Key Points
Question
Is psychotropic medication use associated with differences in the risk of COVID-19 infection among adults with serious mental illness?
Findings
In this cohort study of 1958 inpatients with serious mental illness in a statewide psychiatric hospital system, the use of second-generation antipsychotic medications was associated with a decreased risk of COVID-19 infection; the largest association was observed with the use of paliperidone. Valproic acid use was associated with an increased risk of infection.
Meaning
These results suggest that individual psychotropic medications are associated with differential risks of COVID-19 infection among patients with serious mental illness.
Abstract
Importance
Individuals with serious mental illness are at increased risk of severe COVID-19 infection. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19 but have not been systematically examined in this population.
Objective
To evaluate the associations between the use of psychotropic medications and the risk of COVID-19 infection among adults with serious mental illness receiving long-term inpatient psychiatric treatment.
Design, Setting, and Participants
This retrospective cohort study assessed adults with serious mental illness hospitalized in a statewide psychiatric hospital system in New York between March 8 and July 1, 2020. The final date of follow-up was December 1, 2020. The study included 1958 consecutive adult inpatients with serious mental illness (affective or nonaffective psychoses) who received testing for SARS-CoV-2 by reverse transcriptase–polymerase chain reaction or antinucleocapsid antibodies and were continuously hospitalized from March 8 until medical discharge or July 1, 2020.
Exposures
Psychotropic medications prescribed prior to COVID-19 testing.
Main Outcomes and Measures
COVID-19 infection was the primary outcome, defined by a positive SARS-CoV-2 reverse transcriptase–polymerase chain reaction or antibody test result. The secondary outcome was COVID-19–related death among patients with laboratory-confirmed infection.
Results
Of the 2087 adult inpatients with serious mental illness continuously hospitalized during the study period, 1958 (93.8%) underwent testing and were included in the study; 1442 (73.6%) were men, and the mean (SD) age was 51.4 (14.3) years. A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 infection that occurred while they were hospitalized; of those, 38 (3.9%) died. The use of second-generation antipsychotic medications, as a class, was associated with decreased odds of infection (odds ratio [OR], 0.62; 95% CI, 0.45-0.86), whereas the use of mood stabilizers was associated with increased odds of infection (OR, 1.23; 95% CI, 1.03-1.47). In a multivariable model of individual medications, the use of paliperidone was associated with decreased odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and the use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76). Clozapine use was associated with reduced odds of mortality in unadjusted analyses (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
Conclusions and Relevance
In this cohort study of adults hospitalized with serious mental illness, the use of second-generation antipsychotic medications was associated with decreased risk of COVID-19 infection, whereas the use of valproic acid was associated with increased risk. Further research is needed to assess the mechanisms that underlie these findings.
This cohort study evaluates the associations between the use of psychotropic medications and the risk of COVID-19 infection among adults with serious mental illness receiving long-term inpatient psychiatric treatment.
Introduction
Individuals with serious mental illness are especially vulnerable to COVID-19. Patients with psychiatric disorders are more likely to have medical comorbidities associated with worse outcomes and have a higher mortality rate from COVID-19 independent of these medical risk factors.1,2,3,4,5 Among psychiatric diagnoses, schizophrenia is associated with the greatest increase in mortality risk.1,3,5,6 Although increased mortality risk after COVID-19 infection has been consistently observed among patients with psychiatric disorders, some studies have found lower rates of COVID-19 infection among patients with major psychiatric disorders.3,6,7 This finding may reflect a true decrease in infection rates, possibly owing to social isolation, or failure to detect infection among individuals who do not receive testing.8 In contrast, inpatients residing in psychiatric treatment facilities are at high risk of viral exposure and often have greater access to testing.
Identifying factors associated with the risk of infection among inpatients with serious mental illness is of critical importance given their susceptibility to severe infection. A study of adults with serious mental illness infected with COVID-19 found equivalent mortality rates among those taking antipsychotic medications compared with those not taking antipsychotic medications,9 suggesting that antipsychotic treatment is unlikely to account for increased mortality risk. However, individual medications within and across pharmacologic classes may differ in their associations with infection and adverse outcomes. Several psychotropic medications—including some first-generation antipsychotics (haloperidol10 and chlorpromazine11) and antidepressants (fluvoxamine, in particular12) were identified as potential therapeutic agents based on in vitro evidence of anti–SARS-CoV-2 activity. Clinical evidence to support these in vitro findings has been mixed. Although several studies have established an association between the use of antidepressants13,14,15 and a less severe course of infection in patients with COVID-19, small observational studies have found no association between the use of haloperidol16 or chlorpromazine17 and the severity of COVID-19 infection. These medications and several other psychotropic drugs, including second-generation antipsychotics, may affect the host response to COVID-19 by modifying the balance between proinflammatory and anti-inflammatory cytokines and through other mechanisms.18,19 Several of these medications are common treatments for adults with serious mental illness, but their associations with the risk of COVID-19 infection have not been systematically examined in this population, to our knowledge.
The primary aim of this study was to assess the risk of COVID-19 infection associated with psychopharmacologic treatments among adult long-term inpatients with serious mental illness in a statewide psychiatric hospital system in New York. We also aimed to assess the risk of COVID-19 mortality among patients with laboratory-confirmed infection.
Methods
Study Design and Participants
In this retrospective cohort study, we used data from the electronic health record (EHR) and a centralized COVID-19 registry used by all psychiatric hospitals operated by the New York State (NYS) Office of Mental Health (OMH). The study protocol was reviewed by the institutional review board of the Nathan S. Kline Institute for Psychiatric Research and deemed exempt from the requirement of informed consent based on use of deidentified data and designation of non–human participants research. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
The NYS-OMH operates psychiatric hospitals across the state that provide treatment for adults with serious mental illness in civil units as well as specially designated forensic centers. Adults admitted to civil units meet criteria for involuntary commitment under the Mental Hygiene Law and are typically referred from inpatient psychiatric units in community hospitals for treatment of severe and persistent psychiatric symptoms. In contrast to the relatively brief length of stay at acute care hospitals, the mean length of stay in NYS-OMH facilities varies from 6 months to several years. All hospitals have nursing staff coverage 24 hours a day, 7 days a week, and ensure medication adherence through direct observation while patients are taking prescribed medication.
