Abstract
Indigo naturalis, a herbal medicine purified from indigo-containing plants, such as Strobilanthes cusia, Isatis tinctoria, and Polygonum tinctorium, has been reported to be useful in the treatment of ulcerative colitis by activating the aryl hydrocarbon receptor. However, the aryl hydrocarbon receptor pathway causes crucial side effects, such as pulmonary arterial hypertension. Although P. tinctorium is one of the plant derivatives of indigo naturalis, it is not identical to it. To date, the pure leaves of P. tinctorium have not been reported to ameliorate ulcerative colitis. Therefore, we investigated the effect of pure P. tinctorium leaves, which are consumed in some regions, on experimental colitis induced in mice using sodium dextran sulfate. We found that P. tinctorium leaves ameliorated weight loss (P < 0.01) and pathological inflammatory changes in the colon (P < 0.05), enhanced mRNA expression of interleukin-10 (P < 0.05), and decreased expression of tumor necrosis factor-in colonic tissues (P < 0.05), as determined using quantitative real-time reverse transcription polymerase chain reaction. The intraperitoneal administration of an aryl hydrocarbon receptor antagonist did not antagonize the inhibition of mucosal destruction, whereas an anti-interleukin-10 receptor antibody did. These results suggest that P. tinctorium ameliorate sodium dextran sulfate-induced intestinal inflammation via interleukin-10-related pathway, independent of the aryl hydrocarbon receptor pathway. P. tinctorium leaves have the potential to be a new, safe treatment for ulcerative colitis.
Keywords: Aryl hydrocarbon receptor pathway, Indigo naturalis, Interleukin-10, Polygonum tinctorium, ulcerative Colitis
Abbreviation List: AhR, aryl hydrocarbon receptor; CD, Crohn's disease; DSS, sodium dextran sulfate; EDTA, ethylenediaminetetraacetic acid; H & E, hematoxylin and eosin; HBSS, Hank's balanced salt solution; HPLC, high performance liquid chromatography; IBD, inflammatory bowel disease; IECs, intestinal epithelial cells; IL, interleukin; IFN, interferon; LP, lamina propria; PAH, pulmonary arterial hypertension; qRT-PCR, quantitative real-time reverse transcription polymerase chain reaction; SEM, standard error of the mean; TGF, transforming growth factor; TNF, tumor necrosis factor; UC, ulcerative colitis
Highlights
-
•
P. tinctorium leaves ameliorate DSS-induced colitis via IL-10-related pathway.
-
•
The protective effect for intestinal epithelial damage of P. tinctorium leaves is independent of the AhR pathway.
-
•
P. tinctorium leaves inhibit TNF-α expression but do not upregulate IL-22.
1. Introduction
The pathogenesis of inflammatory bowel disease (IBD), which includes Crohn's disease (CD) and ulcerative colitis (UC), has not yet been ascertained. However, environmental and genetic factors and immune responses are considered to be the major etiologies of IBD [1,2]. During the past decade, the number of patients with IBD has increased significantly [2], with many cases of refractory disease showing repeated remission and relapse cycles, despite significant progress in medical treatment, such as the use of targeted anti-tumor necrosis factor (TNF)-α antibodies. In addition, the rising cost of medical care is a major problem for patients with IBD.
In China, patients suffering from UC have often been treated with traditional herbal medicines. Previous reports showed that indigo naturalis (IN, also referred to as Qing-Dai), obtained by fermenting plants containing indigo (e.g., Strobilanthes cusia, Isatis tinctoria, and Polygonum tinctorium), is also effective against intractable UC [3,4]. In Japan, a multicenter, double-blind, prospective trial investigated the safety and efficacy of IN patients with UC and demonstrated the high efficacy of IN and its short-term safety [5]. Indigo-containing preparations, such as IN, are thought to act as ligands for the aryl hydrocarbon receptor (AhR) that induces the production of anti-inflammatory cytokines, such as interleukin (IL)-22 and IL-10, to ameliorate colitis [6]. However, pulmonary arterial hypertension (PAH) has been reported as a critical side effect of IN treatment [7,8], with reports associating the AhR pathway with PAH [9,10]. Considering its potential safety concerns, IN may be difficult to use clinically as a therapeutic agent.
On the other hand, P. tinctorium is a herb that has been known, in Oriental countries, to have anti-bacterial and anti-inflammatory activities since ancient times. Although P. tinctorium is one of the plant species providing IN, it is not identical to IN, in terms of its composition and biological activity. Therefore, we hypothesized that pure P. tinctorium might have the potential to be used clinically as a therapeutic agent for UC. However, the leaves of P. tinctorium have not been reported to ameliorate UC.
