Abstract
Severe coronavirus disease 2019 (COVID-19) has been associated with cytokine storms and hyperinflammation. In a recent study, Junqueira et al. provide evidence that antibody-mediated uptake of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus by monocytes and macrophages may contribute to this inflammation by activating inflammasomes which trigger pyroptosis.
Monocytes play a key role in fueling the cytokine storm in patients with severe COVID-19 [1]. This leads to hyperinflammation, severe respiratory distress, and even death. It was recently shown that active SARS-CoV-2 infection in primary human monocytes can activate inflammasomes and trigger pyroptosis [2]. Pyroptosis is a highly inflammatory cell death process which begins when pathogen-infected cells recruit apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and cytoplasmic sensors such as absent in melanoma 2 (AIM2) for the assembly of inflammasomes [3]. These inflammasomes in turn activate caspase-1, which then cleaves the pore-forming gasdermin D (GSDMD) and proinflammatory cytokines such as pro-interleukin-1β (pro-IL-1β) into their mature states [3,4]. Consequently, the infected cell rapidly swells and loses cell-membrane integrity before rupturing and releasing its proinflammatory intracellular contents. This contributes to the rapid recruitment of immune cells and further perpetuates the inflammatory cascade. Thus, although pyroptosis unleashes swift immune responses, it can also cause damaging hyperinflammation [3].
In a recent study, Junqueira et al. isolated mononuclear cells from COVID-19 patients and found that only monocytes expressing FcγRIIIa (CD16) – a key receptor for antibody-dependent Fc-mediated phagocytosis – stained positive for SARS-CoV-2 nucleocapsid (N) protein, indicating virus internalization [5]. Furthermore, ~95% of COVID-19 N+ monocytes stained positive for J2 [an antibody to double-stranded (ds)RNA], indicating active infection. To determine whether antibodies mediate infection, the authors used an engineered infectious clone (icSARS-CoV-2-mNG) containing a NeonGreen fluorescent reporter to indicate viral replication. Plasma from COVID-19 patients but not healthy donors mediated the infection of monocytes. COVID-19 plasma-mediated monocyte SARS-CoV-2 infection could be inhibited by remdesivir (viral RNA-dependent RNA polymerase inhibitor), further confirming active viral replication. On the one hand, heat inactivation of plasma did not impact on infection, ruling out the involvement of complement. On the other hand, IgG- but not IgA-depleted COVID-19 plasma abrogated viral infection, suggesting that SARS-CoV-2-specific IgG antibodies could be key to infecting CD16+ monocytes (Figure 1). SARS-CoV-2 infection of monocytes was not prevented by camostat mesylate (an inhibitor of the spike protein priming protease TMPRSS2) or by blocking antibodies against the SARS-CoV-2 receptor ACE2 [6], suggesting an ACE-2 independent route of entry [5]. Instead, blocking antibodies against the Fc receptors CD16 (FcγRIIIa) and CD64 (FcγRI), but not CD32 (FcγRIIa), were effective in inhibiting SARS-CoV-2 infection of monocytes, highlighting possible entry routes for antibody-opsonized virus.