At the beginning of the COVID-19 pandemic in March 2020, the NYS-OMH implemented a centralized COVID-19 registry that included COVID-19 test results and clinical status for every patient in a NYS-OMH facility, including medical transfers to other facilities for COVID-19 treatment and COVID-19–related deaths. The registry was updated daily, with required reporting retroactive to March 1, 2020. SARS-CoV-2 reverse transcriptase–polymerase chain reaction (RT-PCR) testing of suspected cases began March 1, 2020, using multiple commercial laboratories; as of March 24, 2020, RT-PCR testing was performed by a single laboratory (Bioreference Laboratories Inc). The NYS-OMH made universal testing available to all hospitalized patients beginning May 7, 2020; patients were offered screening by RT-PCR and antibody testing using the ARCHITECT SARS CoV-2 antinucleocapsid protein immunoglobulin G (IgG) assay (Abbott). This assay has a reported sensitivity of 100% and specificity of 99.6% for detecting SARS-CoV-2 within several days after infection,20 with detectable antibodies for at least 3 to 6 months after infection.21 As of July 1, 2020, 93% of patients in a NYS-OMH facility had received COVID-19 testing.
Included in this study were adult inpatients (≥18 years of age) with serious mental illness (schizophrenia, schizoaffective disorder, bipolar I disorder, or depression with psychotic features) who received testing for SARS-CoV-2 by RT-PCR or by serum IgG assay and were continuously hospitalized from March 8, 2020, until medical discharge for COVID-19 or July 1, 2020. Patients who were admitted within 30 days prior to testing were excluded because we assessed medication exposure during the 30 days prior to testing, and information about medications could not be confirmed for patients admitted during this assessment window (eFigure in the Supplement). The primary outcome was infection; patients with any positive test result were considered positive. The secondary outcome was COVID-19–related deaths among patients with laboratory-confirmed infection, as reported in the registry and confirmed in the EHR. Deaths were monitored through December 1, 2020.
Procedures
The exposure of interest was psychotropic medications taken prior to COVID-19 testing. Medication data were extracted from the EHR. Patients were considered exposed to a medication if it was (1) prescribed prior to their index test date, (2) used for at least 7 days, and (3) received via scheduled administration (as-needed medications were excluded). Medications were grouped by pharmacologic class: first-generation antipsychotic, second-generation antipsychotic, mood stabilizer, benzodiazepine, or antidepressant (eTable 1 in the Supplement).
The medication exposure period was defined based on the date of SARS-CoV-2 test and the type of test (RT-PCR or antibody). The date of the first positive test result was used as the index date for patients with any positive test result. A positive RT-PCR test result was considered a proxy of active infection, and the exposure period was limited to 30 days. Patients who were transferred to a medical facility for COVID-19 treatment prior to receiving confirmatory testing were also considered to have active infection, and the exposure period included 30 days prior to transfer. For patients without a positive RT-PCR test result or medical transfer for COVID-19 treatment, the date of the SARS-CoV-2 IgG test was used as the index date. Because this was considered a measure of prior infection, the exposure period included the full study period prior to testing (starting from March 8, 2020).
The following covariates were extracted from the EHR and assessed as potential confounders based on their known or hypothesized association with medication exposure and COVID-19 infection and/or death: age, sex, race and ethnicity, body mass index, smoking status, medical comorbid conditions, and psychiatric diagnoses. Race and ethnicity were based on patient self-report and categorized as Asian or Pacific Islander, Black, Latinx, White, and other (including Native American, multiple races, or “other race”) or unknown. Missing data for race and ethnicity and smoking status were assigned to unknown categories. Medical comorbid conditions included chronic respiratory disease, diabetes, heart disease, and hypertension, using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision medical billing category codes (eTable 2 in the Supplement). Psychiatric diagnoses were determined based on admission diagnosis and grouped into nonaffective psychotic disorders (schizophrenia and delusional disorder) and affective psychotic disorders (schizoaffective disorder, bipolar I disorder, and major depressive disorder with psychotic features). Hospital size (number of inpatients) as well as geographic location were also included as model covariates.
Statistical Analysis
We first used unadjusted binary logistic regression to estimate associations between baseline characteristics and psychopharmacologic classes, with COVID-19 infection as the primary outcome (Table 1) and COVID-19–related death (Table 2) as the secondary outcome.
Table 1. Baseline Characteristics of All Psychiatric Inpatients and COVID-19–Positive Patients.
