Abstract
Common and distinct neural bases of Schizophrenia (SZ) and bipolar disorder (BP) have been explored using resting-state fMRI (rs-fMRI) functional connectivity (FC). However, fMRI is an indirect measure of neural activity, which is a convolution of the hemodynamic response function (HRF) and latent neural activity. The HRF, which models neurovascular coupling, varies across the brain within and across individuals, and is altered in many psychiatric disorders. Given this background, this study had three aims: quantifying HRF aberrations in SZ and BP, measuring the impact of such HRF aberrations on FC group differences, and exploring the genetic basis of HRF aberrations. We estimated voxel-level HRFs by deconvolving rs-fMRI data obtained from SZ (N = 38), BP (N = 19), and matched healthy controls (N = 35). We identified HRF group differences (P < .05, FDR corrected) in many regions previously implicated in SZ/BP, with mediodorsal, habenular, and central lateral nuclei of the thalamus exhibiting HRF differences in all pairwise group comparisons. Thalamus seed-based FC analysis revealed that ignoring HRF variability results in false-positive and false-negative FC group differences, especially in insula, superior frontal, and lingual gyri. HRF was associated with DRD2 gene expression (P < .05, 1.62 < |Z| < 2.0), as well as with medication dose (P < .05, 1.75 < |Z| < 3.25). In this first study to report HRF aberrations in SZ and BP, we report the possible modulatory effect of dopaminergic signalling on HRF, and the impact that HRF variability can have on FC studies in clinical samples. To mitigate the impact of HRF variability on FC group differences, we suggest deconvolution during data preprocessing.
Keywords: resting-state fMRI, deconvolution, Schizophrenia, bipolar disorder, hemodynamic response function (HRF), seed-based functional connectivity
Introduction
Although schizophrenia (SZ) and bipolar disorder (BP) have long been viewed as distinct disorders based on differing clinical presentations; 1–3 there is substantial evidence suggesting that these disorders share neuropsychological features4 and cognitive dysfunction.5,6 SZ is a psychotic disorder characterized by altered perception, thought processes, and behaviors7; while BP is a mood disorder involving prolonged states of depression and mania.8 A popular noninvasive technique for characterizing the neural bases of SZ and BP has been resting-state functional magnetic resonance imaging (rs-fMRI) based on the blood-oxygen-level dependent (BOLD) contrast. A primary reason for the popularity of rs-fMRI is that it does not require the subjects to perform an explicit task, which can be difficult in clinical populations. Rs-fMRI also allows us to investigate interactions between neurophysiological events in spatially remote brain regions by assessing the temporal correlation of their respective BOLD signals (i.e. functional connectivity [FC]),9 which can characterize large-scale brain networks both in healthy and clinical populations. To date, several FC studies have attempted to distinguish the neural aberrations in SZ from BP, but the results have been inconsistent. While most studies have identified more extensive disconnection in SZ than BP10,11,12 regional diagnostic specificity has not been evident on a consistent basis For example, cingulate and thalamic FC has been reported to be abnormal in both patients groups13–15 in some but not other studies16–18 Nevertheless, functional connectivity patterns have been considered to hold great promise in differentiating the various disorders in the psychosis continuum19,20 and resting-state FC studies are commonplace due to the ease of pooling large datasets, as well as the lack of task performance, effort, or sensory processing confounds when investigating psychosis.
Interpreting findings from rs-fMRI FC is not straightforward given that fMRI BOLD contrast is an indirect measure of neural activity.21–23 The measured fMRI signal is considered as a convolution of latent neural activity with a transfer function – the hemodynamic response function (HRF).24 As such, the fMRI signal contains variance arising from the variability of the HRF as well. The issue, however, is that HRF shape has been shown to vary not only across subjects and populations, but also across different brain regions within the same subject.25,26 Different factors contribute to this variability. First, it is partly driven by factors affecting neurovascular coupling including neurotransmitters (e.g. γ-aminobutyric acid (GABA), glutamate, dopamine, serotonin, and nitric oxide)27 and components of the neurovascular unit (glial cells, endothelia), both of which are affected by genetic factors.28 Second, nonneural factors such as baseline cerebral blood flow, vasculature differences, caffeine/alcohol/lipid ingestion, partial volume imaging of veins, hematocrit, pulse or respiration differences, global magnetic susceptibilities, and slice timing differences29–31 can also affect the HRF.
The vascular hypothesis in psychosis suggests disruption of appropriate vascular response to changes in cerebral metabolic activity,32,33 possibly due to a dysfunction of the neurovascular unit or neurotransmitters that are vasoactive (e.g. dopamine34–36,37). Neurovascular uncoupling may result in insufficient support for synaptic activity, which in turn may trigger synaptic loss or promote an inflammatory response,38 induce mitochondrial dysfunction and oxidative stress leading to neuronal atrophy.39 Characterizing HRF variability provides an opportunity to systematically study the putative neurovascular uncoupling. Furthermore, HRF variability could confound FC estimates obtained from fMRI data, as a time series pair may appear to be synchronized when the underlying latent neural signals are not and vice versa21,40,41 (figure 1). Thus systematic FC aberrations differentiating clinical groups (SZ and BP) could be driven by factors affecting neurovascular coupling and hence HRF shape.22,42–44
Fig. 1.
The effect of HRF variability on Pearson’s correlation between two-time series obtained from experimental fMRI data. The measured fMRI time series, the estimated HRF and the deconvolved fMRI (or latent neural) time series are shown in the top, middle, and bottom rows respectively. We specifically illustrate two scenarios: (A) The acquired fMRI time series are highly correlated while the correlation between underlying latent neural signals is low (after minimizing HRF variability), and (B) The acquired fMRI time series are uncorrelated while the correlation between underlying latent neural signals is high (after minimizing HRF variability).
Deconvolution methods are capable of estimating voxel-specific HRFs from the measured fMRI signal, and subsequently recovering the latent neural time series.24,45 The procedure is relatively straightforward for fMRI task paradigms because the timing of external events driving neural activity is known.46,47 For rs-fMRI, which lacks explicit timing of neural events,48,49 Wu et al.48 proposed a deconvolution method to identify pseudo-events from rs-fMRI time series, and estimate HRF and latent neural time series using it. We have used this method in the current work. Voxel-specific HRFs were estimated from 3D+time fMRI data (after completing all the preprocessing steps), which were characterized by three shape parameters38: time-to-peak (TTP), response height (RH), and full-width at half-max (FWHM) (see figure 2).
Fig. 2.
Typical HRF with its three characteristic shape parameters.
Response Height; Time-To-Peak; Full-Width At Half Max
This work aimed to address three important questions. (1) The first aim was to identify brain regions with systematic HRF differences between SZ, BP, and matched healthy controls, wherein the neurovascular coupling mechanisms are likely to differ. (2) The second aim was to identify possible confounds introduced by HRF variability in identified functional networks, for which we compared FC estimated from deconvolved (HRF-variability-minimized) and nondeconvolved (HRF-variability ignored) data. (3) The third aim was to understand the relationship between dopamine system (especially D2 receptor) that is therapeutically highly relevant for SZ and BP, by studying the DRD2 gene expression as well as the effect of antipsychotic dose on the HRF aberrations. Probing these questions has been undertaken for the first time in the literature, with potential impact on better mechanistic understanding of shared and distinguishing neurovascular coupling mechanisms of SZ and BP and reduced confounds during studying functional brain networks in these disorders.