In the present study, we aimed to clarify the protective effects of pure P. tinctorium leaves in sodium dextran sulfate (DSS)-induced colitis in a mouse model to determine their potential mechanism of action. Considering safety, we used a P. tinctorium called “Aomori Blue” that is cultivated without pesticide in the northern-most prefecture of Japan. “Aomori Blue”is not only used as a dye but is also distributed as a safe food ingredient.
2. Materials and methods
2.1. Mice
Female BALB/c mice (age, 8 weeks; weight, 18–22 g) were purchased from CLEA Japan (Tokyo, Japan) and maintained in a controlled environment (22 °C ± 2 °C with a 12-h light/dark cycle). All animal experiments complied with the appropriate guidelines, and were performed in accordance with the U.K. Animals (Scientific Procedures) Act, 1986. The study design was approved by Animal Research Committee of Hirosaki University (Permit number: M16015).
2.2. Induction of colitis
Colitis was induced in the mice by the addition of 3% DSS (molecular weight, 1000–9000 g/mol; FUJIFILM Wako Pure Chemical, Osaka, Japan) to their drinking water.
2.3. Administration of P. tinctorium leaves
The leaves of P. tinctorium, cultivated without pesticide use, were provided by Aomoriai-Sangyo Corporation (Aomori, Japan). The mice were fed powdered food (CLEA Rodent Diet CE-2, CLEA Japan) that contained 0.5% by weight of dry powdered P. tinctorium leaves for 12 days after the initial DSS administration.
2.4. Evaluation of colitis activity
The body weights of the mice were measured daily, and at Day 12, the mice were euthanized by cervical dislocation and the colon was isolated. The colon lengths were measured and fixed in 10% formalin, embedded in paraffin, and sliced along the long axis. The specimens were stained with hematoxylin and eosin (H & E) for histological evaluation, which allowed the measurement of the inflammatory cell infiltration, crypt loss, and mucosal epithelial loss at a height just above the muscularis mucosae. To evaluate colitis activity, we compared the body weight, colon length, and histological changes in two groups: the colitis group (DSS positive [+], P. tinctorium negative [−]) (n = 8) and the P. tinctorium group (DSS positive [+], P. tinctorium positive [+]) (n = 8).
2.5. Isolation of intestinal epithelial cells and lamina propria
Intestinal epithelial cells (IECs) were purified as described by Mizoguchi et al. [11]. On Day 4, the mice were anesthetized by exposure to isoflurane, and the thoracic cavity was opened. The left ventricle was perfused with 15 mL of 30-mmol/L ethylenediaminetetraacetic acid (EDTA) in Hank's Balanced Salt Solution (HBSS), which was calcium free and magnesium free. Following perfusion, the entire colon (excluding the cecum) was removed, inverted, and placed in a cold tube containing 2 mL of cold 2-mmol/L EDTA in HBSS. The tube was shaken for 20 s using a mini-beadbeater (BioSpec Products, Bartlesville, OK, USA). The suspension was centrifuged at 15,000×g (4 °C) for 20 min, and the pellet containing IECs was suspended in RNA Later solution (Thermo Fisher Scientific, Waltham, MA, USA). The remaining tissue, which comprised the lamina propria (LP), was placed in an RNA solution.
2.6. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
The total RNA contents from the IECs and LPs were isolated using the RNeasy Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer's instructions, and were reverse transcribed to cDNA using the iScript Advanced cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). The following TaqMan gene expression assays (Thermo Fisher Scientific) were used to quantify the following target genes: Ifng (Mm00801778_m1), Tgfb1 (Mm01178820_m1), TNF (Mm99999068_m1), IL22ra1 (Mm01192943_m1), IL22 (Mm0122672_g1), IL1b (Mm00434228_m1), Foxp3 (Mm00475156_m1), IL10 (Mm00439615_g1), and Actb (Mm00607939_s1). qRT-PCR was performed using a CFX Connect Real-Time System (Bio-Rad Laboratories) and Brilliant III Ultra-Fast QPCR Master Mix solution (Agilent Technologies, Santa Clara, CA, USA). These results were then normalized to Actb RNA expression.
In the qRT-PCR analysis, we compared the expression of mRNA in three groups of mice: the control group (DSS negative [−], P. tinctorium negative [−]) (n = 6), colitis group (n = 6), and P. tinctorium group (n = 6).
