Abstract
People with schizophrenia die 15‐20 years prematurely. Understanding mortality risk and aggravating/attenuating factors is essential to reduce this gap. We conducted a systematic review and random‐effects meta‐analysis of prospective and retrospective, nationwide and targeted cohort studies assessing mortality risk in people with schizophrenia versus the general population or groups matched for physical comorbidities or groups with different psychiatric disorders, also assessing moderators. Primary outcome was all‐cause mortality risk ratio (RR); key secondary outcomes were mortality due to suicide and natural causes. Other secondary outcomes included any other specific‐cause mortality. Publication bias, subgroup and meta‐regression analyses, and quality assessment (Newcastle‐Ottawa Scale) were conducted. Across 135 studies spanning from 1957 to 2021 (schizophrenia: N=4,536,447; general population controls: N=1,115,600,059; other psychiatric illness controls: N=3,827,955), all‐cause mortality was increased in people with schizophrenia versus any non‐schizophrenia control group (RR=2.52, 95% CI: 2.38‐2.68, n=79), with the largest risk in first‐episode (RR=7.43, 95% CI: 4.02‐13.75, n=2) and incident (i.e., earlier‐phase) schizophrenia (RR=3.52, 95% CI: 3.09‐4.00, n=7) versus the general population. Specific‐cause mortality was highest for suicide or injury‐poisoning or undetermined non‐natural cause (RR=9.76‐8.42), followed by pneumonia among natural causes (RR=7.00, 95% CI: 6.79‐7.23), decreasing through infectious or endocrine or respiratory or urogenital or diabetes causes (RR=3 to 4), to alcohol or gastrointestinal or renal or nervous system or cardio‐cerebrovascular or all natural causes (RR=2 to 3), and liver or cerebrovascular, or breast or colon or pancreas or any cancer causes (RR=1.33 to 1.96). All‐cause mortality increased slightly but significantly with median study year (beta=0.0009, 95% CI: 0.001‐0.02, p=0.02). Individuals with schizophrenia <40 years of age had increased all‐cause and suicide‐related mortality compared to those ≥40 years old, and a higher percentage of females increased suicide‐related mortality risk in incident schizophrenia samples. All‐cause mortality was higher in incident than prevalent schizophrenia (RR=3.52 vs. 2.86, p=0.009). Comorbid substance use disorder increased all‐cause mortality (RR=1.62, 95% CI: 1.47‐1.80, n=3). Antipsychotics were protective against all‐cause mortality versus no antipsychotic use (RR=0.71, 95% CI: 0.59‐0.84, n=11), with largest effects for second‐generation long‐acting injectable antipsychotics (SGA‐LAIs) (RR=0.39, 95% CI: 0.27‐0.56, n=3), clozapine (RR=0.43, 95% CI: 0.34‐0.55, n=3), any LAI (RR=0.47, 95% CI: 0.39‐0.58, n=2), and any SGA (RR=0.53, 95% CI: 0.44‐0.63, n=4). Antipsychotics were also protective against natural cause‐related mortality, yet first‐generation antipsychotics (FGAs) were associated with increased mortality due to suicide and natural cause in incident schizophrenia. Higher study quality and number of variables used to adjust the analyses moderated larger natural‐cause mortality risk, and more recent study year moderated larger protective effects of antipsychotics. These results indicate that the excess mortality in schizophrenia is associated with several modifiable factors. Targeting comorbid substance abuse, long‐term maintenance antipsychotic treatment and appropriate/earlier use of SGA‐LAIs and clozapine could reduce this mortality gap.
Keywords: Schizophrenia, psychosis, mortality, suicide, first‐episode schizophrenia, antipsychotics, comorbidity, substance use disorder, cardiovascular disease, physical health, long‐acting injectable antipsychotics, clozapine
Schizophrenia is associated with one of the highest mortality risks of all psychiatric disorders 1 . While it is well recognized that individuals with this disorder die prematurely compared to the general population, reasons for the estimated life expectancy gap of 15‐20 years are less clear 2 .
Modifiable risk factors reportedly associated with greater and earlier mortality in individuals with schizophrenia include poorer lifestyle behaviors, reduced access to physical care, frequent comorbid illnesses, and use – or lack thereof – of antipsychotic medications 3 , 4 . However, it is unclear whether mortality risk changes in new‐onset incident cases or evolves in established prevalent cases. A larger mortality gap has been reported in younger people, not only for suicide but also for physical health causes 5 .
In a nationwide study from Finland that compared 34,809‐42,712 individuals with schizophrenia with 3,877,129‐4,515,838 people from the general population between 1984 and 2014, the higher all‐cause standardized mortality ratio for those with schizophrenia compared to the general population remained stable during the 30 years of follow‐up (1984=2.6; 2014=2.7) 6 . However, in a Danish nationwide cohort study, the standardized mortality gap appeared to be increasing by 0.03 annually between 1995 and 2014 7 .
There is growing evidence supporting the protective effect of antipsychotic treatment versus non‐use of antipsychotics in people with schizophrenia 8 , 9 , 10 . Notably, although antipsychotics have been associated with adverse cardiometabolic effects that can increase the risk of cardiovascular death 11 , 12 , 13 , 14 – which represents the largest absolute risk for mortality associated with schizophrenia 15 , 16 , 17 , 18 , 19 – antipsychotic use versus non‐use has not been associated with a greater risk of hospitalization for any physical disease (hazard ratio, HR=1.00, 95% CI: 0.98‐1.03), including cardiovascular disorders (HR=1.00, 95% CI: 0.92‐1.07) 10 . Rather, antipsychotic use versus non‐use has been associated with a significantly decreased risk for death from cardiovascular illness in individuals with schizophrenia (HR=0.62, 95% CI: 0.57‐0.67) 10 .
This apparent paradox has been explained by healthier lifestyle behaviors, less psychosis‐related stress/cortisol increase, and better help‐seeking behaviors in antipsychotic‐treated individuals. Recently, adherence versus non‐adherence to antipsychotics has also been associated with decreased discontinuation risk of antidiabetics (adjusted hazard ratio, aHR=0.56, 95% CI: 0.47‐0.66), statins (aHR=0.61, 95% CI: 0.53‐0.70), anti‐hypertensives (aHR=0.63, 95% CI: 0.56‐0.71), and beta‐blockers (aHR=0.79, 95% CI: 0.73‐0.87) in within‐subject analyses 20 .
Additionally, among antipsychotic medications, differential risk attenuation of mortality risk in individuals with schizophrenia has been described 8 , 9 , 10 . For example, a Swedish prospective nationwide study on a register‐based cohort followed for a median of 5.7 years reported an approximately 33% reduced mortality risk among individuals who received long‐acting injectable antipsychotics (LAIs) compared with equivalent oral antipsychotics 9 . This greater protective effect of LAIs versus oral antipsychotics was substantiated in a Taiwanese nationwide cohort study with a median of 14 years of follow‐up, which reported a 34% decreased all‐cause mortality risk with LAIs, with an even stronger protective effect (i.e., 47% decreased mortality risk) in subjects switched to an LAI within the first two years of diagnosis of schizophrenia 8 .
Finally, use of clozapine, one of the agents with the highest cardiometabolic risk burden 21 , 22 , has also been associated with decreased all‐cause mortality risk, such as in a Finnish nationwide database study with a median of 14.1 years of follow‐up, where all‐cause mortality was reduced by 61% and cardiovascular death risk was decreased by 45% versus non‐use of antipsychotics 10 . Consistent with the previously noted association between antipsychotic use and adherence to cardiometabolic treatments, clozapine was associated with the largest reduction among all second‐generation antipsychotics (SGAs) regarding discontinuation of statins, antidiabetics and beta‐blockers 20 .
Increased mortality in individuals with schizophrenia appears to be associated to a large degree with comorbid physical conditions and unhealthy lifestyle behaviors. These individuals have higher rates of cardiovascular risk factors than the general population, including (components of) metabolic syndrome 13 and diabetes 14 , as well as sedentary behavior 2 and smoking 23 , yet are less likely to receive education regarding smoking cessation and may not receive preventive or acute care for comorbid illnesses comparable to patients without schizophrenia 24 , 25 , 26 , 27 . Moreover, in addition to increased cardiovascular risk factors, individuals with schizophrenia also receive lower quality of care for cardiovascular disease 28 .
The role of antipsychotics in specific‐cause mortality in schizophrenia has not been definitively clarified, and there is still an ongoing debate regarding whether antipsychotic agents reduce overall mortality largely due to decreasing suicide‐related mortality risk, while tending to increase natural‐cause mortality risk owing to their adverse impact on cardiac repolarization, body weight and other cardiometabolic risk factors 4 , 29 , 30 , a risk that may be aggravated in older age 31 .
To the best of our knowledge, there has been no large‐scale, comprehensive meta‐analysis that has included several control groups, most relevant specific causes of mortality and antipsychotic treatments, as well as an analysis of factors aggravating or attenuating mortality in individuals with schizophrenia. Most of the prior meta‐analyses included fewer than 30 studies. Many studies focused either on one specific causative factor (such as suicide, cardiovascular disease, or use of specific antipsychotic agents) or included schizophrenia among other severe mental illnesses.
To fill this gap, we performed a systematic review and meta‐analysis examining risk of all‐cause and specific‐cause mortality in individuals with schizophrenia versus several control groups, as well as factors associated with increased or attenuated mortality risk in these persons, focusing also on representativeness of the sample, study quality and time trends.
METHODS
Search methods for identification of studies
We conducted a PRISMA 2020‐compliant systematic review 32 searching Medline, PubMed and PsycINFO until September 9, 2021, using the search key (schizophrenia AND (mortal* OR death* OR fatal*)) NOT (animals [mesh] NOT humans [mesh]), and complemented it with manual search. The PRISMA 2020 checklist and abstract checklist are provided in the supplementary information.
Study eligibility criteria
Peer‐reviewed publications of a cohort study (prospective or retrospective; nationwide or not) were eligible. We included only studies in which ≥70% of the participants had a diagnosis of schizophrenia and in which a minimum of 100 patients with this diagnosis were recruited. Publications had to include quantified reporting – e.g., odds ratio (OR), risk ratio (RR), HR, or raw numbers – of the relationship between schizophrenia diagnosis versus control group and any type of mortality. When a risk or protective factor was present that defined a subgroup of people with schizophrenia, such as cardiac illness or diabetes or substance use disorder comorbidity, only studies where the schizophrenia and control group were matched on that risk or protective factor were included.
We excluded non‐cohort studies, such as case‐control studies, reviews, meta‐analyses and systematic reviews. Publications were also excluded if they did not provide mortality data, quantitative data, or if the data were not meta‐analyzable. Publications that contained non‐peer‐reviewed data (such as proceedings, poster abstracts or posters) were not considered. No language or time restrictions were applied.
Four independent raters (GC, LKS, MS, NS) selected studies and extracted outcome data as well as information on potential effect modifiers. The Newcastle‐Ottawa Scale 33 was used to classify quality/risk of bias. When discrepancies occurred, a further rater (CUC) was consulted. Original study authors were contacted to provide missing data.
Outcomes
The primary outcome was RR of all‐cause mortality in individuals with schizophrenia versus any control group. Key secondary outcomes were mortality due to suicide and natural causes. Additional secondary outcomes included other specific‐cause mortality.
Analyses examined incident plus prevalent cohorts together and either prevalent or incident cohorts separately. Prevalent cases include all individuals living with schizophrenia within a specified timeframe, regardless of when the person was diagnosed with or developed the condition. Incident cases encompass all individuals who are newly identified within the period of observation as having schizophrenia, or all new cases of schizophrenia. Control groups consisted of the general population, regardless of underlying comorbid physical diseases (from here on, “general population”), or control samples matched by physical disease. Patients with schizophrenia were compared with both control populations combined, and with each one separately, whenever possible.
Extraction methodology
Whenever results for different degrees of adjustment of RR were presented, we always used the result that was adjusted for the largest number of variables. Whenever data for both prevalent and incident cohorts were presented, we extracted both. For studies where data were only presented graphically, we extracted the data from the respective figures. For studies that only provided data on the point estimates but did not include the standard deviation or 95% CI, we imputed the 95% CI as the mean of all studies with the available data.
Whenever only raw mortality data were reported, we calculated the mortality ratio by dividing the mortality rate for schizophrenia subjects by the rate for controls. When authors presented data by narrow or broad definitions, we picked the broad definition, to be more conservative and include as many potential deaths as possible. Whenever data on samples overlapping by at least 50% were reported in different publications, we used the data including 95% CIs from the larger sample.
Whenever a subgroup of patients with schizophrenia with a specific condition was the subject of a study (for example, schizophrenia with type 2 diabetes mellitus), the control group had to have that same condition. Whenever the exact number of the control group was not specified, but rather the group was defined by a region, state or country, we took the size of that population at the midpoint of the study period. When the sample size of the control group in subgroup analyses was not specified, we imputed it by applying the same ratio of the group with schizophrenia (e.g., same male to female ratio). In representative studies, if the control group was not provided, we extracted data from census sources matching the time of study.
Data analysis
We conducted a random‐effects meta‐analysis 34 and calculated the RR of primary and secondary outcomes. Given that the outcome of interest, mortality, is rare (i.e., less than 10%), and that all included studies used the same design and evaluated the same population of interest, we pooled ORs, RRs, HRs and standardized mortality ratios. When an association measure was not available, we used the raw data (i.e., number of events and sample sizes in schizophrenia and control groups) and calculated the unadjusted RR. When both adjusted and unadjusted effect sizes were available, we prioritized adjusted ones.
I 2 was used to measure heterogeneity 35 , and Egger's test to assess publication bias 36 . When Egger's test revealed publication bias (i.e., p<0.1), we conducted trim and fill analyses, and calculated the fail‐safe number 37 .
Sources of heterogeneity were explored with meta‐regression, sensitivity and subgroup analyses. Random‐effects meta‐regression analyses were conducted with follow‐up time, median study year, number of variables adjusted for, mean age, gender, and sample size as moderator variables. Sensitivity analyses were conducted in studies comparing schizophrenia with the general population, and in studies matching control groups by underlying physical conditions, as well as in incident and prevalent schizophrenia samples. Subgroup analyses were also conducted by use of nationwide versus more restricted samples, Newcastle‐Ottawa Scale quality score, adjustment of results, mean age of the sample, incident or prevalent sample, and antipsychotic class prescribed. We chose to analyze the effect of treatment with antipsychotics using subgroups (by class or formulation or specific medication) as the unit of analysis, instead of using the pooled result of the overall study as the unit of analysis, since a single study might have reported on several different antipsychotic subgroups. Comprehensive Meta Analysis Version 2.0 was used for all analyses.
RESULTS
Search results
An initial search retrieved 8,345 abstracts; removal of duplicates resulted in 6,390 abstracts for review. Of these, a total of 135 studies 5 , 6 , 7 , 8 , 9 , 10 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 were included, after excluding 463 articles upon full text assessment (see Figure 1, Table 1 and supplementary information). We ultimately included 4,536,447 individuals with schizophrenia who were compared with 1,115,600,059 control subjects from the general population.
Figure 1.
PRISMA flow chart. RCT – randomized controlled trial
Table 1.
