Abstract
Bakuchiol was isolated from the seeds of Psoralea corylifolia, a tree native to China with various uses in traditional medicine, followed by extraction with ether and column chromatography combined with silica gel and octyldecyl silane. In this study, the antimicrobial activities of bakuchiol against some oral microorganisms were evaluated in vitro. The cell growth of Streptococcus mutans was inhibited in a bakuchiol concentration-dependent manner, and growth of S. mutans was completely prevented by 20 μg of bakuchiol per ml. The bactericidal effect of bakuchiol on S. mutans was dependent on temperature and stable under the following conditions: sucrose, 0 to 10% (wt/vol); pH, 3.0 to 7.0; organic acids (3% [wt/vol] citric and malic acids). Bakuchiol showed bactericidal effects against all bacteria tested, including S. mutans, Streptococcus sanguis, Streptococcus salivarius, Streptococcus sobrinus, Enterococcus faecalis, Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus plantarum, Actinomyces viscosus, and Porphyromonas gingivalis, with MICs ranging from 1 to 4 μg/ml and the sterilizing concentration for 15 min ranging from 5 to 20 μg/ml. Furthermore, bakuchiol was also effective against adherent cells of S. mutans in water-insoluble glucan in the presence of sucrose and inhibited the reduction of pH in the broth. Thus, bakuchiol would be a useful compound for development of antibacterial agents against oral pathogens and has great potential for use in food additives and mouthwash for preventing and treating dental caries.
Dental plaque, a film of microorganisms on the tooth surface, plays an important part in the development of caries and periodontal diseases (20). Mutans streptococci can colonize the tooth surface and initiate plaque formation by their ability to synthesize extracellular polysaccharides from sucrose, mainly water-insoluble glucan, using glucosyltransferase (4, 5, 7). De novo synthesis of water-insoluble glucan is essential for the adherence of Streptococcus mutans and other oral microorganisms to the tooth surface, forming a barrier that prevents the diffusion of acids produced by the bacteria. The acids accumulate in situ and decalcify minerals in the enamel. This sucrose-dependent adherence and accumulation of cariogenic streptococci is critical to the development of pathogenic plaque. The further accumulation of plaque around the gingival margin and subgingival region may lead to a shift in its microbial composition from streptococcus-dominated to a larger number of Actinomyces spp. and increased numbers of capnophilic and obligatory anaerobic bacteria, such as Porphyromonas gingivalis (21). These microorganisms seem to be involved in root caries and periodontal disease, respectively (26, 27).
To avoid dental caries due to cariogenic bacteria, inhibition of glucosyltransferase activity by specific enzyme inhibitors (9, 31), inhibition of initial cell adhesion of S. mutans by polyclonal and monoclonal antibodies (24), and inhibition of cell growth of S. mutans by antibacterial agents have been investigated. This third line of research in particular has attracted a great deal of attention, and effective antimicrobial agents against these oral pathogens could play an important part in the prevention of dental caries and periodontal diseases, particularly those that affect plaque formation (1, 12, 13, 14, 29, 30).
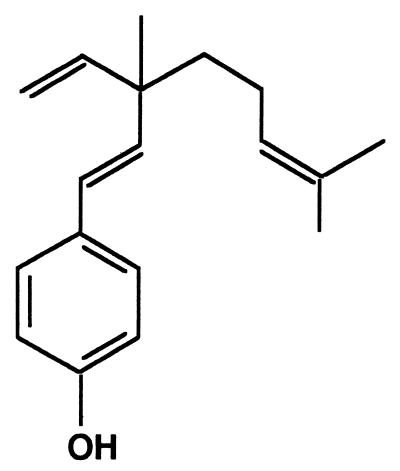
A few recent studies have demonstrated antimicrobial activity against selected oral pathogens from natural sources. From the native American plant Ceanothus americanus, ceanothic acid and ceanothetric acid demonstrated growth-inhibitory effects against S. mutans, Actinomyces viscosus, and P. gingivalis (19). Ethanolic extracts of propolis, a resinous hive product, showed antimicrobial activity against these three oral microorganisms (10). Natural products have been used for thousands of years in folk medicine for several purposes. For example, bakuchiol (Fig. 1) isolated from the seeds and leaves of Psoralea corylifolia Linn, a tree native to China with various uses in traditional Oriental medicine, is a phenolic isoprenoid and exhibits antimutagenic, antimicrobial, and insect juvenile hormone activities (16, 22, 25). However, these activities were assayed using crude extracts from the seeds of P. corylifolia, and the antimicrobial activity of an oily hexane extract from the seeds against only Staphylococcus aureus was reported (8). Thus, the antimicrobial activity of bakuchiol has not been thoroughly investigated, and little is known about its antimicrobial activity against oral microorganisms or its effects on dental plaque formation in vitro.
