Abstract
Background
The clinical efficacy of SARS-CoV-2 vaccines according to antibody response in immunosuppressed patients such as hematological patients has not yet been established.
Patients and methods
A prospective multicenter registry-based cohort study conducted from December 2020 to December 2021 by the Spanish transplant and cell therapy group was used to analyze the relationship of antibody response at 3–6 weeks after full vaccination (2 doses) with breakthrough SARS-CoV-2 infection in 1394 patients with hematological disorders.
Results
At a median follow-up of 165 days after complete immunization, 37 out of 1394 (2.6%) developed breakthrough SARS-CoV-2 infection at median of 77 days (range 7–195) after full vaccination. The incidence rate was 6.39 per 100 persons-year. Most patients were asymptomatic (19/37, 51.4%), whereas only 19% developed pneumonia. The mortality rate was 8%. Lack of detectable antibodies at 3–6 weeks after full vaccination was the only variable associated with breakthrough infection in multivariate logistic regression analysis (Odds Ratio 2.35, 95% confidence interval 1.2–4.6, p = 0.012). Median antibody titers were lower in cases than in non-cases [1.83 binding antibody units (BAU)/mL (range 0–4854.93) vs 730.81 BAU/mL (range 0–56,800), respectively (p = 0.007)]. We identified 250 BAU/mL as a cutoff above which incidence and severity of the infection were significantly lower.
Conclusions
Our study highlights the benefit of developing an antibody response in these highly immunosuppressed patients. Level of antibody titers at 3 to 6 weeks after 2-dose vaccination links with protection against both breakthrough infection and severe disease for non-Omicron SARS-CoV-2 variants.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01275-7.
Keywords: SARS-CoV-2 vaccines, Breakthrough SARS-CoV-2 infection, Correlates of protection, Hematological malignancies, Allogeneic stem cell transplantation, Autologous stem cell transplantation, COVID-19, Vaccine, Immunocompromised patients, Moderna mRNA-1273, Pfizer-BioNTech BNT162b2
Introduction
The coronavirus infectious disease 2019 (COVID-19) pandemic caused by the new coronavirus (SARS-CoV-2) can have a dreadful impact in hematological patients, with mortality rates exceeding 25% [1–6]. SARS-CoV-2 vaccination is expected to reduce the severity of COVID-19 in these immunocompromised patients [7–11]. Although the antibody response after full SARS-CoV-2 vaccination in hematological patients is of a lower magnitude than in the general population [12–18], a clinical benefit is still expected, as is the case with influenza vaccination in allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients [19]. Since no prospective randomized SARS-CoV-2 vaccine trials have been conducted in these patients, vaccine efficacy data are lacking in this scenario.
The current study analyzes the clinical benefit of full SARS-CoV-2 vaccination (2 doses) through an observational registry conducted by the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC) aimed at monitoring the response to SARS-CoV-2 vaccine and the breakthrough SARS-CoV-2 infections over time in a large cohort of 1394 patients with hematological disorders.
Patients and methods
Study population
This is a prospective multicenter registry-based cohort study conducted by the Infectious Complications Subcommittee (GRUCINI) of the GETH-TC in collaboration with the Spanish Society of Hematology and Hemotherapy (SEHH). Details of this registry have been previously described elsewhere [20, 21]. In brief, the registry included consecutive adult patients with a prior history of hematological disorders who were fully vaccinated against SARS-CoV-2 between December 30, 2020, and June 30, 2021, in 21 participating Spanish centers. Patients were followed and monitored for development of SARS-CoV-2-reactive IgG antibodies (SCoV2-R-A) and breakthrough SARS-CoV-2 infection at different time points (3–6 weeks, 3, 6 and 12 months) after the complete vaccination schedule (defined as two vaccine doses). The status of all included patients was updated on December 1, 2021. All patients included in this registry gave their signed informed consent in accordance with the declaration of Helsinki. The local research ethical committee of the Hospital Clínico Universitario of Valencia approved the registry and study protocol (reference code 35.21).
Inclusion criteria and cohort selection
As of December 1, 2021, the GETH-TC registry included 1683 patients with different hematological disorders who had been fully vaccinated against COVID-19. With the aim of assessing the risk of breakthrough SARS-CoV-2 infection according to antibody detection at 3–6 weeks after the second vaccine dose, the current study focused on patients with available serological testing 3 to 6 weeks after the second vaccine dose. We excluded 289 patients from 3 centers, initially included with limited data (only filiation data), that did not obtain the institutional approval for serological testing and 1394 hematological patients were included in the final study analysis.