Included studies reporting on risk of all‐cause and specific‐cause mortality in schizophrenia versus control group, and on mitigating/risk factors
| Country | Years | Comparison | Incident/ prevalent | Number of patients | Number of controls | Mortality outcomes | NOS | |
|---|---|---|---|---|---|---|---|---|
| Alleback & Wistedt 38 | Sweden | 1971‐1981 | Schizophrenia vs. general population | P | 1,190 | 16,902 | All‐cause, suicide, various specific causes, undetermined | 9 |
| Amaddeo et al 39 | Italy | 1982‐1991 | Schizophrenia vs. general population | P | 3,172 | 153,352 | All‐cause | 9 |
| Attar et al 40 | Denmark | 1995‐2013 | Schizophrenia vs. general population | P | 726 | 2,178 | Cardio‐cerebrovascular | 9 |
| Bagewadi et al 41 | India | 2009‐2011 | Schizophrenia vs. general population | P | 325 | NA | All‐cause | 9 |
| Berardi et al 42 | Italy | 2008‐2017 | Schizophrenia vs. general population | P | 7,940 | 4,250,075 | All‐cause, natural, various specific causes | 9 |
| Bitter et al 5 | Hungary | 2005‐2013 | Schizophrenia vs. general population | P | 65,165 | 390,599 | All‐cause | 9 |
| Black & Fisher 43 | US | 1970‐1988 | Schizophrenia vs. general population | P | 356 | 2,869,448 | All‐cause, natural, undetermined | 9 |
| Bouza et al 44 | Spain | 2004‐2004 | Schizophrenia vs. general population | P | 16,776 | 3,951,000 | All‐cause | 9 |
| Bralet et al 45 | France | 1991‐1999 | Schizophrenia vs. general population | P | 150 | 552,303 | All‐cause | 8 |
| Brown et al 46 | UK | 1981‐2006 | Schizophrenia vs. general population | P | 370 | 24,328,853 | All‐cause, suicide, natural, various specific causes, undetermined | 9 |
| Buda et al 47 | US | 1934‐1974 | Schizophrenia vs. general population | P | 332 | NA | Suicide, natural, various specific causes, undetermined | 9 |
| Castagnini et al 48 | Denmark | 1995‐2008 | Schizophrenia vs. general population | I | 4,576 | 3,565,833 | All‐cause, suicide, natural, various specific causes, undetermined | 9 |
| Chan et al 49 | Hong Kong | 2006‐2016 | Schizophrenia vs. general population | I | 3,105 | 13,545 | Natural, various specific causes | 9 |
| Chen et al 50 | Taiwan | 2000‐2016 | Schizophrenia vs. general population | P | 170,322 | 22,710,322 | Cardiovascular | 9 |
| Chen et al 51 | Taiwan | 1999‐2010 | Schizophrenia vs. general population | P | 7,531 | 22,547,531 | All‐cause | 9 |
| Chen et al 52 | Taiwan | 1998‐2004 | Schizophrenia vs. general population | I | 5,515 | 24,238 | All‐cause, natural, undetermined | 9 |
| Cheng et al 53 | Taiwan | 1998‐2008 | Schizophrenia vs. general population | P | 2,457 | 22,561,450 | All‐cause, natural, various specific causes, undetermined | 9 |
| Crump et al 54 | Sweden | 2001‐2008 | Schizophrenia vs. general population | P | 25,359 | 6,908,922 | All‐cause, injury, other | 9 |
| Curkendall et al 55 | Canada | 1994‐1998 | Schizophrenia vs. general population | P | 3,022 | 13,110 | All‐cause, natural | 8 |
| Daumit et al 56 | US | 1992‐2001 | Schizophrenia vs. general population | P | 2,303 | 5,171,640 | Cardiovascular | 8 |
| Dickerson et al 57 | US | 1999‐2009 | Schizophrenia vs. general population | P | 517 | 2,448,017 | Natural | 7 |
| Dickerson et al 58 | US | 1999‐2012 | Schizophrenia vs. general population | P | 710 | 182,165,000 | Natural | 9 |
| Enger et al 59 | US | 1995‐1999 | Schizophrenia vs. general population | P | 1,920 | 11,520 | All‐cause, natural, cardiovascular | 9 |
| Fors et al 60 | Sweden | 1991‐2000 | Schizophrenia vs. general population | P | 255 | 1,530 | All‐cause, natural, cardiovascular, undetermined | 9 |
| Gatov et al 61 | Canada | 1993‐2012 | Schizophrenia vs. general population | P | 34,338 | 8,793,478 | All‐cause | 9 |
| Girardi et al 62 | Italy | 2008‐2018 | Schizophrenia vs. general population | P | 12,196 | 9,787,004 | Suicide, natural, various specific causes | 9 |
| Guan et al 63 | The Netherlands | 1999‐2007 | Schizophrenia vs. general population | P | 4,590 | 23,062 | All‐cause, suicide, natural, other | 9 |
| Haugland et al 64 | US | 1975‐1978 | Schizophrenia vs. general population | P | 351 | NA | All‐cause | 9 |
| Hayes et al 65 | UK | 2000‐2014 | Schizophrenia vs. general population | P | 22,497 | 241,884 | All‐cause, suicide, cardiovascular | 9 |
| Heila et al 66 | Finland | 1980‐1996 | Schizophrenia vs. general population | P | 58,761 | 7,314,595 | All‐cause, suicide | 9 |
| Hellemose et al 67 | Denmark | 1970‐2011 | Schizophrenia vs. general population | I | 17,530 | 5,389,084 | Other | 9 |
| Hennessy et al 68 | US | 1993‐1996 | Schizophrenia vs. general population | P | 136,927 | 29,086 | Cardiovascular | 7 |
| Hewer & Rössler 69 | Germany | 1984‐1986 | Schizophrenia vs. general population | P | 8,927 | 61,057,927 | All‐cause, suicide, natural | 9 |
| Kilbourne et al 70 | US | 1999‐2006 | Schizophrenia vs. general population | P | 22,817 | 38,859 | Cardiovascular | 9 |
| Kim et al 71 | Korea | 2002‐2013 | Schizophrenia vs. general population | I | 9,387 | 1,025,340 | All‐cause | 9 |
| Kiviniemi et al 72 | Finland | 1995‐2001 | Schizophrenia vs. general population | I | 7,591 | 5,120,000 | All‐cause, suicide, natural, various specific causes, undetermined | 9 |
| Kredentser et al 73 | Canada | 1999‐2008 | Schizophrenia vs. general population | P | 9,038 | 978,128 | All‐cause, suicide, natural, various specific causes | 9 |
| Kugathasan et al 74 | Denmark | 1995‐2015 | Schizophrenia vs. general population | P | 30,210 | 5,432,821 | All‐cause, natural, various specific causes | 9 |
| Kugathasan et al 75 | UK | 2013‐2017 | Schizophrenia vs. general population | P | 36,425 | 218,297 | Various specific causes | 9 |
| Kurdyak et al 76 | Canada | 2007‐2010 | Schizophrenia vs. general population | I | 13,385 | 12,851,821 | All‐cause, suicide, injury, other | 9 |
| Lahti et al 77 | Finland | 1969‐2004 | Schizophrenia vs. general population | I | 204 | 12,735 | Cardio‐cerebrovascular | 9 |
| Laursen et al 78 | Denmark, Finland, Sweden | 2000‐2007 | Schizophrenia vs. general population | P | 66,088 | 19,691,360 | All‐cause, natural, cardio‐cerebrovascular, undetermined | 9 |
| Laursen et al 79 | Denmark | 1992‐2006 | Schizophrenia vs. general population | P | 30,614 | 8,999,225 | Cardiovascular | 9 |
| Laursen et al 80 | Denmark | 1995‐2007 | Schizophrenia vs. general population | P | 16,079 | 4,873,115 | Natural | 9 |
| Lomholt et al 7 | Denmark | 1995‐2014 | Schizophrenia vs. general population | P | 38,500 | 6,176,414 | All‐cause | 9 |
| Luo et al 81 | China | 2007‐2010 | Schizophrenia vs. general population | P | 2,071 | 1,909,205 | All‐cause | 9 |
| Meesters et al 82 | The Netherlands | 2008‐2012 | Schizophrenia vs. general population | P | 157 | 25,788 | All‐cause | 9 |
| Mortensen & Juel 83 | Denmark | 1957‐1986 | Schizophrenia vs. general population | P | 6,178 | 2,494,178 | All‐cause, suicide, natural, various specific causes | 6 |
| Mortensen & Juel 84 | Denmark | 1970‐1987 | Schizophrenia vs. general population | I | 9,156 | 5,131,156 | All‐cause, suicide, natural, various specific causes | 6 |
| Newman & Bland 85 | Canada | 1976‐1985 | Schizophrenia vs. general population | P | 3,623 | 4,479,623 | All‐cause, suicide, natural, various specific causes | 6 |
| Nielsen et al 86 | Denmark | 1980‐2010 | Schizophrenia vs. general population | P | 14,974 | 1,326,393 | All‐cause | 9 |
| Olfson et al 87 | US | 2001‐2007 | Schizophrenia vs. general population | I | 1,138,853 | 173,699,853 | All‐cause, suicide, natural, various specific causes | 9 |
| Olfson et al 88 | US | 2007‐2016 | Schizophrenia vs. general population | P | 668,836 | 311,580,000 |
Suicide, other non‐natural |
9 |
| Ösby et al 89 | Sweden | 1973‐1995 | Schizophrenia vs. general population | I | 7,784 | 1,792,216 | All‐cause, suicide, natural, various specific causes, undetermined | 9 |
| Pan et al 90 | Taiwan | 2001‐2016 | Schizophrenia vs. general population | P | 170,322 | 23,000,000 | Suicide, other non‐natural | 9 |
| Pan et al 91 | Taiwan |
2005‐2008 2010‐2013 |
Schizophrenia vs. general population | P |
95,632 104,561 |
2,292,000 229,200 |
All‐cause, suicide, natural, various specific causes | 9 |
| Phillippe et al 92 | France | 1993‐2002 | Schizophrenia vs. general population | P | 3,470 | 33,264,661 | All‐cause, natural | 6 |
| Phillips et al 93 | China | 1995‐1999 | Schizophrenia vs. general population | P | 102 | 19,121 | Suicide, natural | 9 |
| Ran et al 94 | China | 1994‐2004 | Schizophrenia vs. general population | P | 500 | 123,562 | All‐cause, suicide, injury, natural | 9 |
| Ruschena et al 95 | Australia | 1995‐1995 | Schizophrenia vs. general population | P | 25,202 | 35,361,211 | All‐cause, suicide, injury, natural, undetermined | 7 |
| Talaslahti et al 96 | Finland | 1992‐2008 | Schizophrenia vs. general population | P | 9,461 | 1,891,543 | All‐cause, suicide, natural, various specific causes | 9 |
| Tanskanen et al 6 | Finland |
1984 1994 2014 |
Schizophrenia vs. general population | P | 159,858 | 16,701,991 | Suicide, natural, cardiovascular, other | 9 |
| Teferra et al 97 | Ethiopia | 2001‐2005 | Schizophrenia vs. general population | P | 307 | 68,685 | All‐cause | 9 |
| Tenback et al 98 | The Netherlands | 2006‐2008 | Schizophrenia vs. general population | P | 7,415 | 105,141 | All‐cause | 9 |
| Tokuda et al 99 | Japan | 1987‐2004 | Schizophrenia vs. general population | P | 1,108 | 190,157 | All‐cause | 9 |
| Tornianen et al 100 | Sweden | 2006‐2010 | Schizophrenia vs. general population | I | 48,441 | 1,032,760 | All‐cause, suicide, various specific causes | 9 |
| Tran et al 101 | France | 1993‐2003 | Schizophrenia vs. general population | P | 3,434 | 3,434 | Cardiovascular | 9 |
| Westman et al 102 | Sweden | 1987‐2010 | Schizophrenia vs. general population | P | 46,911 | 10,678,728 | All‐cause, suicide, injury, cardio‐cerebrovascular, other | 9 |
| Wood et al 103 | US | 1972‐1976 | Schizophrenia vs. general population | P | 8,779 | 235,558 | All‐cause | 9 |
| Yung et al 104 | China | 2006‐2016 | Schizophrenia vs. general population | P | 817 | 8,987 | All‐cause, cerebrovascular | 9 |
| Yung et al 105 | Hong Kong | 2006‐2016 | Schizophrenia vs. general population | P | 46,896 | 7,500,000 | All‐cause, various specific causes | 9 |
| Zilber et al 106 | Israel | 1978‐1983 | Schizophrenia vs. general population | P | 9,282 | NA | All‐cause, suicide, natural, various specific causes | 9 |
| Attar et al 107 | Sweden | 2000‐2018 | Schizophrenia vs. general population with acute myocardial infarction | P | 1,008 | 285,325 | All‐cause | 9 |
| Babidge et al 108 | Australia | 1988‐1998 | Schizophrenia vs. no schizophrenia homeless | P | 455 | 708 | All‐cause | 9 |
| Bodén et al 109 | Sweden | 1997‐2010 | Schizophrenia vs. general population with acute myocardial infarction | P | 541 | 209,592 | All‐cause, cardiovascular | 9 |
| Bradford et al 110 | US | 2001‐2005 | Schizophrenia vs. general population with lung cancer | P | 835 | 34,644 | All‐cause | 9 |
| Chan et al 111 | Hong Kong | 2001‐2016 | Schizophrenia vs. general population with diabetes mellitus | P | 6,991 | 75,673 | All‐cause, diabetes mellitus | 9 |
| Chong et al 112 | Singapore | 2000‐2006 | Schizophrenia with vs. without tardive dyskinesia | P | 241 | 561 | All‐cause, natural, various specific causes | 9 |
| Chou et al 113 | Taiwan | 2000‐2008 | Schizophrenia vs. no schizophrenia with cancer | P | 1,131 | 6,377 | All‐cause | 9 |
| Chou et al 114 | Taiwan | 2000‐2008 | Schizophrenia vs. general population with pneumonia | P | 6,040 | 13,878 | All‐cause | 9 |
| Closson et al 115 | Canada | 1998‐2012 | Schizophrenia vs. general population with HIV | P | 835 | 13,331 | All‐cause | 9 |
| Crump et al 116 | Sweden | 2003‐2009 | Schizophrenia vs. general population with ischemic heart disease or cancer | P | 8,277 | 6,097,834 | All‐cause | 9 |
| Druss et al 117 | US | 1994‐1995 | Schizophrenia vs. general population with acute myocardial infarction | P | 161 | 88,241 | All‐cause | 9 |
| Fleetwood et al 118 | UK | 1991‐2014 | Schizophrenia vs. no schizophrenia with acute myocardial infarction | P | 923 | 235,310 | Cardiovascular | 9 |
| Fond et al 119 | France | 2020‐2020 | Schizophrenia vs. general population with COVID | P | 823 | 50,750 | COVID | 9 |
| Guerrero Fernandez de Alba et al 120 | Spain | 2012‐2015 | Schizophrenia vs. general population with diabetes mellitus | P | 931 | 52,266 | All‐cause | 9 |
| Hauck et al 121 | Canada | 2008‐2015 | Schizophrenia vs. general population with myocardial infarction | P | 1,145 | 108,610 | All‐cause | 9 |
| Jeon et al 122 | Korea | 2019‐2020 | Schizophrenia vs. general population with COVID | P | 159 | 2,976 | COVID | 9 |
| Kang et al 123 | Taiwan | 2002‐2004 | Schizophrenia vs. general population with stroke | P | 485 | 2,910 | Cerebrovascular | 9 |
| Kapral et al 124 | Canada | 2002‐2017 | Schizophrenia vs. no schizophrenia with stroke | P | 612 | 52,473 | Cerebrovascular, other | 9 |
| Kershenbaum et al 125 | UK | 2013‐2019 | Schizophrenia vs. anxiety disorders | P | 238 | 1,115 | All‐cause | 9 |
| Kugathasan et al 126 | Denmark | 1995‐2015 | Schizophrenia vs. general population with myocardial infarction | P | 631 | 101,510 | All‐cause | 9 |
| Kurdyak et al 127 | Canada | 2002‐2006 | Schizophrenia vs. general population with acute myocardial infarction | P | 842 | 71,668 | Cardiovascular | 9 |
| Laursen et al 128 | Denmark | 1998‐2008 | Schizophrenia vs. general population with stroke | P | 3,660 | 877,507 | All‐cause, cardiovascular, undetermined | 9 |
| Liao et al 129 | Taiwan | 2004‐2007 | Schizophrenia vs. general population with surgery | P | 8,967 | 44,835 | Other | 9 |
| Mohamed et al 130 | US | 2004‐2014 | Schizophrenia vs. other severe mental illness vs. no severe mental illness with myocardial infarction | P | 23,582 | 6,322,796 | Cardiovascular | 9 |
| Shen et al 131 | Taiwan | 2005‐2007 | Schizophrenia vs. general population in intensive care unit | P | 203 | 2,239 | All‐cause | 9 |
| Sögaard et al 132 | Denmark | 2000‐2015 | Schizophrenia vs. general population with atrial fibrillation | P | 534 | 2,552,772 | Cardiovascular | 9 |
| Toender et al 133 | Denmark | 1999‐2017 | Schizophrenia vs. general population with diabetes mellitus | P | 1,004 | 184,470 | All‐cause, diabetes mellitus, other | 9 |
| Tsai et al 134 | Taiwan | 1999‐2008 | Schizophrenia vs. general population with stroke | P | 1,377 | 4,329 | All‐cause | 9 |
| Tsai et al 135 | Taiwan | 1999‐2010 | Schizophrenia vs. general population with osteoporotic fractures | P | 30,335 | 151,675 | All‐cause | 9 |