Materials and Methods
Participants
The subject sample consisted of 38 patients satisfying DSM-IV criteria for SZ, 19 patients having BP with psychosis, and 35 matched healthy controls. Details about the sample and recruitment process are provided in supplementary sections S1 and section S11 and supplementary table S9. 54 out of 57 patients were receiving psychotropic medications at the time of scan. We calculated the median Defined Daily Dose (DDD) for antipsychotics.50 A detailed breakdown of the prescribed medications is presented in supplementary section S11 and supplementary table S8.
fMRI Data Acquisition
Subjects were scanned in a 3T Philips Achieva MRI scanner (Philips, the Netherlands), using an 8-channel SENSE head coil with SENSE factor 2 in anterior–posterior direction. To enhance sensitivity, dual-echo gradient-echo echo-planar images (GE-EPI) were acquired.51 The sequence parameters are provided in supplementary section S2.
fMRI Data Preprocessing
After following a standard resting state fMRI preprocessing procedure (details in Supplementary Section S3), the pipeline was split in two. In the first pipeline, no further processing was pursued and the preprocessed data was designated as the nondeconvolved (NDC) dataset. In the second pipeline, voxel-level HRF deconvolution48 was performed and the resulting data (voxel-level latent neural time series) was designated as the deconvolved (DC) dataset. Finally, both DC and NDC datasets were temporally bandpass filtered (0.01–0.1 Hz).
Deconvolution and HRF Estimation
We applied a blind deconvolution technique developed for rs-fMRI by Wu et al.48 to retrieve latent neural signals as well as estimate voxel-specific HRFs. This method is based on the increasing evidence that the resting-state BOLD spikes can be seen as the response to spontaneous neuronal events and the dynamics of the brain at resting state is governed by nonrandom patterns. Spontaneous neural events were detected by identifying BOLD fluctuations of relatively large amplitude (with a threshold of mean + 1 SD, after despiking and removing sources of noise that might lead to spurious spikes) and modeling spontaneous neuronal events as pseudo-events in the rs-fMRI time series (using a point process model).52,53 A mask with relative head motion <0.3 mm was imposed to further avoid motion-driven events.48 The estimated neural events were convolved with the canonical HRF with different values of RH, TTP, and FWHM varying within their biologically plausible range, and the HRF resulting in the best fit (in the least squares sense) between the resulting time series and experimental data was chosen as the final estimated HRF for each voxel. Finally, latent neural time series were estimated from the HRF and raw BOLD signal using Wiener deconvolution.24 As shown in figure 2, the HRF shape is characterized by RH, TTP, and FWHM, representing the amplitude, timing, and spread of the response, respectively. More details are provided in Supplementary Section S4.
HRF Differences Between Schizophrenia, Bipolar Disorder, and Healthy Controls
We applied a blind deconvolution technique developed for rs-fMRI by Wu et al.48,52,53 to retrieve latent neural signals as well as estimate voxel-specific HRFs. Further details about deconvolution and HRF estimation are provided in Supplementary Section S5. To address the first aim of identifying impaired neurovascular coupling in SZ and BP, we compared HRF parameters between the groups. For each of the three HRF parameters, two sample two-tailed t-tests were conducted for three comparisons separately: SZ vs control, BP vs control, and SZ vs BP. With each comparison, whole-brain maps that indicate voxels showing significant (FDR corrected P-value < .05, cluster-size > 50) group differences in three HRF parameters were obtained. This resulted in 6 maps (SZ < control, SZ > control, BP < control, BP > control, SZ < BP, and SZ > BP) per HRF parameter and 18 maps overall. Finally, we sought to identify voxels that exhibited significantly different HRFs across all three pairwise group comparisons (with at least one HRF parameter). This relied on a conjunction approach that is more stringent than the group comparison maps, as the threshold required passing 3 levels of FDR correction, one for each comparison.
We acknowledge that FDR is an adaptive approach that is seen as more liberal than family-wise error correction methods for type 1 errors. We chose FDR correction over other familywise error correction methods as the problem of interest (differences in HRF parameters) demands avoidance of false negatives over the acceptance of false positives.54 Furthermore, this approach avoided the issue of arbitrary cluster inclusion55 when studying the three HRF properties whose spatial properties (“ground truth”) are not known a priori. Nevertheless, to ensure the inadvertent false positives are kept minimal, we used a cluster extent threshold as well (50 voxels) that takes into account the possible dependencies among adjacent voxels.
Impact of HRF Variability on Seed-Based Functional Connectivity Group Differences
To address the second aim of identifying the effect of HRF variability on group differences in FC, we compared seed-based FC across the groups using DC and NDC datasets. Specifically, we identified the seed region(s) from our earlier analysis as that whose HRF shape (i.e. at least one of the three HRF parameters) was different across all the three groups (only the thalamus was found from that analysis, which was defined as the seed region of interest [ROI]). For each participant, the mean time series within the thalamus seed ROI was obtained. FC between the thalamus seed ROI time series and the remaining gray matter voxels in the brain were evaluated using Pearson’s correlation coefficient. The correlation coefficients were transformed by Fisher’s z-transform to ensure Gaussianity for statistical comparisons.56,57 This procedure for obtaining FC maps was implemented separately for the two datasets: (1) NDC: data processed without deconvolution, and (2) DC: data processed with deconvolution.
For each group, the FC maps from individual subjects were fed to a random effects one-sample t-test (FDR corrected P-value < .05, cluster-size > 50) to determine voxels whose connectivity with the thalamus seed were significantly different from zero. Subsequently, the FC maps from individual subjects were also fed to a random effects two-sample t-test (FDR corrected P-value < 0.05, cluster-size > 50) to identify brain regions showing significantly different FC with the thalamus seed ROI in three comparisons separately (SZ vs control, BP vs control, and SZ vs BP). These procedures were also implemented separately for both DC and NDC datasets.
With the aim of investigating the effect of HRF variability on between-group differences in FC, we performed a two-way repeated-measures ANOVA using SPSS software (version 20, IBM Inc., USA). Here the groups (SZ, BP, and Control) were considered as one factor and data with/without deconvolution as the other factor. We then identified the voxels showing a significant interaction between the two factors (FDR corrected P-value < 0.05, cluster size > 50 voxels).
Association Between HRF Parameters and Gene Expression
The Allen Human Brain Atlas of gene expression has been mapped to58 Freesurfer’s Desikan–Killianey (DK) whole-brain parcellation with 62 ROIs,59 allowing for the exploration of associations between gene expression and imaging measures. To address our third aim of understanding the relationship between gene expression and HRF aberrations in these disorders, we probed the associations between DK parcellation gene expression map and the maps of HRF parameters in the DK parcellation space. For this, we found mean HRF parameters among all the voxels within each DK parcellation ROI, giving us one RH value (and one TTP and one FWHM value) for each DK-ROI in each subject. We subsequently obtained group difference maps for the HRF parameters for three group comparisons: BP vs Control, SZ vs Control, and BP vs SZ, in DK-space.
We then examined the association between the HRF difference values from each NDC-DC data pair and gene expressions of Dopamine Receptor D2 (DRD2) gene, which encodes the D2 subtype of the dopamine receptor, highly relevant to the pathophysiology as well as treatment response in schizophrenia60–63 and BP.64 As a control for the gene expression – HRF relationship, we studied the expression of the Polybromo 1 (PBRM1) gene which encodes a subunit of ATP-dependent chromatin-remodeling complexes and implicated in both BP and SZ65,66 but no known direct effects on the vasoactive neuromodulators.
We specifically chose DRD2 expression as regional cerebral blood flow changes that occur on administration of antipsychotics have been shown to be directly proportional to brain D2R densities and DRD2 mRNA expression measures (Selvaggi et al.34).