| Characteristic | All patients, No. (%) (N = 1958) | Patients with COVID-19a | |
|---|---|---|---|
| No. (%) (n = 969 [49.5%]) | Crude OR (95% CI) | ||
| Clinical characteristics | |||
| Age, mean (SD), y | 51.4 (14.3) | 52.8 (14.2) | 1.01 (1.01-1.02)b |
| Age group, y | |||
| 18-44 | 634 (32.4) | 269 (27.8) | 1 [Reference] |
| 45-54 | 371 (19.0) | 186 (19.2) | 1.36 (1.06-1.77)b |
| 55-64 | 570 (29.1) | 295 (30.4) | 1.46 (1.16-1.83)b |
| ≥65 | 383 (19.6) | 219 (22.6) | 1.81 (1.40-2.34)b |
| Sex | |||
| Male | 1442 (73.6) | 723 (74.6) | 1 [Reference] |
| Female | 516 (26.4) | 246 (25.4) | 0.91 (0.74-1.11) |
| Race and ethnicity | |||
| Asian or Pacific Islander | 83 (4.2) | 54 (5.6) | 2.43 (1.51-3.90)b |
| Black | 761 (38.9) | 410 (42.3) | 1.53 (1.25-1.87)b |
| Latinx | 303 (15.5) | 156 (16.1) | 1.39 (1.06-1.81)b |
| White | 747 (38.2) | 324 (33.4) | 1 [Reference] |
| Other or unknownc | 64 (3.3) | 25 (2.6) | 0.84 (0.50-1.41) |
| Smoking status | |||
| Nonsmoker | 1021 (52.1) | 492 (50.8) | 1 [Reference] |
| Smoker | 419 (21.4) | 172 (17.8) | 0.75 (0.60-0.94)b |
| Status not available | 518 (26.5) | 305 (31.5) | 1.54 (1.24-1.91)b |
| BMI group | |||
| Normal (≤25) | 530 (27.1) | 283 (29.2) | 1 [Reference] |
| Overweight (26-30) | 735 (37.5) | 372 (38.4) | 0.89 (0.72-1.12) |
| Low-risk obesity (31-35) | 457 (23.3) | 206 (21.3) | 0.72 (0.56-0.92)b |
| Moderate- to high-risk obesity (>35) | 236 (12.1) | 108 (11.1) | 0.74 (0.54-1.00)b |
| Chronic respiratory disease | |||
| Yes | 136 (6.9) | 80 (8.3) | 1.50 (1.05-2.14)b |
| No | 1822 (93.1) | 889 (91.7) | 1 [Reference] |
| Diabetes | |||
| Yes | 240 (12.3) | 136 (14.0) | 1.39 (1.06-1.82)b |
| No | 1718 (87.7) | 833 (86.0) | 1 [Reference] |
| Heart disease | |||
| Yes | 70 (3.6) | 47 (4.9) | 2.14 (1.29-3.55)b |
| No | 1888 (96.4) | 922 (95.1) | 1 [Reference] |
| Hypertension | |||
| Yes | 368 (18.8) | 214 (22.1) | 1.54 (1.22-1.93)b |
| No | 1590 (81.2) | 755 (77.9) | 1 [Reference] |
| Affective psychotic disorder | |||
| Yesd | 911 (46.5) | 452 (46.7) | 1.01 (0.85-1.21) |
| Noe | 1047 (53.5) | 517 (53.4) | 1 [Reference] |
| Facility size, mean (SD) No. of patients | 166.8 (74.3) | 199.8 (60.9) | 1.01 (1.01-1.02)b |
| Hospital region | |||
| New York City and Long Island | 1099 (56.1) | 653 (67.4) | 1 [Reference] |
| Hudson River | 508 (25.9) | 307 (31.7) | 1.04 (0.84-1.29) |
| Central or Western New York | 351 (17.9) | 9 (0.9) | 0.02 (0.01-0.04)b |
| Psychotropic medication class | |||
| First-generation antipsychotic | |||
| Yes | 1198 (61.2) | 611 (63.1) | 1.17 (0.97-1.40)b |
| No | 760 (38.8) | 358 (36.9) | 1 [Reference] |
| Second-generation antipsychotic | |||
| Yes | 1794 (91.6) | 870 (89.8) | 0.62 (0.45-0.86)b |
| No | 164 (8.4) | 99 (10.2) | 1 [Reference] |
| Mood stabilizers | |||
| Yes | 1043 (53.3) | 541 (55.8) | 1.23 (1.03-1.47)b |
| No | 915 (46.7) | 428 (44.2) | 1 [Reference] |
| Benzodiazepines | |||
| Yes | 1016 (51.9) | 502 (51.8) | 0.99 (0.83-1.19) |
| No | 942 (48.1) | 467 (48.2) | 1 [Reference] |
| Antidepressants | |||
| Yes | 547 (27.9) | 261 (26.9) | 0.91 (0.74-1.10) |
| No | 1411 (72.1) | 708 (73.1) | 1 [Reference] |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); OR, odds ratio.
Estimates show the crude associations between clinical characteristics, psychotropic medication class, and COVID-19 infection from binary logistic regression.
P < .10.
Includes Native American, multiple races, or “other race.”
Includes schizoaffective disorder, bipolar I disorder, and major depressive disorder with psychotic features.
Includes schizophrenia, delusional disorder, and schizotypal disorder.
Table 2. Baseline Characteristics of All COVID-19–Positive Psychiatric Inpatients and COVID-19–Related Deaths.