| Tzur Bitan et al 136 | Israel | 2020‐2021 | Schizophrenia vs. no schizophrenia with COVID | P | 25,539 | 51,078 | COVID | 8 |
| Wellejus Albertsen et al 137 | Denmark | 2000‐2013 | Schizophrenia vs. general population with acute myocardial infarction | P | 1,160 | 36,685 | Cardiovascular | 9 |
| Alaräisänen et al 138 | Finland | 1997‐2005 | Schizophrenia vs. other mental disorder | I | 100 | 422 | Suicide | 9 |
| Dickerson et al 139 | US | 1999‐2018 | Schizophrenia vs. bipolar disorder or major depressive disorder | P | 861 | 1,745 | Natural | 9 |
| Hayes et al 140 | UK | 2007‐2010 | Schizophrenia vs. bipolar disorder | P | 4,270 | 6,109 | All‐cause | 9 |
| Kodesh et al 141 | Israel | 2002‐2012 | With vs. without very late onset schizophrenia | P | 329 |
94,120 |
All‐cause | 9 |
| Chen et al 142 | Taiwan | 1998‐2008 | Schizophrenia on SGA vs. FGA | I | 812 | 1,624 | All‐cause | 9 |
| Cho et al 143 | UK | 2008‐2015 | TRS with vs. without clozapine | TRS | 1,025 | 2,817 | All‐cause | 9 |
| Cullen et al 144 | US | 1994‐2004 | Schizophrenia with or without annual antipsychotic continuity | P | 2,132 | ‐ | All‐cause, suicide, cardiovascular | 9 |
| Dickerson et al 145 | US | 1999‐2004 | Schizophrenia with vs. without Toxoplasma | P | 358 | ‐ | Natural | 9 |
| Fontanella et al 146 | US | 2006‐2013 | Schizophrenia with vs. without benzodiazepines with or without antipsychotics | P | 5,212 | 32,694 | All‐cause, suicide, natural | 9 |
| Funayama et al 147 | Japan | 1999‐2016 | Schizophrenia with vs. without catatonia | P | 140 | 1,710 | All‐cause | 9 |
| Hayes et al 148 | UK | 2007‐2011 | TRS with vs. without clozapine | TRS | 617 | 9,437 | All‐cause | 9 |
| Hjorthoj et al 149 | Denmark | 1969‐2013 | Schizophrenia with vs. without substance use disorder | P | 29,549 | 41,470 | All‐cause, suicide, various specific causes | 9 |
| Horsdal et al 150 | Denmark | 2000‐2012 | Schizophrenia with vs. without abnormal C‐reactive protein or white blood cell levels | I | 208 | 1,025 | All‐cause | 9 |
| Huang et al 8 | Taiwan | 2002‐2017 | Schizophrenia with oral vs. LAI antipsychotic | I | 2,614 | 2,614 | Suicide, natural | 9 |
| Kadra et al 151 | UK | 2007‐2014 | Schizophrenia vs. bipolar disorder | P | 5,896 | 7,782 | All‐cause | 9 |
| Kiviniemi et al 152 | Finland | 1998‐2003 | First‐episode schizophrenia with or without antipsychotics | I | 5,266 | 6,713 | All‐cause, suicide, cardiovascular | 9 |
| Kugathasan et al 153 | Denmark | 1980‐2015 | Schizophrenia with vs. without physical health multimorbidity | P | 9,775 | 1,798 | All‐cause | 9 |
| Lahteenvuo et al 154 |
Finland, Sweden |
1972‐2007 2006‐2016 |
Schizophrenia with vs. without substance use disorder | P |
8,110 4,514 |
30,860 14,616 |
Suicide, injury, natural | 9 |
| Liu et al 155 | China | 2006‐2010 | Schizophrenia vs. other mental disorders | P | 7,628 | 3,810,782 | All‐cause | 9 |
| Oh et al 156 | Korea | 2003‐2017 | Schizophrenia with vs. without antipsychotics | P | 77,139 | 86,923 | All‐cause, suicide, various specific causes | 9 |
| Pridan et al 157 | Israel | 2007‐2012 | TRS with vs. without clozapine | TRS | 43 | 527 | All‐cause | 9 |
| Ran et al 158 | China | 1994‐2015 | Men vs. women and older vs. younger people with schizophrenia | P | 510 | 123,062 | All‐cause, suicide, natural, other | 9 |
| Strom et al 159 | Multicountry | 2002‐2006 | Schizophrenia on ziprasidone vs. olanzapine | P | 9,077 | 18,154 | All‐cause, suicide, cardiovascular, other | 9 |
| Strømme et al 160 | Norway | 2005‐2014 | Schizophrenia with vs. without antipsychotics | P | 101 | 696 | All‐cause | 9 |
| Stroup et al 161 | US | 2001‐2009 | TRS with vs. without clozapine | TRS | 3,123 | 6,246 | All‐cause | 9 |
| Taipale et al 9 | Sweden | 2006‐2013 | Schizophrenia with vs. without antipsychotics |
P I |
34,426 | ‐ | All‐cause | 9 |
| Taipale et al 10 | Finland | 1996‐2015 | Schizophrenia with vs. without antipsychotics |
P I |
62,250 | ‐ | All‐cause, suicide, cardiovascular | 9 |
| Tang et al 162 | Taiwan | 2001‐2015 | Schizophrenia on oral vs. LAI antipsychotics | P | 58,615 | 87,247 | Cardiovascular | 9 |
| Taub et al 163 | Israel | 2012‐2014 | Schizophrenia on clozapine with vs. without physical illness | P | 2,406 | 1,817 | All‐cause | 9 |
| Tiihonen et al 164 | Finland | 2000‐2007 | Schizophrenia with vs. without antipsychotics, antidepressants or benzodiazepines | I | 2,192 | 2,588 | All‐cause | 9 |
| Wimberley et al 165 | Denmark | 1996‐2013 | TRS with vs. without clozapine | TRS | 1,372 | 2,370 | All‐cause, suicide, natural, other | 9 |
| Wu & Shur‐Fen Gau 166 | Taiwan | 2001‐2012 | Schizophrenia with vs. without antipsychotics or benzodiazepines | P | 32,512 | 68,718 | All‐cause | 9 |
NOS – Newcastle‐Ottawa Scale, I – incident, P – prevalent, TRS – treatment‐resistant schizophrenia, NA – not available, SGA – second generation antipsychotic, FGA – first generation antipsychotic, LAI – long‐acting injectable antipsychotic
Studies compared subjects with schizophrenia (N=3,494,716) versus the general population (N=1,097,856,754) (n=72); schizophrenia subjects (N=29,616) versus general population groups matched for physical comorbidities (N=17,733,923) (n=30); and schizophrenia individuals (N=19,011) versus groups with other mental disorders (N=3,827,955) (n=6). Additionally, 27 studies (N=994,273) investigated the association between present/absent risk/protective factors and mortality within two groups of subjects with schizophrenia.
Studies were conducted in the US (n=20), Denmark (n=19), Taiwan (n=17), Sweden (n=10), Finland (n=9), Canada (n=9), the UK (n=9), China (n=6), Israel (n=5), France (n=4); 3 each in Italy, Hong Kong, the Netherlands, Korea, or multiple countries; 2 each in Australia, Japan and Spain; and one each in Ethiopia, Germany, Hungary, India, Norway and Singapore.
There were 22 (16.3%) prospective and 113 (83.7%) retrospective cohort studies, with 85 (63.0%) being nationwide database studies. Study periods ranged from 1957 to 2021.
Nearly one‐third of the studies (32.6%) included in the meta‐analysis did not report an age range. When an age range was provided, 23 studies (17.0%) reported the minimal age as >15 years and another 22 studies (16.3%) used >18 years. The remaining 46 studies listed widely heterogeneous age ranges, with upper and lower extremes ranging from 10 to 109 years old.
Altogether, 20 studies (14.8%) exclusively or also included incident (i.e., earlier‐phase) cases with schizophrenia, two studies (1.5%) included first‐episode patients, and five studies (3.7%) focused on treatment‐resistant schizophrenia. Regarding outcomes, 49 studies (36.3%) only reported on all‐cause mortality, 25 (18.5%) only on a specific cause of mortality, and 63 (46.7%) on both (see Table 1).
Primary outcome: all‐cause mortality
Across 79 studies, schizophrenia was associated with significantly higher all‐cause mortality as compared with any control group (RR=2.52, 95% CI: 2.38‐2.68, I2=99.7%) (see Table 2). Patients with schizophrenia had substantially higher all‐cause mortality versus the general population (RR=2.94, 95% CI: 2.75‐3.13, I2=99.7%, n=57) (see Table 2 and Figure 2). The association was the highest in two studies specifically including individuals with first‐episode schizophrenia (RR=7.43, 95% CI: 4.02‐13.75, I2=93.0%), and significantly higher in incident than prevalent schizophrenia (RR=3.52, 95% CI: 3.09‐4.00, I2=97.1%, n=7 vs. RR=2.86, 95% CI: 2.62‐3.12, I2=99.67, n=50, p=0.009) (see Table 2, Figures 3, 4 and supplementary information).
Table 2.
All‐cause and cause‐specific mortality risk in schizophrenia versus control groups
| Incident/prevalent | N. studies | Risk ratio | 95% CI | p | I2 | Egger's p | |
|---|---|---|---|---|---|---|---|
| All‐cause mortality | |||||||
| Schizophrenia vs. any other population | I + P | 79 | 2.523 | 2.377‐2.678 | 0.000 | 99.7 | 0.001 |
| P | 72 | 2.432 | 2.253‐2.626 | 0.000 | 99.591 | 0.690 | |
| First‐episode schizophrenia vs. general population | I | 2 | 7.433 | 4.017‐13.754 | 0.000 | 92.965 | NA |
| Schizophrenia vs. general population | I + P | 57 | 2.938 | 2.753‐3.135 | 0.000 | 99.733 | 0.050 |
| I | 7 | 3.516 | 3.092‐3.998 | 0.000 | 97.114 | 0.840 | |
| P | 50 | 2.859 | 2.622‐3.117 | 0.000 | 99.669 | 0.360 | |
| Schizophrenia vs. no schizophrenia (all matched) | P | 22 | 1.664 | 1.425‐1.943 | 0.000 | 97.226 | 0.530 |
| Schizophrenia vs. no schizophrenia (matched for acute myocardial infarction) | P | 6 | 1.821 | 1.491‐2.224 | 0.000 | 83.146 | 0.840 |
| Schizophrenia vs. no schizophrenia (matched for diabetes mellitus) | P | 4 | 1.913 | 1.082‐3.380 | 0.026 | 99.414 | 0.500 |
| Schizophrenia vs. no schizophrenia (matched for stroke) | P | 2 | 1.351 | 1.219‐1.498 | 0.000 | 0.000 | NA |
| Schizophrenia vs. other mental disorder | I + P | 5 | 2.130 | 0.648‐7.002 | 0.213 | 99.349 | 0.110 |
| P | 5 | 2.130 | 0.648‐7.002 | 0.213 | 99.349 | 0.110 | |
| Schizophrenia vs. bipolar disorder | P | 3 | 1.257 | 1.031‐1.533 | 0.023 | 25.362 | 0.210 |
| Schizophrenia with vs. without substance use disorder | P | 3 | 1.625 | 1.467‐1.799 | 0.000 | 57.443 | 0.680 |
| Mortality due to suicide | |||||||
| Schizophrenia vs. general population | I + P | 28 | 9.764 | 7.598‐12.549 | 0.000 | 99.478 | 0.030 |
| I | 5 | 12.654 | 5.245‐30.530 | 0.000 | 99.802 | 0.050 | |
| P | 23 | 9.281 | 7.311‐11.782 | 0.000 | 98.793 | 0.680 | |
| Mortality due to natural cause | |||||||
| Schizophrenia vs. any other population | I + P | 59 | 1.996 | 1.851‐2.153 | 0.000 | 99.464 | 0.020 |
| P | 53 | 1.967 | 1.793‐2.158 | 0.000 | 99.201 | 0.040 | |
| Schizophrenia vs. general population | I + P | 44 | 2.162 | 1.985‐2.355 | 0.000 | 99.571 | 0.004 |
| I | 6 | 2.149 | 1.861‐2.481 | 0.000 | 94.602 | 0.270 | |
| P | 38 | 2.154 | 1.961‐2.367 | 0.000 | 99.182 | 0.140 | |
| Schizophrenia vs. no schizophrenia (all matched) | P | 16 | 1.565 | 1.346‐1.821 | 0.000 | 94.001 | 0.030 |
| Schizophrenia vs. no schizophrenia (matched for acute myocardial infarction) | P | 5 | 1.659 | 1.238‐2.223 | 0.001 | 96.379 | 0.070 |
| Mortality due to cardio‐cerebrovascular diseases | |||||||
| Schizophrenia vs. any other population | I + P | 30 | 2.028 | 1.678‐2.452 | 0.000 | 99.470 | 0.020 |
| Schizophrenia vs. general population | I + P | 28 | 2.099 | 1.797‐2.451 | 0.000 | 99.008 | 0.001 |
| I | 4 | 3.470 | 1.792‐6.719 | 0.000 | 97.883 | 0.570 | |
| P | 24 | 1.984 | 1.729‐2.275 | 0.000 | 97.690 | 0.210 | |
| Schizophrenia vs. no schizophrenia (all matched) | P | 2 | 1.329 | 0.907‐1.946 | 0.144 | 97.625 | NA |
| Mortality due to cardiovascular diseases | |||||||
| Schizophrenia vs. any other population | I + P | 25 | 2.089 | 1.764‐2.474 | 0.000 | 99.289 | 0.020 |
| P | 20 | 1.963 | 1.653‐2.331 | 0.000 | 98.841 | 0.220 | |
| Schizophrenia vs. general population | I + P | 19 | 2.205 | 1.824‐2.666 | 0.000 | 99.412 | 0.050 |
| I | 5 | 2.701 | 1.802‐4.050 | 0.000 | 98.514 | 0.250 | |
| P | 14 | 2.058 | 1.680‐2.522 | 0.000 | 99.120 | 0.370 | |
| Schizophrenia vs. no schizophrenia (all matched) | P | 7 | 1.855 | 1.392‐2.473 | 0.000 | 91.665 | 0.480 |
| Schizophrenia vs. no schizophrenia (matched for acute myocardial infarction) | P | 4 | 1.847 | 1.515‐2.252 | 0.000 | 73.575 | 0.360 |
| Mortality due to cerebrovascular diseases | |||||||
| Schizophrenia vs. any other population | I + P | 16 | 1.458 | 1.168‐1.822 | 0.001 | 97.435 | 0.090 |
| P | 11 | 1.386 | 0.993‐1.936 | 0.055 | 98.027 | 0.260 | |
| Schizophrenia vs. general population | I + P | 13 | 1.598 | 1.250‐2.042 | 0.000 | 97.748 | 0.220 |
| I | 5 | 1.764 | 1.357‐2.292 | 0.000 | 72.580 | 0.090 | |
| P | 8 | 1.583 | 1.062‐2.359 | 0.024 | 98.505 | 0.490 | |
| Schizophrenia vs. no schizophrenia (all matched) | P | 3 | 0.972 | 0.520‐1.817 | 0.929 | 91.905 | 0.240 |
| Schizophrenia vs. no schizophrenia (matched for stroke) | P | 2 | 0.724 | 0.173‐3.038 | 0.659 | 95.719 | NA |
| Mortality due to diabetes mellitus | |||||||
| Schizophrenia vs. any other population | I + P | 7 | 2.512 | 1.623‐3.889 | 0.000 | 99.121 | 0.170 |
| P | 6 | 2.271 | 1.444‐3.572 | 0.000 | 98.201 | 0.920 | |
| Schizophrenia vs. general population | I + P | 5 | 3.159 | 2.420‐4.123 | 0.000 | 94.848 | 0.270 |
| P | 4 | 2.878 | 1.858‐4.458 | 0.000 | 94.485 | 0.630 | |
| Schizophrenia vs. no schizophrenia (matched for diabetes mellitus) | P | 2 | 1.483 | 1.032‐2.131 | 0.033 | 95.695 | NA |
| Mortality due to any cancer | |||||||
| Schizophrenia vs. general population | I + P | 25 | 1.327 | 1.187‐1.482 | 0.000 | 97.942 | 0.001 |
| I | 5 | 1.315 | 0.982‐1.760 | 0.066 | 93.121 | 0.060 | |
| P | 20 | 1.328 | 1.157‐1.524 | 0.000 | 97.109 | 0.420 | |
| Mortality due to endocrine diseases | |||||||
| Schizophrenia vs. general population | I + P | 9 | 3.802 | 1.750‐8.262 | 0.001 | 97.438 | 0.500 |
| I | 3 | 4.217 | 1.747‐10.179 | 0.001 | 76.243 | 0.390 | |
| P | 6 | 3.519 | 1.216‐10.185 | 0.020 | 98.350 | 0.640 | |
| Mortality due to gastrointestinal diseases | |||||||
| Schizophrenia vs. general population | I + P | 12 | 2.859 | 2.069‐3.950 | 0.000 | 96.838 | 0.930 |
| I | 4 | 2.384 | 1.939‐2.932 | 0.000 | 0.000 | 0.910 | |
| P | 8 | 3.060 | 2.046‐4.577 | 0.000 | 97.959 | 0.800 | |
| Mortality due to any infectious diseases | |||||||
| Schizophrenia vs. general population | I + P | 10 | 3.840 | 2.103‐7.012 | 0.000 | 97.025 | 0.460 |
| P | 8 | 4.344 | 2.228‐8.471 | 0.000 | 97.679 | 0.410 | |
| Mortality due to any liver diseases | |||||||
| Schizophrenia vs. general population | I + P | 2 | 1.964 | 1.899‐2.032 | 0.000 | 0.000 | NA |
| Mortality due to any neurological diseases | |||||||
| Schizophrenia vs. general population | I + P | 8 | 2.347 | 1.942‐2.838 | 0.000 | 6.879 | 0.400 |
| I | 4 | 1.972 | 1.126‐3.452 | 0.018 | 25.381 | 0.270 | |
| P | 4 | 2.435 | 2.245‐2.641 | 0.000 | 0.000 | 0.840 | |
| Mortality due to any respiratory diseases | |||||||
| Schizophrenia vs. general population | I + P | 15 | 3.748 | 2.989‐4.699 | 0.000 | 97.563 | 0.790 |
| I | 4 | 3.267 | 2.365‐4.515 | 0.000 | 60.784 | 0.430 | |
| P | 11 | 3.860 | 2.963‐5.029 | 0.000 | 98.217 | 0.720 | |
| Mortality due to any urogenital diseases | |||||||
| Schizophrenia vs. general population | I + P | 9 | 3.328 | 2.062‐5.372 | 0.000 | 98.032 | 0.640 |
| P | 7 | 3.752 | 2.183‐6.450 | 0.000 | 98.518 | 0.560 | |
Significant values of risk ratio are highlighted in bold. I – incident, P – prevalent, TRS – treatment‐resistant schizophrenia, FGA – first‐generation antipsychotic, SGA, second‐generation antipsychotic, LAI – long‐acting injectable antipsychotic, NA – not available
Figure 2.