With respect to the gene expression analysis, our aim was to demonstrate that HRF changes may not be on the primary mechanistic pathways that lead to the clinical expression of schizophrenia or bipolar disorder; but neuromodulators such as dopamine, which are directly relevant to the treatments used in practice, can affect the HRF. We did not have any specific hypothesis on DRD2’s effects being higher than the other genetic loci; thus, we do not study or report the interaction effects (i.e. difference in strength of slopes between 2 genetic loci). To this end, we selected a control locus that has susceptibility signal for multiple psychiatric disorders (see Cross-Disorder Group of the PGC, 201467; Liu et al.68 and Yang et al69; also see Golovino70), but not directly linked to dopamine or other neuromodulators, exhibit low endothelial profile in line with DRD2 (based on cerebral cortex tissue expression summary from proteinatlas.org) despite having established cortical and thalamic expression profile (based on Allen Human Brain Tissue Gene Expression Profiles).
Relationship Between HRF Parameters and DDD of Medications
Finally, we probed the association between DDD of medications with HRF parameters. Specifically, we probed DDD values for antipsychotics in SZ. The association between DDD measures and the three HRF parameters at each voxel were assessed. Significant clusters are reported (P < 0.05 FDR corrected, cluster size > 50).
Results
Between-Group HRF Differences
In the SZ vs control comparison, we found that bilateral thalamus, midbrain, and precuneus had significantly wider FWHM (figure 3A) in the SZ group, while bilateral cerebellum posterior lobe, left middle frontal gyrus (MFG), and bilateral precentral gyrus had significantly wider FWHM (figure 3A) in the control group. Right ACC and right parahippocampal gyrus had significantly higher RH (figure 3A) in the SZ group, while bilateral cerebellum posterior lobe, posterior cingulate cortex (PCC), precuneus, and lingual gyrus had significantly higher RH (figure 3A) in the control group. Bilateral midbrain had significantly longer TTP (figure 3A) in the SZ group, while bilateral cerebellum posterior lobe had significantly longer TTP (figure 3A) in the control group.
Fig. 3.
Spatial maps showing regions with significantly different HRF parameters in the three comparisons: (A) schizophrenia (SZ) vs control, (B) bipolar disorder (BP) vs control, and (C) BP vs SZ.
In the BP vs control comparison, we found that bilateral ACC and precuneus had significantly wider FWHM (figure 3B) in the BP group, while bilateral cerebellum posterior lobe, left middle temporal gyrus (MTG) and bilateral precentral gyrus had significantly wider FWHM (figure 3B) in the control group. Bilateral ACC, bilateral cingulate gyrus, bilateral mPFC, and right supramarginal gyrus had significantly higher RH (figure 3B) in the BP group, while bilateral cerebellum posterior lobe, bilateral cerebellum anterior lobe, bilateral thalamus and bilateral mPFC had significantly higher RH (figure 3B) in the control group. Bilateral cingulate gyrus had significantly longer TTP (figure 3B) in the BP group, while bilateral cerebellum posterior lobe, left MTG, and bilateral MFG had significantly longer TTP (figure 3A) in control group.
In the BP vs SZ comparison, we found that bilateral cerebellum posterior lobe had significantly wider FWHM (figure 3C) in the BP group, while bilateral thalamus, cerebellum anterior lobe, and midbrain had significantly wider FWHM (figure 3C) in the SZ group. Bilateral mPFC, left lingual gyrus, bilateral PCC, and bilateral inferior parietal lobule had significantly higher RH (figure 3C) in the BP group, while bilateral parahippocampal gyrus and bilateral superior frontal gyrus (SFG) had significantly higher RH (figure 3C) in the SZ group. Bilateral cerebellum posterior lobe had significantly longer TTP (figure 3C) in the BP group, while bilateral pons and thalamus had significantly shorter TTP (figure 3C) in the BP group.
These results support the notion of HRF aberrations (and thus neurovascular coupling impairments) in SZ and BP, and provide a mechanistic map of such aberrations. These results also illustrate certain distinct HRF aberrations between SZ and BP populations. Detailed information such as cluster sizes, cluster centroids etc. are shown in table 1.
Table 1.
Voxel Clusters that had Significantly Different (FDR Corrected P-Value < .05, Cluster Size > 50 Voxels) HRF Parameters in Three Comparisons: BP vs Control, SZ vs Control, and BP vs SZ. The Anatomical Labels, MNI Coordinates of Cluster Centroids, Cluster Size in Terms of the Number of Voxels, T Score, and Corresponding Brodmann Areas (Where Appropriate) Are Listed
| Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comparison | Cluster Anatomical Location | x | y | Z | Cluster Size | Hemisphere | BA | T Score |
| Full-width at half max SZ > Control |
Thalamus | −20 | −16 | 0 | 4678 | Bilateral | 4.19 | |
| Midbrain | 0 | −32 | −14 | 3.91 | ||||
| Precuneus | 14 | −56 | 32 | 31 | 3.52 | |||
| Full-width at half max SZ < Control |
Cerebellum Posterior Lobe | −62 | −40 | −12 | 4352 | Bilateral | 21 | −4.47 |
| Middle Frontal Gyrus | −36 | 44 | 26 | 208 | Left | 10 | −4.84 | |
| Precentral Gyrus | 18 | −28 | 68 | 320 | Right | 4 | −3.91 | |
| Precentral Gyrus | −20 | −28 | 66 | 715 | Left | 4 | −4.48 | |
| Response height SZ > Control |
Anterior Cingulate Cortex | 18 | 36 | 18 | 104 | Right | 32 | 4.15 |
| Parahippocampal Gyrus | 20 | −4 | −28 | 326 | Right | 28 | 4.71 | |
| Response height SZ < Control |
Cerebellum Posterior Lobe | −30 | −64 | −50 | 2378 | Bilateral | −3.96 | |
| Posterior Cingulate | 18 | −60 | 4 | 527 | Bilateral | 30 | −4.44 | |
| Precuneus | −6 | −52 | 30 | 1236 | Bilateral | 31 | −3.85 | |
| Lingual Gyrus | −4 | −76 | 0 | 18 | −3.55 | |||