| Characteristic | All patients with COVID-19, No. (%) (N = 969) | COVID-19–related deathsa | ||
|---|---|---|---|---|
| No. (%) (n = 38 [3.9%]) | Crude OR (95% CI) | |||
| Clinical characteristics | ||||
| Age, mean (SD), y | 52.8 (14.2) | 67.0 (10.6) | 1.12 (1.08-1.16)b | |
| Age group, y | ||||
| 18-44 | 269 (27.8) | 1 (2.6) | 1 [Reference] | |
| 45-54 | 186 (19.2) | 2 (5.3) | 2.91 (0.26-32.36) | |
| 55-64 | 295 (30.4) | 9 (23.7) | 8.43 (1.06-67.00)b | |
| ≥65 | 219 (22.6) | 26 (68.4) | 36.10 (4.86-268.28)b | |
| Sex | ||||
| Male | 723 (74.6) | 29 (76.3) | 1 [Reference] | |
| Female | 246 (25.4) | 9 (23.7) | 0.91 (0.42-1.95) | |
| Race and ethnicity | ||||
| Asian or Pacific Islander | 54 (5.6) | 5 (13.2) | 2.44 (0.83-7.15) | |
| Black | 410 (42.3) | 13 (34.2) | 0.78 (0.36-1.71) | |
| Latinx | 156 (16.1) | 5 (13.2) | 0.79 (0.28-2.26) | |
| White | 324 (33.4) | 13 (34.2) | 1 [Reference] | |
| Other or unknownc | 25 (2.6) | 2 (5.3) | 2.08 (0.44-9.78) | |
| Smoking status | ||||
| Nonsmoker | 492 (50.8) | 15 (39.5) | 1 [Reference] | |
| Smoker | 172 (17.8) | 6 (15.8) | 1.15 (0.44-3.01) | |
| Status not available | 305 (31.5) | 17 (44.7) | 1.88 (0.92-3.82)b | |
| BMI group | ||||
| Normal (≤25) | 283 (29.2) | 11 (28.9) | 1 [Reference] | |
| Overweight (26-30) | 372 (38.4) | 16 (42.1) | 1.11 (0.51-2.43) | |
| Low-risk obesity (31-35) | 206 (21.3) | 6 (15.8) | 0.74 (0.27-2.04) | |
| Moderate- to high-risk obesity (>35) | 108 (11.1) | 5 (13.2) | 1.20 (0.41-3.54) | |
| Chronic respiratory disease | ||||
| Yes | 80 (8.3) | 4 (10.5) | 1.32 (0.46-3.83) | |
| No | 889 (91.7) | 34 (89.5) | 1 [Reference] | |
| Diabetes | ||||
| Yes | 136 (14.0) | 12 (31.6) | 3.00 (1.48-6.11)b | |
| No | 833 (86.0) | 26 (68.4) | 1 [Reference] | |
| Heart disease | ||||
| Yes | 47 (4.9) | 8 (21.1) | 6.09 (2.63-14.17)b | |
| No | 922 (95.1) | 30 (78.9) | 1 [Reference] | |
| Hypertension | ||||
| Yes | 214 (22.1) | 17 (44.7) | 3.02 (1.56-5.83)b | |
| No | 755 (77.9) | 21 (55.3) | 1 [Reference] | |
| Affective psychotic disorder | ||||
| Yesd | 452 (46.6) | 16 (42.1) | 0.83 (0.43-1.59) | |
| Noe | 517 (53.4) | 22 (57.9) | 1 [Reference] | |
| Facility size, mean (SD) No. of patients | 199.8 (60.9) | 213.8 (54.2) | 1.00 (1.00-1.01) | |
| Hospital region | ||||
| New York City and Long Island | 653 (67.4) | 23 (60.5) | 1 [Reference] | |
| Hudson River | 307 (31.7) | 15 (39.5) | 1.41 (0.72-2.74) | |
| Central or Western New York | 9 (0.9) | 0 | NA | |
| Psychotropic medication class | ||||
| First-generation antipsychotic | ||||
| Yes | 611 (63.0) | 23 (60.5) | 0.89 (0.46-1.74) | |
| No | 358 (37.0) | 15 (39.5) | 1 [Reference] | |
| Second-generation antipsychotic | ||||
| Yes | 870 (89.8) | 33 (86.8) | 0.74 (0.28-1.94) | |
| No | 99 (10.2) | 5 (13.2) | 1 [Reference] | |
| Mood stabilizers | ||||
| Yes | 541 (55.8) | 19 (50.0) | 0.78 (0.41-1.50) | |
| No | 428 (44.2) | 19 (50.0) | 1 [Reference] | |
| Benzodiazepines | ||||
| Yes | 502 (51.8) | 16 (42.1) | 0.67 (0.35,1.28) | |
| No | 467 (48.2) | 22 (57.9) | 1 [Reference] | |
| Antidepressants | ||||
| Yes | 261 (26.9) | 6 (15.8) | 0.50 (0.21-1.20)b | |
| No | 708 (73.1) | 32 (84.2) | 1 [Reference] | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; OR, odds ratio.
Estimates show the crude associations between clinical characteristics, psychotropic medication class, and COVID-19 death among inpatients with COVID-19.
P < .10.
Includes Native American, multiple races, or “other race.”
Includes schizoaffective disorder, bipolar I disorder, and major depressive disorder with psychotic features.
Includes schizophrenia, delusional disorder, and schizotypal disorder.
Statistical tests were 2-sided. Multivariable statistical modeling retained individual medications with at least 5% exposure in the study population from psychopharmacologic classes identified as being associated with infection at the P < .10 significance level.22 Binary logistic regression was used to estimate the association between use of each medication and infection using odds ratios (ORs) and 95% CIs. Three types of ORs were estimated: (1) unadjusted, (2) adjusted for age and sex, and (3) fully adjusted for age and sex in addition to covariates that were significantly associated with infection in binary logistic regression (P < .05). Because patients could have been exposed to multiple medications within and across drug classes, medications were retained in each step to empirically adjust for polypharmacy. A Bonferroni-type correction was used to adjust for multiple comparisons within medication classes.
A similar approach was used to assess the association between medication exposure and mortality among patients with a positive SARS-CoV-2 test result. Medications retained for statistical modeling included those from drug classes associated with mortality (P < .10) and medications significantly associated with infection (P < .05) after adjusting for age and sex. All analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Results
A total of 2087 adults with serious mental illness were continuously inpatients at 18 NYS-OMH psychiatric hospitals between March 8 and July 1, 2020; of these 2087 inpatients, 125 (6.0%) were excluded owing to lack of testing information, and 4 were excluded owing to admission within 30 days prior to the test result (eFigure in the Supplement). The eligible cohort included 1958 patients with a mean (SD) age of 51.4 (4.3) years; 1442 (73.6%) were male and 516 (26.4%) were female (Table 1). Of these 1958 patients, 969 (49.5%) had laboratory-confirmed COVID-19 infection; 572 cases (59.0%) were identified by antibody testing. Among the 969 inpatients with confirmed infections, 38 (3.9%) were determined to have had a COVID-19–related death (Table 2).
