Highlights
-
•
Hemp seed protein (HPI) stabilized hemp seed oil (HSO) emulsion was prepared.
-
•
The emulsion was treated with high intensity ultrasonic (HIU) treatment.
-
•
The emulsion has the optimal properties at a HIU power of 450 W.
-
•
The emulsion has good stability and tetrahydrocannabinol retention.
Keywords: Hemp seed protein (HPI), Hemp seed oil (HSO), High-intensity ultrasonic (HIU), Emulsion, Oxidative stability
Abstract
In this study, hemp seed oil (HSO) emulsions stabilized with hemp seed protein (HPI) were prepared and treated with high intensity ultrasonic (HIU). The effects of different treatment powers (0, 150, 300, 450, 600 W) on the properties, microstructure and stability of emulsions were investigated. HIU-treated emulsions showed improved emulsifying activity index and emulsifying stability index, reduced particle size, and increased absolute values of ζ-potential, with the extreme points of these indices occurring at a treatment power of 450 W. Here, the emulsion showed the best dispersion and the smallest particle size in fluorescence microscopy observation, with the highest adsorbed protein content (30.12%), and the highest tetrahydrocannabinol (THC) retention rate (87.64%). The best thermal and oxidative stability of the emulsions were obtained under HIU treatment with a power of 450 W. The D43 and the peroxide values (POV) values after 30 d storage were the smallest at 985.74 ± 64.89 nm and 4.6 μmol/L, respectively. Therefore, 450 W was optimal HIU power to effectively improve the properties of HPI-stabilized HSO emulsion and promote the application of HSO and its derivatives in food processing production.
1. Introduction
Hemp is an industrial crop that is commonly used in the textile, pharmaceutical, and paper industries. Hemp seeds are a by-product of hemp processing and contain 25–30% oil [1], 20–30% protein and other nutritional substances[2]. Hemp seed oil (HSO) contains more than 80% unsaturated fatty acids and a high content of functional components, including linoleic acid (LA), linolenic acid (LNA) and other essential fatty acids, as well as tocopherols, vitamin A, minerals, etc., which have a positive impact on cardiovascular, mental and immune diseases and can be added to foods as functional fats [3], [4]. HSO contains low levels of the psychoactive compound tetrahydrocannabinol (THC) (<0.2%) in compliance with European Union and American standards [5]. THC is a polyphenol with high antioxidant properties, which enhances the antioxidant properties of the product. Therefore, encapsulating HSO in emulsions can improve its bioavailability[6], avoid oxidation reactions, and broaden its application scope [7]. Mikulcová et al. prepared HSO oil-in-water emulsion with emulsifier Tween to improve its stability and antimicrobial activity [8]. Jarzebski et al. used pea protein as a stabilizer for HSO emulsions and the emulsions showed good results in terms of particle size and encapsulation efficiency [9].
Proteins with amphiphilic properties can be used as emulsifiers, and similarly, hemp seed proteins (HPI) can be used to stabilize HSO emulsions. Compared to common high-quality proteins (Soy protein, egg white protein, rice bran protein, etc.), HPI is hypoallergenic, highly digestibility, has a good composition of essential amino acids (with high levels of arginine and glutamine at 12% and 15.99%, respectively). This means a more nutritionally superior amino acid pattern [10]. In addition, its solubility is low, and its emulsification ability can be affected by external factors such as pH during treatment [11].
HPI-stabilized sunflower oil emulsions were prepared by Dapčević-Hadnađevd et al [12]. It was found that the interaction between HPIs affected the transient flocculation network and contributed to the emulsion stabilization, and the emulsification activity of HPIs was similar to the protein solubility curve. Tang et al. have compared the functional properties of different proteins, where HPI has lower emulsifying activity and emulsifying stability than soybean protein [13], rice protein [49] and pea protein. Therefore, external more energy-efficient means or devices are needed to assist in the preparation of stable emulsions, such as ultrasonic, microwave, microjet, etc.
High-intensity ultrasonic (HIU) is an emerging processing method with frequencies typically in the range of 20–100 kHz and intensities in the range of 10–1000 W/cm2 [14]. HIU treatment can reduce the particle size, increase solubility and improve the emulsification characteristics of protein. In Zhao et al.'s study, the solubility, foaming and emulsification of perilla protein isolate (PPI) were improved after ultrasonic treatment [15]. Meanwhile, the interfacial waves and cavitation bubbles generated by the sound field broke up the oil droplets, reducing the emulsion droplet size [16], [17]. In addition, the mechanical shearing, thermal effects, dynamic stirring and turbulence generated by ultrasonic would further process the emulsion and change the emulsion properties [18]. Taha et al.[19] investigated ultrasonic-assisted emulsions stabilized with different plant and animal proteins and found that ultrasonic treatment increased the interfacial protein content and thermal stability. Wang et al. [20] has studied the effect of different HIU powers on soybean isolate protein-pectin emulsions and the its stability was highest at 450 W. HIU has been considered as a green, safe and efficient method to assist in the preparation of emulsions.
In this study, the HSO emulsions stabilized with hemp seed protein were prepared and the effects of different HIU treatment powers on the physicochemical properties, microstructure and stability of the emulsions were investigated. The emulsification properties, particle size and potential of the emulsions under different conditions were explored in the study, and the effects of HIU treatment on the thermal and oxidative stability of the emulsions were discussed. We expect the HIU treatment to improve the emulsification performance of HPI, enhance the stability of HSO emulsions, improve the retention of THC, and further expand the application of processed products from hemp seeds in the food industry.
2. Materials and methods
2.1. Materials
Hemp seeds were provided by Shanxi Qinchang Seed Co., Ltd. (Shanxi, China). Other reagents used in this work were of analytical grade.