| Time-to-peak SZ > Control |
Midbrain | 2 | −30 | −14 | 540 | Bilateral | 3.70 | |
| Time-to-peak SZ < Control |
Cerebellum Posterior Lobe | −26 | −74 | −50 | 1850 | Bilateral | −5.08 | |
| Full-width at half max BP > Control |
Anterior Cingulate Cortex | 4 | 30 | −8 | 1752 | Bilateral | 32 | 3.91 |
| Precuneus | −18 | −46 | 30 | 329 | Left | 31 | 4.12 | |
| Precuneus | 14 | −54 | 32 | 999 | Right | 31 | 4.57 | |
| Full-width at half max BP < Control |
Cerebellum Posterior Lobe | −40 | −54 | −50 | 1229 | Bilateral | −3.28 | |
| Middle Temporal Gyrus | −62 | −40 | −12 | 86 | Left | 21 | −3.58 | |
| Precentral Gyrus | −18 | −32 | 70 | 4352 | Right | 4 | −4.69 | |
| Precentral Gyrus | 14 | −38 | 72 | Left | 3 | −3.93 | ||
| Response height BP > Control |
Cingulate Gyrus | −16 | 4 | 30 | 167 | Left | 3.66 | |
| Cingulate Gyrus | −16 | −26 | 40 | 228 | Left | 31 | 3.92 | |
| Cingulate Gyrus | 16 | −58 | 28 | 96 | Right | 31 | 4.27 | |
| Medial Prefrontal Cortex | 4 | −10 | 66 | 624 | Bilateral | 6 | 4.01 | |
| Supramarginal Gyrus | 42 | −48 | 34 | 438 | Right | 40 | 3.92 | |
| Response height BP < Control |
Cerebellum Posterior Lobe | 2 | −58 | −48 | 524 | Bilateral | −4.03 | |
| Cerebellum Anterior Lobe | 20 | −42 | −26 | −3.59 | ||||
| Cerebellum Anterior Lobe | −20 | −40 | −26 | −3.39 | ||||
| Thalamus | 4 | −20 | 4 | 80 | Bilateral | −4.65 | ||
| Medial Prefrontal Cortex | −2 | −10 | 66 | 481 | Bilateral | 6 | −3.22 | |
| Time-to-peak BP > Control |
Cingulate Gyrus | −10 | 12 | 34 | 262 | Left | 24 | 3.72 |
| Cingulate Gyrus | 10 | −6 | 38 | 150 | Right | 24 | 4.29 | |
| Time-to-peak BP < Control |
Cerebellum Posterior Lobe | −10 | −66 | −50 | 909 | Bilateral | −3.29 | |
| Middle Temporal Gyrus | −36 | 2 | −50 | 59 | Left | 20 | −3.25 | |
| Middle Frontal Gyrus | −26 | −6 | 60 | 1157 | Left | 6 | −4.12 | |
| Middle Frontal Gyrus | 32 | −8 | 60 | 231 | Right | 6 | −3.88 | |
| Full-width at half max BP > SZ |
Cerebellum Posterior Lobe | −22 | −80 | −44 | 267 | Left | 4.36 | |
| Cerebellum Posterior Lobe | 16 | −74 | −50 | 81 | Right | 4.02 | ||
| Full-width at half max BP < SZ |
Thalamus | −6 | −24 | 0 | 1126 | Bilateral | −4.63 | |
| Cerebellum Anterior Lobe | −16 | −32 | −18 | −3.91 | ||||
| Midbrain | −6 | −32 | −20 | −3.52 | ||||
| Response height BP > SZ |
Medial Prefrontal Cortex | 10 | 36 | −20 | 196 | Bilateral | 11 | 4.26 |
| Lingual Gyrus | −12 | −90 | −2 | 477 | Left | 17 | 4.21 | |
| Posterior Cingulate | −4 | −60 | 18 | 23 | 3.84 | |||
| Posterior Cingulate | 18 | −66 | 8 | 280 | Right | 30 | 3.76 | |
| Inferior Parietal Lobule | 40 | −40 | 26 | 358 | Right | 4.13 | ||
| Inferior Parietal Lobule | −42 | −40 | 24 | 527 | Left | 4.24 | ||
| Response height BP < SZ |
Parahippocampal Gyrus | 34 | −6 | −26 | 231 | Right | −3.81 | |
| Parahippocampal Gyrus | −16 | −14 | −26 | 103 | Left | 28 | −4.06 | |
| Medial Prefrontal Cortex | −8 | −12 | 68 | 218 | Bilateral | 6 | −4.26 | |
| Time-to-peak BP > SZ |
Cerebellum Posterior Lobe | −32 | −78 | −42 | 1193 | Bilateral | 3.56 | |
| Time-to-peak BP < SZ |
Pons | −16 | −26 | −30 | 997 | −3.96 | ||
| Thalamus | −8 | −24 | 0 | −3.71 | ||||
| Intersection | Thalamus | 2 | −22 | 4 | 101 | Bilateral | ||
Coordinates indicate location of maximum Z-scores for clusters or location of local maxima. For clusters with more than one peak, local maxima are listed. Cluster size in voxels. Coordinates are in standard MNI space. BA, Brodmann Area.
We found that a thalamic cluster showed significant differences in at least one HRF parameter in all 3 comparisons: BP vs control, SZ vs control, and BP vs SZ. (figure 4A). Note that in the identified thalamus seed ROI, the differences in HRF parameters exhibited the following pattern – FWHM: SZ > Control, RH: BP < Control, and FWHM: SZ > BP. The overlap of our thalamic seed (shown in white, figure 4B) with thalamic zones as described in the Oxford thalamic atlas based on diffusion tractography71 revealed that our seed had the highest probability of structural connectivity with the temporal and prefrontal cortex. By registering our seed ROI to the digital 3D Morel atlas,72,73 we found that the thalamic seed ROI encompassed the mediodorsal (MD) nucleus, habenular (Hb) nucleus, and the central lateral (CL) nucleus (figure 4C).
Fig. 4.
Thalamus seed. (A) Voxels within the thalamus that showed significant differences in all three HRF parameters in the following three comparisons: BP vs control, SZ vs control, and BP vs SZ. Sagittal (x = 2), and axial (z = 4) views are shown in standard MNI space. In the thalamus seed ROI, alterations in HRF parameters exhibited the following pattern: FWHM: SZ > control; RH: BP < control, and FWHM: SZ > BP. (B) The overlap of our thalamic seed (shown in white) with thalamic zones as described in the Oxford thalamic atlas.71 (C) The overlap of our thalamic seed (shown in white) with thalamic nuclei described in the Morel thalamic atlas.72,73 The seed encompassed the mediodorsal nucleus, habenular nucleus, and the central lateral nucleus.
Group Differences in Seed-Based Thalamic Functional Connectivity
Within group FC patterns using the thalamic seed as well as group differences in thalamic FC patterns were assessed (SZ vs controls, BP vs controls, and SZ vs BP) separately using DC and NDC datasets. The corresponding maps and regional statistics have been provided in Supplementary Section S6. A summary of those observations has been depicted in figure 5.
Fig. 5.
Summary of between-group differences in seed-based thalamic functional connectivity for (A) deconvolved, and (B) nondeconvolved datasets. The regions in red boxes displayed functional connectivity differences between groups that varied by whether the data was deconvolved or not. (C) The 3D positions of involved regions. Note that the Insula is shown as an interior region within a semitransparent rendering of the brain.