Patients prescribed second-generation antipsychotics were less likely to test positive for COVID-19 infection than those not prescribed second-generation antipsychotics (OR, 0.62; 95% CI, 0.45-0.86), whereas patients prescribed mood stabilizers were more likely to test positive than patients who were not exposed to medications in this class (OR, 1.23; 95% CI, 1.03-1.47) (Table 1). First-generation antipsychotic use was associated with a nonsignificant increase in odds of infection (OR, 1.17; 95% CI, 0.97-1.40; P = .08). No difference in the rate of infection was observed in association with benzodiazepine or antidepressant exposure.
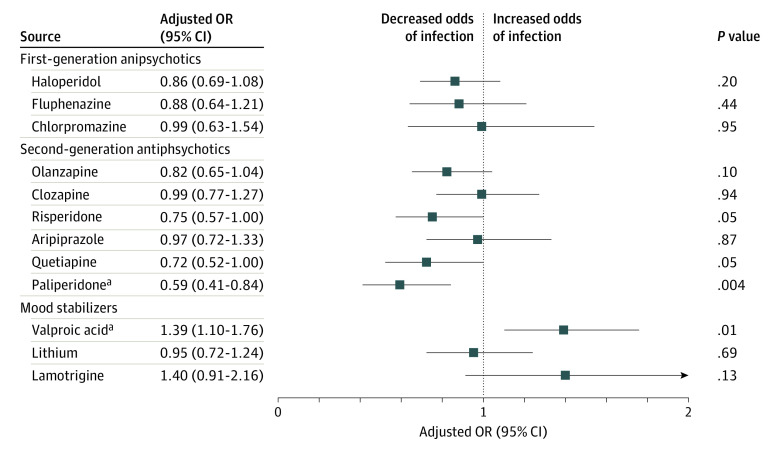
Each of the second-generation antipsychotics (olanzapine, clozapine, risperidone, aripiprazole, quetiapine, and paliperidone), mood stabilizers (valproic acid, lithium, and lamotrigine), and first-generation antipsychotics (haloperidol, fluphenazine, and chlorpromazine) with at least 5% exposure in the study population were retained for regression modeling of infection (Table 3). After adjustment for age and sex, decreased odds of infection were observed in association with use of chlorpromazine (OR, 0.59; 95% CI, 0.40-0.86), clozapine (OR, 0.79; 95% CI, 0.64-0.98), paliperidone (OR, 0.59; 95% CI, 0.42-0.81), risperidone (OR, 0.67; 95% CI, 0.53-0.86), and olanzapine (OR, 0.70; 95% CI, 0.58-0.86). Conversely, valproic acid use was associated with increased odds of infection after adjusting for age and sex (OR, 1.36; 95% CI, 1.12-1.65). After full adjustment for sociodemographic characteristics (including age, sex, race and ethnicity, facility size, and region), medical risk factors (including body mass index, chronic respiratory disease, diabetes, hypertension, and heart disease), and exposure to other medications, use of paliperidone remained significantly associated with decreased infection (OR, 0.59; 95% CI, 0.41-0.84), whereas use of valproic acid remained significantly associated with increased infection (OR, 1.39; 95% CI, 1.10-1.76) (Figure). Results of a sensitivity analysis limited to RT-PCR testing, with a 30-day medication exposure period for all participants, can be found in eTable 3 in the Supplement.
Table 3. Incidence and Adjusted Odds Ratios of COVID-19 Infection by Psychotropic Medication Exposures.
| Psychotropic medication | All patients, No. (N = 1958) | Patients with COVID-19, No. (%) (n = 969 [49.5%]) | Odds ratio (95% CI) | ||
|---|---|---|---|---|---|
| Medications only | Adjusted for age and sex | Fully adjusteda | |||
| First-generation antipsychotics | |||||
| Haloperidol | 886 | 453 (51.1) | 1.08 (0.90-1.31) | 1.14 (0.94-1.38) | 0.86 (0.69-1.08) |
| Fluphenazine | 274 | 138 (50.4) | 1.01 (0.77-1.32) | 1.04 (0.79-1.37) | 0.88 (0.64-1.21) |
| Chlorpromazine | 134 | 47 (35.1) | 0.55 (0.38-0.81)b | 0.59 (0.40-0.86)b | 0.99 (0.63-1.54) |
| Second-generation antipsychotics | |||||
| Olanzapine | 860 | 398 (46.3) | 0.69 (0.57-0.84)c | 0.70 (0.58-0.86)c | 0.82 (0.65-1.04) |
| Clozapine | 712 | 341 (47.9) | 0.73 (0.59-0.90)c | 0.79 (0.64-0.98) | 0.99 (0.77-1.27) |
| Risperidone | 377 | 157 (41.6) | 0.66 (0.52-0.84)c | 0.67 (0.53-0.86)c | 0.75 (0.57-1.00) |
| Aripiprazole | 266 | 131 (49.2) | 0.97 (0.74-1.27) | 1.03 (0.79-1.35) | 0.97 (0.72-1.33) |
| Quetiapine | 262 | 126 (48.1) | 0.84 (0.64-1.10) | 0.85 (0.64-1.11) | 0.72 (0.52-1.00) |
| Paliperidone | 186 | 67 (36.0) | 0.57 (0.41-0.79)c | 0.59 (0.42-0.81)c | 0.59 (0.41-0.84)c |
| Mood stabilizers | |||||
| Valproic acid | 688 | 363 (52.8) | 1.34 (1.10-1.63)b | 1.36 (1.12-1.65)b | 1.39 (1.10-1.76)b |
| Lithium | 375 | 190 (50.7) | 1.14 (0.90-1.44) | 1.17 (0.92-1.48) | 0.95 (0.72-1.24) |
| Lamotrigine | 136 | 74 (54.4) | 1.35 (0.94-1.94) | 1.36 (0.94-1.94) | 1.40 (0.91-2.16) |
The fully adjusted odds ratios controlled for age, sex, race and ethnicity, facility size, hospital region, body mass index, chronic respiratory disease, diabetes, hypertension, heart disease, and exposure to medications in the model.