FIG. 1.
Structure of bakuchiol.
In this study, we purified bakuchiol from the seeds of P. corylifolia and evaluated the in vitro antimicrobial activity of bakuchiol against some oral microorganisms, especially the effects on adherent mutans streptococci.
MATERIALS AND METHODS
Microorganisms.
The following 18 oral microorganisms were used in this study: Streptococcus mutans JCM 5175, Streptococcus mutans IFO 13955, Enterococcus faecalis IFO 3989, Enterococcus faecium IFO 3826, Lactobacillus acidophilus AKU 1122, Lactobacillus acidophilus AKU 1124, Lactobacillus plantarum AKU 1130, and Porphyromonas gingivalis ATCC 33277 were obtained from stock culture collections. Streptococcus sanguis 179-3, Streptococcus sanguis 254-4, Streptococcus salivarius 70-2, Streptococcus salivarius 160-2, Actinomyces viscosus 19246, Lactobacillus plantarum 8016, and Lactobacillus casei 4646 were obtained from the Department of Conservative Dentistry, School of Dentistry, Tokushima University, Tokushima, Japan. Streptococcus mutans GS5, Streptococcus mutans JC2, and Streptococcus sobrinus 6715 were obtained from the Department of Oral Bacteriology, School of Dentistry, Hokkaido University, Sapporo, Japan.
Extraction and isolation of bakuchiol.
Seeds of P. corylifolia Linn were purchased from Tochimoto Tenkai-do, Osaka, Japan. Bakuchiol was extracted and isolated from the seeds as described by Mehta et al. (22). Percolation of whole seeds (1 kg) with diethyl ether (5 liters) at room temperature followed by removal of solvent gave a dark brown gummy residue (30 g). This extract was taken up in diethyl ether (200 ml), and the strongly acidic compounds were removed by washing with 1% (wt/vol) KOH solution (50 ml, three times). The organic phase was washed with water followed by drying on Na2SO4 and evaporated to furnish a bakuchiol-rich residue (20 g), which was chromatographed on a silica gel column (BW-200, 100 cm by 4.2 cm; Fuji Silysia Chemical Ltd., Kasugai, Japan), gradually eluting with 10 to 30% (vol/vol) ethyl acetate (EtOAc) in n-hexane (a total of 9 liters). The aliquots of each fraction were subjected to thin-layer chromatography (TLC) (silica gel, high-performance TLC [HPTLC] plate, 1 mm; Merck) in a solvent system consisting of 20% (vol/vol) EtOAc in n-hexane, with monitoring of absorption at 254 nm. Crude bakuchiol (12 g) was recovered from the active fractions at 254 nm in the silica gel column and then purified with a reversed-phase octyldecyl silane (ODS) column (DM1020T, 100-200 mesh, 50 cm by 2.2 cm; Fuji Silysia Chemical Ltd.) with chromatography, eluting with 90% (vol/vol) methanol (MeOH). Each fraction was subjected to TLC (RP18, HPTLC plate, 1 mm; Merck) in a solvent system of 90% (vol/vol) MeOH, with monitoring of absorption at 254 nm, followed by recovery, evaporation and drying in a desiccator to furnish pure bakuchiol as a colorless liquid (8 g). The purity of bakuchiol was examined by high-pressure liquid chromatography (HPLC) and mass spectra as described (15, 16, 22). The purified bakuchiol was dissolved in the mobile-phase solvent and applied to an ODS column (YMC-Pack ODS-A, S-5 μm, 120 A, A-312, 6 mm by 150 mm; YMC Co. Ltd., Kyoto, Japan). Bakuchiol was eluted by 85% (vol/vol) MeOH at 1.0 ml/min and analyzed with a UV detector (SPD-6AU; Shimadzu, Kyoto, Japan) at 263 nm. Mass spectrometry (MS) of the purified bakuchiol was carried out on a JMS-AM II (Jeol Ltd., Tokyo, Japan) recorded at 70 eV with a source temperature of 250°C: MS: m/z 256 (M+, 8%), 213 (13%), 173 (100%), 158 (20%), 145 (36%), 107 (28%), and 83 (17%). Pure bakuchiol (>98%) was dissolved in dimethyl sulfoxide (DMSO) at 5% (wt/vol), and the bakuchiol solution was used as an antimicrobial agent in the experiments described below.