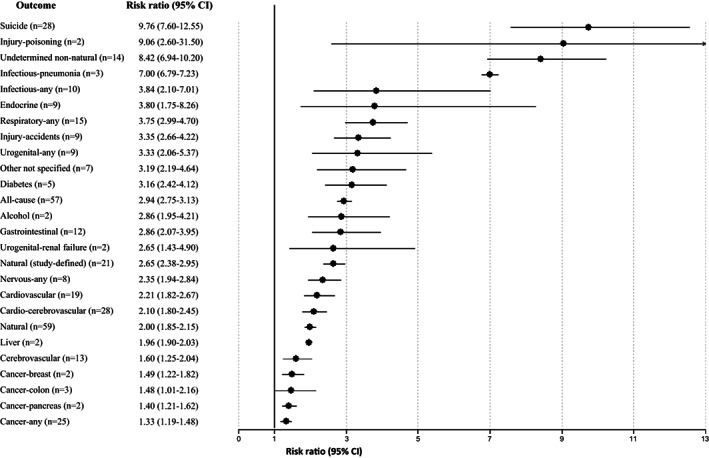
Significant findings for all‐cause and cause‐specific mortality risk in incident plus prevalent schizophrenia versus the general population
Figure 3.
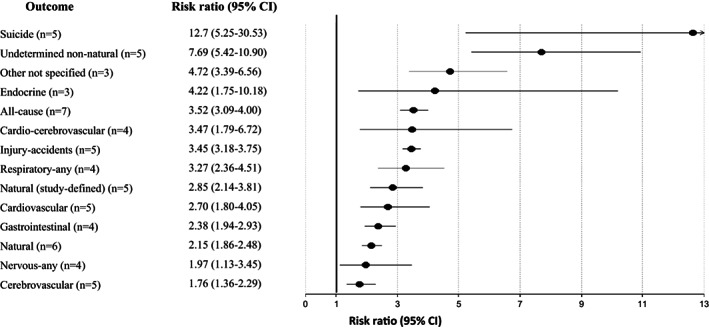
Significant findings for all‐cause and cause‐specific mortality risk in incident schizophrenia versus the general population
Figure 4.
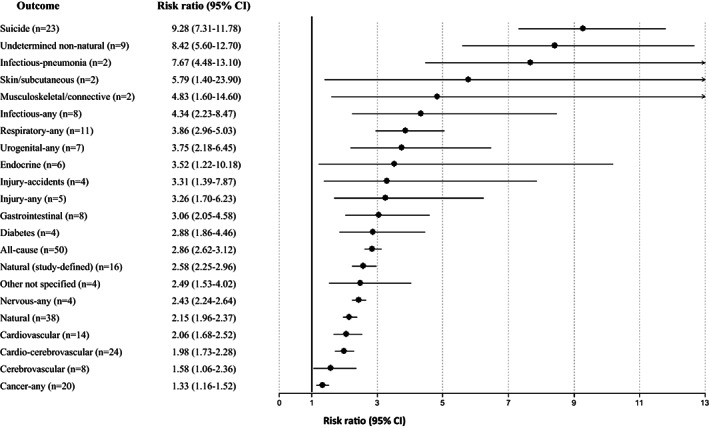
Significant findings for all‐cause and cause‐specific mortality risk in prevalent schizophrenia versus the general population
Compared with controls matched for physical diseases, the mortality risk of individuals with schizophrenia was attenuated but still significant (RR=1.66, 95% CI: 1.42‐1.94, I2=97.2%, n=22) (see Table 2). Specifically, individuals with schizophrenia had significantly higher mortality compared with controls matched for acute myocardial infarction (RR=1.82, 95% CI: 1.49‐2.22, I2=83.1%, n=6), diabetes mellitus (RR=1.91, 95% CI: 1.08‐3.38, I2=99.4, n=4), and stroke (RR=1.35, 95% CI: 1.22‐1.50, I2=0%, n=2) (see Table 2).
No significantly increased mortality risk emerged when schizophrenia was compared with other psychiatric disorders, except for bipolar disorder (RR=1.26, 95% CI: 1.03‐1.53, I2=25.4%, n=3) (see Table 2).
Regarding risk and protective factors for all‐cause mortality, having a substance use disorder comorbid with schizophrenia increased mortality (RR=1.62, 95% CI: 1.47‐1.80, I2=57.4%, n=3) (see Table 2).
Wherever publication bias was detected, we conducted trim and fill analyses, which confirmed the magnitude and significance of the findings in the primary analyses, with a fail‐safe N ranging from 545 to 27,164,601 (see also supplementary information).
Key secondary outcomes: suicide‐related mortality and natural causes of mortality
Suicide‐related mortality
Across 28 studies, schizophrenia was associated with increased mortality by suicide compared with the general population (RR=9.76, 95% CI: 7.60‐12.55, I2=99.5%) (see Table 2 and Figure 2), suggesting that suicide is the greatest relative risk factor for mortality in individuals with schizophrenia. There was a numerically but not statistically significantly greater suicide‐related mortality among the incident versus prevalent cohort (RR=12.7, 95% CI: 5.25‐30.53, I2=99.8, n=5 vs. RR=9.28, 95% CI: 7.31‐11.78, I2=98.8%, n=23, p=0.51) (see Table 2, Figures 3, 4 and supplementary information).
Wherever publication bias was detected, we conducted trim and fill analyses, which confirmed the magnitude and significance of the primary findings, with a fail‐safe N ranging from 25,581 to 229,490 (see also supplementary information).
Natural causes of mortality
Across 59 studies, schizophrenia was associated with higher natural‐cause mortality (which excludes mortality due to suicide or accident or poisoning) compared with either the general population or control groups matched for a physical disease (RR=2.00, 95% CI: 1.85‐2.15, I2=99.5%) (see Table 2).
Higher natural‐cause mortality was confirmed across 44 studies involving comparisons with the general population (RR=2.16, 95% CI: 1.99‐2.36, I2=99.6%), without differences between incident and prevalent schizophrenia (RR=2.15, 95% CI: 1.86‐2.48, I2=94.6, n=6 vs. RR=2.15, 95% CI: 1.96‐2.37, I2=99.1%, n=38, p=0.939) (see Table 2, Figures 3, 4 and supplementary information).
Across 16 studies involving prevalent populations with physical disease‐matched controls, natural‐cause mortality risk was also significantly increased (RR=1.56, 95% CI: 1.35‐1.82, I2=94.0%), including specifically matched patients with acute myocardial infarction (RR=1.66, 95% CI: 1.24‐2.22, I2=96.4%, n=5) (see Table 2).
Wherever publication bias was detected, we conducted trim and fill analyses, which confirmed the magnitude and significance of the primary findings (with a fail‐safe N ranging from 235 to 282,469), except for a slight reduction of the effect size in comparison with physical disease‐matched controls (four studies trimmed, RR=1.35, 95% CI: 1.17‐1.56) (see also supplementary information).
Additional secondary outcomes: other specific‐cause mortality
Cardiovascular and/or cerebrovascular diseases
Across 30 studies, schizophrenia was associated with higher cardio‐cerebrovascular‐related mortality compared with either the general population or control groups matched for a physical illness (RR=2.03, 95% CI: 1.68‐2.45, I2=99.5%) (see Table 2). Separating causes, higher mortality from cardiovascular diseases (RR=2.09, 95% CI: 1.76‐2.47, I2=99.3%, n=25) as well as from cerebrovascular diseases (RR=1.46, 95% CI: 1.17‐1.82, I2=97.4%, n=16) was observed among individuals with schizophrenia (see Table 2).
Comparing schizophrenia with the general population, significant findings emerged for the composite mortality outcome (RR=2.10, 95% CI: 1.80‐2.45, I2=99.0%, n=28), as well as for mortality due to cardiovascular diseases (RR=2.21, 95% CI: 1.82‐2.67, I2=99.4%, n=19) and to cerebrovascular diseases (RR=1.60, 95% CI: 1.25‐2.04, I2=97.7%. n=13). Mortality due to cardio‐cerebrovascular diseases was substantially higher in incident (RR=3.47, 95% CI: 1.79‐6.72, I2=97.9%, n=4) than in prevalent schizophrenia (RR=1.98, 95% CI: 1.73‐2.27, I2=97.7%, n=24) (see Table 2 and Figure 2).
Compared with physical disease‐matched controls, patients with schizophrenia had significantly higher mortality from cardiovascular diseases (RR=1.86, 95% CI: 1.39‐2.47, I2=91.7%, n=7), including cohorts that were specifically matched for acute myocardial infarction (RR=1.85, 95% CI: 1.52‐2.25, I2=73.6%, n=4) (see Table 2).
Other specific causes
Individuals with schizophrenia had significantly higher mortality than the general population from pneumonia (RR=7.00, 95% CI: 6.79‐7.23, n=3), any infectious diseases (RR=3.84, 95% CI: 2.10‐7.01, n=10), any endocrine diseases (RR=3.80, 95% CI: 1.75‐8.26, n=9), any respiratory diseases (RR=3.75, 95% CI: 2.99‐4.70, n=15), any urogenital diseases (RR=3.33, 95% CI: 2.06‐5.37, n=9), diabetes mellitus (RR=3.16, 95% CI: 2.42‐4.12, n=5), any gastrointestinal diseases (RR=2.86, 95% CI: 2.07‐3.95, n=12), any neurological diseases (RR=2.35, 95% CI: 1.94‐2.84, n=8), any liver diseases (RR=1.96, 95% CI: 1.90‐2.03, n=2), and any cancer (RR=1.33, 95% CI: 1.19‐1.48, n=25) (see Table 2 and Figure 2).
Among individuals with schizophrenia, mortality was significantly higher than the general population also from injury‐poisoning (RR=9.06, 95% CI: 2.60‐31.50, n=2) and undetermined non‐natural causes (RR=8.42, 95% CI: 6.94‐10.20, n=14) (see Figure 2 and supplementary information).
In incident schizophrenia, no significant association was found with death due to cancer (RR=1.31, 95% CI: 0.98‐1.76, n=5), whereas the association was observed in prevalent schizophrenia (RR=1.33, 95% CI: 1.16‐1.52, n=20) (see Table 2 and Figure 4). There was instead a significantly increased risk of mortality in both incident and prevalent schizophrenia cohorts due to endocrine diseases (incident: RR=4.22, 95% CI: 1.75‐10.18, n=3; prevalent: RR=3.52, 95% CI: 1.22‐10.18, n=6), gastrointestinal diseases (incident: RR=2.38, 95% CI: 1.94‐2.93, n=4; prevalent: RR=3.06, 95% CI: 2.04‐4.58, n=8), neurological diseases (incident: RR=1.97, 95% CI: 1.13‐3.45, n=4; prevalent: RR=2.43, 95% CI: 2.24‐2.64, n=4) and respiratory diseases (incident: RR=3.27, 95% CI: 2.36‐4.51, n=4; prevalent: RR=3.86, 95% CI: 2.96‐5.03, n=11) (see Table 2 and Figures 2, 3, 4).