In vivo administration of chemical antagonist.
To confirm AhR involvement, the mice received intraperitoneal injections of an AhR antagonist (CH223191; Selleck Chemicals, Houston, TX, USA; 10 mg/kg) every day from the initial DSS administration. We compared the colon length and histological changes were evaluated in two groups, namely the colitis + CH223191 group (n = 6) and the P. tinctorium + CH223191 group (n = 6).
To confirm the involvement of IL-10, mice received intraperitoneal injections of mouse anti-IL-10 receptor antibody (anti-IL-10R Ab, Clone 1B1.3A; Bio X Cell, Lebanon, KS, USA; 25 mg/kg) at the start of DSS administration and on Day 5. We compared the colon lengths and histological changes in two groups: the colitis + anti-IL-10R group (n = 6) and the P. tinctorium + anti-IL-10R group (n = 6).
2.7. High-performance liquid chromatography analysis
P. tinctorium leaves were extracted by soaking in n-hexane, at 25 °C, for 24 h, twice. The extracts were concentrated, dried, dissolved in dimethyl sulfoxide, and subjected to high-performance liquid chromatography (HPLC) to determine the major components. The indigo, indirubin, and tryptanthrin in P. tinctorium leaves were analyzed using HPLC with the following conditions: column, COSMOSIL 5 PE-MS Packed Column (i.d. 4.6 × 250 mm; Nacalai Tesque, Kyoto, Japan); mobile phase, 40% CH3CN; flow rate, 1.0 mL/min; detection 254 nm; and temperature, 25 °C. The data were collected by Chromato-PRO (RTC, Tokyo, Japan). The standard reagent for tryptanthrin was purchased from Sigma-Aldrich Japan (Tokyo, Japan). All the other reagents were purchased from Fujifilm-Wako Pure Chemicals, Co.
2.8. Statistical analysis
All data are presented as means ± standard errors of the means (SEMs). The data, when they were not normal probability distribution, were analyzed with a Mann-Whitney U test using GraphPad Prism (Version 8.2.1, GraphPad Software, San Diego, CA, USA). A p-value of <0.05 was considered statistically significant.
3. Results
3.1. P. tinctorium leaves ameliorated DSS-induced colitis
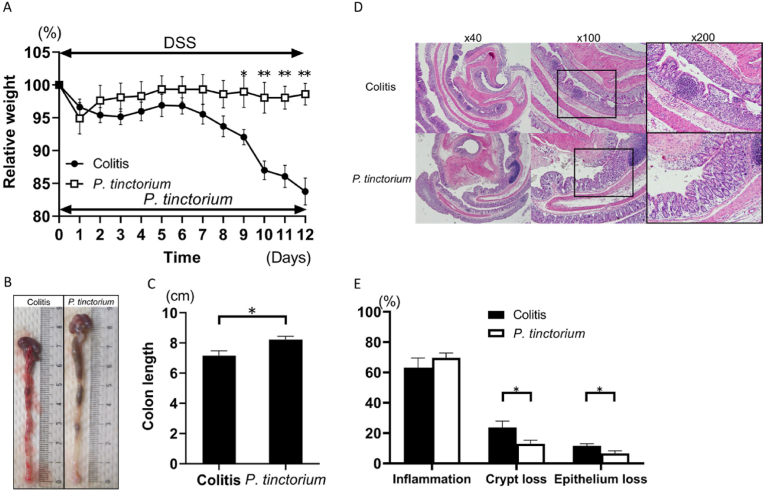
Compared with that in the colitis group (n = 8), the animals’ weight loss was markedly reduced in the P. tinctorium group (n = 8) from Day 9 to Day 12 (Fig. 1A). The colons obtained on Day 12 showed that those from the P. tinctorium group were longer than those from the colitis group (Fig. 1B, C). Histological evaluation demonstrated that the crypt and mucosal epithelial losses were lower in the P. tinctorium group than in the colitis group (Fig. 1D, E).
Fig. 1.
Polygonum tinctorium suppresses dextran sulfate sodium (DSS)-induced colitis.
(A) A graphical representation of the average percent weight loss in the colitis and P. tinctorium groups, relative to the weights at the start of DSS treatment. ∗P < 0.05; ∗∗P < 0.01. (B) Colons harvested from the mice in the colitis and P. tinctorium groups on Day 12. (C) A graphical representation of the colon lengths harvested from the mice in the colitis and P. tinctorium groups on Day 12. (D) Histological samples of hematoxylin and eosin-stained colons observed at 40 × , 100 × , and 200 × magnifications. (E) The extent of inflammatory cell infiltration and crypt and mucosal epithelial losses. Data are expressed as the means ± standard error of the mean of groups of 8 mice.