Impact of HRF Variability on Group Differences in Functional Connectivity
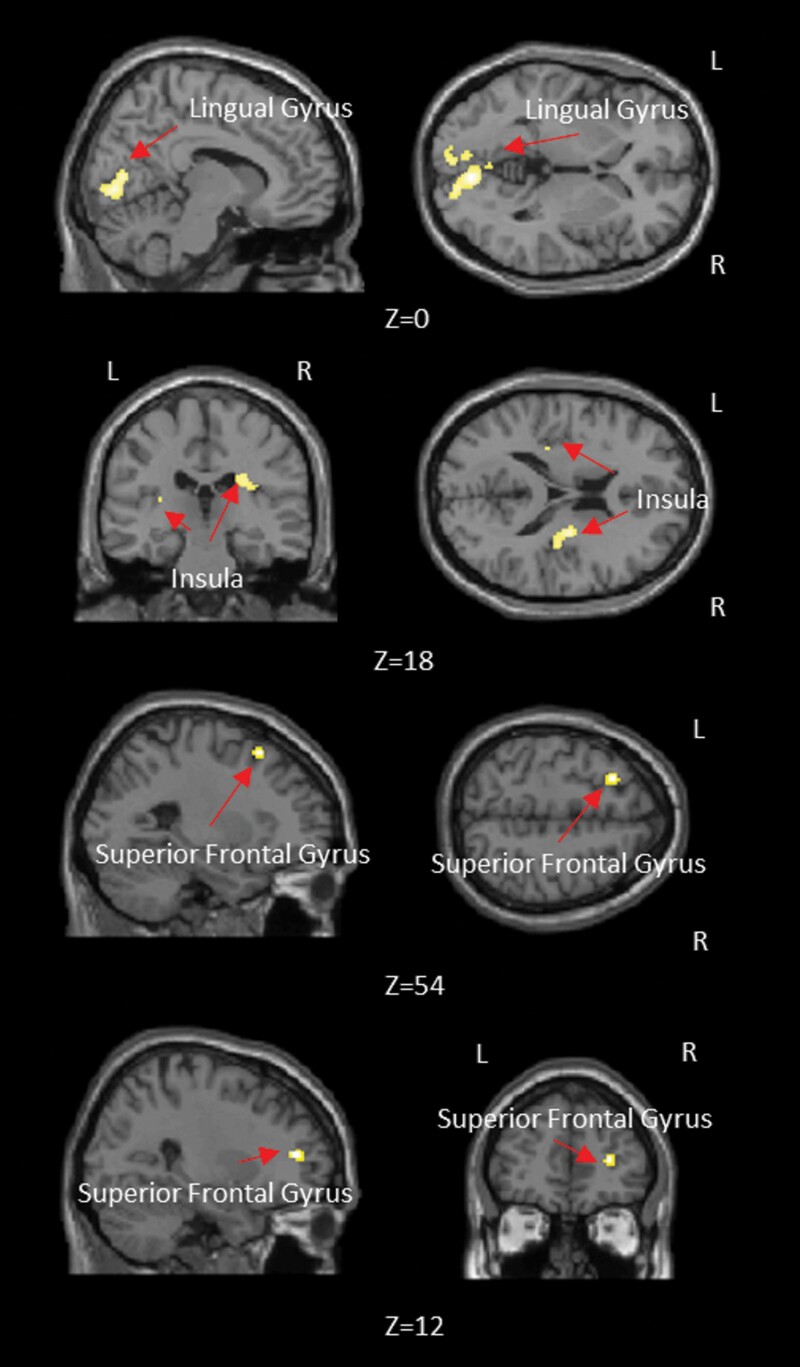
Voxel clusters in bilateral lingual gyrus, bilateral insula, and bilateral SFG showed a significant interaction effect between the group factor (control vs BP vs SZ) and the deconvolution factor (data with and without deconvolution) (figure 6, table 2). This implied that seed-based FC differences between the three groups (SZ, BP, and controls) in these regions were influenced by HRF variability, and thus resulted in differing group differences with DC and NDC datasets.
Fig. 6.
Brain regions showing significant interaction between the group factor (control vs BP vs SZ) and the deconvolution factor (data with and without deconvolution).
Table 2.
Anatomical Labels, Cluster Size, and P-Value of Local Maxima in Brain Areas Showing Significant Interaction Between the Group Factor (Control vs BP vs SZ) and the Deconvolution Factor (Data With and Without Deconvolution)
| Peak Coordinates | |||||
|---|---|---|---|---|---|
| Cluster Anatomical Location | x | y | z | Cluster Size | Hemisphere |
| Lingual Gyrus | −8 | 84 | −4 | 728 | Bilateral |
| Insula | −30 | −28 | 12 | 42 | Left |
| Insula | 30 | −18 | 22 | 190 | Right |
| Superior Frontal Gyrus | −24 | 20 | 54 | 45 | Left |
| Superior Frontal Gyrus | 28 | 44 | 10 | 41 | Right |
Coordinates referring to the peak of the cluster in MNI Space.
Anatomical labels based on Automated Anatomical Labeling (AAL).
Relationship Between HRF Parameters and Gene Expression
In order to understand the basis of HRF group differences, our third aim was to assess the association between gene expression (DRD2 and PBRM1 genes) and the significant differences observed in HRF parameters. Figure 7 shows the difference value of HRF parameters between the groups (BP-minus-control, SZ-minus-control, and BP-minus-SZ) in DK-space, which were used to test associations with gene expressions. Figure 8 shows the gene expression maps for DRD2 and PBRM1 genes. The association of gene expression values with HRF difference values is presented in table 3. We found that DRD2 gene expression was significantly positively associated with RH and TTP difference values for SZ-minus-control, and with RH difference value for BP-minus-SZ (table 3). No significant associations with the PBRM1 gene expression were observed.
Fig. 7.
The difference value in HRF parameters (RH, FWHM, and TTP) between the groups. The difference values have been normalized across the whole brain (range 0–1). Figure shows HRF difference value for: (A) BP-minus-control, (B) SZ-minus-control, (C) BP-minus-SZ.
Fig. 8.
Gene expression map of the two genes under consideration (normalized to the range 0–1). (A) DRD2 gene expression. (B) PBRM1 gene expression.
Table 3.
The Association (R-Value and Corresponding P-Value) of Regional HRF Parameters with DRD2 Gene and PBRM1 Gene expressions. Before calculating the association, mean group difference maps for the HRF parameters for three group comparisons: BP vs Control, SZ vs Control, and BP vs SZ, were obtained in DK-space. We then examined the association between the mean HRF difference values from each NDC-DC data pair and gene expressions of DRD2 and PBRM1 genes by using corresponding values across all ROIs as the sample
| DRD2(R-Value/P-Value) | PBRM1 (R-Value/P-Value) | |||||
|---|---|---|---|---|---|---|
| RH | TTP | FWHM | RH | TTP | FWHM | |
| BP-Control | −0.02/.87 | 0.15/.24 | 0.04/.74 | −0.16/.21 | −0.19/.14 | −0.17/.20 |
| SZ-Control | 0.35/.005* | 0.31/.015* | 0.22/.09 | −0.15/.25 | 0.03/.80 | −0.01/.91 |
| BP-SZ | −0.29/.02* | 0.14/.28 | 0.17/.19 | 0.005/.99 | 0.20/.12 | 0.17/.20 |
*Significant associations are labeled in bold letters.
Relationship Between HRF Parameters and DDD of Medications
In the SZ group, DDD of antipsychotics was significantly associated with RH in left mPFC (figure 9A, supplementary table S7 in supplementary section S10), with FWHM in right cingulate gyrus and right MFG (figure 9B, supplementary table S7), and with TTP in cerebellum posterior lobe and occipital lobe (figure 9C, supplementary table S7).
Fig. 9.
Brain regions showing significant association between DDDantipsych and HRF parameters (FWHM, RH, and TTP) in the SZ group.
Discussion
This study had three aims: (1) quantifying neurovascular coupling impairments (using the HRF) in SZ and BP, (2) measuring the impact of such neurovascular coupling impairments (i.e. HRF variability as a confound) on the commonly used measure of functional connectivity, and (3) understanding the role of dopamine receptor (DRD2) gene expression and D2-blocking agents on the neurovascular coupling parameters. To this effect, we identified HRF differences between SZ, BP, and control groups, and identified the significant impact of HRF variability on FC group differences hitherto attributed to disease related disruptions of neural level statistical dependencies. In particular, both patient groups had aberrant HRF parameters affecting the thalamus, but differing in degree between the 2 disorders. We also uncovered the likely influence of DRD2 gene expression as well as psychotropic medication use on HRF parameters, highlighting the importance of accounting for HRF variations while estimating functional connectivity in clinical samples.