Antimicrobial activity against S. mutans
JCM 5175 was inoculated into brain heart infusion broth (Difco Laboratories, Detroit, Mich.) in test tubes and grown in stationary culture for 24 h at 37°C. Aliquots of 10 ml of culture of S. mutans JCM 5175 were inoculated into 100 ml of fresh brain heart infusion broth containing bakuchiol at 0, 1.0, 5.0, 10, and 20 μg/ml in 200-ml Erlenmeyer flasks. The 5% (wt/vol) bakuchiol solution in DMSO was diluted in the medium and added to each flask. Solvent controls were included, though no adverse effect had been noted at the highest concentration employed. Culture was continued at 37°C for 24 h, and then the turbidity at various time points was measured at 610 nm.
To determine viable-cell counts in the broth containing bakuchiol at each incubation time point, the culture broth was diluted with sterile water containing 0.1% (wt/vol) Tween 80 for inactivation of bakuchiol, and aliquots were inoculated into 10 ml of fresh brain heart infusion agar containing 0.1% (wt/vol) Tween 80 for inactivation of bakuchiol in plates. The plates were incubated for 48 h at 37°C, and CFU were counted.
Determination of MIC and sterilizing concentration of bakuchiol against oral microorganisms.
All oral microorganisms used in this study were inoculated into brain heart infusion broth in test tubes and grown to stationary phase for 24 h at 37°C up to 2.0 × 108 to 1.0 × 109 CFU/ml. The MIC was estimated as described by Li et al. (19). The culture of each oral microorganism was diluted 10-fold with sterile water. Aliquots of 100 μl of the diluted cultures of each oral microorganism were inoculated at 105 to 106 CFU/ml into 10 ml of fresh brain heart infusion broth containing bakuchiol at 0, 0.4, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 3.0, and 4.0 μg/ml in test tubes, serially diluted bakuchiol, and growth medium. The MIC of bakuchiol was determined by the turbidity at 610 nm after 48 h of incubation at 37°C. The MIC for each oral microorganism was defined as the minimum concentration of bakuchiol limiting turbidity to <0.05 absorbance unit at 610 nm.
The sterilizing concentration for 15 min against each oral microorganism was estimated as described below. Aliquots of 100 μl of culture of each oral microorganism were inoculated at 106 to 107 CFU/ml into 10 ml of fresh brain heart infusion broth containing bakuchiol at 0, 2.5, 5.0, 10, 20, and 40 μg/ml in test tubes, serially diluted bakuchiol, and growth medium. After cultures of each oral microorganism were incubated with bakuchiol at 37°C for 15 min, aliquots of 100 μl of the mixtures were inoculated into 10 ml of fresh brain heart infusion broth containing 0.1% (wt/vol) Tween 80 for inactivation of bakuchiol in test tubes. The sterilizing concentration of bakuchiol for 15 min was determined by the turbidity at 610 nm after 48 h of incubation at 37°C. The sterilizing concentration for 15 min against each oral microorganism was defined as the minimum concentration of bakuchiol limiting turbidity to <0.05 absorbance unit at 610 nm.
Antimicrobial activity against adherent S. mutans in water-insoluble glucan.