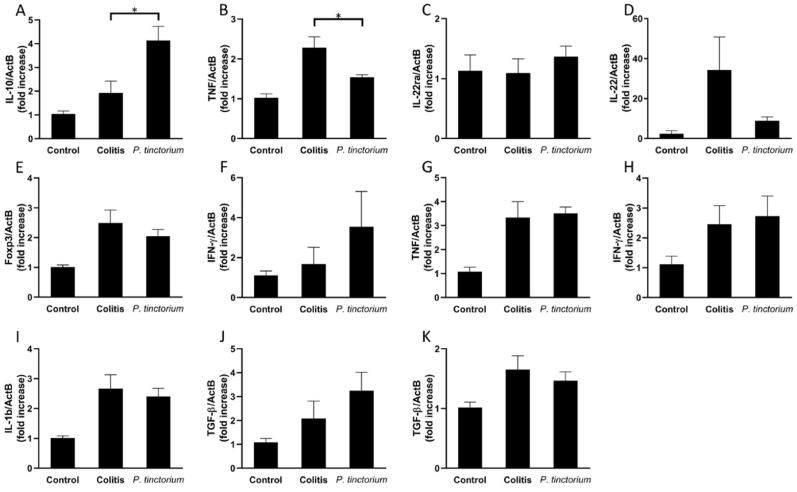
P. tinctorium enhanced IL-10 expression in colonic LP of mice with DSS-induced colitis. Next, we analyzed the mRNA expressions of inflammatory or anti-inflammatory cytokines which are important in pathophysiology of IBD [2] and concern mechanism of indigo naturalis including IL-22 or IL-10 [6]. The qRT-PCR analysis showed that IL-10 mRNA expression in the LP of the P. tinctorium group (n = 6) was significantly enhanced compared with that in the LP of the colitis group (n = 6) (Fig. 2A). Conversely, TNF-α mRNA expression in the LP of the P. tinctorium group was lower than that in the LP of the colitis group (Fig. 2B). The mRNA expression levels of IL22ra in IECs and IL22 and Foxp3 in the LP were similar between the colitis and P. tinctorium groups (Fig. 2C–E). Moreover, there were no significant differences in the mRNA expression levels of several inflammatory cytokines, such as IFN-γ and TNF-α in IECs and IFN-γ and IL-1β in LP (Fig. 2F–I), between the colitis and P. tinctorium groups. The mRNA expression levels of the anti-inflammatory cytokine TGF-β in the IECs and LP of the colitis group were similar to those in the IECs and LP of the P. tinctorium group (Fig. 2J, K).
Fig. 2.
mRNA expressions in IECs and LP.
Polygonum tinctorium increases the expression of IL-10 mRNA and decreases the expression of TNF-α mRNA in the LP. On Day 4 after the induction of colitis, colon cells were separated into IECs and LP, the total RNA was extracted, and mRNA expression was analyzed using qRT-PCR. (A) LP expression of IL-10. (B) LP expression of TNF-α. (C) IEC expression of IL-22ra. (D) LP expression of IL-22. (E) LP expression of Foxp3. (F) IEC expression of IFN-γ (G) IEC expression of TNF-α. (H) LP expression of IFN-γ. (I) LP expression of IL-1β. (J) IEC expression of TGF-β. (K) LP expression of TGF-β; these data are compared between the colitis and P. tinctorium groups and are expressed as means ± SEMs of groups of 6 mice; ∗P < 0.05.
IECs, intestinal epithelial cells; IL, interleukin; IFN, interferon; LP, lamina propria; mRNA, messenger ribonucleic acid; qRT-PCR, quantitative real-time reverse transcription polymerase chain reaction; SEM, standard error of the mean; TGF, transforming growth factor; TNF, tumor necrosis factor.