P < .02, where α′ = .05/3 = .017 to correct for multiple comparisons.
P < .001, where α′ = .05/6 = .0008 to correct for multiple comparisons.
Figure. Adjusted Odds Ratios (ORs) for Infection by Psychotropic Medication Exposure.
The fully adjusted ORs controlled for age, sex, race and ethnicity, hospital region, body mass index, chronic respiratory disease, diabetes, hypertension, heart disease, and exposure to medications in the model.
aSignificant associations at the Bonferroni-adjusted level of significance.
In the secondary analysis of mortality of 969 inpatients with infection, antidepressant medications were retained for statistical modeling based on bivariate analysis of medication class (OR, 0.50; 95% CI, 0.21-1.20; P = .10). Chlorpromazine, clozapine, paliperidone, risperidone, olanzapine, and valproic acid were retained based on their significant association with infection after adjusting for age and sex. Clozapine was the only medication associated with decreased odds of mortality in the unadjusted model (OR, 0.25, 95% 0.10-0.62); this association did not remain statistically significant after adjusting for age and sex (OR, 0.40; 95% CI, 0.16-1.03) or after additional adjustment for medical risk factors (OR, 0.43; 95% CI, 0.17-1.12) (Table 4). There were no deaths among COVID-19–positive patients prescribed chlorpromazine (n = 53) or escitalopram (n = 48). A list of individual psychotropic medications prescribed to the full study cohort and by outcomes (infection and mortality) is included in eTable 1 in the Supplement.
Table 4. Case Fatality and Adjusted Odds Ratios of COVID-19 Mortality by Psychotropic Medication Exposures.
| Psychotropic medication | Patients with COVID, No. (n = 969) | COVID-19–related deaths, No. (%) (n = 38 [3.9%]) | Odds ratio (95% CI) | ||
|---|---|---|---|---|---|
| Medications only | Adjusted for age and sex | Fully adjusteda | |||
| First-generation antipsychotic | |||||
| Chlorpromazineb | 47 | 0 | NA | NA | NA |
| Second-generation antipsychotic | |||||
| Olanzapine | 398 | 14 (3.5) | 0.54 (0.26-1.09) | 0.61 (0.30-1.27) | 0.61 (0.29-1.29) |
| Clozapine | 341 | 6 (1.8) | 0.25 (0.10-0.62)c | 0.40 (0.16-1.03) | 0.43 (0.17-1.12) |
| Risperidone | 157 | 3 (1.9) | 0.32 (0.10-1.07) | 0.35 (0.10-1.20) | 0.42 (0.12-1.45) |
| Paliperidone | 67 | 2 (3.0) | 0.62 (0.14-2.71) | 0.51 (0.11-2.32) | 0.54 (0.12-2.49) |
| Mood stabilizers | |||||
| Valproic acid | 363 | 16 (4.4) | 1.33 (0.68-2.59) | 1.58 (0.78-3.16) | 1.48 (0.72-3.03) |
| Antidepressants | |||||
| Sertraline | 62 | 1 (1.6) | 0.36 (0.05-2.67) | 0.46 (0.06-3.52) | 0.53 (0.07-4.08) |
| Citalopram | 70 | 1 (1.4) | 0.36 (0.05-2.67) | 0.38 (0.05-2.94) | 0.46 (0.06-3.57) |
| Escitalopramb | 47 | 0 | NA | NA | NA |
Abbreviation: NA, not applicable.
The fully adjusted odds ratios controlled for age, sex, diabetes, hypertension, heart disease, and other medications in the model.
Could not be retained for statistical modeling owing to 0 deaths.
P < .01, where α′ = .05/4 = .0125 to correct for multiple comparisons.
Discussion
We investigated COVID-19 infection and mortality among long-term inpatients with serious mental illness in a NYS-operated psychiatric hospital system during the first wave of the pandemic, with a focus on the association between exposures to psychotropic medications and adverse outcomes. Nearly half of the patients in this cohort had laboratory-confirmed COVID-19 infection, and infection-related fatality was more than 4 times higher than estimates from the general population in New York during the same time period.23 This finding is consistent with prior studies that have found increased rates of infection in congregate settings24,25 and increased mortality after infection among patients with serious mental illness.1,5,6,7 The potential harms and benefits associated with several psychotropic medications have been explored in preclinical and clinical studies since the start of the COVID-19 pandemic,19 but, to our knowledge, this is the largest study to systematically assess associations between the use of individual medications and the risk of COVID-19 infection among inpatients with serious mental illness.
The use of second-generation antipsychotics, as a class, was associated with decreased infection. The largest effect size was observed in association with paliperidone. The use of second-generation antipsychotics was also found to have a protective association with mortality, although the association was not statistically significant, likely owing to insufficient sample size. These findings are contrary to what would be expected based on in vitro evidence showing that first-generation antipsychotics (specifically, haloperidol), but not second-generation antipsychotics, interact with sigma-1-receptors to reduce SARS-CoV-2 replication.10 However, there are other drug-protein interactions that may interfere with the viral life cycle. SARS-CoV-2 3c-like protease and viral RNA-dependent polymerase are critical enzymes for viral protein processing and genome replication, respectively. An in silico screening analysis identified paliperidone as one of the leading drugs to bind these enzymes with high affinity.26
Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection,27 clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association. This finding stands in contrast to prior EHR studies that found an increased risk of COVID-19 infection associated with clozapine treatment28,29 but is consistent with a prior study limited to inpatients that found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.30 One potential explanation for this discrepancy is surveillance bias because patients receiving clozapine in the general community may be more likely than other outpatients to undergo testing for COVID-19. Because 94% of patients in this cohort underwent testing, surveillance bias was less likely. Furthermore, this is the largest study to date of COVID-19–infected patients prescribed clozapine. Clozapine is unique among antipsychotics in its ability to enhance the T helper cell type 1 response that supports antiviral immune response and is blunted in schizophrenia.31 Further research is needed to determine whether clozapine may protect against severe COVID-19 infection.