Subgroup analyses and meta‐regression
Use of any antipsychotic versus non‐use was associated with a reduction of all‐cause mortality in patients with incident plus prevalent schizophrenia (RR=0.71, 95% CI: 0.59‐0.84, I2=97.7%, n=11). Reduction of all‐cause mortality risk versus no antipsychotic treatment differed significantly across antipsychotic subgroups (p=0.0001), in descending order as follows: any SGA LAI (RR=0.39, 95% CI: 0.27‐0.56, I2=81.0%, n=3), clozapine (RR=0.43, 95% CI: 0.34‐0.55, I2=77.9%, n=3), any LAI (RR=0.47, 95% CI: 0.39‐0.58, I2=91.8%, n=2), any oral SGA (RR=0.47, 95% CI: 0.45‐0.50, I2=18.9%, n=4), any first‐generation antipsychotic (FGA) LAI (RR=0.50, 95% CI: 0.43‐0.57, I2=68.9%, n=3), any SGA (RR=0.53, 95% CI: 0.44‐0.63, I2=91.0%, n=4), any oral antipsychotic (RR=0.64, 95% CI: 0.51‐0.80, I2=95.9%, n=4), and any FGA (RR=0.73, 95% CI: 0.55‐0.97, I2=97.0%, n=5). There was a borderline significant all‐cause mortality reduction among individuals with treatment‐resistant schizophrenia who received clozapine compared with other medications (RR=0.70, 95% CI: 0.49‐1.00, I2=57.9%, n=5) (see Figure 5 and supplementary information).
Figure 5.
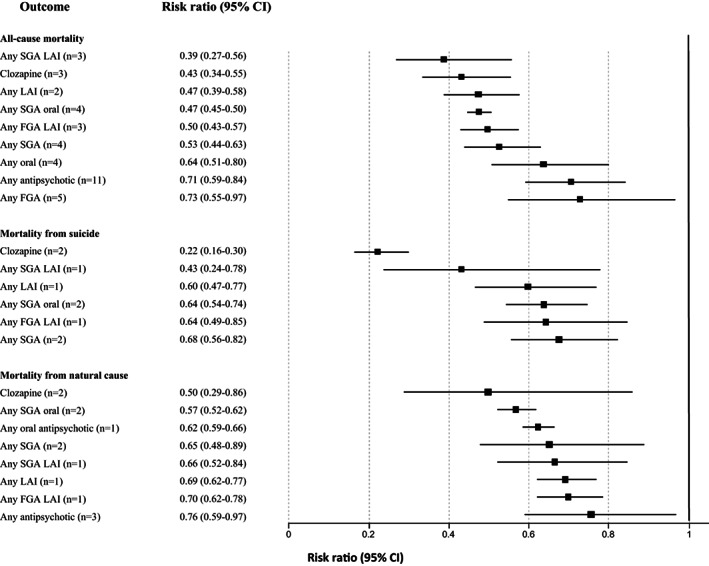
Findings in subgroup analyses of mortality risk due to any cause, suicide, and natural death by antipsychotic treatment within incident plus prevalent schizophrenia versus no antipsychotic. FGA – first‐generation antipsychotic, SGA – second‐generation antipsychotic, LAI – long‐acting injectable antipsychotic
In incident schizophrenia, the largest protective association emerged for SGA LAIs (RR=0.15, 95% CI: 0.04‐0.55, n=1), whereas the protective effect was not significant for any oral antipsychotics, or FGA in any formulation (p=0.07 for comparison across antipsychotics). In prevalent schizophrenia, the largest association emerged for SGA LAIs again (RR=0.42, 95% CI: 0.29‐0.59, n=2), and the smallest for any antipsychotic (RR=0.69, 95% CI: 0.57‐0.84, n=7) (p=0.0001 for comparison across antipsychotics) (see supplementary information).
Use of any antipsychotic versus non‐use was not associated with a reduction of suicide‐related mortality in patients with incident plus prevalent schizophrenia (RR=0.73, 95% CI: 0.47‐1.12, I2=94.4%, n=4). Reduction of suicide‐related mortality versus no antipsychotic treatment differed significantly across antipsychotic subgroups (p=0.0001), in descending order as follows: clozapine (RR=0.22, 95% CI: 0.16‐0.30, I2=0%, n=2), any SGA LAI (RR=0.43, 95% CI: 0.24‐0.78, I2 not available, n=1), any LAI (RR=0.60, 95% CI: 0.47‐0.77, I2 not available, n=1), any SGA oral (RR=0.64, 95% CI: 0.54‐0.74, I2=0, n=2), any FGA LAI (RR=0.64, 95% CI: 0.49‐0.85, I2 not available, n=1), and any SGA (RR=0.68, 95% CI: 0.56‐0.82, I2=44.2%, n=2). In contrast, compared to no antipsychotic, any FGA (RR=1.05, 95% CI: 0.37‐2.99, I2=97.2%, n=2) and oral FGAs (RR=1.13, 95% CI: 0.33‐3.93, I2=95.7%, n=2) did not protect individuals with schizophrenia against suicide‐related mortality (see Figure 5 and supplementary information).
In incident schizophrenia, the largest protective association regarding suicide‐related mortality emerged for clozapine (RR=0.29, 95% CI: 0.14‐0.62, n=1), while, in contrast, oral FGAs were associated with increased mortality (RR=2.17, 95% CI: 1.36‐3.48, n=1) (p=0.0001 for comparison across antipsychotics). In prevalent schizophrenia, the lowest risk of suicide‐related mortality emerged for clozapine (RR=0.21, 95% CI: 0.15‐0.29, n=1), and the closest to null effect emerged for any antipsychotic (RR=0.73, 95% CI: 0.36‐1.49, n=2) (p=0.0001 for comparison across antipsychotics) (see supplementary information).
In incident plus prevalent schizophrenia, any antipsychotic versus no antipsychotic use was protective against natural causes of mortality (RR=0.76, 95% CI: 0.59‐0.97, I2=90.7%, n=3). Reduction of natural‐cause mortality versus no antipsychotic treatment differed significantly across antipsychotic subgroups (p=0.04), in descending order as follows: clozapine (RR=0.50, 95% CI: 0.29‐0.86, I2=21.3%, n=2), any oral SGA (RR=0.57, 95% CI: 0.52‐0.62, I2=0%, n=2), any oral antipsychotic (RR=0.62, 95% CI: 0.59‐0.66, I2 not available, n=1), any SGA (RR=0.65, 95% CI: 0.48‐0.89, I2=71.4%, n=2), any SGA LAI (RR=0.66, 95% CI: 0.52‐0.84, I2 not available, n=1), any LAI (RR=0.69, 95% CI: 0.62‐0.77, I2 not available, n=1), any FGA LAI (RR=0.70, 95% CI: 0.62‐0.78, I2 not available, n=1). In contrast, any FGA or any oral FGA were not associated with lower natural‐cause mortality (see Figure 5 and supplementary information).
In incident schizophrenia, no significant reduction of natural‐cause mortality emerged for any antipsychotic subgroup versus no antipsychotic use. Oral FGAs were associated with increased natural‐cause mortality (RR=2.20, 95% CI: 1.29‐3.77, n=1) (p=0.0004 for comparison across antipsychotics). In prevalent schizophrenia, the largest protective effect emerged for clozapine (RR=0.55, 95% CI: 0.47‐0.64, n=1), and the smallest for FGA LAIs (RR=0.70, 95% CI: 0.62‐0.78, n=1) (p=0.0005 for comparison across antipsychotics) (see supplementary information).
In subgroup analyses of incident plus prevalent schizophrenia cohorts by age, the risk of all‐cause mortality was significantly higher for patients aged <40 vs. ≥40 years (RR=3.93, 95% CI: 3.34‐4.63 vs. RR=2.66, 95% CI: 2.18‐3.26, p=0.003). A similar difference was observed for suicide‐related mortality (RR=17.58, 95% CI: 12.36‐24.99 vs. RR=4.69, 95% CI: 1.77‐12.45, p=0.01). There was no significant difference between the two age groups for natural‐cause mortality (see supplementary information).
No consistent and significant differences emerged from subgroup analyses considering nationwide versus other samples, quality of studies, and adjustment of results, suggesting that findings concerning mortality are not systematically influenced by these moderators (see supplementary information).
In meta‐regression analyses, we found in incident plus prevalent schizophrenia a significant increase of all‐cause mortality (beta=0.0009, 95% CI: 0.001‐0.02, p=0.02) and of natural‐cause mortality (beta=0.01, 95% CI: 0.006‐0.02, p=0.0002) with increasing median year of study publication, without a significant time trend for suicide‐related mortality (beta=0.006, 95% CI: –0.01 to 0.03, p=0.56) (see supplementary information).
For all‐cause mortality, in incident plus prevalent schizophrenia, more recent study year moderated a larger protective effect of any antipsychotic (beta=–0.11, 95% CI: –0.15 to –0.06) and of oral FGA versus no antipsychotic (beta=–0.11, 95% CI: –0.17 to –0.05). Similarly, for suicide‐related mortality, more recent study year moderated a larger protective effect of any FGA versus no antipsychotic in incident plus prevalent schizophrenia (beta=–0.27, 95% CI: –0.36 to –0.18).
Longer duration of follow‐up and more variables used to adjust the analyses increased the protective effect against suicide‐related mortality of any antipsychotic in prevalent schizophrenia (beta=–0.14, 95% CI: –0.24 to –0.04, and beta =–0.23, 95% CI: –0.40 to –0.06, respectively). Higher percentage of females increased the risk of suicide‐related mortality in incident schizophrenia (beta=0.36, 95% CI: 0.23‐0.49, p<0.0001).
For natural‐cause mortality, the protective effect of any FGA versus no antipsychotic in incident plus prevalent schizophrenia was increased by more recent study year (beta=–0.23, 95% CI: –0.33 to –0.13) and more variables used to adjust the analyses (beta=–0.12, 95% CI: –0.17 to –0.07). Natural‐cause mortality versus any other population was greater in higher quality studies in incident plus prevalent schizophrenia (beta=0.11, 95% CI: 0.04‐0.18). Natural‐cause mortality versus the general population was also greater in higher quality studies in incident plus prevalent schizophrenia (beta=0.13, 95% CI: 0.06‐0.20), as well as in incident schizophrenia (beta=0.20, 95% CI: 0.08‐0.31) and in prevalent schizophrenia (beta=0.11, 95% CI: 0.02‐0.19). Natural‐cause mortality was also larger, in incident schizophrenia, with higher number of variables that analyses were adjusted for (beta=0.12, 95% CI: 0.06‐0.18).
DISCUSSION
Schizophrenia is one of the mental disorders with the highest mortality risk. This meta‐analysis of 135 cohort studies comparing 4.5 million schizophrenia patients with about 1.11 billion people from the general population comprehensively quantified this increased risk. Specifically, we observed a 2.9‐fold increased all‐cause mortality in patients with schizophrenia versus the general population, and a somewhat lower but still significantly 1.6‐fold increased risk versus physical disease‐matched general population controls.
In addition, we identified significantly greater specific‐cause mortality among individuals with schizophrenia versus the general population, which was particularly pronounced for suicide (9.7‐fold); other non‐natural causes, including poisoning (8‐ to 9‐fold); and pneumonia (7‐fold). The mortality risk remained greater for infectious, endocrine and respiratory diseases (3.7‐3.8‐fold); injury or accidents (3.3‐fold); diabetes mellitus (3.2‐fold); alcohol use and gastrointestinal diseases (2.9‐fold); urogenital diseases (2.6‐fold); neurological diseases (2.3‐fold); cardiovascular diseases (2.2‐fold); liver diseases (2‐fold); and cerebrovascular diseases (1.6‐fold); also extending to breast, colon, pancreas and any cancer (1.3‐ to 1.5‐fold).
The relative increase in mortality compared to the general population was larger in incident (i.e., earlier‐phase) than prevalent (i.e., more chronic) schizophrenia cohorts. Moreover, all‐cause and suicide‐related mortality were higher in patients <40 years old, whereas this was not the case for natural‐cause mortality. Comorbid substance use disorder increased the all‐cause mortality gap, while antipsychotic treatment versus no treatment decreased this gap. The largest protective effect was observed with SGA LAIs and clozapine. In contrast to this protective effect, FGAs increased suicide‐related and natural‐cause mortality in incident schizophrenia.
We found that first‐episode schizophrenia was associated with a 7.4‐fold higher all‐cause mortality risk versus the general population, indicating the critical importance of providing a swift and accurate diagnosis followed by initiating effective treatment. The lifetime prevalence of completed suicide in patients with schizophrenia has been reported to be 5.6%, with the majority of these suicides occurring near illness onset 167 . Moreover, suicide attempts have been found to be predicted by greater severity of psychotic illness and of depressive symptoms 168 , two factors that should prompt clinicians to screen for and guard against suicide attempts in the early phase of the illness. Furthermore, our finding that females with schizophrenia have a significantly higher risk increase than males for suicide‐related mortality compared to the general population should prompt clinicians to extend the focus from males, who are still at the highest risk for completed suicide 169 , to this additional high‐risk group.
All‐cause mortality was increased in persons with schizophrenia even when they were matched with general population controls for many relevant physical diseases. These included cardiovascular, cerebrovascular, endocrine, gastrointestinal, infectious, liver, neurological, respiratory and urogenital diseases, diabetes mellitus and cancer. Importantly, the relative mortality risk for cardio‐cerebrovascular diseases was substantially greater in the incident (RR=3.47) versus prevalent (RR=1.98) cohorts, which is perhaps reflective of the lower overall frequency of these diseases in the younger general population and of their earlier onset in people with schizophrenia, likely due to poorer lifestyle behaviors 170 , 171 , 172 and to the effect of antipsychotic and other medications 21 , 173 .
Disparities between individuals with schizophrenia and the general population with respect to the implementation of screening procedures (e.g., for cardiovascular risk factors and disorders, and for cancer) and the quality of medical care, including a lack of advice for lifestyle changes such as smoking cessation and physical activity, have been repeatedly reported 2 , 27 , 28 , 43 , 174 , 175 . Addressing smoking is of particular importance, given the 70‐162% increased risk of asthma, chronic obstructive pulmonary disease and pneumonia in subjects with schizophrenia 176 , and considering our finding that pneumonia confers the highest risk of death among natural causes. Thus, to close the mortality gap in individuals with schizophrenia, smoking cessation interventions, cardiovascular and cancer screening and monitoring, consistent healthy lifestyle instructions, as well as early interventions for detected physical diseases, should be regarded as imperative. Since individuals with schizophrenia may be less likely to receive or seek help from a medical health care provider than people from the general population, mental health care providers need to orchestrate physical care for these individuals as part of a comprehensive and collaborative care model 17 .
Comorbid substance use disorders were found in our meta‐analysis to be a significant risk factor for increased mortality in people with schizophrenia. This finding is likely due to the multiple adverse physical as well as intentional or accidental suicide‐related effects of these disorders 177 , 178 , 179 , 180 , 181 . Additionally, comorbid substance use, and cannabis use in particular, can worsen adherence to antipsychotics 182 , 183 , 184 . All these factors point to the need to screen for and address substance use disorders as early as possible when treating patients with schizophrenia 185 , 186 .
This meta‐analysis found that, compared with no antipsychotic use, antipsychotic treatment was associated with reduced all‐cause mortality in patients with schizophrenia. Specifically, factors associated with a reduction in all‐cause mortality included the use of any LAI, any SGA and, especially, of clozapine. These findings support prior research which found that continuous clozapine use was associated with significantly lower long‐term all‐cause mortality compared with other antipsychotics in patients with schizophrenia, despite the adverse impact of clozapine on cardiometabolic risk factors 187 . We also observed a borderline significant reduction in all‐cause mortality among patients with treatment‐resistant schizophrenia who were treated with clozapine compared with other antipsychotics, with lack of significance likely being due to low power of these analyses.