Technical considerations and definitions
Antibody detection or seropositivity was defined when SARS-CoV-2-reactive IgG antibodies (SCoV2-R-A) were detected at any level above the lower limit of detection level for each tests used. We assessed seropositivity using serological ELISA or chemiluminescence immunoassay following manufacturer instructions according to their availability at the microbiology services of each participating center. As recommended by the SEHH, in vaccinated individuals serological testing included the detection of IgG against both the nucleocapsid (N) and surface (S) proteins (anti-N and anti-S IgG, respectively) [7]. Of the 1394 patients included, 1244 (89%) received quantitative assessment, whereas the remainder was assessed through qualitative testing. Antibody levels were normalized according to the WHO standard, and results were reported as SCoV2-R-A binding antibody units per milliliter (BAU/mL). Additional file 1: Table S1 summarizes the technical characteristics of the serological tests used and normalization of antibody titers to BAU/mL according to WHO standards.
Pre-vaccination SARS-CoV-2 infection was defined as patients with prior history of PCR-proven COVID-19 and/or positive SARS-CoV-2 serostatus (IgG and/or IgM) before the first vaccine dose.
Patients with respiratory symptoms underwent PCR screening for the development of SARS-CoV-2 according to the treating physician criteria. Breakthrough SARS-CoV-2 infection was defined as molecular (PCR test) or humoral (anti-N seroconversion between two consecutive serological tests) evidence of SARS-CoV-2 infection 7 days after the second vaccine dose until last follow-up. The PCR tests used are provided in Additional file 1: Table S2.
Endpoints and statistical analysis
The primary objective of the study was to assess the occurrence of breakthrough SARS-CoV-2 infection and its correlation with qualitative and quantitative humoral response at 3–6 weeks after full COVID-19 vaccination. We also analyzed the effect of different cutoff values of quantitative SCoV2-R-A titers on development and severity of breakthrough SARS-CoV-2 infection.
The main patient characteristics were reported by descriptive statistics on the total available information: Medians and ranges were used for continuous variables, while absolute and percentage frequencies were used for categorical variables. For comparisons, Fisher exact test or Mann–Whitney’s U test was used when appropriate. Univariate and multivariate analyses were tested using logistic regression models. Variables with a p value ≤ 0.1 in the univariate model were included in the multivariate analysis. A p value < 0.05 was considered statistically significant. All p values are two-sided. A median test sub-analysis to check the protective effect of the amount of SCoV2-R-A was carried out in patients with available quantitative SCoV2-R-A titers normalized to BAU/mL. All analyses were performed using the statistical software SPSS v. 25(IBM SPSS Statistics, Armonk, New York, USA).
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Most patients (n = 1345, 96.5%) received complete vaccination with mRNA vaccines, and their median age was 63 years (range 18–97). Overall, the most common hematological diseases were B-cell non-Hodgkin’s lymphoma (B-cell NHL) (n = 302, 21.6%), plasma cell disorders (PCD) (n = 236, 16.9%), acute myeloid leukemia (AML) (n = 179, 12.8%), chronic lymphocytic leukemia (CLL) (n = 158, 11.3%) and chronic myeloproliferative neoplasms (cMPN) (n = 139, 10%). Among the cell therapy procedures, the most common was allo-HSCT (n = 369, 26.5%) followed by autologous stem cell transplantation (n = 110, 8%) and chimeric antigen receptor of T cell (CAR-T) therapy (n = 21, 1.5%). Note that this series included 109 patients (8.4%) with prior PCR and/or serological proof of SARS-CoV-2 infection before being vaccinated. Median follow-up after the second vaccine dose was 165 days (range 12–269).
Table 1.
Patient characteristics