To our knowledge, this is the first study to assess the association between valproic acid use and COVID-19 risk. Although it has been hypothesized that the use of valproic acid could reduce the risk of COVID-19 infection or reduce viral load by its association with angiotensin-converting enzyme 2 and transmembrane serine protease 2,32 we found a significant increase in infection and a similar (although nonsignificant) increase in mortality. Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells,33 and several studies have shown that the downregulation of membrane-bound angiotensin-converting enzyme 2 may impair immune function and contribute to poor outcomes in the setting of COVID-19 infection.34 Additional factors may confound the association between valproic acid use and COVID-19 risk. For example, patients are typically prescribed mood stabilizers, such as valproic acid, to manage mood lability and other manic symptoms; these symptoms may negatively affect adherence to infection prevention measures, such as mask wearing and social distancing.
Risk of mortality was lower among patients taking antidepressants. Although the association was not statistically significant in this cohort, this finding is consistent with larger studies that found reduced risk of adverse outcomes associated with antidepressant use.13,14,15,35 There were no COVID-19–related deaths among patients prescribed escitalopram (n = 88), venlafaxine (n = 53), bupropion (n = 43), or fluvoxamine (n = 25).
Strengths and Limitations
This study has several strengths. Restricting the sample to inpatients with chronic psychotic disorders minimized differences in risk associated with community exposures, underlying psychiatric diagnosis, medication adherence, and access to care, thus facilitating assessment of the association between psychotropic medications and outcomes. Implementation of universal screening and use of antibody testing to detect prior infection helped identify patients who may not have received confirmatory testing by RT-PCR when infected owing to lack of test availability or other factors.
This study also has several limitations. During the study period, the pandemic was at its first peak in New York. Interventions, including regular testing of staff and a statewide vaccination campaign, which successfully limited the spread of COVID-19 during the second surge of the virus,36 had not yet been implemented. The availability of tests and criteria for testing evolved during the study period and varied across facilities. Duration of medication use, medication dose, and polypharmacy may have been associated with differences in outcomes and were not explored in this analysis. In addition, medication exposure after infection could not be evaluated for patients who were transferred to outside facilities for medical treatment. Medication adherence, although likely high, was not directly assessed. Additionally, the study population represents a subset of adults with serious mental illness: those with persistent and severe illness, chronically exposed to psychotropic medications, who reside in long-term state psychiatric hospitals. The association between the use of psychotropic medications and the risk of COVID-19 infection may differ between patients with less severe psychiatric disease and those without prior medication exposure.
Conclusions
Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second-generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection. Further research is needed to replicate our findings, evaluate their generalizability, and explore underlying mechanisms.
eFigure. Study Profile
eTable 1. Psychotropic Medications Exposure in Full Cohort and by Infection and Death
eTable 2. ICD-9/10 Category Codes Used to Identify Medical Conditions and Psychiatric Diagnoses
eTable 3. Incidence and Adjusted Odds Ratios of COVID-19 Infection by Psychotropic Medication Exposure in Patients with PCR Testing
References
- 1.Vai B, Mazza MG, Delli Colli C, et al. Mental disorders and risk of COVID-19–related mortality, hospitalisation, and intensive care unit admission: a systematic review and meta-analysis. Lancet Psychiatry. 2021;8(9):797-812. doi: 10.1016/S2215-0366(21)00232-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Li F, Fortunati F, Krystal JH. Association of a prior psychiatric diagnosis with mortality among hospitalized patients with coronavirus disease 2019 (COVID-19) infection. JAMA Netw Open. 2020;3(9):e2023282. doi: 10.1001/jamanetworkopen.2020.23282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teixeira AL, Krause TM, Ghosh L, et al. Analysis of COVID-19 infection and mortality among patients with psychiatric disorders, 2020. JAMA Netw Open. 2021;4(11):e2134969. doi: 10.1001/jamanetworkopen.2021.34969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20(1):124-130. doi: 10.1002/wps.20806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fond G, Nemani K, Etchecopar-Etchart D, et al. Association between mental health disorders and mortality among patients with COVID-19 in 7 countries: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(11):1208-1217. doi: 10.1001/jamapsychiatry.2021.2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386. doi: 10.1001/jamapsychiatry.2020.4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzur Bitan D, Krieger I, Kridin K, et al. COVID-19 prevalence and mortality among schizophrenia patients: a large-scale retrospective cohort study. Schizophr Bull. 2021;47(5):1211-1217. doi: 10.1093/schbul/sbab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892. doi: 10.1001/jamapsychiatry.2020.0894 [DOI] [PubMed] [Google Scholar]