For formation of water-insoluble glucan by S. mutans JCM 5175, aliquots of 100 μl of a culture of S. mutans JCM 5175 were inoculated into 10 ml of fresh brain heart infusion broth containing 2% (wt/vol) sucrose in test tubes and incubated at 37°C for 24 h at an angle of 30°. The fluid was gently removed, and then the cells in water-insoluble glucan in test tubes were gently washed in 10 ml of sterile water. To the cells of S. mutans JCM 5175 in water-insoluble glucan in test tubes was then added 10 ml of citrate buffer (10 mM, pH 6.0) containing 100 μg of bakuchiol per ml, followed by incubation at 37°C for 5 min. The mixture was gently removed and rinsed twice with 10 ml of sterile water containing 0.1% (wt/vol) Tween 80 to inactivate bakuchiol. To the bakuchiol-treated cells of S. mutans JCM 5175 in water-insoluble glucan in test tubes was added 10 ml of fresh brain heart infusion broth containing both 2% (wt/vol) sucrose and 0.1% (wt/vol) Tween 80 for inactivation of bakuchiol, followed by incubation at 37°C for 24 h. After incubation for 7.5, 16, or 24 h, the acidogenicity of the cultures was measured with a pH meter. The fluid containing free cells of S. mutans JCM 5175 was gently removed, and 10 ml of sterile water was added to the test tube. The water-insoluble glucan formed in the test tube was homogenized by five 30-s bursts of ultrasonication (UT-204, Silent Sonic; Sharp, Osaka, Japan), and then the turbidity was measured at 610 nm.
RESULTS
Antimicrobial activity against S. mutans.
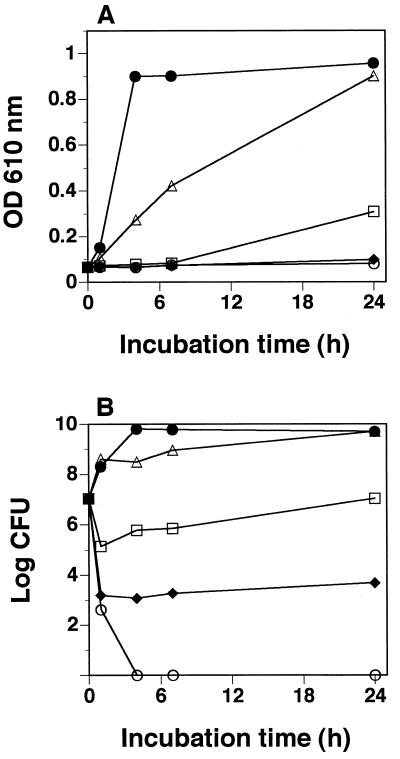
Figure 2A shows the inhibitory effect of bakuchiol on growth of S. mutans JCM 5175. Growth was inhibited in a bakuchiol concentration-dependent manner. At 5 μg/ml, bakuchiol inhibited growth of S. mutans JCM 5175 for 7 h before cell growth started. At 10 μg/ml, cell growth was inhibited even after 24 h of incubation. To examine viable cells in the cultures, CFU of S. mutans JCM 5175 were counted in plates containing 0.1% (wt/vol) Tween 80 for inactivation of bakuchiol (Fig. 2B). After incubation with bakuchiol at 5 μg/ml, CFU did not increase in plates, and thus growth of S. mutans JCM 5175 was bacteriostatically inhibited by bakuchiol at 5 μg/ml. After incubation with bakuchiol at 10 μg/ml, CFU decreased to the order of 103 from 107, and thus the growth was bactericidally partially blocked by bakuchiol at 10 μg/ml. After incubation with bakuchiol at 20 μg/ml, growth was completely blocked, and thus the cell was sterilized by bakuchiol at 20 μg/ml.
FIG. 2.
Cell growth (A) and viable-cell counts (B) of S. mutans JCM 5175 in the presence and absence of bakuchiol. Bakuchiol concentration: ▵, 1 μg/ml; □, 5 μg/ml; ⧫, 10 μg/ml; ○, 20 μg/ml; ●, none.
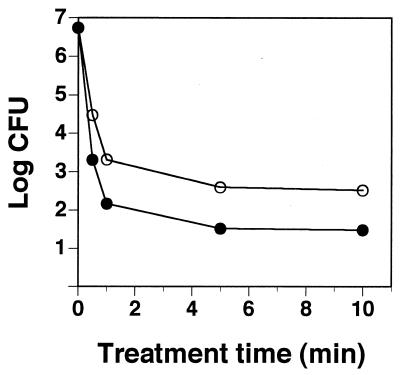
Figure 3 shows the relationship between bakuchiol treatment period and bakuchiol concentration for the bactericidal effect against S. mutans JCM 5175. After incubation with bakuchiol for each treatment time, CFU of S. mutans JCM 5175 were counted in plates containing 0.1% (wt/vol) Tween 80 for inactivation of bakuchiol. The bactericidal effect of bakuchiol was treatment time dependent. The required time for the bactericidal effect against S. mutans JCM 5175 was shorter with bakuchiol at 20 μg/ml than at 5 μg/ml.