2.2. Preparation of HPI
The extraction method of HPI was referred to the method described by Tang et al [21]. Hemp seed powder was dispersed in deionized water (1:20, w/v) and the pH was adjusted to 10.0 with 2 mol/L NaOH to dissolve the protein. After stirring for 2 h at 37 °C and centrifugation (7000× g for 60 min at 4 °C), the supernatant was adjusted to pH 5.0 with 2 mol/L HCL, the protein was precipitated and centrifuged again (7000× g for 60 min at 4 °C). The Kjeldahl method determined the protein content to be 96.62% ± 0.74%. The precipitate was dispersed in deionized water, pH adjusted to 7.0 with 2 M NaOH, and freeze-dried to obtain HPI.
2.3. Extraction of HSO
The extraction of HSO was referred to the method of Shi et al. and used for subsequent experiments [22].
2.4. Preparation of emulsion
HPI was dissolved in phosphate buffer solution (0.1 M, pH = 7.0), HSO (oil-water ratio 9:1) was added, and the HSO oil-in-water emulsion stabilized by HPI was continuously mixed with a homogenizer (Ultra-Turrax T18, Angi Co., Shanghai, China) at 12000 rpm for 5 min. The beaker containing the emulsion was then placed in ice water and kept at a temperature below 20 °C. The solution was then treated with an ultrasonic generator (TL-650Y, Jiangsu Tianling Instrument Co., Ltd., Yancheng, China). Different ultrasound powers (0,150,300,450,600 W) were used for 10 min of treatment at 20 kHz ultrasound frequency. The ultrasonic titanium probe was immersed in the emulsion at a depth of 1 cm from the bottom of the emulsion, and the ultrasonic pulse system was operated for 2 s with a 2 s rest. Store the emulsion at 4 °C until further analysis.
2.5. Determination of emulsifying properties
The emulsification properties as measured by the method of Li et al [19] with some modifications [23]. 50 μL of HSO emulsions treated with different power HIU were taken separately and diluted 0.1% (w/v) sodium dodecyl sulfate solution was added. The absorbance at 500 nm was measured and the emulsification activity index (EAI) and emulsion stability index (ESI) were calculated.
where A0 is the absorbance at 0 min, D and F are dilution factors (100), C is the protein concentration before emulsification (g/mL), θ is the oil volume fraction of the emulsion (v/v), L is the optical path (1 cm), and A10 is the absorbance at 10 min.
2.6. Determination of particle size and ζ-potential
The particle size, volume mean diameter (D43) and ζ-potential of the emulsions treated with different ultrasonic powers were measured by a laser light diffraction particle size analyzer (Mastersizer 2000; Malvern Instrument Co., Ltd., UK.). The phosphate buffer (0.01 M, pH 7.0) was selected as the solvent to dilute the emulsion samples HIU-treated with different power. The refractive indices of the particles and dispersant were set to 1.46 and 1.33, respectively. The D43 and ζ-potentia of emulsions were measured at 25 °C.
2.7. Fluorescence microscopy observation
Fluorescence microscopy was selected to observe the type of emulsions with different powers of HIU treatment. Nile Red was dissolved in isopropyl alcohol and 0.1% of the staining solution was prepared, mixed well and filtered. Add 20 μL of Nile Red staining solution to 0.5 mL of emulsion treated with different ultrasonic power and stain for 30 min. The stained samples were placed on microscope slides and covered with coverslips to obtain images.
2.8. Distribution of interfacial proteins
The HSO emulsion was centrifuged at 10,000× g for 30 min to separate the non-adsorbed and adsorbed proteins [24]. The top cream layer was removed with a disposable syringe and filtered with a 0.45 μm filter membrane. The amounts of non-adsorbed and adsorbed proteins were subsequently calculated using the Lowry method.
2.9. Determination of thermal stability
The thermal stability of HSO emulsions ultrasonicated at different powers were studied by heating at 100 °C for 20 min and cooling to room temperature, respectively, and the particle size distribution was determined.
2.10. The THC retention rate
THC retention studies were performed on HSO emulsions treated with different power sonication. The THC content was measured and THC retention was calculated by keeping the emulsions in a thermostat at 20 °C for 0, 5, 10, 15, 20, 25 and 30 days. The samples were prepared with reference to the study of Shi et al [22]. The separation was performed on an Acquity UPLC HSS C18 column (2.1 mm × 150 mm, 1.8 μm) and the THC content was determined by ultra-performance liquid chromatography-mass spectrometry (UHPLC-MS/MS) system [25].
where E is the THC content of HSO emulsion at the nth day under different power HIU treatment and E0 is the THC content of HSO emulsion at the 0th day under different power HIU treatment.
2.11. Determination of oxidative stability
The oxidative stability of HSO emulsions treated with different powers of ultrasonic was studied and the peroxide values (POV) of the emulsions were determined by keeping them in a thermostat at 20 °C for 0, 5, 10, 15, 20, 25 and 30 d, where POV was determined according to the American Oil Chemists Society (AOCS) official method Cd 8–53 [26].
2.12. Statistical analysis
All the tests were repeated three times. The average and standard error values of the test results were taken. The basic data were collated, analyzed and plotted by Origin 2018 statistical analysis software. Data were subjected to one-way analysis of variance (ANOVA) using SPSS 26.0, and the differences were tested by Duncan Multiple Range Test (P < 0.05).