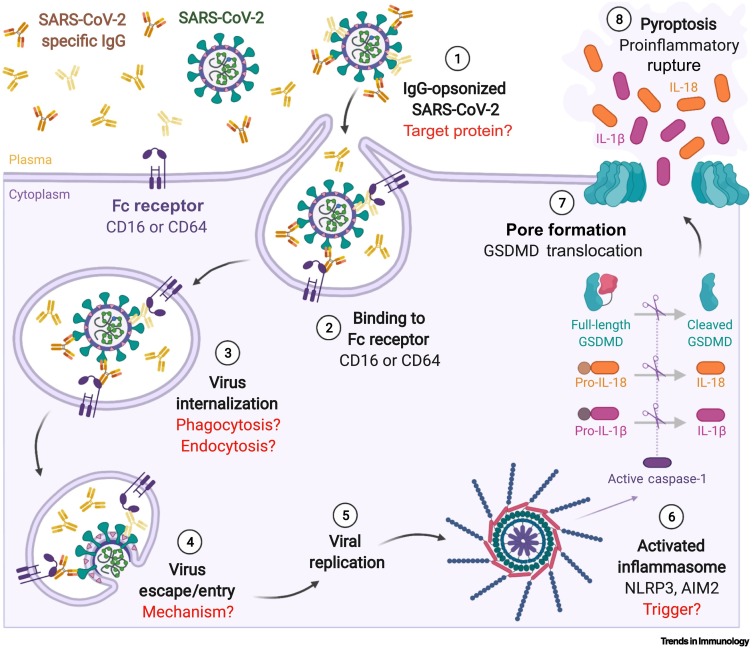
Figure 1.
Fc-receptor mediated uptake of IgG-opsonized SARS-CoV-2 triggers pyroptosis in monocytes.
(Anticlockwise) ① SARS-CoV-2-specific antibodies (IgG) elicited by infection or vaccination can opsonize SARS-CoV-2 virions. ② Opsonized SARS-CoV-2 virions then bind to Fc receptors CD16 and/or CD64 on monocytes, triggering internalization ③. This leads to infection of monocytes ④ and viral replication ⑤. However, active infections within monocytes are abruptly terminated because activated inflammasomes in turn activate caspase-1, leading to gasdermin (GSDMD) cleavage ⑥. Cleaved N-terminal GSDMD translocates to the plasma membrane where it assembles into pores ⑦. This causes the infected monocytes to undergo pyroptosis and rupture instead, releasing mature, active proinflammatory cytokines such as IL-18 and IL-1β into the milieu ⑧. This in turn could recruit more immune cells to the site, perpetuating the inflammatory cascade and leading to a cytokine storm [5]. Steps which remain unclear after the current study [5] and warrant further research are indicated in red. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Figure created with BioRender.com.
Of note, although anti-spike monoclonal antibodies (non-neutralizing C1A-H12 and neutralizing C1A-B12) could induce some monocyte infection, plasma from healthy mRNA spike vaccinees (two doses of Pfizer-BioNTech vaccine, N = 6) did not promote monocyte infection despite having twofold higher anti-RBD (receptor-binding domain) plasma IgG than COVID-19 patients. The authors also investigated the contribution of afucosylated SARS-CoV-2-specific IgG, which is elevated in severe infection and is associated with enhanced CD16 binding [7]; however, they did not observe any significant differences in infection, potentially because of limited sample sizes (N = 11) and assay sensitivity [5]. More work needs to be done to better understand what antigen(s) are being targeted by SARS-CoV-2-specific plasma IgG, as well as their biophysical properties, which may contribute towards this antibody-mediated monocyte infection.
Junqueira et al. also note that, although ~10% of monocytes from COVID-19 patients were infected (J2+, N+), a portion were undergoing pyroptosis [5]. Notably, ~6% of monocytes from COVID-19 patients displayed damaged plasma membranes (staining Zombie Yellow-positive) but did not undergo apoptosis (annexin V-negative). Most Zombie+ cells (62 ± 9%) instead had ASC specks. Importantly, almost all SARS-CoV-2-infected (J2+, N+) monocytes displayed ASC specks and ~80% of monocytes with ASC specks were infected. Moreover, ~4% of monocytes from COVID-19 patients stained positive for both activated caspase-1 [fluorescent inhibitor of caspases (FLICA)-positive] and ASC specks, 80% of which exhibited colocalized staining. These COVID-19 monocytes with ASC specks were ballooning and relocated pore-forming GSDMD from the cytoplasm to the cell membrane. GSDMD C-terminal fragments were also detected in COVID-19 monocytes, confirming the presence of cleaved GSDMD. Together, these data suggest that the SARS-CoV-2-infected monocytes are undergoing pyroptosis. Of interest, ASC specks in COVID-19 monocytes colocalized with canonical NLRP3 and AIM2 inflammasomes. Although traditionally a sensor for dsDNA, the AIM2 inflammasome was recently shown to play a key role in RNA virus infections too, including chikungunya, Zika, and influenza viruses [3]. Antibody-complexed adenoviruses capsids have also been shown to trigger pyroptosis through AIM2 on monocyte-derived dendritic cells [8].