FIG. 3.
Relationship between bakuchiol treatment time and bakuchiol concentration for the bactericidal effect against S. mutans JCM 5175. Bakuchiol concentration: ○, 5 μg/ml; ●, 20 μg/ml.
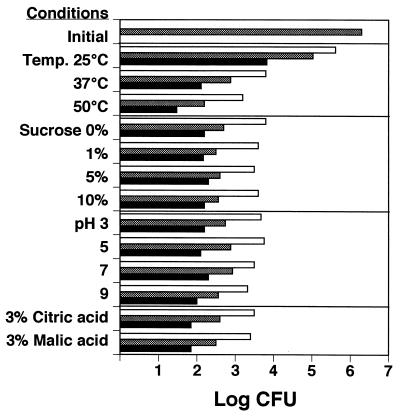
Figure 4 shows the antimicrobial activity of bakuchiol against S. mutans JCM 5175 under several treatment conditions, such as different temperatures, pHs, sugars, and organic acids. The bactericidal effect of bakuchiol on S. mutans JCM 5175 was dependent on temperature of incubation and stable under the following conditions: sucrose, 0 to 10% (wt/vol); pH, 3.0 to 7.0; organic acids, 3% (wt/vol) citric and malic acids).
FIG. 4.
Antimicrobial activity of bakuchiol against S. mutans JCM 5175 under several treatment conditions, including variations in temperature, pH, sucrose, and organic acids (citric and malic acids). Standard treatment conditions: temperature, 37°C; pH, 7.0; sucrose, 0% (wt/vol); organic acid, 0% (wt/vol). Bars (bakuchiol concentration): open, 2.5 μg/ml; stippled, 5.0 μg/ml; solid, 20 μg/ml.
MIC and sterilizing concentration of bakuchiol against oral microorganisms.
To evaluate the antimicrobial activity of bakuchiol against oral microorganisms, the MICs and the sterilizing concentrations for 15 min were determined (Table 1). The bacteriostatic effects of bakuchiol required concentrations of 1 to 4 μg/ml, and the bactericidal effects required sterilizing concentrations for 15 min of 5 to 20 μg/ml for all microorganisms tested. The antimicrobial activity of bakuchiol was effective for both gram-negative anaerobic periodontal pathogens, such as P. gingivalis, and gram-positive cariogenic bacteria, such as S. mutans and A. viscosus.
TABLE 1.
Antimicrobial activity of bakuchiol against oral microorganisms
| Species | MIC (μg/ml) | Sterilizing concn (μg/ml) |
|---|---|---|
| Streptococcus mutans JCM 5175 | 1.0 | 20.0 |
| Streptococcus mutans GS5 | 1.2 | 20.0 |
| Streptococcus mutans JC2 | 1.4 | 5.0 |
| Streptococcus mutans IFO 13955 | 1.8 | 10.0 |
| Streptococcus sobrinus 6715 | 1.6 | 10.0 |
| Porphyromonas gingivalis ATCC 33277 | 4.0 | 20.0 |
| Enterococcus faecalis IFO 3989 | 2.0 | 10.0 |
| Enterococcus faecium IFO 3826 | 2.0 | 10.0 |
| Lactobacillus acidophilus AKU 1122 | 1.0 | 10.0 |
| Lactobacillus acidophilus AKU 1124 | 1.0 | 5.0 |
| Lactobacillus plantarum AKU 1130 | 1.0 | 5.0 |
| Streptococcus sanguis 179-3 | NDa | 10.0 |
| Streptococcus sanguis 254-4 | ND | 5.0 |
| Streptococcus salivarius 70-2 | ND | 5.0 |
| Streptococcus salivarius 160-2 | ND | 5.0 |
| Actinomyces viscosus 19246 | ND | 5.0 |
| Lactobacillus plantarum 8016 | ND | 20.0 |
| Lactobacillus casei 4646 | ND | 5.0 |
ND, not determined.
Antimicrobial activity against adherent S. mutans in water-insoluble glucan.