3. Results and discussion
3.1. Emulsifying properties analysis
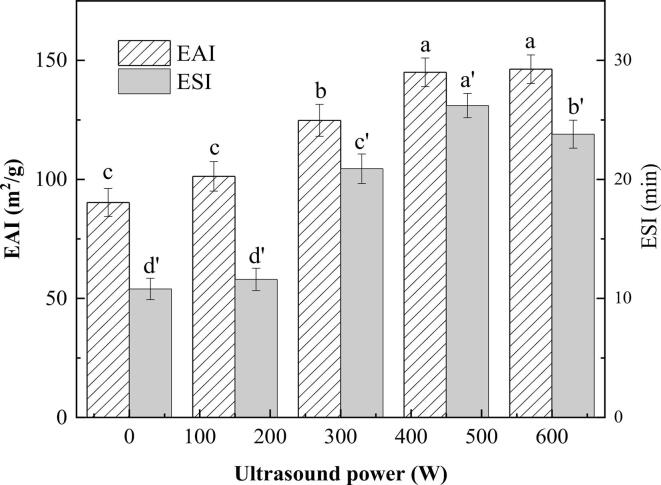
Emulsification properties describe the ability of which proteins form and stabilize emulsions [27]. The EAI and ESI of HPI-stabilized HSO emulsions under HIU treatment with different powers are shown in Fig. 1. Both indicators were improved in all samples following the HIU treatment. The best emulsifying properties of the emulsions were obtained at an ultrasonic power of 450 W. At this power, the EAI and ESI were 145.0 m2/g and 26.2 min, respectively. This improvement may be due to the increased solubility of HPI by HIU treatment, as demonstrated by the study of Feng et al [28], where HIU-treated buckwheat protein had higher solubility and better emulsifying properties. On the other hand, the cavitation of HIU temporarily dispersed or broke the non-covalent bonds that maintain the spatial structure of the protein. In this case, HPI was adsorbed in an orderly arrangement at the oil-water interface. At lower HIU power, the increase of ESI and EAI was not significant, indicating that the effect of HPI at the oil-water interface was different in emulsions treated with different HIU power. At a power of 600 W, too high power may affect the degree of protein aggregation, which was consistent with the study of Wang et al [5]. High power destroyed the spherical structure of proteins, formed protein aggregates, and reduced the emulsifying properties.
Fig. 1.
Emulsifying activity index (EAI) and emulsifying stability index (ESI) of emulsions at different ultrasonic powers. Different letters represent significant differences at p < 0.05.
3.2. Particle size, PDI, and ζ-potential analysis
The HPI-stabilized HSO emulsions showed different particle size, PDI and ζ-potential under HIU treatment at different power levels, as reflected by the values in Table 1. The D43 and PDI of HIU-treated emulsions were significantly lower (P < 0.05) compared with those of non-HIU-treated emulsions.
Table 1.
Particle size, PDI and ζ-potential of emulsions at different ultrasonic powers.
| Ultrasound power (W) | D43 (nm) | PDI | ζ-potential (mV) |
|---|---|---|---|
| 0 | 1593.85 ± 6.45a | 0.832 ± 0.088a | −7.42 ± 0.36a |
| 150 | 1296.78 ± 5.16b | 0.478 ± 0.036b | −11.56 ± 0.27b |
| 300 | 496.66 ± 3.28d | 0.325 ± 0.057c | −14.87 ± 0.43c |
| 450 | 497.66 ± 3.05d | 0.297 ± 0.042c | −17.69 ± 0.16d |
| 600 | 584.52 ± 6.04c | 0.421 ± 0.073bc | −15.23 ± 0.25c |
Note: The different superscript letters in the same column indicate significant differences between the data (p < 0.05).
With the increase of HIU power, the particle size and PDI showed a trend of increasing and then decreasing, reaching the minimum at 450 W. At this power, the D43 of the emulsion was 497.66 ± 3.17 nm and the PDI was 0.297 ± 0.042, and the particles in the emulsion had similar sizes and were uniformly distributed. This reduction may be due to cavitation effects, turbulence and strong shear forces generated by HIU treatment, which disrupt some of the larger insoluble protein aggregates and allow HPI to be more uniformly dispersed in the emulsion [29]. The HIU treatment at high power (600 W) showed a slight but significant increase, a phenomenon that occurred probably owing to the re-agglomeration of particles in the emulsion. Similar findings emerged in the study of Wang et al [30], where cavitation of ultrasound disrupted the spatial structure of rice bran proteins, causing intermolecular collisions and reducing the particle size. The smaller particle size and PDI facilitate more HPI adsorption at the oil-water interface and maintain the stability of the emulsion. The presence of protein charges the emulsion and the ζ-potential measurement can also be used to determine the stability of the emulsion. The absolute value of ζ-potential increased significantly after HIU treatment, reaching a maximum at 450 W. The reason for this phenomenon may be that HIU treatment can unfold the dense structure of HPI, slightly change its surface charge, improve the electrostatic repulsion between HPI particles, limit the occurrence of aggregation, and further improve the stability of the emulsion [17]. Therefore, the 450 W HIU treatment resulted in the emulsion with the smallest particle size and PDI and the highest absolute value of ζ-potential.