HRF Results in Relation to Neurochemicals
HRF variability across brain regions and subjects due to nonneural factors tend to be more randomly distributed25,26,74–76 and are expected to cancel out when comparing different populations. On the other hand, pathological neurochemical alterations are expected to introduce more systematic differences in the HRF between groups. In the current study, we show that D2 receptor expression, but not PBRM1 expression, relates to HRF parameters as predicted. Unlike DRD2, the PBRM1 gene has no known effect on neurotransmitters that modulate the HRF. Furthermore, higher D2 blockade in patients as indexed by higher DDD of antipsychotics related to reduced HRF response height in cingulate cortex, in line with prior reports of D2 receptors being responsible for a decrease of hemodynamic response.35 While we selected a single gene expression profile with therapeutic relevance to both SZ and BP, multiple other neurochemicals such as GABA, serotonin, glutamate, and nitric oxide (NO) also control neurovascular coupling.27Figure 10 shows a schematic of these putative neurovascular coupling mechanisms.
Fig. 10.
A schematic illustrating neurovascular coupling (the hemodynamic coupling between neural activity and blood flow). The HRF is a mathematical function which represents this neurovascular coupling mechanism. Aberrations in neurochemicals in SZ and BP subjects, which may impact neurovascular coupling and hence HRF shape, are shown. Abnormalities in GABA are shown as 1, dopamine as 2, nitric oxide as 3, and glutamate as 4.
HRF Differences Across All Three Groups in the Thalamus
We detected a cluster of voxels within the thalamus (figure 4A) that showed significant HRF differences across all three groups. According to the Morel thalamic atlas,72,73 this thalamic sub-region encompasses MD, Hb, and CL nuclei (figure 4C). Kumar et al77 proposed a thalamic parcellation based on both rs-fMRI FC and structural connectivity of the thalamus. They compared their parcellations with the Morel atlas as well.72,73 They reported that their parcellated thalamic clusters that overlapped with MD and CL thalamic nuclei were functionally connected at rest with the occipital and parietal lobes, the temporoparietal junction, pre- and post-central gyrus, cuneus, inferior, and medial temporal lobes, the ACC, and parts of the medial and lateral prefrontal cortices, in line with our reported observations. Previous studies have also reported aberrations in thalamic connectivity in SZ78,12,79–81 and BP12, 82 similar to those reported here. Taken together, these patterns of connectivity between the MD, CL, and Hb thalamic nuclei and the rest of the brain, as well as the observed aberrations involving the thalamus in SZ and BP disorder are in line with prior reports.
Impact of HRF Variability on Group Differences in Functional Connectivity
It is noteworthy that group differences in FC were different between deconvolved and nondeconvolved data (see figure 6 in main text as well as supplementary figures S1–S3). Although mostly similar overarching clusters were identified with both DC and NDC data, the size, peak coordinates, and corresponding statistics were different. On some instances, certain between-group FC differences were observed only in one of the datasets (DC or NDC). We found significant interaction effects in the FC paths between thalamus seed and the following regions: bilateral lingual gyrus, bilateral insula, and bilateral SFG (figure 6, table 2). This implies that FC differences across groups between the thalamic seed and these regions would be inferred differently in the NDC and DC datasets. This finding suggests that if FC results are to be interpreted purely in terms of underlying neural processing rather than in terms of confounding nonneural and neural (viz. neurovascular signaling mechanisms) components affecting the HRF, then it would be preferable to characterize FC using the latent neural time series (i.e. deconvolved data) rather than nondeconvolved fMRI data. In regions where the interaction effect was not significant, the alteration in numerical FC values due to HRF variability may impact analyses at the single-subject level, including (but not limited) to machine learning analyses used in precision psychiatry.
This result must be viewed in light of other reports21 suggesting that ignoring HRF variability can cause a mean error of 14.7% in connectivity estimates, with a tenth of the connections confounded by over 33%. Also, consistent with Rangaprakash et al's study,21 we found more false positives than false negatives if HRF variability were ignored (i.e. comparing NDC and DC datasets, more clusters and larger cluster sizes in within-group comparisons were found with the former; supplementary figures S1–S3). NDC dataset (that ignored HRF variability) was also generally less sensitive to group differences (i.e., fewer clusters and smaller cluster sizes were seen in most cases in between-group comparisons with NDC dataset, as compared to DC dataset; supplementary figures S4–S6). Given the evidence from previous studies21,22,42–44 and evidence presented in the current study, we suggest that: (1) HRF variability cannot be ignored while performing fMRI connectivity modeling as it confounds within-group FC estimates as well as between-group FC differences; (2) HRF variability can be minimized by performing a deconvolution step after completing all other preprocessing steps and prior to postprocessing steps such as connectivity modeling.
Among the limitations of this study were the fact that we investigated the effect of HRF variability on FC only with the thalamus seed; the magnitude and characteristics of HRF confound on FC elsewhere in the brain could be different. A similar analysis at the whole brain level, though computationally expensive, may provide a more complete picture. We selected this seed based on a stringent selection approach that required at least three significant “hits” in the group comparison maps of the 3 parameters. We used a multiple testing correction approach aimed at improving sensitivity over specificity83 in this exploratory work; we acknowledge that this is not the gold standard correction for mass univariate search approaches. The regions highlighted here could inform further motivated studies of HRF in schizophrenia in the future. Second, the discussion points we provided regarding how neurochemical factors might affect the HRF is still speculative, given that there is yet very limited literature directly investigating the relationship between HRF and these factors. In the absence of direct measurement of neurochemicals (using in vivo MR spectroscopy, positron emission tomography or single-photon emission computed tomography) in regions showing alterations in the shape of the HRF, it is not possible to make definitive claims regarding how these neurochemicals might control different aspects of the HRF shape. Third, in both deconvolved and nondeconvolved data, we estimated the correlation between HRF parameters and FC as well as clinical variables at the individual subject level. None of the correlations were significant after correcting for multiple comparisons, while a couple of associations approached trend level. While we agree that this type of analysis would provide interesting insights, we feel that the variability in the data as well as the sample size must be larger for this type of individual difference analysis. This might be an interesting research direction for future studies. Third, the sample size used in this study is modest, especially for bipolar disorder. Therefore, the conclusions obtained from this study must be replicated in relatively larger samples in order to inform best practices in the use of functional MRI in studies involving subjects with schizophrenia or bipolar disorder. Finally, we used gene expression maps obtained from the Allen Human Brain Atlas. However, more definitive conclusions can be reached if one had gene expression and neurochemical data from the same subjects (SZ, BP and Control) undergoing the MRI.
To conclude, neurovascular coupling is highly variable among brain regions within an individual, as well as between different individuals. These variations relate to psychosis, dopamine receptor distribution, and psychotropic dose exposure. In psychotic disorders, functional disconnection among brain regions may result from factors differentially affecting regional neurovascular coupling. Parsing this uncoupling could potentially improve the consistency of mechanistic studies based on functional connectivity.
Supplementary Material
Acknowledgments
The data for this study was acquired from a study originally funded by the Medical Research Council (UK) Grant Number: G0601442. LP acknowledges salary support from the Tanna Schulich Chair of Neuroscience and Mental Health, Schulich School of Medicine and Dentistry. PFL acknowledges support from the Wellcome Trust. GD acknowledges salary support from the Auburn University’s Samuel Ginn College of Engineering. WY acknowledges support from Beijing Natural Science Foundation (Grant Number 4202014), Humanity and Social Science Youth Foundation of Ministry of Education of China (Grant Number 20YJCZH229), National Natural Science Foundation of China (Grant Number 61873005) and Young teachers’ scientific research ability improvement program supported by Beijing Technology and Business University (Grant Number PXM2019_014213_000007). LP reports personal fees from Otsuka Canada, SPMM Course Limited, UK, Canadian Psychiatric Association; book royalties from Oxford University Press; investigator-initiated educational grants from Janssen Canada, Sunovion, and Otsuka Canada outside the submitted work. All other authors report no relevant conflicts.