The investigators also reported that plasma markers for pyroptosis (GSDMD, IL-1β, IL-1RA, IL-18, LDH activity) were significantly elevated in COVID-19 patients versus controls, and these correlated with disease severity [5]. Pyroptosis might therefore play a key role in severe COVID-19. However, the authors noted that, although plasma IL-1β was significantly higher in COVID-19 samples than in healthy controls, it was still low overall. IL-1β is produced without a signal sequence, and the release of mature IL-1β into the plasma is closely linked to efficient pyroptosis. Previous research has shown that SARS-CoV-2 N protein can bind to the C-terminal linker of GSDMD, blocking its cleavage by caspase-1 and thus preventing pyroptosis [9]. This impairs the secretion of IL-1β by monocytes, even in response to stronger stimuli such as lipopolysaccharide (LPS) and nigericin. Thus, there might be a delicate interplay between pyroptosis activation by SARS-CoV-2 antibody complexes and inhibition by SARS-CoV-2 N protein in infected monocytes. This might also explain why the authors observed ASC-containing inflammations only in infected lung macrophages (CD14+) and not in infected lung epithelial (E-cadherin+) and endothelial cells (CD31+ CD14−) [5], despite previous research showing that RNA viruses, such as influenza A, can indeed trigger pyroptosis in respiratory epithelial cells [10].
Overall, although this study by Junqueira and colleagues [5] has further bridged the relationship between SARS-CoV2 infection and the resulting cytokine storm during severe disease progression, there are still several gaps in our knowledge. Is this mechanism enhanced with increasing age, and does it predispose the elderly to a higher risk of developing severe COVID-19? Does it contribute to 'long COVID'? Could antibodies raised against other vaccine types which contain more than the SARS-CoV-2 spike protein – such as inactivated virus or multivalent vaccines – trigger a different response? How do the additional mutations found throughout the newer SARS-CoV-2 variants affect interactions between various components of the inflammasome/pyroptosis pathway? Future studies will be necessary to better understand the implications of antibody complexes for vaccine design and treatment options across different cohorts and age groups.
Acknowledgments
Acknowledgments
A.W.C is supported by a National Health and Medical Research Council investigator grant (2008092).
Declaration of interests
The authors declare no conflicts of interest.
References
- 1.Vanderbeke L., et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat. Commun. 2021;12:4117. doi: 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira A.C., et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Dis. 2021;7:43. doi: 10.1038/s41420-021-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spel L., Martinon F. Detection of viruses by inflammasomes. Curr. Opin. Virol. 2021;46:59–64. doi: 10.1016/j.coviro.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X., et al. The roles of gasdermin D in coronavirus infection and evasion. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.784009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Junqueira C., et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022 doi: 10.1038/s41586-022-04702-4. Published online April 6, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson C.B., et al. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty S., et al. Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severity. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abm7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichholz K., et al. Immune-complexed adenovirus induce AIM2-mediated pyroptosis in human dendritic cells. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma J., et al. SARS-CoV-2 nucleocapsid suppresses host pyroptosis by blocking gasdermin D cleavage. EMBO J. 2021;40 doi: 10.15252/embj.2021108249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S., et al. Influenza A virus infection triggers pyroptosis and apoptosis of respiratory epithelial cells through the type I interferon signaling pathway in a mutually exclusive manner. J. Virol. 2018;92 doi: 10.1128/JVI.00396-18. [DOI] [PMC free article] [PubMed] [Google Scholar]