3.3. Fluorescence microscopy observation
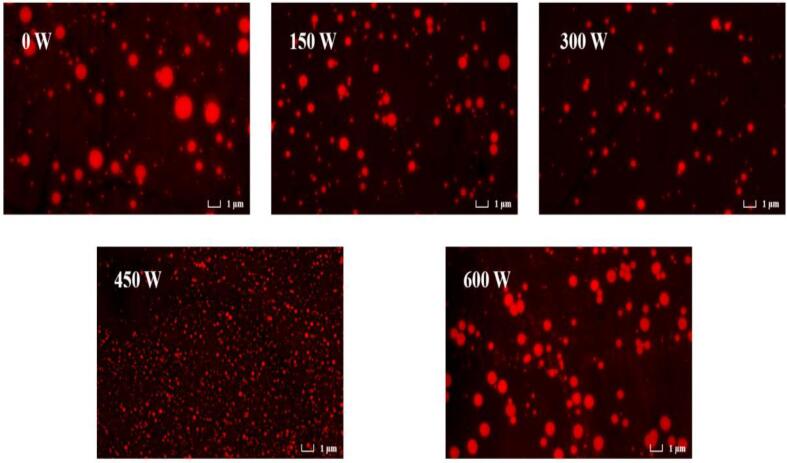
The HSO emulsions treated with different powers were subjected to fluorescence microscopy images, as shown in Fig. 2. It can be seen that HSO (red) is covered by HPI (green), and the HIU treatment increases the dispersibility of the emulsion and avoids aggregation of the emulsion compared to the emulsion without HIU treatment. The smallest particle size of the emulsion was observed at a HIU power of 450 W, a phenomenon that remained consistent with our previous study [20]. The particle size reduction is probably caused by the cavitation force generated by the microflow of the ultrasonically treated probe. And it disrupts electrostatic and hydrophobic interactions in proteins, preventing the formation of aggregates and promoting the formation of small particle sizes [31]. In the study by Cui et al [32], the protein was treated by ultrasonic to have a smaller particle size, which was beneficial to improve the emulsification properties of the protein and enhance the stability of the emulsion stabilized by the protein, providing some basis for the next studies.
Fig. 2.
Fluorescence microscopy of emulsions at different ultrasonic powers.
3.4. Distribution of interfacial proteins
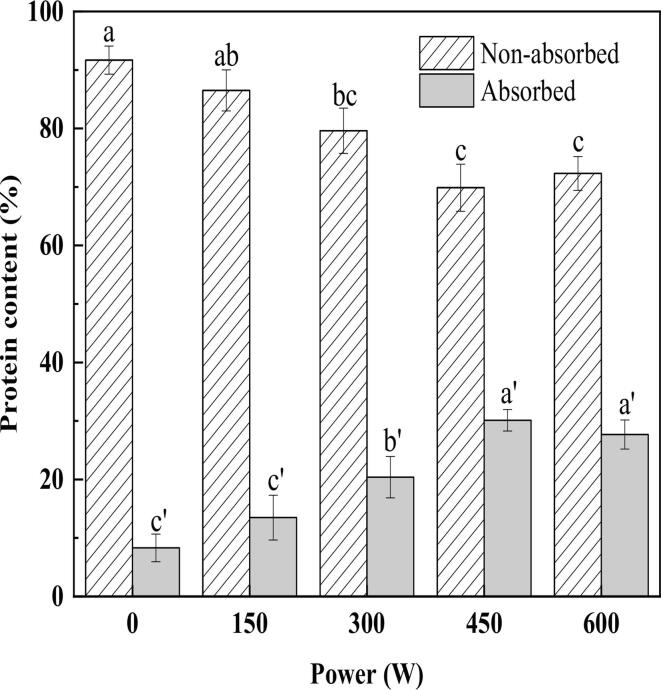
The amount of protein adsorbed and loaded at the emulsion interface is an important indicator of the stability of the emulsion [33]. The higher the amount of protein adsorbed at the interface, the more stable the oil-in-water emulsion will be during storage [34]. The interfacial protein distribution of the emulsions treated with different HIU power can be seen in Fig. 3. With the increase of HIU power, the adsorbed protein content showed a trend of increasing and then slightly decreasing, while the non-adsorbed protein content showed the opposite trend. At a treated power of 450 W, the adsorbed protein content reached 30.12% and the non-adsorbed protein content reached 69.88%. This result proved that the HIU treatment benefited the adsorption of HPI on the HSO surface. At that time, HIU may lead to the decrease of HSO oil droplet size and increase of surface area, which was one of the reasons for the increase of adsorbed protein content [35]. In addition, HIU reduced the particle size of HPI (Trends consistent with those in 3.2 and 3.3), which accelerated the diffusion rate of protein and made it easier to rearrange at the oil-water interface to reduce interfacial tension, which can improve emulsion stability. Interestingly, when the HIU power was increased to 600 W, the adsorbed protein content decreased slightly to 27.69%. This phenomenon was consistent with some existing studies, where too high a power can lead to the phenomenon of “overprocessing” [8], [36], where the emulsion showed a tendency to re-agglomerate, increasing the emulsion particle size, which was detrimental to the stability of the emulsion. The same was found in a study by Li et al [37] on casein emulsified soybean oil, where prolonged sonication reduced the amount of adsorbed protein in the casein emulsion, though not significantly.
Fig. 3.
Non-adsorbed and adsorbed proteins of emulsions at different ultrasonic powers. Different letters represent significant differences at p < 0.05.
