. Selig JP, Little TD. Autoregressive and cross-lagged panel analysis for longitudinal data. In: Laursen B, Little TD, Card NA, eds. Handbook of Developmental Research Methods. New York, NY: The Guilford Press; 2012. [Google Scholar]
- 62. Graham JW, Coffman DL. Structural equation modeling with missing data. In: Hoyle RH, ed. Handbook of Structural Equation Modeling. New York, NY: The Guilford Press; 2012:277–295. [Google Scholar]
- 63. Brown RS. An opening: trauma and transcendence. Psychosis 2015;7(1):72–80. [Google Scholar]
- 64. Weigard A, Clark DA, Sripada C. Cognitive efficiency beats top-down control as a reliable individual difference dimension relevant to self-control. Cognition 2021;215:104818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zucker RA, Fitzgerald HE, Noll RB. Drinking and Drug History. 4th ed. East Lansing, MI: Michigan State University; 1990. [Google Scholar]
- 66. Winton-Brown TT, Fusar-Poli P, Ungless MA, Howes OD. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37(2):85–94. [DOI] [PubMed] [Google Scholar]
- 67. Van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam LJ. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness–persistence–impairment model of psychotic disorder. Psychol Med 2009;39(2):179–95. [DOI] [PubMed] [Google Scholar]
- 68. Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res 2011;125(1):54–61. [DOI] [PubMed] [Google Scholar]
- 69. Bechtold J, Hipwell A, Lewis DA, Loeber R, Pardini D. Concurrent and sustained cumulative effects of adolescent marijuana use on subclinical psychotic symptoms. Am J Psychiat 2016;173(8):781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bourque J, Spechler PA, Potvin S, et al. Functional neuroimaging predictors of self-reported psychotic symptoms in adolescents. Am J Psychiat 2017;174(6):566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.