Recently, a Finnish national database study 20 indicated that patients with schizophrenia who were taking antipsychotics, especially LAIs and clozapine, were significantly less likely to interrupt ongoing treatment with statins, antidiabetic agents, anti‐hypertensive medications, and beta‐blockers. Such an association between the use of antipsychotics and better adherence to medical treatments – and potentially also closer and more regular medical monitoring as might be the case with clozapine and LAIs – is likely to be a mediator of the protective effect of antipsychotic use on mortality risk in people with schizophrenia. Studies that specifically test this hypothesis are warranted.
The use of any SGA or clozapine also had a significant protective effect against suicide‐related mortality in prevalent schizophrenia, compared with no use of antipsychotics, which was not observed with FGAs. While the anti‐suicidal efficacy of clozapine has been established 188 , the differential finding favoring SGAs may be due to the fact that suicide in schizophrenia is often associated with the emergence of depression 168 . FGAs do not improve or even induce depressive symptoms, while many SGAs have been shown to be effective in treating these symptoms 189 , 190 , 191 .
We found that, in incident schizophrenia, FGAs were even associated with an increased mortality risk due to suicide. This finding should caution against the use of these medications as first‐line agents, in particular in earlier‐phase patients. The fact that this increased mortality risk in incident schizophrenia was not found with FGA LAIs points to a potentially mediating effect of poorer adherence with oral FGAs or a protective effect of LAI use due to increased surveillance and, possibly, treatment of emergent depression.
Thus, in addition to underscoring the importance of comprehensive physical health monitoring and integrated or collaborative care to address and improve both physical and mental health problems in patients with schizophrenia, this meta‐analysis points to the need for antipsychotic maintenance treatment, monitoring for and mitigating antipsychotic non‐adherence, also through a broader and earlier consideration of SGA LAIs. Furthermore, our findings point to the need to screen for and treat substance use disorders as well as depression as important clinical strategies to reduce overall and specific‐cause mortality in individuals with schizophrenia.
We found a slight but significant increase of the excess mortality in people with schizophrenia by median study year of investigation (ranging from 1957 to 2021). This finding further emphasizes the urgency with which the mortality gap in these people needs to be addressed.
Among the strengths of this meta‐analysis are the large number of studies (n=135) that met the inclusion criteria, the substantial number of patients with schizophrenia (4,536,447) and general population controls (1,115,600,059); and the high quality of the studies included, with results being consistent and robust even after all trim and fill analyses. Moreover, directions for future research are provided, as analyses adjusted for more potentially relevant confounders and longer follow‐up were associated with greater protective effects of antipsychotic medications against the increased mortality risk.
However, the results of this meta‐analysis have to be interpreted within its limitations. First, meta‐analyzed studies were observational cohort investigations. Their non‐randomized nature cannot imply causality. However, since mortality is a relatively rare and late‐onset/distal event, randomized controlled trials – that generally include relatively few individuals, have a modest follow‐up duration and many dropouts, and that also exclude many patients that may be more severely mentally and physically ill 192 – are not the best or most feasible studies to quantify mortality risk and identify generalizable aggravating and protective factors. For the study of mortality risk, longitudinal cohort and, especially, nationwide database studies represent more appropriate study options. Furthermore, consistent with our meta‐analysis, two smaller meta‐analyses focusing on patients in randomized controlled trials reported similar results – i.e., an about 30‐50% lower mortality among patients randomized to antipsychotics compared with patients randomized to placebo 193 , 194 .
Second, although we were able to include as many as 135 individual studies, with a large number of individuals with schizophrenia and even more control subjects from the general population, some findings were based on five or fewer studies. The need for additional studies is particularly important with respect to the quantitative evaluation of specific factors that increase or decrease the existing mortality gap. Third, there was substantial inconsistency in the definitions of age groups across the included studies, which limited our ability to comprehensively analyze the effect of age on all‐cause and specific‐cause mortality risk. Future studies should report age both categorically across relevant age groups as well as continuously.
Fourth, few studies specifically evaluated mortality risk in patients with first‐episode or treatment‐resistant schizophrenia, two subgroups of considerable clinical interest. Fifth, some studies did not quantify the number of the general population control group, but used instead regional or nationwide control groups restricted to certain time periods and/or age groups. In such instances, we estimated the number of general population controls based on census‐based (sub)population numbers at the time of data collection, which may have introduced some imprecision. Sixth, studies used different metrics to report mortality: in order to pool results, we combined risk estimates that have somewhat different characteristics, which could have led to some imprecision. However, since mortality is a relatively rare event and since all included studies used the same cohort design and evaluated the same population of interest, the degree of imprecision is likely low.
Finally, although we preferred the risk estimate that was adjusted for the most likely potential confounders, we also included unadjusted risk estimates, and adjustments may not have included all/the most relevant covariates that are associated with mortality risk. However, we were not interested in isolating the genetic or narrowly illness‐related effect of schizophrenia on mortality risk, but rather in estimating the differential risk of all‐cause and specific‐cause mortality in individuals with schizophrenia who differ in many psychological, behavioral, social and environmental respects from the general population and other control groups. The potential residual confounding from a statistical standpoint, therefore, represents the reality of individuals living with schizophrenia and ensures the desired generalizability of the findings.
CONCLUSIONS
This meta‐analysis provides the largest and most comprehensive quantitative assessment of the all‐cause and detailed specific‐cause mortality risk of individuals with schizophrenia versus the general population and other control groups, additionally focusing on reported aggravating and protective factors. It confirms that the mortality gap between patients with and without schizophrenia is high, being highest for suicide‐related mortality but extending to multiple other specific‐cause mortality reasons. Results of this mortality gap in individuals with schizophrenia were based on high‐quality data in >97% of the studies and were robust and confirmed in multiple subgroup and meta‐regression analyses. Importantly, the increased mortality was associated with certain modifiable risk factors, which can inform clinical practice.
Consistent and long‐term use of SGAs, SGA LAIs and, if indicated, clozapine in patients with schizophrenia across all stages of illness can reduce the mortality risk, as antipsychotics are protective compared to non‐use of antipsychotics against many kinds of mortality, including that due to cardio‐cerebrovascular disease. This finding indicates that even antipsychotics with elevated cardiometabolic adverse effects, such as clozapine, can reduce overall mortality, which is not counterbalanced by larger but supported by reduced cardiometabolic‐related mortality. Results were confirmed or even stronger in more recent, higher quality, adjusted studies, and those with longer follow‐up. Finally, despite heightened awareness of the mortality gap of people with severe mental illness and especially with schizophrenia, this gap seems to be increasing slightly with time, including data as recent as 2021.
These results underscore the urgency with which the mortality disparity in individuals with schizophrenia need to be addressed at multiple levels. Clinicians should routinely monitor patients with schizophrenia for cardiovascular risk and physical diseases and also screen for and address substance use disorders and depression. In addition, they should screen patients with first‐episode schizophrenia, both males and females, for suicide risk and depression, and avoid FGAs.
Overall, integrated mental and physical health care of individuals with schizophrenia must be at the center of mental health research and policy making agendas. Data from this meta‐analysis point to the responsibility of reducing mortality risk by screening for and optimizing the management of physical as well as psychiatric comorbidities, and by earlier use of LAIs and, if indicated, clozapine in individuals with schizophrenia. This information should be considered by treatment guidelines and incorporated into actionable policies by health care administrators.
ACKNOWLEDGEMENTS
C.U. Correll, M. Solmi and G. Croatto are joint first authors of this paper. Supplementary information on the study is available at https://osf.io/j2ymb/.
REFERENCES
- 1. Vermeulen JM, van Rooijen G, Doedens P et al. Antipsychotic medication and long‐term mortality risk in patients with schizophrenia: a systematic review and meta‐analysis. Psychol Med 2017;47:2217‐28. [DOI] [PubMed] [Google Scholar]
- 2. Vancampfort D, Rosenbaum S, Schuch F et al. Cardiorespiratory fitness in severe mental illness: a systematic review and meta‐analysis. Sports Med 2017;47:343‐52. [DOI] [PubMed] [Google Scholar]
- 3. Björk Brämberg E, Torgerson J, Norman Kjellström A et al. Access to primary and specialized somatic health care for persons with severe mental illness: a qualitative study of perceived barriers and facilitators in Swedish health care. BMC Fam Pract 2018;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia. Arch Gen Psychiatry 2007;64:1123‐31. [DOI] [PubMed] [Google Scholar]
- 5. Bitter I, Czobor P, Borsi A et al. Mortality and the relationship of somatic comorbidities to mortality in schizophrenia. A nationwide matched‐cohort study. Eur Psychiatry 2017;45:97‐103. [DOI] [PubMed] [Google Scholar]
- 6. Tanskanen A, Tiihonen J, Taipale H. Mortality in schizophrenia: 30‐year nationwide follow‐up study. Acta Psychiatr Scand 2018;138:492‐9. [DOI] [PubMed] [Google Scholar]
- 7. Lomholt LH, Andersen DV, Sejrsgaard‐Jacobsen C et al. Mortality rate trends in patients diagnosed with schizophrenia or bipolar disorder: a nationwide study with 20 years of follow‐up. Int J Bipolar Disord 2019;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang CY, Fang SC, Shao YJ. Comparison of long‐acting injectable antipsychotics with oral antipsychotics and suicide and all‐cause mortality in patients with newly diagnosed schizophrenia. JAMA Net Open 2021;4:e218810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taipale H, Mittendorfer‐Rutz E, Alexanderson K et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res 2018;197:274‐80. [DOI] [PubMed] [Google Scholar]
- 10. Taipale H, Tanskanen A, Mehtälä J et al. 20‐year follow‐up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 2020;19:61‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Hert M, Detraux J, van Winkel R et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011;8:114‐26. [DOI] [PubMed] [Google Scholar]
- 12. Galling B, Roldán A, Nielsen RE et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta‐analysis. JAMA Psychiatry 2016;73:247‐59. [DOI] [PubMed] [Google Scholar]
- 13. Vancampfort D, Stubbs B, Mitchell AJ et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vancampfort D, Correll CU, Galling B et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large‐scale meta‐analysis. World Psychiatry 2016;15:166‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Correll CU, Solmi M, Veronese N et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large‐scale meta‐analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16:163‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Hert M, Correll CU, Bobes J et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu NH, Daumit GL, Dua T et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry 2017;16:30‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Firth J, Siddiqi N, Koyanagi A et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019;6:675‐712. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol 2021;18:136‐45. [DOI] [PubMed] [Google Scholar]
- 20. Solmi M, Tiihonen J, Lähteenvuo M et al. Antipsychotics use is associated with greater adherence to cardiometabolic medications in patients with schizophrenia: results from a nationwide, within‐subject design study. Schizophr Bull 2022;48:166‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pillinger T, McCutcheon RA, Vano L et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta‐analysis. Lancet Psychiatry 2020;7:64‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Solmi M, Murru A, Pacchiarotti I et al. Safety, tolerability, and risks associated with first‐ and second‐generation antipsychotics: a state‐of‐the‐art clinical review. Ther Clin Risk Manag 2017;13:757‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Firth J, Solmi M, Wooton RE et al. A meta‐review of “lifestyle psychiatry”: the role of exercise, smoking diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020;19:360‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Attar R, Jensen SE, Nielsen RE et al. Time trends in the use of coronary procedures, guideline‐based therapy, and all‐cause mortality following the acute coronary syndrome in patients with schizophrenia. Cardiology 2020;145:401‐9. [DOI] [PubMed] [Google Scholar]
- 25. Ayerbe L, Forgnone I, Foguet‐Boreu Q et al. Disparities in the management of cardiovascular risk factors in patients with psychiatric disorders: a systematic review and meta‐analysis. Psychol Med 2018;48:2693‐701. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell AJ, Vancampfort D, De Hert M et al. Do people with mental illness receive adequate smoking cessation advice? A systematic review and meta‐analysis. Gen Hosp Psychiatry 2015;37:14‐23. [DOI] [PubMed] [Google Scholar]
- 27. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol 2010;24(Suppl. 4):69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solmi M, Fiedorowicz J, Poddighe L et al. Disparities in screening and treatment of cardiovascular diseases in patients with mental disorders across the world: systematic review and meta‐analysis of 47 observational studies. Am J Psychiatry 2021;178:793‐803. [DOI] [PubMed] [Google Scholar]
- 29. Chou RH, Lo LW, Liou YJ et al. Antipsychotic treatment is associated with risk of atrial fibrillation: a nationwide nested case‐control study. Int J Cardiol 2017;227:134‐40. [DOI] [PubMed] [Google Scholar]
- 30. Khan A, Schwartz K, Stern C et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry 2007;68:1828‐33. [DOI] [PubMed] [Google Scholar]
- 31. Jackson JW, Schneeweiss S, VanderWeele TJ et al. Quantifying the role of adverse events in the mortality difference between first and second‐generation antipsychotics in older adults: systematic review and meta‐synthesis. PLoS One 2014;9:e105376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wells GA, Shea B, O'Connel D et al. The Newcastle‐Ottawa scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 34. Serghiou S, Goodman SN. Random‐effects meta‐analysis: summarizing evidence with caveats. JAMA 2019;321:301‐2. [DOI] [PubMed] [Google Scholar]
- 35. Von Hippel PT. The heterogeneity statistic I2 can be biased in small meta‐analyses. BMC Med Res Methodol 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Egger M, Smith GD, Schneider M et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455‐63. [DOI] [PubMed] [Google Scholar]
- 38. Alleback P, Wistedt B. Mortality in schizophrenia. a ten‐year follow‐up based on the Stockholm County inpatient register. Arch Gen Psychiatry 1986;43:650‐3. [DOI] [PubMed] [Google Scholar]