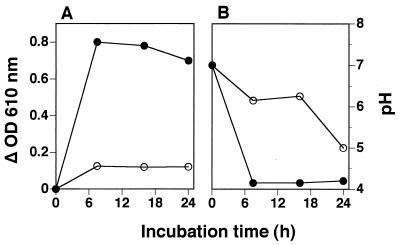
The adherence of cells to a glass surface was evident in test tubes when S. mutans JCM 5175 was grown in broth containing 2% (wt/vol) sucrose for 24 h. After the adherent cells of S. mutans JCM 5175 were treated with bakuchiol at 100 μg/ml for 5 min, the bakuchiol-treated cells were then incubated in broth containing both 2% (wt/vol) sucrose and 0.1% (wt/vol) Tween 80 for inactivation of bakuchiol for 7.5, 16, and 24 h. Figure 5 shows the antimicrobial activity of bakuchiol against the adherent cells of S. mutans JCM 5175 and pH in broth containing sucrose. The adherent cells of S. mutans JCM 5175 without bakuchiol treatment grew well, accompanied by synthesis of water-insoluble glucan in the presence of sucrose. The pH of the broth also decreased rapidly to 4.0 after 7.5 h of incubation. On the other hand, the adherent cells treated with bakuchiol at 100 μg/ml grew slightly, and water-insoluble glucan synthesis was almost completely inhibited in the presence of sucrose. The decrease in pH after 16 h of incubation in the presence of sucrose was negligible, and the pH of the broth finally decreased to 5.0 after 24 h. When the adherent cells were treated with bakuchiol at 20 μg/ml, these effects were weak (data not shown).
FIG. 5.
Inhibitory effects of bakuchiol on the formation of water-insoluble glucan (A) and the decrease in pH in broth (B) in adherent cells of S. mutans JCM 5175 in the presence of sucrose. Bakuchiol concentration: ○, 100 μg/ml; ●, none.
DISCUSSION
It is now clear that S. mutans plays an essential role in the pathogenesis of dental caries and consequently provides the three prime targets for prevention of caries: antimicrobial agents against S. mutans, inhibition of adhesion of S. mutans on the tooth surface, and inhibition of glucosyltransferase of S. mutans from forming water-insoluble glucan. Extensive screening for biologically active compounds from natural sources with these effects has been performed. For example, the ethanolic extract of propolis inhibited both the growth of S. mutans and the activity of glucosyltransferase (10). Tea polyphenol also inhibited the growth of S. mutans as well as the production of insoluble glucans by glucosyltransferases (12, 13, 14). In contrast, mutastein and ribocitrin, which were isolated from culture supernatants of Aspergillus terreus (2) and a Streptomyces sp. (23), respectively, inhibited the glucosyltransferase of S. mutans but did not show antibacterial activity. The methanol extract of the native American plant Ceanothus americanus demonstrated antimicrobial activity against selected oral pathogens (19). Thus, ceanothic acid and ceanothetric acid had growth-inhibitory effects against S. mutans, A. viscosus, and P. gingivalis, with MICs ranging from 42 to 625 μg/ml.
In this study, we evaluated in vitro the antimicrobial activities of bakuchiol against some oral microorganisms and showed that bakuchiol had bactericidal effects against all bacteria tested, including S. mutans, S. sanguis, S. salivarius, S. sobrinus, E. faecalis, E. faecium, L. acidophilus, L. casei, L. plantarum, A. viscosus, and P. gingivalis, with MICs ranging from 1 to 4 μg/ml and sterilizing concentrations for 15 min ranging from 5 to 20 μg/ml (Table 1). The antimicrobial activity of bakuchiol was also effective against adherent cells of S. mutans in water-insoluble glucan in the presence of sucrose (Fig. 5). Bakuchiol was found to have cytotoxic activity against L929 cells in cell culture, and this cytotoxic activity was considered to be due to injury of the cell membrane, based on electron microscopic observation and hemolytic activity (15). Recently, various biological activities of bakuchiol have been reported, e.g., anti-inflammatory effects (3), inhibition of mitochondrial lipid peroxidation (6), stimulation of the immune system (18), inhibition of DNA polymerase (28), inhibition of papilloma formation (17), and prevention of diabetes (11). Therefore, bakuchiol might be a promising lead compound for development of antimicrobial agents against oral pathogens in humans. The mechanism of the antimicrobial activity of bakuchiol is also under investigation in our laboratory.