Contributor Information
Wenjing Yan, Department of Electrical and Computer Engineering, AU MRI Research Center, Auburn University, Auburn, AL, USA; Department of Information Management, School of E-business and Logistics, Beijing Technology and Business University, Beijing, China.
Lena Palaniyappan, Department of Psychiatry, University of Western Ontario, London, ON, Canada; Robarts Research Institute, University of Western Ontario, London, ON, Canada; Department of Medical Biophysics, University of Western Ontario, London, ON, Canada.
Peter F Liddle, Centre for Translational Neuroimaging, Division of Mental Health and Clinical Neuroscience, Institute of Mental Health, University of Nottingham, UK.
D Rangaprakash, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Wei Wei, Department of Information Management, School of E-business and Logistics, Beijing Technology and Business University, Beijing, China.
Gopikrishna Deshpande, Department of Electrical and Computer Engineering, AU MRI Research Center, Auburn University, Auburn, AL, USA; Department of Psychological Sciences, Auburn University, Auburn, AL; Alabama Advanced Imaging Consortium, Birmingham, AL; Center for Neuroscience, Auburn University, AL, USA; School of Psychology, Capital Normal University, Beijing, China; Key Laboratory for Learning and Cognition, Capital Normal University, Beijing, China; Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, India; Centre for Brain Research, Indian Institute of Science, Bangalore, India.
Author Contributions
G.D., L.P., and P.F.L. conceived and designed the study. L.P. and P.F.L. acquired the data. W.Y. performed the bulk of the analysis with some of the analyses performed by G.D. and D.R. All authors wrote and reviewed the manuscript.
Data Availability
Raw and pre-processed data as well as results can be made available upon reasonable request to the corresponding author.
References
- 1. Zhu Y, Womer FY, Leng H, et al. The relationship between cognitive dysfunction and symptom dimensions across schizophrenia, bipolar disorder, and major depressive disorder. Front Psychiatry. 2019;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fischer BA. A review of American psychiatry through its diagnoses: the history and development of the Diagnostic and Statistical Manual of Mental Disorders. J Nerv Ment Dis. 2012;200:1022–1030. [DOI] [PubMed] [Google Scholar]
- 3. Sorella S, Lapomarda G, Messina I, et al. Testing the expanded continuum hypothesis of schizophrenia and bipolar disorder. Neural and psychological evidence for shared and distinct mechanisms. Neuroimage Clin. 2019;23:101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee J, Rizzo S, Altshuler L, et al. Deconstructing bipolar disorder and schizophrenia: a cross-diagnostic cluster analysis of cognitive phenotypes. J Affect Disord. 2017;209:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuswanto C, Chin R, Sum MY, et al. Shared and divergent neurocognitive impairments in adult patients with schizophrenia and bipolar disorder: whither the evidence? Neurosci Biobehav Rev. 2016;61:66–89. [DOI] [PubMed] [Google Scholar]
- 7. Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA Jr. The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010;71:1488–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. [DOI] [PubMed] [Google Scholar]
- 10. Huang CC, Luo Q, Palaniyappan L, et al. Transdiagnostic and illness-specific functional dysconnectivity across schizophrenia, bipolar disorder and major depression and relationships with working memory. SSRN Electron J. 2019. doi: 10.2139/ssrn.3356860 [DOI] [PubMed] [Google Scholar]
- 11. Palaniyappan L, Liddle PF. Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr Bull. 2014;40:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Argyelan M, Ikuta T, DeRosse P, et al. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr Bull. 2014;40:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anticevic A, Savic A, Repovs G, et al. Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr Bull. 2015;41:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tu PC, Bai YM, Li CT, et al. Identification of common thalamocortical dysconnectivity in four major psychiatric disorders. Schizophr Bull. 2019;45:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meda SA, Gill A, Stevens MC, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skåtun KC, Kaufmann T, Brandt CL, et al. Thalamo-cortical functional connectivity in schizophrenia and bipolar disorder. Brain Imaging Behav. 2018;12:640–652. doi: 10.1007/s11682-017-9714-y [DOI] [PubMed] [Google Scholar]
- 18. Birur B, Kraguljac NV, Shelton RC, Lahti AC. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder-a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr. 2017;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmidt A, Diwadkar VA, Smieskova R, et al. Approaching a network connectivity-driven classification of the psychosis continuum: a selective review and suggestions for future research. Front Hum Neurosci. 2014;8:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo S, He N, Liu Z, Linli Z, Tao H, Palaniyappan L. Brain-wide functional dysconnectivity in schizophrenia: parsing diathesis, resilience, and the effects of clinical expression. Can J Psychiatry. 2020;65:21–29. doi: 10.1177/0706743719890174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rangaprakash D, Wu GR, Marinazzo D, Hu X, Deshpande G. Hemodynamic response function (HRF) variability confounds resting-state fMRI functional connectivity. Magn Reson Med. 2018;80:1697–1713. [DOI] [PubMed] [Google Scholar]
- 22. Rangaprakash D, Dretsch MN, Yan W, Katz JS, Denney TS Jr, Deshpande G. Hemodynamic variability in soldiers with trauma: implications for functional MRI connectivity studies. Neuroimage Clin. 2017;16:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. [DOI] [PubMed] [Google Scholar]
- 24. Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. [DOI] [PubMed] [Google Scholar]
- 25. Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21:1639–1651. [DOI] [PubMed] [Google Scholar]
- 26. Handwerker DA, Gonzalez-Castillo J, D’Esposito M, Bandettini PA. The continuing challenge of understanding and modeling hemodynamic variation in fMRI. Neuroimage. 2012;62:1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. [DOI] [PubMed] [Google Scholar]
- 28. Shan ZY, Vinkhuyzen AAE, Thompson PM, et al. Genes influence the amplitude and timing of brain hemodynamic responses. Neuroimage. 2016;124:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buxton RB. Introduction to Functional Magnetic Resonance Imaging: principles and Techniques. Energy. 2002;24:xi, 523. doi: 10.1017/CBO9780511605505 [DOI] [Google Scholar]
- 30. Levin JM, Ross MH, Mendelson JH, et al. Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Res. 1998;82:135–146. [DOI] [PubMed] [Google Scholar]
- 31. Noseworthy MD, Alfonsi J, Bells S. Attenuation of brain BOLD response following lipid ingestion. Hum Brain Mapp. 2003;20:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet. 2005;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sukumar N, Sabesan P, Anazodo U, Palaniyappan L. Neurovascular uncoupling in schizophrenia: a bimodal meta-analysis of brain perfusion and glucose metabolism. Front Psychiatry. 2020;11:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Selvaggi P, Hawkins PCT, Dipasquale O, et al. Increased cerebral blood flow after single dose of antipsychotics in healthy volunteers depends on dopamine D2 receptor density profiles. Neuroimage. 2019;188:774–784. [DOI] [PubMed] [Google Scholar]
- 35. Bruinsma TJ, Sarma VV, Oh Y, et al. The relationship between dopamine neurotransmitter dynamics and the blood-oxygen-level-dependent (BOLD) signal: a review of pharmacological functional magnetic resonance imaging. Front Neurosci. 2018;12:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mandeville JB, Sander CYM, Jenkins BG, et al. A receptor-based model for dopamine-induced fMRI signal. Neuroimage. 2013;75:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krimer LS, Muly EC 3rd, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci. 1998;1:286–289. [DOI] [PubMed] [Google Scholar]