- 39. Amaddeo F, Bisoffi G, Bonizzato P et al. Mortality among patients with psychiatric illness. A ten‐year case register study in an area with a community‐based system of care. Br J Psychiatry 1995;166:783‐8. [DOI] [PubMed] [Google Scholar]
- 40. Attar R, Valentin JB, Freeman P et al. The effect of schizophrenia on major adverse cardiac events, length of hospital stay and prevalence of somatic comorbidities following acute coronary syndrome. Eur Heart J Qual Care Clin Outcomes 2018;5:121‐6. [DOI] [PubMed] [Google Scholar]
- 41. Bagewadi VI, Kumar CN, Thirthalli J et al. Standardized mortality ratio in patients with schizophrenia – findings from Thirthahalli: a rural South Indian community. Indian J Psychol Med 2016;38:202‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berardi D, Stivanello E, Chierzi F et al. Mortality in mental health patients of the Emilia‐Romagna region of Italy: a registry‐based study. Psychiatry Res 2021;296:113702. [DOI] [PubMed] [Google Scholar]
- 43. Black DW, Fisher R. Mortality in DSM‐IIIR schizophrenia. Schizophr Res 1992;7:109‐10. [DOI] [PubMed] [Google Scholar]
- 44. Bouza C, López‐Cuadrado T, Amate JM. Physical disease in schizophrenia: a population‐based analysis in Spain. BMC Public Health 2010;10:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bralet MC, Yon V, Loas G et al. Cause of mortality in schizophrenic patients: prospective study of years of a cohort of 150 chronic schizophrenic patients. Encephale 2000;26:32‐41. [PubMed] [Google Scholar]
- 46. Brown S, Kim M, Mitchell C et al. Twenty‐five year mortality of a community cohort with schizophrenia. Br J Psychiatry 2010;196:116‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buda M, Tsuang MT, Fleming JA. Causes of death in DSM‐III schizophrenics and other psychotics (atypicals): a comparison with the general population. Arch Gen Psychiatry 1988;45:283‐5. [DOI] [PubMed] [Google Scholar]
- 48. Castagnini A, Foldager L, Bertelsen A. Excess mortality of acute and transient psychotic disorders: comparison with bipolar affective disorder and schizophrenia. Acta Psychiatr Scand 2013;128:370‐5. [DOI] [PubMed] [Google Scholar]
- 49. Chan JKN, Wong CSM, Yung NCL et al. Pre‐existing chronic physical morbidity and excess mortality in people with schizophrenia: a population‐based cohort study. Soc Psychiatry Psychiatr Epidemiol 2021; doi: 10.1007/s00127-021-02130-9. [DOI] [PubMed] [Google Scholar]
- 50. Chen P‐H, Tsai S‐Y, Pan C‐H et al. Age effect on incidence, physical, and psychiatric comorbidity for sudden cardiac death in schizophrenia. Can J Psychiatry 2020;66:367‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen W‐Y, Huang S‐J, Chang C‐K et al. Excess mortality and risk factors for mortality among patients with severe mental disorders receiving home care case management. Nord J Psychiatry 2021;75:109‐17. [DOI] [PubMed] [Google Scholar]
- 52. Chen Y‐H, Lee H‐C, Lin H‐C. Mortality among psychiatric patients in Taiwan – Results from a universal national health insurance programme. Psychiatry Res 2010;178:160‐5. [DOI] [PubMed] [Google Scholar]
- 53. Cheng K‐Y, Lina C‐Y, Chang T‐K et al. Mortality among long‐stay patients with schizophrenia during the setting‐up of community facilities under the Yuli model. Health Psych Behav Med 2014;2:602‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crump C, Sundquist K, Winkleby MA et al. Mental disorders and risk of accidental death. Br J Psychiatry 2013;203:297‐302. [DOI] [PubMed] [Google Scholar]
- 55. Curkendall SM, Mo J, Glasser DB et al. Cardiovascular disease in patients with schizophrenia in Saskatchewan, Canada. J Clin Psychiatry 2004;65:715‐20. [DOI] [PubMed] [Google Scholar]
- 56. Daumit GL, Anthony CB, Ford DE et al. Pattern of mortality in a sample of Maryland residents with severe mental illness. Psychiatry Res 2010;176:242‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dickerson F, Stallings C, Origoni A et al. Mortality in schizophrenia: clinical and serological predictors. Schizophr Bull 2013;40:796‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dickerson F, Origoni A, Schroeder J et al. Mortality in schizophrenia and bipolar disorder: clinical and serological predictors. Schizophr Res 2016;170:177‐83. [DOI] [PubMed] [Google Scholar]
- 59. Enger C, Weatherby L, Reynolds RF et al. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis 2004;192:19‐27. [DOI] [PubMed] [Google Scholar]
- 60. Fors BM, Isacson D, Bingefors K et al. Mortality among persons with schizophrenia in Sweden: an epidemiological study. Nord J Psychiatry 2007;61:252‐9. [DOI] [PubMed] [Google Scholar]
- 61. Gatov E, Rosella L, Chiu M et al. Trends in standardized mortality among individuals with schizophrenia, 1993‐2012: a population‐based, repeated cross‐sectional study. CMAJ 2017;189:E1177‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Girardi P, Schievano E, Fedili U et al. Causes of mortality in a large population‐based cohort of psychiatric patients in Southern Europe. J Psychiatr Res 2021;136:167‐72. [DOI] [PubMed] [Google Scholar]
- 63. Guan NC, Termorshuizen F, Laan W et al. Cancer mortality in patients with psychiatric diagnoses: a higher hazard of cancer death does not lead to a higher cumulative risk of dying from cancer. Soc Psychiatry Psychiatr Epidemiol 2013;48:1289‐95. [DOI] [PubMed] [Google Scholar]
- 64. Haugland G, Craig TJ, Goodman AB et al. Mortality in the era of deinstitutionalization. Am J Psychiatry 1983;140:848‐52. [DOI] [PubMed] [Google Scholar]
- 65. Hayes JF, Marston L, Walters K et al. Mortality gap for people with bipolar disorder and schizophrenia: UK based cohort study 2000‐2014. Br J Psychiatry 2017;211:175‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Heila H, Haukka J, Suvisaari J et al. Mortality among patients with schizophrenia and reduced psychiatric hospital care. Psychol Med 2005;35:725‐32. [DOI] [PubMed] [Google Scholar]
- 67. Hellemose AA, Laursen TM, Larsen JT et al. Accidental deaths among persons with schizophrenia: a nationwide population‐based cohort study. Schizophr Res 2018;199:149‐53. [DOI] [PubMed] [Google Scholar]
- 68. Hennessy S, Bilker WB, Knauss JS et al. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ 2002;325:1070‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hewer W, Rössler W. Mortalität von Patienten mit funktionellen psychischen erkrankungen während des Zeitraums stationärer behandlung. Fortschr Neurol Psychiatr 1997;65:171‐81. [DOI] [PubMed] [Google Scholar]
- 70. Kilbourne AM, Morden NE, Austin K et al. Excess heart‐disease‐related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. Gen Hosp Psychiatry 2009;31:555‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim W, Jang S‐Y, Chun SY et al. Mortality in schizophrenia and other psychoses: data from the South Korea National Health Insurance Cohort, 2002‐2013. J Korean Med Sci 2017;32:836‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kiviniemi M, Suvisaard J, Pirkola S et al. Regional differences in five‐year mortality after a first episode of schizophrenia in Finland. Psychiatr Serv 2010;61:272‐9. [DOI] [PubMed] [Google Scholar]
- 73. Kredentser MS, Martens PJ, Chochinov HM et al. Cause and rate of death in people with schizophrenia across the lifespan: a population‐based study in Manitoba, Canada. J Clin Psychiatry 2014;75:154‐61. [DOI] [PubMed] [Google Scholar]
- 74. Kugathasan P, Stubbs B, Aagaard J et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand 2019;140:340‐8. [DOI] [PubMed] [Google Scholar]
- 75. Kugathasan P, Laursen TM, Grøntved S et al. Increased long‐term mortality after myocardial infarction in patients with schizophrenia. Schizophr Res 2018;199:103‐8. [DOI] [PubMed] [Google Scholar]
- 76. Kurdyak P, Mallia E, de Oliveira C et al. Mortality after the first diagnosis of schizophrenia‐spectrum disorders: a population‐based retrospective cohort study. Schizophr Bull 2021;47:864‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lahti M, Tiihonen J, Wildgust H et al. Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med 2012;42:2275‐85. [DOI] [PubMed] [Google Scholar]
- 78. Laursen TM, Wahlbeck K, Hällgren J et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One 2013;8:e67133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laursen TM, Nordentoft M. Heart disease treatment and mortality in schizophrenia and bipolar disorder: changes in the Danish population between 1994 and 2006. J Psychiatr Res 2011;45:29‐35. [DOI] [PubMed] [Google Scholar]
- 80. Laursen TM, Munk‐Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS One 2011;6:e24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Luo Y, Pang L, Guo C et al. Association of urbanicity with schizophrenia and related mortality in China. Can J Psychiatry 2021;66:385‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Meesters PD, Comijs HC, Smit JH et al. Mortality and its determinants in late‐life schizophrenia: a 5‐year prospective study in a Dutch catchment area. Am J Geriatr Psychiatry 2016;24:272‐7. [DOI] [PubMed] [Google Scholar]
- 83. Mortensen PB, Juel K. Mortality and causes of death in schizophrenic patients in Denmark. Acta Psychiatr Scand 1990;81:372‐7. [DOI] [PubMed] [Google Scholar]
- 84. Mortensen PB, Juel K. Mortality and causes of death in first admitted schizophrenic patients. Br J Psychiatry 1993;163:183‐9. [DOI] [PubMed] [Google Scholar]
- 85. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry 1991;36:239‐45. [DOI] [PubMed] [Google Scholar]
- 86. Nielsen RE, Uggerby AS, Jensen SOW et al. Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades – A Danish nationwide study from 1980 to 2010. Schizophr Res 2013;146:22‐7. [DOI] [PubMed] [Google Scholar]
- 87. Olfson M, Gerhard T, Huang C et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015;72:1172‐81. [DOI] [PubMed] [Google Scholar]
- 88. Olfson M, Stroup TS, Huang C et al. Suicide risk in Medicare patients with schizophrenia across the life span. JAMA Psychiatry 2021;78:876‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ösby U, Correia N, Brandt L et al. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res 2000;45:21‐8. [DOI] [PubMed] [Google Scholar]
- 90. Pan CH, Chen PH, Chang HM et al. Incidence and method of suicide mortality in patients with schizophrenia: a nationwide cohort study. Soc Psychiatry Psychiatr Epidemiol 2021;56:1437‐46. [DOI] [PubMed] [Google Scholar]
- 91. Pan Y‐J, Yeh L‐L, Chan H‐Y et al. Excess mortality and shortened life expectancy in people with major mental illnesses in Taiwan. Epidemiol Psychiatr Sci 2020;29:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Phillippe A, Vaiva G, Casadebaig F. Data on diabetes from the French cohort study in schizophrenia. Eur Psychiatry 2005;20:S340‐4. [DOI] [PubMed] [Google Scholar]
- 93. Phillips MR, Yang G, Li S et al. Suicide and the unique prevalence pattern of schizophrenia in mainland China: a retrospective observational study. Lancet 2004;364:1062‐8. [DOI] [PubMed] [Google Scholar]
- 94. Ran M‐S, Chen EY‐H, Conwell Y et al. Mortality in people with schizophrenia in rural China: 10‐year cohort study. Br J Psychiatry 2007;190:237‐42. [DOI] [PubMed] [Google Scholar]
- 95. Ruschena D, Mullen PE, Burgess P et al. Sudden death in psychiatric patients. Br J Psychiatry 1998;172:331‐4. [DOI] [PubMed] [Google Scholar]
- 96. Talaslahti T, Alanen H‐M, Hakko H et al. Mortality and causes of death in older patients with schizophrenia. Int J Geriatr Psychiatry 2012;27:1131‐7. [DOI] [PubMed] [Google Scholar]
- 97. Teferra S, Shibre T, Fekadu A et al. Five‐year mortality in a cohort of people with schizophrenia in Ethiopia. BMC Psychiatry 2011;11:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tenback D, Pijl B, Smeets H et al. All‐cause mortality and medication risk factors in schizophrenia: a prospective cohort study. J Clin Psychopharmacol 2012;31:31‐5. [DOI] [PubMed] [Google Scholar]
- 99. Tokuda Y, Obara H, Nakazato N et al. Acute care hospital mortality of schizophrenic patients. J Hosp Med 2008;3:110‐6. [DOI] [PubMed] [Google Scholar]
- 100. Tornianen M, Mittendorfer‐Rutz E, Tanskanen A et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull 2015;41:656‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tran E, Rouillon F, Loze J‐Y et al. Cancer mortality in patients with schizophrenia: an 11‐year prospective cohort study. Cancer 2009;115:3555‐62. [DOI] [PubMed] [Google Scholar]
- 102. Westman J, Eriksson SV, Gissler M et al. Increased cardiovascular mortality in people with schizophrenia: a 24‐year national register study. Epidemiol Psychiatr Sci 2018;27:519‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wood J B, Evenson RC, Cho DW et al. Mortality variations among public mental health patients. Acta Psychiatr Scand 1985;72:218‐29. [DOI] [PubMed] [Google Scholar]
- 104. Yung NCL, Wong CSM, Chan JKN et al. Excess mortality and life‐years lost in people with schizophrenia and other non‐affective psychoses: an 11‐year population‐based cohort study. Schizophr Bull 2021;47:474‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yung NCL, Wong CSM, Chan JKN et al. Mortality in patients with schizophrenia admitted for incident ischemic stroke: a population‐based cohort study. Eur Neuropsychopharmacol 2019;31:152‐7. [DOI] [PubMed] [Google Scholar]
- 106. Zilber N, Schufman N, Lerner Y. Mortality among psychiatric patients – the groups at risk. Acta Psychiatr Scand 1985;79:248‐56. [DOI] [PubMed] [Google Scholar]
- 107. Attar R, Wester A, Koul S et al. Higher risk of major adverse cardiac events after acute myocardial infarction in patients with schizophrenia. Open Heart 2020;7:e001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Babidge NC, Buhrich N, Butler T. Mortality among homeless people with schizophrenia in Sydney, Australia: a 10‐year follow‐up. Acta Psychiatr Scand 2001;103:105‐10. [DOI] [PubMed] [Google Scholar]
- 109. Bodén R, Molin E, Jernberg T et al. Higher mortality after myocardial infarction in patients with severe mental illness: a nationwide cohort study. J Intern Med 2014;277:727‐36. [DOI] [PubMed] [Google Scholar]
- 110. Bradford DW, Goulet J, Hunt M et al. A cohort study of mortality in individuals with and without schizophrenia after diagnosis of lung cancer. J Clin Psychiatry 2016;77:e1626‐30. [DOI] [PubMed] [Google Scholar]
- 111. Chan JKN, Wong CSM, Or PCF et al. Risk of mortality and complications in patients with schizophrenia and diabetes mellitus: population‐based cohort study. Br J Psychiatry 2021;219:375‐82. [DOI] [PubMed] [Google Scholar]
- 112. Chong S‐A, Tay JAM, Subramaniam M et al. Mortality rates among patients with schizophrenia and tardive dyskinesia. J Clin Psychopharmacol 2009;29:5‐8. [DOI] [PubMed] [Google Scholar]
- 113. Chou FH‐C, Tsai K‐Y, Su C‐Y et al. The incidence and relative risk factors for developing cancer among patients with schizophrenia: a nine‐year follow‐up study. Schizophr Res 2011;129:97‐103. [DOI] [PubMed] [Google Scholar]
- 114. Chou FH‐C, Tsai K‐Y, Chou Y‐M. The incidence and all‐cause mortality of pneumonia in patients with schizophrenia: a nine‐year follow‐up study. J Psychiatr Res 2013;47:460‐6. [DOI] [PubMed] [Google Scholar]
- 115. Closson K, McLinden T, Patterson TL et al. HIV, schizophrenia, and all‐cause mortality: a population‐based cohort study of individuals accessing universal medical care from 1998 to 2012 in British Columbia, Canada. Schizophr Res 2019;209:198‐205. [DOI] [PubMed] [Google Scholar]
- 116. Crump C, Winkleby MA, Sundquist K et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 2013;170:324‐33. [DOI] [PubMed] [Google Scholar]
- 117. Druss BG, Bradford WD, Rosenheck RA et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry 2001;58:565‐72. [DOI] [PubMed] [Google Scholar]
- 118. Fleetwood K, Wild SH, Smith DJ et al. Severe mental illness and mortality and coronary revascularization following a myocardial infarction: a retrospective cohort study. BMC Med 2021;19:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fond G, Pauly V, Leone M et al. Disparities in intensive care unit admission and mortality among patients with schizophrenia and COVID‐19: a national cohort study. Schizophr Bull 2021;47:624‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Guerrero Fernandez de Alba I, Gimeno‐Miguel A, Poblador‐Plou B et al. Association between mental health comorbidity and health outcomes in type 2 diabetes mellitus patients. Sci Rep 2020;10:19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hauck TS, Liu N, Wijeysundera HC et al. Mortality and revascularization among myocardial infarction patients with schizophrenia: a population‐based cohort study. Can J Psychiatry 2020;65:454‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jeon H‐L, Kwon JS, Park S‐H et al. Association of mental disorders with SARS‐CoV‐2 infection and severe health outcomes: nationwide cohort study. Br J Psychiatry 2021;218:344‐51. [DOI] [PubMed] [Google Scholar]