Bakuchiol was isolated from the seeds and leaves of P. corylifolia Linn, a tree native to China with various uses in traditional Oriental medicine (16, 22, 25). However, its safety was not confirmed completely, and the safety of bakuchiol needs to be tested in detail. Since the bactericidal effect of bakuchiol was stable under various environmental conditions simulating those that occur in the mouth, such as different temperatures, sugars, pHs, and organic acids (Fig. 4), bakuchiol is potentially useful for development of antibacterial agents applicable to food additives for candy and chewing gum. Furthermore, bakuchiol will also be applicable for use in mouthwash preparations because of the short treatment time (30 s) required for the bactericidal effect against S. mutans (Fig. 3). Research is in progress in our laboratory to evaluate the antimicrobial effects of bakuchiol against oral microorganisms using in vivo models.
ACKNOWLEDGMENTS
We are very grateful to Hiromichi Yumoto and Takashi Matsuo (Department of Conservative Dentistry, School of Dentistry, Tokushima University) and Ken-ichiro Shibata (Department of Oral Bacteriology, School of Dentistry, Hokkaido University) for providing the oral microorganisms used and for helpful suggestions during this work.
REFERENCES
- 1.Brissette J L, Cabacungan E A, Pieringer R A. Studies on the antibacterial activity of dodecylglycerol, its limited metabolism and inhibition of glycerolipid and lipoteichoic acid biosynthesis in Streptococcus mutans BHT. J Biol Chem. 1986;261:6338–6345. [PubMed] [Google Scholar]
- 2.Endo A, Hayashida O, Murakawa S. Mutastein, a new inhibitor of adhesive-insoluble glucan synthesis by glucosyltransferases of Streptococcus mutans. J Antibiot (Tokyo) 1983;36:203–207. doi: 10.7164/antibiotics.36.203. [DOI] [PubMed] [Google Scholar]
- 3.Ferrandiz M L, Gil B, Sanz M J, Ubeda A, Erazo S, Gonzalez E, Negrete R, Pacheco S, Paya M, Alcaraz M J. Effect of bakuchiol on leukocyte functions and some inflammatory responses in mice. J Pharm Pharmacol. 1996;48:975–980. doi: 10.1111/j.2042-7158.1996.tb06016.x. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons R J, van Houte J. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- 5.Hamada S, Slade H D. Biology, immunology and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haraguchi H, Inoue J, Tamura Y, Mizutani K. Inhibition of mitochondrial lipid peroxidation by bakuchiol, a meroterpene from Psolalea corylifolia. Planta Med. 2000;66:569–571. doi: 10.1055/s-2000-8605. [DOI] [PubMed] [Google Scholar]
- 7.Jacquelin L F, Brisset L, Lemagrex E, Carquin J, Gelle M P, Choisy C. Preventation of carcinogenic dental plaque. Study of the structures implicated in the Streptococcus mutans and Streptococcus sobrinus adhesion and coaggregation. Pathol Biol. 1995;43:371–379. [PubMed] [Google Scholar]
- 8.Kaul R. Kinetics of the antistaphylococcal activity of bakuchiol in vitro. Arzneimittelforschung. 1976;26:486–489. [PubMed] [Google Scholar]
- 9.Koga T, Hamada S, Murakawa S, Endo A. Effect of a glucosyltransferase inhibitor on glucan synthesis and cellular adherence of Streptococcus mutans. Infect Immun. 1982;38:882–886. doi: 10.1128/iai.38.3.882-886.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo H, Gomes B P F A, Rosalen P L, Ambrosano G M B, Park Y K, Cury J A. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch Oral Biol. 2000;45:141–148. doi: 10.1016/s0003-9969(99)00117-x. [DOI] [PubMed] [Google Scholar]
- 11.Krenisky J M, Luo J, Reed M J, Carney J R. Isolation and antihyperglycemic activity of bakuchiol from Otholobium pubescens (Fabaceae), a Peruvian medicinal plant used for the treatment of diabetes. Biol Pharm Bull. 1999;22:1137–1140. doi: 10.1248/bpb.22.1137. [DOI] [PubMed] [Google Scholar]