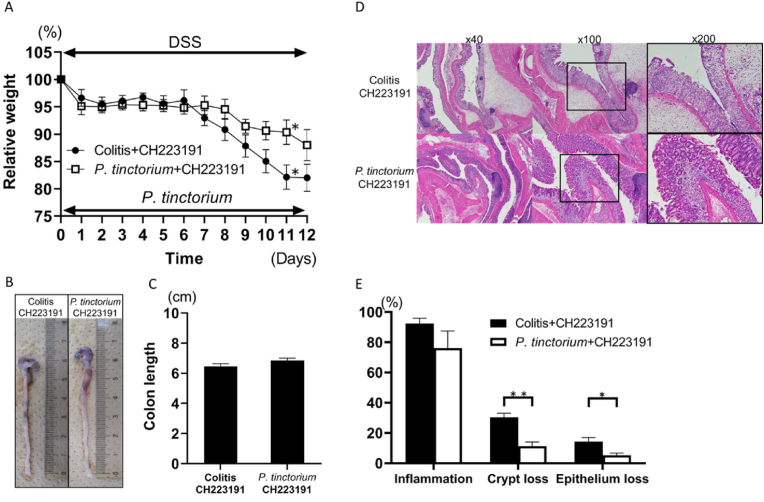
AhR antagonist did not antagonize the protective effect of P. tinctorium leaves in DSS-induced colitis.
In animals receiving intraperitoneal administration of the AhR antagonist, the mean body weight in the colitis + CH223191 group (n = 6) was significantly less than that in the P. tinctorium + CH223191 group (n = 6) by Day 11 (Fig. 3A). Additionally, the mean colon length in the P. tinctorium + CH223191 group tended to be longer than that in the colitis + CH223191 group, although the difference was not significant (Fig. 3B, C). Notably, histological evaluations of crypt and mucosal epithelial loss showed that the effects of P. tinctorium leaves were significant, even when the mice received an AhR antagonist (Fig. 3D, E).
Fig. 3.
Weight loss, colon length, and extent of inflammatory cell infiltration and crypt and mucosal epithelial losses between the colitis and Polygonum tinctorium groups after administration of an AhR antagonist.
Intraperitoneal administration of an AhR antagonist did not attenuate the mucosal improvement effect of P. tinctorium leaves. (A) AhR antagonist was intraperitoneally administered to the colitis and P. tinctorium groups every day from the start of DSS administration. The average percent weight loss in the two groups of mice is compared with the weights at the start of DSS treatment. (B) The colon samples from both groups, harvested on Day 12. (C) Graphical representation of the colon lengths of both groups on Day 12. (D) Histological samples of the colon tissue stained with H&E and observed under 40 × , 100 × , and 200 × magnifications. (E) Graphical representation of the extent of inflammatory cell infiltration and crypt and mucosal epithelial losses; data are expressed as means ± SEM in groups of 6 mice; ∗P < 0.05 and ∗∗P < 0.01.
AhR, aryl hydrocarbon receptor; DSS, sodium dextran sulfate; H&E, hematoxylin and eosin; SEM, standard error of the mean.
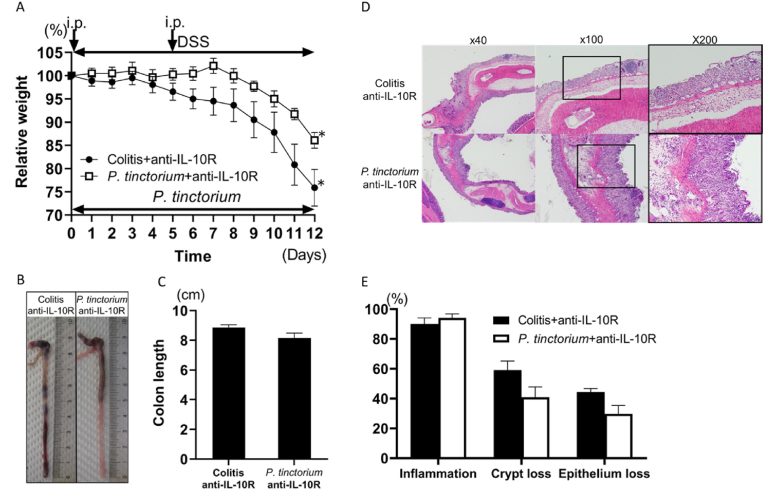
3.2. IL-10-neutralizing Ab counteracted the ameliorating effect of P. tinctorium leaves in DSS-induced colitis
After intraperitoneal administration of anti-IL-10R, the weight loss in the P. tinctorium + anti-IL-10R group was similar to that in the colitis + anti-IL-10R group. One of the six mice in the colitis + anti-IL-10R group was unable to survive until Day 12. In the P. tinctorium + anti-IL-10R group (n = 6) and the colitis + anti-IL-10R group (n = 5), weight loss was exacerbated after the second administration of anti-IL-10R Ab (Fig. 4A). The colon lengths were similar between the P. tinctorium + anti-IL-10R and colitis + anti-IL-10R groups after the administration of anti-IL-10R Ab (Fig. 4B, C). Additionally, histological evaluation demonstrated that anti-IL-10R Ab administration to the P. tinctorium + anti-IL-10R group exacerbated inflammatory cell infiltration and increased crypt and mucosal epithelial loss; these inflammatory changes were similar to those observed in the colitis group (Fig. 4D, E).