3.5. Thermal stability
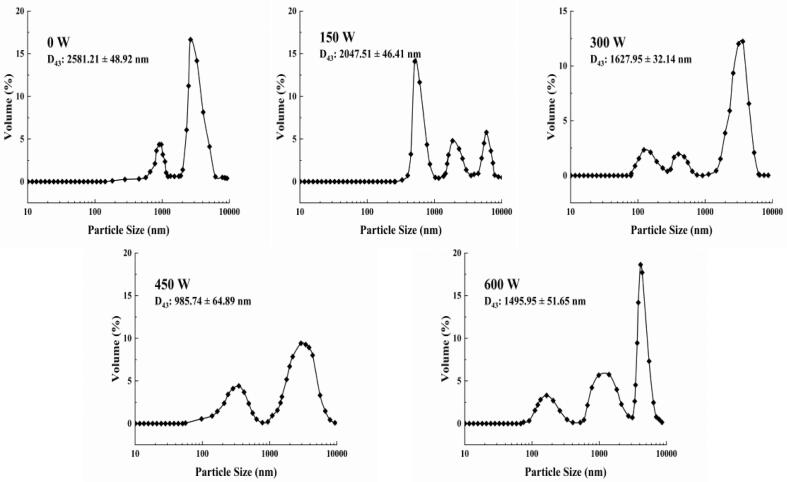
HIU treatment alters the emulsifying properties while also improving the stability of the emulsion. In processing and production, changes in temperature may change the interfacial behavior and the aggregation degree of proteins, so the evaluation of the thermal stability of HIU-assisted prepared emulsion samples is necessary. The effect of different HIU power on the particle size distribution of the heat-treated emulsions is shown in Fig. 4. The emulsions without HIU treatment have the largest D43, and poor dispersion. This phenomenon implied that heat treatment led to the formation of protein aggregates, which resulted in larger emulsion sizes and inhomogeneous particle size distributions. As a control group, the emulsions without ultrasonic treatment, formed protein aggregates under heating conditions, resulting in larger emulsion sizes and non-uniform particle size distribution [38]. Meanwhile, the D43 of the emulsion droplets decreased significantly after the HIU treatment. Among them, the smallest D43 of the emulsion was 985.74 ± 64.89 nm at a power of 450 W. The dispersion of the emulsion was also improved at this time, and the thermal stability reached the best compared with the other treatment powers selected. This conclusion was also demonstrated in the study by Li et al. The ultrasonicated oat proteins showed smoother fluctuations in particle size during heating and had better resistance to thermal aggregation [39]. At a power of 600 W, the D43 of the emulsion showed a rebound, which was in agreement with the results in 3.4, probably owing to the excessive ultrasonic intensity affecting the emulsion stability.
Fig. 4.
Thermal stability analysis under different ultrasound powers.
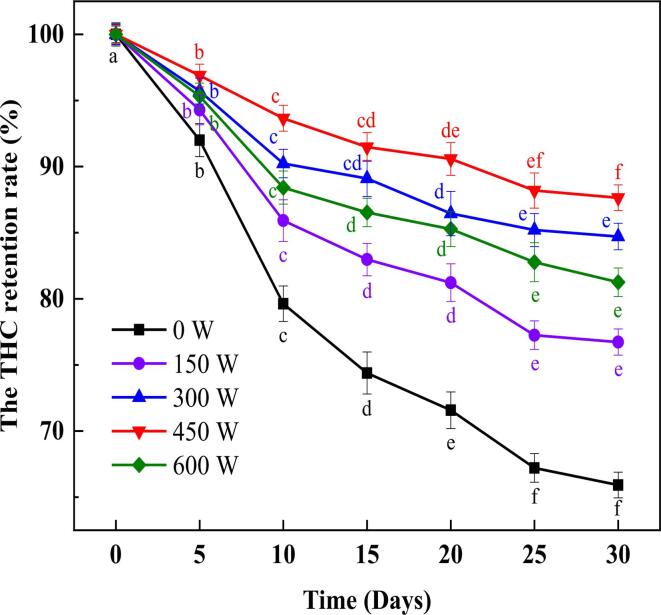
3.6. The THC retention rate
THC is an unstable polyphenol in HSO and is subject to oxidative degradation during storage, thus the retention rate of THC in emulsions can be used as an indicator to evaluate the stability of emulsions. The THC retention rates of the emulsions under different power HIU treatments after storage for 0, 5, 10, 15, 20, 25, and 30 d are shown in Fig. 5. The THC retention rate of all emulsions showed a decreasing trend as the storage time increased, and the retention rate of the emulsions without HIU treatment decreased to 65.92% after 30 days of storage, while the rest of them were above 75%. Furthermore, the THC retention rate for 30 Days was the first to increase and then decrease with increasing ultrasonic power, and the highest value occurred at a power of 450 W with 87.64%. After increasing the power to 600 W, the THC retention decreased to 81.25%. It can be concluded that HIU treatment promoted the formation of a denser and thicker interfacial layer of the emulsion, which hindered the diffusion of oxygen and oxidant, thereby avoiding the oxidative degradation of THC [40]. This was also discussed in Sun et al.'s study, where ultrasonic treatment-assisted α-tocopherol/casein-stabilized grape seed oil emulsions were effective in inhibiting the formation of oxidative radicals in the oil and avoiding molecular degradation [41]. At an ultrasonic power of 600 W, a decrease in THC retention rate was observed. The appearance of this phenomenon was associated with the HIU treatment promoting the dissociation of water molecules and the production of free radicals. The rupture of cavitation bubbles generated by ultrasonics can produce free radicals, and the amounts of free radicals would be approximately proportional to the ultrasonic power [50]. Thus, at a treatment power of 600 W, free radicals caused oxidative degradation of THC, leading to a decrease in THC retention rate.
Fig. 5.
The THC retention rate of emulsions at different ultrasonic powers during storage. Different letters represent significant differences at p < 0.05.