- 12.Kubo I, Muroi H, Himejima M. Antimicrobial activity of green tea flavor components and their combination effects. J Agric Food Chem. 1992;40:245–248. [Google Scholar]
- 13.Kubo I, Muroi H, Himejima M. Antimicrobial activity against Streptococcus mutans of mate tea flavor components. J Agric Food Chem. 1993;41:107–111. [Google Scholar]
- 14.Kubo I, Muroi H, Kubo A. Antimicrobial activity of long-chain alcohols against Streptococcus mutans. J Agric Food Chem. 1993;41:2447–2450. [Google Scholar]
- 15.Kubo M, Dohi T, Odani T, Tanaka H, Iwamura J. Cytotoxicity of Corylifoliae Fructus. I. Isolation of the effective compound and the cytotoxicity. Yakugaku Zasshi. 1989;109:926–931. doi: 10.1248/yakushi1947.109.12_926. [DOI] [PubMed] [Google Scholar]
- 16.Labbé C, Faini F, Coll J, Connolly J D. Bakuchiol derivatives from the leaves of Psoralea glandulosa. Phytochemistry. 1996;42:1299–1303. [Google Scholar]
- 17.Latha P G, Panikkar K R. Inhibition of chemical carcinogenesis by Psoralea corylifolia seeds. J Ethnopharmacol. 1999;68:295–298. doi: 10.1016/s0378-8741(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 18.Latha P G, Evans D A, Panikkar K R, Jayavardhanan K K. Immunomodulatory and antitumor properties of Psoralea corylifolia seeds. Fitoterapia. 2000;71:223–231. doi: 10.1016/s0367-326x(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 19.Li X-C, Cai L, Wu C D. Antimicrobial compounds from Ceanothus americanus against oral pathogens. Phytochemistry. 1997;46:97–102. doi: 10.1016/s0031-9422(97)00222-7. [DOI] [PubMed] [Google Scholar]
- 20.Marsh P D. Microbiological aspects of the chemical control of plaque and gingivitis. J Dent Res. 1992;71:1431–1438. doi: 10.1177/00220345920710071501. [DOI] [PubMed] [Google Scholar]
- 21.Marsh P D. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 22.Mehta G, Nayak U R, Dev S. Meroterpenoids. 1. Psoralea Corylifolia Linn. 1. Bakuchiol, a novel monoterpene phenol. Tetrahedron. 1973;29:1119–1125. [Google Scholar]
- 23.Okami Y, Takashio M, Umezawa H. Ribocitrin, a new inhibitor of dextransucrase. J Antibiot (Tokyo) 1981;34:344–345. doi: 10.7164/antibiotics.34.344. [DOI] [PubMed] [Google Scholar]
- 24.Raamsdonk M, Mei H C, Soet J J, Busscher H J, Graaff J. Effect of polyclonal and monoclonal antibodies on surface properties of Streptococcus sobrinus. Infect Immun. 1995;63:1698–1702. doi: 10.1128/iai.63.5.1698-1702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakakiyama S, Yamamoto K, Asaoka M. A new synthesis of (+)-bakuchiol. Nat Prod Lett. 1999;14:1–4. [Google Scholar]
- 26.Schüpbach P, Osterwalder V, Guggenheim B. Human root caries: microbiota in plaque covering sound, carious and arrested carious root surfaces. Caries Res. 1995;29:382–395. doi: 10.1159/000262097. [DOI] [PubMed] [Google Scholar]
- 27.Slots J, Rams T E. Microbiology of periodontal disease. In: Slots J, Taubman M A, editors. Contemporary oral microbiology and immunology. St. Louis, Mo: Mosby Year Book; 1992. pp. 425–443. [Google Scholar]
- 28.Sun N J, Woo S H, Cassady J M, Snapka R M. DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia. J Nat Prod. 1998;61:362–366. doi: 10.1021/np970488q. [DOI] [PubMed] [Google Scholar]
- 29.Tsutiya H, Sato M, Iinuma M, Yokoyama J, Ohyama M, Tanaka T, Takase I, Namikawa I. Inhibition of the growth of carcinogenic bacteria in vitro by plant flavanones. Experientia. 1994;50:846–849. doi: 10.1007/BF01956469. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Katayama S, Matsubara M, Honda Y, Kuwahara M. Antibacterial carbohydrate monoesters suppressing cell growth of Streptococcus mutans in the presence of sucrose. Curr Microbiol. 2000;41:210–213. doi: 10.1007/s002840010121. [DOI] [PubMed] [Google Scholar]
- 31.Yanagida A, Kanda T, Tanabe M, Matsudaira F, Cordeiro J G O. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J Agric Food Chem. 2000;48:5666–5671. doi: 10.1021/jf000363i. [DOI] [PubMed] [Google Scholar]