Fig. 4.
Weight loss, colon length, and extent of inflammatory cell infiltration and crypt and mucosal epithelial cell losses between the colitis and Polygonum tinctorium groups after administration of an anti-IL10R Ab.
Intraperitoneal administration of an anti-IL10R Ab attenuated the mucosal improvement effect of P. tinctorium. At the start of DSS initiation and on Day 5, the anti-IL10R Ab was intraperitoneally administered to the colitis and P. tinctorium groups. (A) The average percent weight loss in the two groups is compared with the weights at the start of DSS treatment. (B) Colon samples from both groups, harvested on Day 12. (C) Graphical representation of the colon lengths of both groups on Day 12. (D) Histological samples of the H&E-stained colon tissue observed under 40 × , 100 × , and 200 × magnifications. (E) Graphical representation of the extent of inflammatory cell infiltration and crypt and mucosal epithelial losses; data are expressed as means ± SEM of groups of 5–6 mice; ∗P < 0.05.
Ab, antibody; DSS, sodium dextran sulfate; H&E, hematoxylin and eosin; i.p., intraperitoneal administration of Ab; SEM, standard error of the mean.
3.3. Components of P. tinctorium leaves
Since P. tinctorium contains multiple constituents, quantitative analysis was performed using HPLC, revealing the amounts of tryptanthrin, indirubin, and indigo in P. tinctorium to be 28.11, 14.23, and 4.6 ng/g, respectively.
4. Discussion
To the best of our knowledge, this is the first report suggesting that P. tinctorium leaves have a protective effect against DSS-induced mucosal destruction and revealing the main mechanism involved in IL-10-related pathway, but not the AhR pathway.
UC is a disease in which the intestinal mucosal epithelium is repeatedly damaged, and its etiology is not fully understood. Despite advances in medical therapy and treatment outcomes that have decreased the proportion of patients undergoing surgery [12,13], the occurrence of refractory cases and the high cost of treatment have become social problems [14,15]. The high efficacy of IN cases of refractory UC has been demonstrated in many studies, to date [4,16]. Moreover, indigo and indirubin (the active ingredients of IN) act as ligands for the AhR pathway and stimulate innate lymphoid cell 3 in the intestinal mucosa to induce IL-22 and promote healing of mucosal damage [17,18].
From our results, oral consumption of P. tinctorium leaves could inhibit the weight loss, colon shortening, and mucosal damage caused by DSS-induced colitis. Notably, this study demonstrates a new mechanism through which P. tinctorium leaves inhibit mucosal damage: enhancement of IL-10 expression and inhibition of TNF-α expression, without inducing IL-22. In addition, the treatment with anti-IL-10R antibody lessened the protective effect of P. tinctorium in vivo. The protective effect of P. tinctorium on mucosal damage that was maintained during treatment with AhR-antagonist suggests that the mechanism of inhibiting the protective effect is AhR-independent. However, colon shortening was decreased following treatment with the AhR-antagonist. These results suggest that the AhR pathway partially influences the protective effect of P. tinctorium leaves.
The main components of IN are indigo and indirubin (2.0% and 0.13% or more, respectively) [19]. Although IN also contains tryptanthrin and other alkaloids, the ratios of some of these components are reportedly lower than those in other indigo-containing plants [20]. In P. tinctorium leaves, the ratios of indigo, indirubin, and tryptanthrin extracted with n-hexane showed tryptanthrin to be the most abundant component, followed by indirubin (about one-half), and then indigo (about one-sixth). These data suggest that tryptanthrin or other alkaloids, not indigo or indirubin, are responsible for the protective effect through IL-10-related pathway. Previous reports demonstrated that tryptanthrin act as therapeutic agent through production IL-10 in RA model mice [21], and that tryptanthrin ameliorates dextran sodium sulfate-induced colitis [22]. Tryptanthrin might be one of the key components of P. tinctorium leaves.