- 123. Kang J‐H, Xirasagar S, Lin H‐C. Lower mortality among stroke patients with schizophrenia: a nationwide population‐based study. Psychosom Med 2011;73:106‐11. [DOI] [PubMed] [Google Scholar]
- 124. Kapral MK, Kurdyak P, Casaubon LK et al. Stroke care and case fatality in people with and without schizophrenia: a retrospective cohort study. BMJ Open 2021;11:e044766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kershenbaum A, Cardinal RN, Chen S et al. Investigation of risk of dementia diagnosis and death in patients in older people's secondary care mental health services. Int J Geriatr Psychiatry 2020;36:573‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kugathasan P, Horsdal HT, Aagaard J et al. Association of secondary preventive cardiovascular treatment after myocardial infarction with mortality among patients with schizophrenia. JAMA Psychiatry 2018;85:1261‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kurdyak P, VIgod S, Calzavara A et al. High mortality and low access to care following incident acute myocardial infarction in individuals with schizophrenia. Schizophr Res 2012;142:52‐57. [DOI] [PubMed] [Google Scholar]
- 128. Laursen TM, Mortensen PB, MacCabe JH et al. Cardiovascular drug use and mortality in patients with schizophrenia or bipolar disorder: a Danish population‐based study. Psychol Med 2014;44:1625‐37. [DOI] [PubMed] [Google Scholar]
- 129. Liao C‐C, Shen WW, Chang C‐C et al. Surgical adverse outcomes in patients with schizophrenia: a population‐based study. Ann Surg 2013;257:433‐8. [DOI] [PubMed] [Google Scholar]
- 130. Mohamed MO, Rashid M, Farooq S et al. Acute myocardial infarction in severe mental illness: prevalence, clinical outcomes, and process of care in U.S. hospitalizations. Can J Cardiol 2019;35:821‐30. [DOI] [PubMed] [Google Scholar]
- 131. Shen H‐N, Lu C‐L, Yang H‐H. Increased risks of acute organ dysfunction and mortality in intensive care unit patients with schizophrenia: a nationwide population‐based study. Psychosom Med 2011;73:620‐6. [DOI] [PubMed] [Google Scholar]
- 132. Søgaard M, Skjøth F, Kjaeldgaard JN et al. Atrial fibrillation in patients with severe mental disorders and the risk of stroke, fatal thromboembolic events and bleeding: a nationwide cohort study. BMJ Open 2017;7:e018209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Toender A, Vestergaard M, Munk‐Olsen T et al. Risk of diabetic complications and subsequent mortality among individuals with schizophrenia and diabetes – a population‐based register study. Schizophr Res 2020;218:99‐106. [DOI] [PubMed] [Google Scholar]
- 134. Tsai K‐Y, Lee C‐C, Chou Y‐M et al. The incidence and relative risk of stroke in patients with schizophrenia: a five‐year follow‐up study. Schizophr Res 2012;138:41‐7. [DOI] [PubMed] [Google Scholar]
- 135. Tsai K‐Y, Lee C‐C, Chou Y‐M et al. The risks of major osteoporotic fractures in patients with schizophrenia: a population‐based 10‐year follow‐up study. Schizophr Res 2014;159:322‐8. [DOI] [PubMed] [Google Scholar]
- 136. Tzur Bitan D, Kridin K, Cohen AD et al. COVID‐19 hospitalization, mortality, vaccination, and postvaccination trends among people with schizophrenia in Israel: a longitudinal cohort study. Lancet Psychiatry 2021;8:901‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wellejus Albertsen LW, Heide‐Jørgensen U, Schmidt SAJ et al. The DANish Comorbidity Index for Acute Myocardial Infarction (DANCAMI): development, validation and comparison with existing comorbidity indices. Clin Epidemiol 2020;12:1299‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Alaräisänen A, Miettunen J, Räsänen P et al. Suicide rate in schizophrenia in the Northern Finland, 1966 Birth Cohort. Soc Psychiatry Psychiatr Epidemiol 2009;44:1107‐10. [DOI] [PubMed] [Google Scholar]
- 139. Dickerson F, Origoni A, Rowe K et al. Risk factors for natural cause mortality in a cohort of 1494 persons with serious mental illness. Psychiatry Res 2021;298:113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hayes RD, Chang C‐K, Fernandes A et al. Associations between symptoms and all‐cause mortality in individuals with serious mental illness. J Psychosom Res 2012;72:114‐9. [DOI] [PubMed] [Google Scholar]
- 141. Kodesh A, Goldberg Y, Rotstein A et al. Risk of dementia and death in very‐late‐onset schizophrenia‐like psychosis: a national cohort study. Schizophr Res 2020;223:220‐6. [DOI] [PubMed] [Google Scholar]
- 142. Chen VC‐H, Liao Y‐T, Lai T‐J et al. Survival analysis of the use of first and second‐generation antipsychotics among patients suffering schizophrenia: a nationwide population‐based cohort study. Schizophr Res 2015;169:406‐11. [DOI] [PubMed] [Google Scholar]
- 143. Cho J, Hayes RD, Jewell A et al. Clozapine and all‐cause mortality in treatment‐resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand 2019;139:237‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cullen BA, McGinty EE, Zhang Y et al. Guideline‐concordant antipsychotic use and mortality in schizophrenia. Schizophr Bull 2013;39:1159‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dickerson F, Boronow J, Stallings C et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull 2007;33:737‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Fontanella CA, Campo JV, Phillips GS et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry 2016;77:661‐7. [DOI] [PubMed] [Google Scholar]
- 147. Funayama M, Takata T, Koreki A et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosom Med 2018;80:370‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hayes RD, Downs J, Chang C‐K et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull 2014;41:644‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hjorthoj C, Östergaard MLD, Benros ME et al. Association between alcohol and substance use disorders and all‐cause and cause‐specific mortality in schizophrenia, bipolar disorder, and unipolar depression: a nationwide, prospective, register‐based study. Lancet Psychiatry 2015;2:801‐8. [DOI] [PubMed] [Google Scholar]
- 150. Horsdal HT, Köhler‐Forsberg O, Benros ME et al. C‐reactive protein and white blood cell levels in schizophrenia, bipolar disorders and depression – associations with mortality and psychiatric outcomes: a population‐based study. Eur Psychiatry 2017;44:164‐72. [DOI] [PubMed] [Google Scholar]
- 151. Kadra G, Stewart R, Shetty H et al. Long‐term antipsychotic polypharmacy prescribing in secondary mental health care and the risk of mortality. Acta Psychiatr Scand 2018;138:123‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kiviniemi M, Suvisaari J, Koivumaa‐Honkanen H et al. Antipsychotics and mortality in first‐onset schizophrenia: prospective Finnish study with 5‐year follow‐up. Schizophr Res 2013;159:274‐80. [DOI] [PubMed] [Google Scholar]
- 153. Kugathasan P, Wu H, Gaughran F et al. Association of physical health multimorbidity with mortality in people with schizophrenia spectrum disorders: using a novel semantic search system that captures physical diseases in electronic patient records. Schizophr Res 2020;216:408‐15. [DOI] [PubMed] [Google Scholar]
- 154. Lähteenvuo M, Batalla A, Luykx JJ et al. Morbidity and mortality in schizophrenia with comorbid substance use disorders. Acta Psychiatr Scand 2021;144:42‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liu T, Song X, Chen G et al. Prevalence of schizophrenia disability and associated mortality among Chinese men and women. Psychiatr Res 2014;220:181‐7. [DOI] [PubMed] [Google Scholar]
- 156. Oh J, Nam H, Park S et al. Decreased cardiovascular death in schizophrenia patients treated with antipsychotics: a Korean national cohort study. Schizophr Res 2021;228:417‐24. [DOI] [PubMed] [Google Scholar]
- 157. Pridan S, Swartz M, Baruch Y et al. Effectiveness and safety of clozapine in elderly patients with chronic resistant schizophrenia. Int Psychogeriatr 2015;27:131‐4. [DOI] [PubMed] [Google Scholar]
- 158. Ran M‐S, Ziao Y, Fazel S et al. Mortality and suicide in schizophrenia: 21‐year follow‐up in rural China. BJPsych Open 2020;6:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Strom BL, Eng SM, Faich G et al. Comparative mortality associated with ziprasidone and olanzapine in real‐world use among 18,154 patients with schizophrenia: the Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC). Am J Psychiatry 2011;168:193‐201. [DOI] [PubMed] [Google Scholar]
- 160. Strømme MF, Mellesdal LS, Bartz‐Johannesen C et al. Mortality and non‐use of antipsychotic drugs after acute admission in schizophrenia: a prospective total‐cohort study. Schizophr Res 2021;235:29‐35. [DOI] [PubMed] [Google Scholar]
- 161. Stroup TS, Gerhard T, Crystal S et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry 2016;173:166‐73. [DOI] [PubMed] [Google Scholar]
- 162. Tang CH, Ramcharran D, Yang CW et al. A nationwide study of the risk of all‐cause, sudden death, and cardiovascular mortality among antipsychotic‐treated patients with schizophrenia in Taiwan. Schizophr Res 2021;237:9‐19. [DOI] [PubMed] [Google Scholar]
- 163. Taub S, Hoshen M, Balicer R et al. Metabolic predictors for mortality among patients treated with long‐term clozapine – A longitudinal study. Eur Neuropsychopharmacol 2020;41:63‐9. [DOI] [PubMed] [Google Scholar]
- 164. Tiihonen J, Suokas JT, Suvisaari JM et al. Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry 2012;69:476‐83. [DOI] [PubMed] [Google Scholar]
- 165. Wimberley T, MacCabe H, Laursen TM et al. Mortality and self‐harm in association with clozapine in treatment‐resistant schizophrenia. Am J Psychiatry 2017;174:990‐8. [DOI] [PubMed] [Google Scholar]
- 166. Wu C‐S, Shur‐Fen Gau S. Association between antipsychotic treatment and advanced diabetes complications among schizophrenia patients with Type 2 diabetes mellitus. Schizophr Bull 2016;42:703‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia. A reexamination. Arch Gen Psychiatry 2005;62:247‐53. [DOI] [PubMed] [Google Scholar]
- 168. Díaz‐Caneja CM, Pina‐Camacho L, Rodríguez‐Quiroga A et al. Predictors of outcome in early‐onset psychosis: a systematic review. NPJ Schizophr 2015;1:14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Miranda‐Mendizabal A, Castellví P, Parés‐Badell O et al. Gender differences in suicidal behavior in adolescents and young adults: systematic review and meta‐analysis of longitudinal studies. Int J Public Health 2019;64:265‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Pillinger T, D'Ambrosio E, McCutcheon R et al. Is psychosis a multisystem disorder? A meta‐review of central nervous system, immune, cardiometabolic, and endocrine alterations in first‐episode psychosis and perspective on potential models. Mol Psychiatry 2019;24:776‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Pillinger T, Beck K, Stubbs B et al. Cholesterol and triglyceride levels in first‐episode psychosis: systematic review and meta‐analysis. Br J Psychiatry 2017;211:339‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Pillinger T, Beck K, Gobjila C et al. Impaired glucose homeostasis in first‐episode schizophrenia: a systematic review and meta‐analysis. JAMA Psychiatry 2017;74:261‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Correll CU, Robinson DG, Schooler NR et al. Cardiometabolic risk in patients with first‐episode schizophrenia spectrum disorders: baseline results from the RAISE‐ETP study. JAMA Psychiatry 2014;71:1350‐63. [DOI] [PubMed] [Google Scholar]
- 174. Solmi M, Firth J, Miola A et al. Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta‐analysis including 4 717 839 people. Lancet Psychiatry 2020;7:52‐63. [DOI] [PubMed] [Google Scholar]
- 175. Shao M, Tian H, Wang L et al. Mortality risk following acute coronary syndrome among patients with schizophrenia: a meta‐analysis. Prog Neuropsychopharmacol Biol Psychiatry 2020;96:108737. [DOI] [PubMed] [Google Scholar]
- 176. Suetani S, Honarparvar F, Siskind D et al. Increased rates of respiratory disease in schizophrenia: a systematic review and meta‐analysis including 619,214 individuals with schizophrenia and 52,159,551 controls. Schizophr Res 2021;237:131‐40. [DOI] [PubMed] [Google Scholar]
- 177. Forray A, Yonkers KA. The collision of mental health, substance use disorder, and suicide. Obstet Gynecol 2021;137:1083‐90. [DOI] [PubMed] [Google Scholar]
- 178. Harris BR, Tracy M, Comber KG et al. Suicide safer care in behavioral health settings: a comparative analysis of perceptions, training completion, and practice between mental health and substance use disorder treatment providers. J Subst Abuse Treat 2021;126:108330. [DOI] [PubMed] [Google Scholar]
- 179. Miller KA, Hitschfeld MJ, Lineberry TW et al. How does active substance use at psychiatric admission impact suicide risk and hospital length‐of‐stay? J Addict Dis 2016;35:291‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ostergaard MLD, Nordentoft M, Hjorthoj C. Associations between substance use disorders and suicide or suicide attempts in people with mental illness: a Danish nation‐wide, prospective, register‐based study of patients diagnosed with schizophrenia, bipolar disorder, unipolar depression or personality disorder. Addiction 2017;112:1250‐9. [DOI] [PubMed] [Google Scholar]
- 181. Volkow ND, Torrens M, Poznyak V et al. Managing dual disorders: a statement by the Informal Scientific Network, UN Commission on Narcotic Drugs. World Psychiatry 2020;19:396‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ameller A, Gorwood P. Attributable risk of co‐morbid substance use disorder in poor observance to pharmacological treatment and the occurrence of relapse in schizophrenia. Encephale 2015;41:174‐83. [DOI] [PubMed] [Google Scholar]
- 183. Foglia E, Schoeler T, Klamerus E et al. Cannabis use and adherence to antipsychotic medication: a systematic review and meta‐analysis. Psychol Med 2017;47:1691‐705. [DOI] [PubMed] [Google Scholar]
- 184. Schoeler T, Petros N, Di Fort M et al. Effect of continued cannabis use on medication adherence in the first two years following onset of psychosis. Psychiatr Res 2017;255:36‐41. [DOI] [PubMed] [Google Scholar]
- 185. Masroor A, Khorochkov A, Prieto J et al. Unraveling the association between schizophrenia and substance use disorder – predictors, mechanisms and treatment modifications: a systematic review. Cureus 2021;13:e16722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Maj M, van Os J, De Hert M et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry 2021;20:4‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Vermeulen JM, van Rooijen G, van de Kerkhof MPJ et al. Clozapine and long‐term mortality risk in patients with schizophrenia: a systematic review and meta‐analysis of studies lasting 1.1‐12.5 years. Schizophr Bull 2019;45:315‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Wilkinson ST, Trujillo Diaz D, Rupp ZW et al. Pharmacological and somatic treatment effects on suicide in adults: a systematic review and meta‐analysis. Depress Anxiety 2022;39:100‐12. [DOI] [PubMed] [Google Scholar]
- 189. Miura I, Nosaka T, Yabe H et al. Antidepressive effect of antipsychotics in the treatment of schizophrenia: meta‐regression analysis of randomized placebo‐controlled trials. Int J Neuropsychopharmacol 2021;24:200‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kadakia A, Dembek C, Heller V et al. Efficacy and tolerability of atypical antipsychotics for acute bipolar depression: a network meta‐analysis. BMC Psychiatry 2021;21:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Nuñez NA, Joseph B, Pahwa M et al. Augmentation strategies for treatment resistant major depression: a systematic review and network meta‐analysis. J Affect Disord 2022;302:385‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Taipale H, Schneider‐Thoma J, Pinzón‐Espinosa J et al. Representation and outcomes of individuals with schizophrenia seen in everyday practice who are ineligible for randomized clinical trials. JAMA Psychiatry 2022; doi: 10.1001/jamapsychiatry.2021.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Khan A, Faucett J, Morrison S et al. Comparative mortality risk in adult patients with schizophrenia, depression, bipolar disorder, anxiety disorders, and attention‐deficit/hyperactivity disorder participating in psychopharmacology clinical trials. JAMA Psychiatry 2013;70:1091‐9. [DOI] [PubMed] [Google Scholar]
- 194. Schneider‐Thoma J, Efthimiou O, Huhn M et al. Second‐generation antipsychotic drugs and short‐term mortality: a systematic review and meta‐analysis of placebo‐controlled randomised controlled trials. Lancet Psychiatry 2018;5:653‐63. [DOI] [PubMed] [Google Scholar]