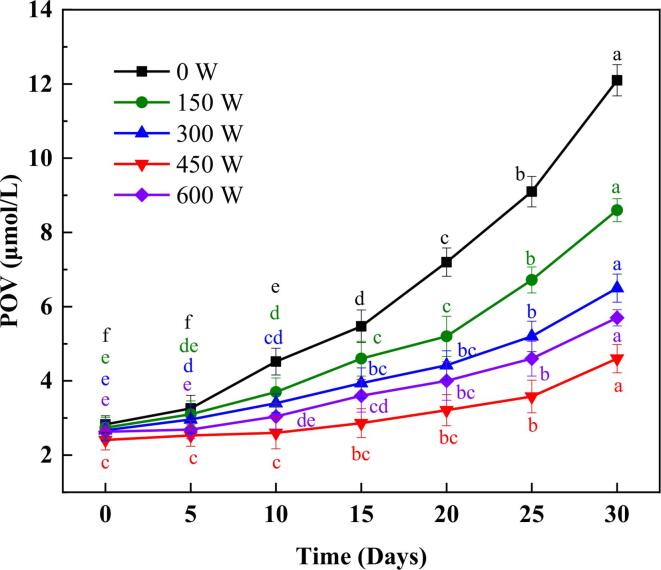
3.7. Oxidative stability
The POV value is the degree of oil oxidation, which mainly determined the hydroperoxides produced by the reaction of unsaturated fatty acids with oxygen, and can be used to evaluate the oxidative stability of the emulsion. The POV of HSO emulsions treated with different power HIU at days 0, 5, 10, 15, 20, 25 and 30 are shown in Fig. 6. The POV of all HSO emulsions increased with the storage time within 30 d. It can be observed that the POV values during storage of the emulsions were significantly reduced after treatment compared to those of the non-HIU treated emulsions during storage 10–30 d (p < 0.05). This phenomenon demonstrated that the HIU treatment was able to inhibit the increase of hydroperoxides in HSO and improve the oxidative stability of the emulsions. This inhibition effect was optimal at a power of 450 W. The POV value of 4.6 μmol/L was the lowest value after 30 d storage. The reason for this phenomenon is in agreement with the findings in 3.2 and 3.4 above, that the 450 W-treated emulsion has the smallest particle size and the highest adsorbed protein content, where the reduction in particle size may enhance the vulnerability of the emulsion to oxygen reaction [42], [43]; Increased adsorption proteins allow faster scavenging of free radicals to inhibit lipid oxidation [44], [45], thus improving the oxidative stability. This same phenomenon was also found in the study by Li et al. Appropriate sonication time helped to improve the oxidative stability of sodium caseinate – soybean oil emulsion [37], [46]. The POV value is 5.7 μmol/L at 600 W, which is higher than the emulsion at 450 W. This may be caused by the high sound field of HIU and the strong cavitation effect promoting the formation of highly reactive radicals (hydrogen and hydroxyl radicals), which further synthesize hydrogen peroxide and oxidize HSO [47], [48]. Thus, while HIU treatment resulted in an increase in hydrogen peroxide content. At 450 W treatment power, it still decreased the POV value during storage and improved the oxidative stability of the emulsion.
Fig. 6.
The oxidation stability of emulsions at different ultrasonic powers during storage. Different letters represent significant differences at p < 0.05.
4. Conclusion
In this study, HPI-stabilized HSO emulsions were prepared by HIU treatment, so that HPI was adsorbed at the oil-water interface and arranged in an orderly manner, and the effects of treatment power on the physicochemical properties, microstructure and stability of the emulsions were discussed. The HIU treatment improved the emulsifying properties, obtaining a more uniform particle distribution and higher potentials. The particle size of the HIU treated emulsion in the fluorescence microscope image was small and well dispersed. Meanwhile, HIU treatment significantly increased the amount of adsorbed protein, THC retention rate, thermal stability and oxidative stability in the emulsion. In summary, HIU treatment is considered as a suitable technique to assist in the preparation of HSO emulsions stabilized by HPI. The treatment at appropriate ultrasonic power helps to obtain HSO emulsions with desirable properties, which effectively protect the bioactive components in the oil. This further expands its application in the food industry.
CRediT authorship contribution statement
Na Li: Methodology, Investigation, Writing – original draft. Tong Wang: Investigation, Validation, Data curation. Xinrun Yang: Formal analysis. Jiayao Qu: Supervision. Ning Wang: Visualization. Liqi Wang: Conceptualization, Writing – review & editing. Dianyu Yu: Funding acquisition. Cuiping Han: Methodology, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by a grant from the 14th Five-Year National Key R&D Program: R&D of key technologies for flexible processing of bulk oilseed protein (No: 2021YFD2100401).
Contributor Information
Dianyu Yu, Email: dyyu2000@126.com.
Cuiping Han, Email: cphan2006@126.com.