In this study, the mice receiving P. tinctorium leaves were protected from DSS-induced mucosal damage through the upregulation of IL-10. Conversely, blocking the IL-10 receptor restricted this protective effect, demonstrating that the protective effect of P. tinctorium leaves is associated with the upregulation of IL-10. The major source of IL-10 is CD4-positive regulatory T-cells that express Foxp3. However, the cells that are stimulated by P. tinctorium leaves to upregulate IL-10. Remain unclear. Previous reports have shown that kaempferol, 3,5,4′-trihydroxy-6,7-methylenedioxyflavone-O-glycosides, and their aglycones from indigo upregulate IL-10 in cultured macrophages [23,24]. Another study showed that IN induces the expansion of CD4-positive, Foxp3-negative, IL-10 producing T-cells, known as type I regulatory T-cells [6]. In our study, the expression of Foxp3 mRNA was not upregulated in the P. tinctorium-treated mice, suggesting that P. tinctorium might upregulate macrophage-derived IL-10 or CD4-positive, Foxp3-negative, IL-10-producing T cell-derived IL-10. In DSS-induced colitis, mucosal inflammation is known to develop via loss of intestinal epithelial cells as a steppingstone [25]. Loss of intestinal epithelial cells leads to mucosal inflammation by entry of luminal bacteria. The inflammation occurs in the absence of T-cells and B-cells [26], suggesting that this model of colitis is the best for research of innate immune systems including intestinal epithelial cells and macrophages. As for IL-10-related pathway in innate immune systems, Toll-like receptors-mediated IL-10 expression by macrophages play an important role to protect from mucosal injury. Therefore, mucosal macrophages may be a key player in the IL-10-related protective effect of P. tinctorium leaves. On the other hands, IL-10R-signaling pathway in intestinal epithelial cells including JAK/STAT pathway is also important for gut mucosal homeostasis [27]. To confirm the opinions, further studies are needed.
This study had some limitations. Notably, all the components of P. tinctorium leaves were not identified, in detail, and only one type of P. tinctorium leaves (Aomori Blue) was used. It is unknown whether one particular ingredient included in P. tinctorium leaves or a particular proportional mixture shows the protective effect. We demonstrated the effect of P. tinctorium leaves involving IL-10 pathway in vivo, while the protein level of IL-10 was not shown.
In conclusion, P. tinctorium leaves were found to ameliorate DSS-induced intestinal inflammation via IL-10-related pathway. The protective effect of P. tinctorium leaves on mucosal damage is independent of the AhR pathway, which is known to cause PAH. Therefore, P. tinctorium leaves are a potential new, safe treatment for UC.
Funding
This work was supported by a Hirosaki University Grant for Exploratory Research by Young Scientists and Newly Appointed Scientists, the Hirosaki University Guroukaru Fund, and an Interdisciplinary Collaborative Research Grant for Young Scientists from Hirosaki University.
Declaration of competing interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgments
We would like to thank the Aomoriai-Sangyo Corporation and the organic farmers of P. tinctorium for providing the Aomori Blue used in this study. English language editing was provided by Editage (https://www.editage.jp/).
Data availability
The data that has been used is confidential.
References
- 1.Piovani D., Danese S., Peyrin-Biroulet L., Nikolopoulos G.K., et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. 2019;157:647–659. doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 3.Jiang X.L., Cui H.F. An analysis of 10218 ulcerative colitis cases in China. World J. Gastroenterol. 2002;8:158–161. doi: 10.3748/wjg.v8.i1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimoto S., Naganuma M., Kiyohara H., Arai M., et al. Clinical efficacy and safety of oral Qing-Dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion. 2016;93:193–201. doi: 10.1159/000444217. [DOI] [PubMed] [Google Scholar]
- 5.Naganuma M., Sugimoto S., Mitsuyama K., Kobayashi T., et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018;154:935–947. doi: 10.1053/j.gastro.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Kawai S., Iijima H., Shinzaki S., Hiyama S., et al. Indigo naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J. Gastroenterol. 2017;52:904–919. doi: 10.1007/s00535-016-1292-z. [DOI] [PubMed] [Google Scholar]
- 7.Nishio M., Hirooka K., Doi Y. Chinese herbal drug natural indigo may cause pulmonary artery hypertension. Eur. Heart J. 2016;37:1992. doi: 10.1093/eurheartj/ehw090. [DOI] [PubMed] [Google Scholar]