- 9.Nemani K, Conderino S, Marx J, Thorpe LE, Goff DC. Association between antipsychotic use and COVID-19 mortality among people with serious mental illness. JAMA Psychiatry. 2021;78(12):1391-1393. doi: 10.1001/jamapsychiatry.2021.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon DE, Hiatt J, Bouhaddou M, et al. Comparative host–coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370(6521):eabe9403. doi: 10.1126/science.abe9403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plaze M, Attali D, Petit A-C, et al. Repurposing chlorpromazine to treat COVID-19: the reCoVery study. Encephale. 2020;46(3):169-172. doi: 10.1016/j.encep.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharmacol. 2021;12:652688. doi: 10.3389/fphar.2021.652688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. ; TOGETHER investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10(1):e42-e51. doi: 10.1016/S2214-109X(21)00448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oskotsky T, Maric I, Tang A, et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4(11):e2133090. doi: 10.1001/jamanetworkopen.2021.33090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoertel N, Sánchez-Rico M, Vernet R, et al. ; AP-HP/Universities/INSERM COVID-19 Research Collaboration and AP-HP COVID CDR Initiative . Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26(9):5199-5212. doi: 10.1038/s41380-021-01021-4 [DOI] [PubMed] [Google Scholar]
- 16.Hoertel N, Sánchez-Rico M, Vernet R, et al. ; AP-HP/Universities/INSERM COVID-19 research collaboration and AP-HP COVID CDR Initiative . Observational study of haloperidol in hospitalized patients with COVID-19. PLoS One. 2021;16(2):e0247122. doi: 10.1371/journal.pone.0247122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoertel N, Sánchez-Rico M, Vernet R, et al. ; AP-HP/Universities/INSERM COVID-19 Research Collaboration and AP-HP COVID CDR Initiative . Observational study of chlorpromazine in hospitalized patients with COVID-19. Clin Drug Investig. 2021;41(3):221-233. doi: 10.1007/s40261-021-01001-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prokopez CR, Farinola R, Vallejos M, et al. Olanzapine, risperidone and quetiapine: do these atypical antipsychotics have a protective effect for SARS-CoV-2? Schizophr Res. 2022;241:140-141. doi: 10.1016/j.schres.2022.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stip E, Arnone D, Abdel Aziz K, Javaid SF. Diversity of mechanism of action of psychotropic drugs in their anti-COVID-19 properties. Mol Psychiatry. 2021;26(12):7093-7097. doi: 10.1038/s41380-021-01222-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. EUA authorized serology test performance. Accessed October 3, 2021. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 21.Maine GN, Lao KM, Krishnan SM, et al. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J Clin Virol. 2020;133:104663. doi: 10.1016/j.jcv.2020.104663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. John Wiley & Sons; 2013. [Google Scholar]
- 23.Yang W, Kandula S, Huynh M, et al. Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis. Lancet Infect Dis. 2021;21(2):203-212. doi: 10.1016/S1473-3099(20)30769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy BS, Richeson RP, Houde AJ. Risk factors for SARS-CoV-2 in a statewide correctional system. N Engl J Med. 2020;383(25):2479-2480. doi: 10.1056/NEJMc2029354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael TM, Currie DW, Clark S, et al. ; Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team . Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. doi: 10.1056/NEJMoa2005412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gul S, Ozcan O, Asar S, Okyar A, Barıs I, Kavakli IH. In silico identification of widely used and well-tolerated drugs as potential SARS-CoV-2 3C-like protease and viral RNA-dependent RNA polymerase inhibitors for direct use in clinical trials. J Biomol Struct Dyn. 2021;39(17):6772-6791. doi: 10.1080/07391102.2020.1802346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dotson S, Hartvigsen N, Wesner T, Carbary TJ, Fricchione G, Freudenreich O. Clozapine toxicity in the setting of COVID-19. Psychosomatics. 2020;61(5):577-578. doi: 10.1016/j.psym.2020.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Govind R, Fonseca de Freitas D, Pritchard M, Hayes RD, MacCabe JH. Clozapine treatment and risk of COVID-19 infection: retrospective cohort study. Br J Psychiatry. 2021;219(1):368-374. doi: 10.1192/bjp.2020.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okusaga OO, Mitchell BG, Bernard JD, Walder A. Clozapine is associated with higher COVID-19 infection rate in veterans with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2021;82(5):21br14028. [DOI] [PubMed] [Google Scholar]
- 30.Prokopez CR, Vallejos M, Lopredo LS, et al. An analysis of the possible protective effect of antipsychotics for SARS-CoV-2 in patients under treatment for severe mental illnesses. Schizophr Res. 2021;233:99-100. doi: 10.1016/j.schres.2021.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandurangi AK, Buckley PF. Inflammation, antipsychotic drugs, and evidence for effectiveness of anti-inflammatory agents in schizophrenia. Curr Top Behav Neurosci. 2020;44:227-244. doi: 10.1007/7854_2019_91 [DOI] [PubMed] [Google Scholar]
- 32.Pitt B, Sutton NR, Wang Z, Goonewardena SN, Holinstat M. Potential repurposing of the HDAC inhibitor valproic acid for patients with COVID-19. Eur J Pharmacol. 2021;898:173988. doi: 10.1016/j.ejphar.2021.173988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S, Singh KK. Valproic acid in prevention and treatment of COVID-19. Int J Respir Pulm Med. 2020;7:138. doi: 10.23937/2378-3516/1410138 [DOI] [Google Scholar]
- 34.Vieira C, Nery L, Martins L, Jabour L, Dias R, Simões E Silva AC. Downregulation of membrane-bound angiotensin converting enzyme 2 (ACE2) receptor has a pivotal role in COVID-19 immunopathology. Curr Drug Targets. 2021;22(3):254-281. doi: 10.2174/1389450121666201020154033 [DOI] [PubMed] [Google Scholar]
- 35.Hoertel N, Sánchez-Rico M, Gulbins E, et al. ; AP-HP/Université de Paris/INSERM COVID-19 research collaboration, AP-HP COVID CDR Initiative, “Entrepôt de Données de Santé” AP-HP Consortium . Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational multicenter study. Clin Pharmacol Ther. 2021;110(6):1498-1511. doi: 10.1002/cpt.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith TE, Rodgers IT, Silverman DJ, et al. COVID-19 case rates after surveillance and vaccinations in a statewide psychiatric hospital system. Am J Public Health. 2021;111(10):1780-1783. doi: 10.2105/AJPH.2021.306444 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Study Profile
eTable 1. Psychotropic Medications Exposure in Full Cohort and by Infection and Death
eTable 2. ICD-9/10 Category Codes Used to Identify Medical Conditions and Psychiatric Diagnoses
eTable 3. Incidence and Adjusted Odds Ratios of COVID-19 Infection by Psychotropic Medication Exposure in Patients with PCR Testing