References
- 1.Callaway J.C. Hempseed as a nutritional resource: An overview. Euphytica. 2004;140(1-2):65–72. [Google Scholar]
- 2.House J.D., Neufeld J., Leson G. Evaluating the Quality of Protein from Hemp Seed (Cannabis sativa L.) Products Through the use of the Protein Digestibility-Corrected Amino Acid Score Method. J Agric Food Chem. 2010;58:11801–11807. doi: 10.1021/jf102636b. [DOI] [PubMed] [Google Scholar]
- 3.Montserrat-De L.P.S., Marín-Aguilar F., García-Giménez M.D., Fernández-Arche M.A. Hemp (Cannabis sativa L.) Seed Oil: Analytical and Phytochemical Characterization of the Unsaponifiable Fraction. Journal of Agricultural & Food Chemistry. 2014;62:1105–1110. doi: 10.1021/jf404278q. [DOI] [PubMed] [Google Scholar]
- 4.International Journal of Food Science & Technology. 2020 [Google Scholar]
- 5.Wang T., Chen K., Zhang X., Yu Y., Yu D., Jiang L., Wang L. Effect of ultrasound on the preparation of soy protein isolate-maltodextrin embedded hemp seed oil microcapsules and the establishment of oxidation kinetics models. Ultrasonics Sonochemistry. 2021;77 doi: 10.1016/j.ultsonch.2021.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Current Opinion in Colloid & Interface Science. 2009;14:3–15. [Google Scholar]
- 7.Raikos V., Ranawana V. Designing emulsion droplets of foods and beverages to enhance delivery of lipophilic bioactive components – a review of recent advances. International Journal of Food Science & Technology. 2017;52:68–80. [Google Scholar]
- 8.Desrumaux A., Marcand J. Formation of sunflower oil emulsions stabilized by whey proteins with high-pressure homogenization (up to 350MPa): effect of pressure on emulsion characteristics. International Journal of Food Science & Technology. 2002;37(3):263–269. [Google Scholar]
- 9.Jarzębski M., Fathordoobady F., Guo Y., Xu M., Singh A., Kitts D.D., Kowalczewski P.Ł., Jeżowski P., Singh A.P. Pea Protein for Hempseed Oil Nanoemulsion Stabilization. Molecules. 2019;24(23):4288. doi: 10.3390/molecules24234288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X.S., Tang C.H., Yang X.Q., Gao W.R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chemistry. 2008;107:11–18. [Google Scholar]
- 11.Nishinari K., Fang Y., Guo S., Phillips G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocolloids. 2014;39:301–318. [Google Scholar]
- 12.Dapcevic-Hadnadev T., Dizdar M., Pojic M., Krstonosic V., Zychowski L.M., Hadnadev M. Emulsifying properties of hemp proteins: Effect of isolation technique. Food Hydrocolloids. 2019;89:912–920. [Google Scholar]
- 13.Tang C.H., Zi T., Wang X.S., Yang X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J Agric Food Chem. 2006;54:8945–8950. doi: 10.1021/jf0619176. [DOI] [PubMed] [Google Scholar]
- 14.Kentish S., Feng H. Applications of Power Ultrasound in Food Processing. Annual Review of Food Science & Technology. 2014;5(1):263–284. doi: 10.1146/annurev-food-030212-182537. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q., Xie T., Hong X., Zhou Y., Fan L., Liu Y., Li J. Modification of functional properties of perilla protein isolate by high-intensity ultrasonic treatment and the stability of o/w emulsion. Food Chemistry. 2022;368 doi: 10.1016/j.foodchem.2021.130848. [DOI] [PubMed] [Google Scholar]
- 16.Ashokkumar M. Applications of ultrasound in food and bioprocessing. Ultrasonics Sonochemistry. 2015;25:17–23. doi: 10.1016/j.ultsonch.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Gul O., Saricaoglu F.T., Besir A., Atalar I., Yazici F. Effect of ultrasound treatment on the properties of nano-emulsion films obtained from hazelnut meal protein and clove essential oil. Ultrasonics Sonochemistry. 2018;41:466–474. doi: 10.1016/j.ultsonch.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Meng Y., Liang Z., Zhang C., Hao S., Han H., Du P., Li A., Shao H., Li C., Liu L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT. 2021;152 [Google Scholar]
- 19.Taha A., Ahmed E., Hu T., Xu X., Pan S., Hu H. Effects of different ionic strengths on the physicochemical properties of plant and animal proteins-stabilized emulsions fabricated using ultrasound emulsification. Ultrasonics Sonochemistry. 2019;58 doi: 10.1016/j.ultsonch.2019.104627. [DOI] [PubMed] [Google Scholar]
- 20.Wang T., Wang N., Li N., Ji X., Zhang H., Yu D., Wang L. Effect of high-intensity ultrasound on the physicochemical properties, microstructure, and stability of soy protein isolate-pectin emulsion. Ultrasonics Sonochemistry. 2022;82 doi: 10.1016/j.ultsonch.2021.105871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang C., Ten Z., Wang X., Yang X. Physicochemical and Functional Properties of Hemp (Cannabis sativa L.) Protein Isolate. Journal of Agricultural and Food Chemistry. 2006;54:8945–8950. doi: 10.1021/jf0619176. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y., Wang W., Zhu X., Wang B., Hao Y., Wang L., Yu D., Elfalleh W. Preparation and physicochemical stability of hemp seed oil liposomes. Industrial Crops and Products. 2021;162:113283. [Google Scholar]
- 23.Li C., Huang X., Peng Q., Shan Y., Xue F. Physicochemical properties of peanut protein isolate-glucomannan conjugates prepared by ultrasonic treatment. Ultrasonics – Sonochemistry. 2014;21:1722–1727. doi: 10.1016/j.ultsonch.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J., Zhu B.o., Liu Y., Xiong Y.L. Interfacial Structural Role of pH-Shifting Processed Pea Protein in the Oxidative Stability of Oil/Water Emulsions. J. Agric. Food Chem. 2014;62(7):1683–1691. doi: 10.1021/jf405190h. [DOI] [PubMed] [Google Scholar]
- 25.Pacifici R., Marchei E., Salvatore F., Guandalini L., Busardò F.P., Pichini S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clinical chemistry and laboratory medicine. 2017;55:1555. doi: 10.1515/cclm-2016-1060. [DOI] [PubMed] [Google Scholar]
- 26.AOCS, Official Method and Recommended Practices of the American Oil Chemists’ Society, American Oil Chemists’ Society. Champaign (2009).