- 38. Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab. 2012;32:1207–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watts ME, Pocock R, Claudianos C. Brain energy and oxygen metabolism: emerging role in normal function and disease. Front Mol Neurosci. 2018;11:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ward PGD, Orchard ER, Oldham S, et al. Individual differences in haemoglobin concentration influence bold fMRI functional connectivity and its correlation with cognition. Neuroimage. 2020;221:117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rangaprakash D, Deshpande G, Daniel TA, et al. Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and posttraumatic stress disorder. Hum Brain Mapp. Published online 2017. doi: 10.1002/hbm.23551 [DOI] [PMC free article] [PubMed]
- 42. Yan W, Rangaprakash D, Deshpande G. Aberrant hemodynamic responses in autism: implications for resting state fMRI functional connectivity studies. Neuroimage Clin. 2018;19:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yan W, Rangaprakash D, Deshpande G. Estimated hemodynamic response function parameters obtained from resting state BOLD fMRI signals in subjects with autism spectrum disorder and matched healthy subjects. Data Brief. 2018;19:1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rangaprakash D, Dretsch MN, Yan W, Katz JS, Denney TS Jr, Deshpande G. Hemodynamic response function parameters obtained from resting-state functional MRI data in soldiers with trauma. Data Brief. 2017;14:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aquino KM, Robinson PA, Schira MM, Breakspear M. Deconvolution of neural dynamics from fMRI data using a spatiotemporal hemodynamic response function. Neuroimage. 2014;94:203–215. [DOI] [PubMed] [Google Scholar]
- 46. Havlicek M, Friston KJ, Jan J, Brazdil M, Calhoun VD. Dynamic modeling of neuronal responses in fMRI using cubature Kalman filtering. Neuroimage. 2011;56:2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sreenivasan KR, Havlicek M, Deshpande G. Nonparametric hemodynamic deconvolution of FMRI using homomorphic filtering. IEEE Trans Med Imaging. 2015;0062(c):1–1. [DOI] [PubMed] [Google Scholar]
- 48. Wu GR, Liao W, Stramaglia S, Ding JR, Chen H, Marinazzo D. A blind deconvolution approach to recover effective connectivity brain networks from resting state fMRI data. Med Image Anal. 2013;17:365–374. [DOI] [PubMed] [Google Scholar]
- 49. Wu GR, Marinazzo D. Point-process deconvolution of fMRI BOLD signal reveals effective connectivity alterations in chronic pain patients. Brain Topogr. 2015;28:541–547. [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization (WHO) International Working Group for Drug Statistics Methodology. WHO Collaborating Centre for Drug Statistics Methodology, WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services, Introduction to Drug Utilization Research, WHO, Oslo, Norway, 2003. [Google Scholar]
- 51. Gowland PA, Bowtell R. Theoretical optimization of multi-echo fMRI data acquisition. Phys Med Biol. 2007;52:1801–1813. [DOI] [PubMed] [Google Scholar]
- 52. Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Criticality in large-scale brain FMRI dynamics unveiled by a novel point process analysis. Front Physiol. 2012;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petridou N, Gaudes CC, Dryden IL, Francis ST, Gowland PA. Periods of rest in fMRI contain individual spontaneous events which are related to slowly fluctuating spontaneous activity. Hum Brain Mapp. 2013;34:1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindquist MA, Mejia A. Zen and the art of multiple comparisons. Psychosom Med. 2015;77:114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C (2nd Ed.): The Art of Scientific Computing. New York, United States: Cambridge University Press; 1992. doi: 10.2307/1269484 [DOI] [Google Scholar]
- 57. Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. [DOI] [PubMed] [Google Scholar]
- 58. French L, Paus T. A FreeSurfer view of the cortical transcriptome generated from the Allen Human Brain Atlas. Front Neurosci. 2015;9:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 60. Dubertret C, Gouya L, Hanoun N, et al. The 3’ region of the DRD2 gene is involved in genetic susceptibility to schizophrenia. Schizophr Res. 2004;67:75–85. [DOI] [PubMed] [Google Scholar]
- 61. Kaalund SS, Newburn EN, Ye T, et al. Contrasting changes in DRD1 and DRD2 splice variant expression in schizophrenia and affective disorders, and associations with SNPs in postmortem brain. Mol Psychiatry. 2014;19:1258–1266. [DOI] [PubMed] [Google Scholar]
- 62. Tomasella E, Bechelli L, Ogando MB, et al. Deletion of dopamine D2 receptors from parvalbumin interneurons in mouse causes schizophrenia-like phenotypes. Proc Natl Acad Sci U S A. 2018;115:3476–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pergola G, Di Carlo P, D’Ambrosio E, et al. DRD2 co-expression network and a related polygenic index predict imaging, behavioral and clinical phenotypes linked to schizophrenia. Transl Psychiatry. 2017;7:e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zou YF, Wang F, Feng XL, et al. Association of DRD2 gene polymorphisms with mood disorders: a meta-analysis. J Affect Disord. 2012;136:229–237. [DOI] [PubMed] [Google Scholar]
- 65. Iles MM. Genome-Wide Association Studies. In: Genetic Epidemiology. Methods in Molecular Biology (Methods and Protocols), Totowa, NJ: Humana Press. 2011. 10.1007/978-1-60327-416-6_7 [DOI] [Google Scholar]
- 66. Piletz JE, Zhang X, Ranade R, Liu C. Database of genetic studies of bipolar disorder. Psychiatr Genet. 2011;21:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Smoller JW, Kendler K, Craddock N, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu J, Chen J, Perrone-Bizzozero NI, Turner JA, Calhoun VD. Regional enrichment analyses on genetic profiles for schizophrenia and bipolar disorder. Schizophr Res. 2018;192:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang Z, Zhou D, Li H, et al. The genome-wide risk alleles for psychiatric disorders at 3p21.1 show convergent effects on mRNA expression, cognitive function, and mushroom dendritic spine. Mol Psychiatry. 2020;25:48–66. [DOI] [PubMed] [Google Scholar]
- 70. Golovina E, Vickers MH, Erb CD, O’Sullivan JM. GWAS SNPs Impact shared regulatory pathways amongst multimorbid psychiatric disorders and cognitive functioning. Front Psychiatry. 2020;11:560751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. [DOI] [PubMed] [Google Scholar]
- 72. Morel A. Stereotactic Atlas of the Human Thalamus and Basal Ganglia. New York: Informa Healthcare USA; 2007. doi: 10.3109/9781420016796. [DOI] [Google Scholar]
- 73. Krauth A, Blanc R, Poveda A, Jeanmonod D, Morel A, Székely G. A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage. 2010;49:2053–2062. [DOI] [PubMed] [Google Scholar]
- 74. Aguirre GK, Zarahn E, D’esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. [DOI] [PubMed] [Google Scholar]
- 75. Chang C, Thomason ME, Glover GH. Mapping and correction of vascular hemodynamic latency in the BOLD signal. Neuroimage. 2008;43:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kemna LJ, Posse S. Effect of respiratory CO(2) changes on the temporal dynamics of the hemodynamic response in functional MR imaging. Neuroimage. 2001;14:642–649. [DOI] [PubMed] [Google Scholar]
- 77. Kumar VJ, van Oort E, Scheffler K, Beckmann CF, Grodd W. Functional anatomy of the human thalamus at rest. Neuroimage. 2017;147:678–691. [DOI] [PubMed] [Google Scholar]
- 78. Andreasen NC, O’Leary DS, Cizadlo T, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93:9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marenco S, Stein JL, Savostyanova AA, et al. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Noble S, Scheinost D, Constable RT. Cluster failure or power failure? Evaluating sensitivity in cluster-level inference. Neuroimage. 2020;209:116468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and pre-processed data as well as results can be made available upon reasonable request to the corresponding author.