- 8.Misumi K., Ogo T., Ueda J., Tsuji A., et al. Development of pulmonary arterial hypertension in a patient treated with qing-dai (Chinese herbal medicine) Intern. Med. 2019;58:395–399. doi: 10.2169/internalmedicine.1523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean A., Gregorc T., Docherty C.K., Harvey K.Y., et al. Role of the aryl hydrocarbon receptor in Sugen 5416-induced experimental pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2018;58:320–330. doi: 10.1165/rcmb.2017-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiraide T., Teratani T., Uemura S., Yoshimatsu Y., et al. Pulmonary arterial hypertension caused by AhR signal activation protecting against colitis. Am. J. Respir. Crit. Care Med. 2021;203:385–388. doi: 10.1164/rccm.202009-3385LE. [DOI] [PubMed] [Google Scholar]
- 11.Mizoguchi E., Mizoguchi A., Takedatsu H., Cario E., et al. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122:134–144. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 12.Uchino M., Ikeuchi H., Hata K., Okada S., et al. Changes in the rate of and trends in colectomy for ulcerative colitis during the era of biologics and calcineurin inhibitors based on a Japanese nationwide cohort study. Surg. Today. 2019;49:1066–1073. doi: 10.1007/s00595-019-01845-2. [DOI] [PubMed] [Google Scholar]
- 13.Barnes E.L., Jiang Y., Kappelman M.D., Long M.D., et al. Decreasing colectomy rate for ulcerative colitis in the United States between 2007 and 2016: a time trend analysis, Inflamm. Bowel Dis. 2020;26:1225–1231. doi: 10.1093/ibd/izz247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Valk M.E., Mangen M.J., Leenders M., Dijkstra G., et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72–79. doi: 10.1136/gutjnl-2012-303376. [DOI] [PubMed] [Google Scholar]
- 15.Saito S., Nakazawa K., Suzuki K., Ishikawa T., et al. Paradigm shift of healthcare cost for patients with inflammatory bowel diseases: a claims data-based analysis in Japan. Gastrointest. Disord. 2019;1:120–128. doi: 10.3390/gidisord1010009. [DOI] [Google Scholar]
- 16.Suzuki H., Kaneko T., Mizokami Y., Narasaka T., et al. Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis. World J. Gastroenterol. 2013;19:2718–2722. doi: 10.3748/wjg.v19.i17.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimoto S., Naganuma M., Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J. Gastroenterol. 2016;51:853–861. doi: 10.1007/s00535-016-1220-2. [DOI] [PubMed] [Google Scholar]
- 18.Qiu J., Guo X., Chen Z.M., He L., et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinese Pharmacopoeia Commission . China Medical Science Press; Beijing: 2015. Chinese Pharmacopoeia Part 1. [Google Scholar]
- 20.Chiang Y., Li A., Leu Y., Fang J.Y., et al. An in vitro study of the antimicrobial effects of indigo naturalis prepared from Strobilanthes formosanus Moore. Molecules. 2013;18:14381–14396. doi: 10.3390/molecules181114381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirpotina L.N., Schepetkin I.A., Hammaker D., Kuhs A., et al. Therapeutic effects of tryptanthrin and tryptanthrin-6-oxime in models of rheumatoid arthritis. Front. Pharmacol. 24 July 2020 doi: 10.3389/fphar.2020.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micallef M.J., Iwaki K., Ishihara T., Ushio S., et al. The natural plant product tryptanthrin ameliorates dextran sodium sulfate-induced colitis in mice. Int. Immunopharm. 2002;2:565–578. doi: 10.1016/s1567-5769(01)00206-5. [DOI] [PubMed] [Google Scholar]
- 23.Palacz-Wrobel M., Borkowska P., Paul-Samojedny M., Kowalczyk M., et al. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 2017;93:1205–1212. doi: 10.1016/j.biopha.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 24.Kimura H., Tokuyama-Nakai S., Hirabayashi Y., Jisaka M., et al. Anti-inflammatory and bioavailability studies on dietary 3,5,4’-trihydroxy-6,7-methylenedioxyflavone-O-glycosides and their aglycone from indigo leaves in a murine model of inflammatory bowel disease. J. Pharm. Biomed. Anal. 2021;193:113716. doi: 10.1016/j.jpba.2020.113716. [DOI] [PubMed] [Google Scholar]
- 25.Kiesler P., Fuss I.J., Strober W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015;1:154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieleman L.A., Ridwan B.U., Tennyson G.S., Beagley K.W., et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 27.Willson T.A., Jurickova I., Collins M., Denson L.A. Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm. Bowel Dis. 2013;19:512–525. doi: 10.1097/MIB.0b013e31828028ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.