- 27.Malik M.A., Sharma H.K., Saini C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrasonics Sonochemistry. 2017;39:511–519. doi: 10.1016/j.ultsonch.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 28.F. Xue, Z. Wu, J. Tong, J. Zheng, and C. Li, Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Bioscience, Biotechnology, and Biochemistry (2017). [DOI] [PubMed]
- 29.Wang N., Zhou X., Wang W., Wang L., Jiang L., Liu T., Yu D. Effect of high intensity ultrasound on the structure and solubility of soy protein isolate-pectin complex. Ultrasonics Sonochemistry. 2021;80 doi: 10.1016/j.ultsonch.2021.105808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T., Chen X., Wang W., Wang L., Jiang L., Yu D., Xie F. Effect of ultrasound on the properties of rice bran protein and its chlorogenic acid complex. Ultrasonics Sonochemistry. 2021;79 doi: 10.1016/j.ultsonch.2021.105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Sullivan J., Murray B., Flynn C., Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocolloids. 2016;53:141–154. [Google Scholar]
- 32.Cui Q., Wang L., Wang G., Zhang A., Wang X., Jiang L. Ultrasonication effects on physicochemical and emulsifying properties of Cyperus esculentus seed (tiger nut) proteins. LWT. 2021;142 [Google Scholar]
- 33.Hollingsworth K.G., Johns M.L. Measurement of emulsion droplet sizes using PFG NMR and regularization methods. Journal of Colloid & Interface Science. 2003;258:383–389. doi: 10.1016/s0021-9797(02)00131-5. [DOI] [PubMed] [Google Scholar]
- 34.Peng W., Kong X., Chen Y., Zhang C., Yang Y., Hua Y. Effects of heat treatment on the emulsifying properties of pea proteins. Food Hydrocolloids. 2016;52:301–310. [Google Scholar]
- 35.Ma W., Wang J., Wu D., Xu X., Wu C., Du M. Physicochemical properties and oil/water interfacial adsorption behavior of cod proteins as affected by high-pressure homogenization. Food Hydrocolloids. 2020;100 [Google Scholar]
- 36.Kentish S., Wooster T.J., Ashokkumar M., Balachandran S., Mawson R., Simons L. The use of ultrasonics for nanoemulsion preparation. Innovative Food Science & Emerging Technologies. 2008;9(2):170–175. [Google Scholar]
- 37.Li K.e., Li Y., Liu C.-L., Fu L., Zhao Y.-Y., Zhang Y.-Y., Wang Y.-T., Bai Y.-H. Improving interfacial properties, structure and oxidative stability by ultrasound application to sodium caseinate prepared pre-emulsified soybean oil. LWT- Food Science and Technology. 2020;131:109755. [Google Scholar]
- 38.Ma W., Wang J., Xu X., Qin L., Wu C., Du M. Ultrasound treatment improved the physicochemical characteristics of cod protein and enhanced the stability of oil-in-water emulsion. Food Research International. 2019;121:247–256. doi: 10.1016/j.foodres.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Li R., Xiong Y.L. Ultrasound-induced structural modification and thermal properties of oat protein. LWT- Food Science and Technology. 2021;149:111861. [Google Scholar]
- 40.Xu L., Yan W., Zhang M., Hong X., Liu Y., Li J. Application of ultrasound in stabilizing of Antarctic krill oil by modified chickpea protein isolate and ginseng saponin. LWT. 2021;149 [Google Scholar]
- 41.Sun L., Wang H., Li X., Lan S., Wang J., Yu D. Ultrasonic-assisted preparation of α-Tocopherol/casein nanoparticles and application in grape seed oil emulsion. Ultrasonics Sonochemistry. 2021;80 doi: 10.1016/j.ultsonch.2021.105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.A. Aq, L.A. Meng, A. Rs, A. Ab, A. Mag, A. Mh, B. Mi, C. Swas, A. Zj, and A. Jh, Laccase cross-linking of sonicated α-Lactalbumin improves physical and oxidative stability of CLA oil in water emulsion. Ultrasonics Sonochemistry 71. [DOI] [PMC free article] [PubMed]
- 43.O'Sullivan J.J., Norwood E., O'Mahony J.A., Kelly A.L. Atomisation technologies used in spray drying in the dairy industry: A review. Journal of Food Engineering. 2019;243:57–69. [Google Scholar]
- 44.Hu J., Zheng H., Chen X., Li X., Xu Y., Xu M. Synergetic effects of whey protein isolate and naringin on physical and oxidative stability of oil-in-water emulsions. Food Hydrocolloids. 2020;101 [Google Scholar]
- 45.O'Sullivan J.J., Espinoza C.J.U., Mihailova O., Alberini F. Characterisation of flow behaviour and velocity induced by ultrasound using particle image velocimetry (PIV): Effect of fluid rheology, acoustic intensity and transducer tip size. Ultrasonics Sonochemistry. 2018;48:218–230. doi: 10.1016/j.ultsonch.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 46.Malik M.A., Saini C.S. Heat treatment of sunflower protein isolates near isoelectric point: Effect on rheological and structural properties. Food Chemistry. 2019;276:554–561. doi: 10.1016/j.foodchem.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 47.Younis K., Ahmad S., Osama K., Malik M.A. Optimization of de-bittering process of mosambi (Citrus limetta) peel: Artificial neural network, Gaussian process regression and support vector machine modeling approach. Journal of Food Process Engineering. 2019;42(6) [Google Scholar]
- 48.Fu X., Belwal T., Cravotto G., Luo Z. Sono-physical and sono-chemical effects of ultrasound: primary applications in extraction and freezing operations and influence on food components. Ultrasonics Sonochemistry. 2020;60:104726. doi: 10.1016/j.ultsonch.2019.104726. [DOI] [PubMed] [Google Scholar]
- 49.Marcin A.K., Anubhav P. Plant-Based (Hemp, Pea and Rice) Protein-Maltodextrin Combinations as Wall Material for Spray-Drying Microencapsulation of Hempseed (Cannabis sativa) Oil. Foods. 2020;9(11):1707. doi: 10.3390/foods9111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holst A., Rolfsen W., Svensson B., Ollinger K., Lundgren B. Formation of free radicals during phacoemulsification. Current Eye Research. 1993;12:359–365. doi: 10.3109/02713689308999460. [DOI] [PubMed] [Google Scholar]