| Characteristics | (n = 1394) |
|---|---|
| Prior COVID-19, n (%) | 109 (7.9) |
| Diagnosed by PCR | 95 (7) |
| Positive serostatus prior to vaccination | 37 (2.6) |
| Negative serostatus prior to vaccination | 13 (1) |
| Detected by pre-vaccine serological test | 14 (1.5) |
| Median time from COVID-19 to vaccination, days (range) | 185 (33–460) |
| Serological status prior to vaccination, n (%) | |
| Positive | 50 (4) |
| Negative | 422 (30) |
| Not tested | 922 (66) |
| Median time from serology to vaccination, days (range) | 0 (0–386) |
| Type of vaccine, n (%) | |
| Moderna mRNA-1273 | 983 (70.5) |
| Pfizer-BioNTech BNT162b2 | 362 (26) |
| Adenoviral vector-based | 49 (3.5) |
| Age (years), median (range) | 63 (18–97) |
| 18–40 years, n (%) | 143 (10) |
| 41–60 years, n (%) | 496 (35.5) |
| 61–70 years, n (%) | 373 (26.8) |
| > 71 years, n (%) | 382 (27.4) |
| Male, n (%) | 784 (56.3) |
| ECOG 0–1 at vaccination | 1351 (97) |
| Baseline disease, n (%) | |
| AML | 179 (12.8) |
| ALL | 46 (3.3) |
| MDS | 158 (11.3) |
| B-cell NHL | 302 (21.6) |
| T cell NHL | 38 (2.7) |
| Plasma cell disorders | 236 (16.9) |
| CLL | 158 (11.3) |
| HD | 103 (7.4) |
| cMPN | 139 (10) |
| Aplastic anemia | 16 (1) |
| Non-malignant disorders | 18 (1.3) |
| Type of cell therapy | |
| Allo-HSCT | 369 (26.5) |
| ASCT | 110 (8) |
| CAR-T | 21 (1.5) |
| Status disease at vaccination, n (%) | |
| Complete remission | 824 (59.2) |
| Partial remission | 162 (11.6) |
| Active disease | 408 (29.2) |
| Time last treatment to COVID-19 vaccine, months (range) | |
| Untreated | 172 (12.3) |
| Active treatment | 509 (36.5) |
| ≥ 6 month to 1 year | 92 (6.6) |
| ≥ 1 year | 621 (44.5) |
| Immunosuppressant drugs at vaccination, n (%) | 300 (21.5) |
| Corticosteroids at vaccination, n (%) | 255 (18.6) |
| Daratumumab, n (%) | 46 (3.3) |
| Venetoclax, n (%) | 14 (1) |
| Anti-CD-20 moAb, n (%) | 241 (17.3) |
| < 6 months before 1st vaccine dose | 87 (6.2) |
| 6 to 1 year before 1st vaccine dose | 25 (1.8) |
| > 1 year before 1st vaccine dose | 129 (9.3) |
| BTK inhibitor therapy, n (%) | 63 (4.5) |
| TKI therapy, n (%) | 40 (2.9) |
| Lenalidomide maintenance, n (%) | 120 (8.6) |
| Ruxolitinib therapy, n (%) | 14 (1) |
| Blood count before vaccination (× 109/mL) | |
| Absolute neutrophile counts, median (range) | 3.1 (0–46.7) |
| Absolute lymphocyte counts, median (range) | 1.73 (0.14–262.1) |
| Absolute lymphocyte counts < 1 × 109/L | 265 (18.6) |
| Time from 2nd dose to first serologies, median days (range) | 21 (12–62) |
| Median time between vaccine doses, median days (range) | 28 (17–115) |
| SCoV2-R-A detection at 3–6 weeks after full vaccination, n (%) | 1090 (78.2) |
| Patient with SCoV2-R-A titers at 3–6 weeks in BAU/mL, n (%) | 1244 (89%) |
| Median SCoV2-R-A titers at 3–6 weeks in BAU/mL, (range) | 715 (0–56,800) |
| Third vaccine dose given, n (%) | 550 (39.5) |
| Time from 2nd dose to 3rd dose, days (range) | 153 (39–269) |
| Median follow-up after full vaccination, days (range) | 165 (12–269) |
| COVID-19 after vaccination, n (%) | 37 (2.7) |
| Median time from vaccination to SARS-CoV-2 infection, days (range) | 77 (7–195) |
PCR, Polymerase chain reaction AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; B-cell NHL, B-cell non-Hodgkin lymphoma; T cell NHL, T cell non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; HD, Hodgkin disease; MPN, chronic myeloproliferative neoplasm; Allo-HSCT, allogeneic stem cell transplantation; ASCT, autologous stem cell transplantation; CAR-T, T cell chimeric antigen receptor; moAb, monoclonal antibody; BTK inhibitor, Bruton’s tyrosine kinase inhibitor; TKIs, tyrosine kinase inhibitors; and SCoV2-R-A, SARS-CoV-2-reactive IgG antibodies
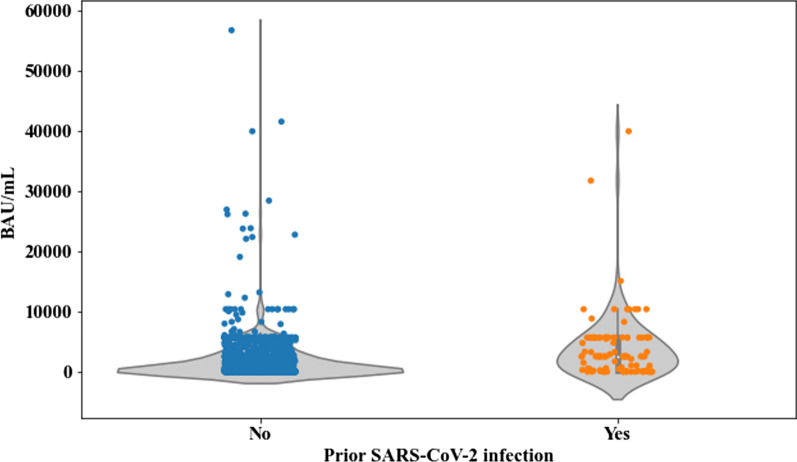
Overall, the SCoV2-R-A detection rate at 3–6 weeks after the complete vaccination was 78.2%. Among those with quantitative antibody testing, the median SCoV2-R-A titer was 720.26 BAU/mL (range 0–58,600). We compared SCoV2-R-A titers at 3–6 weeks after full vaccination in patients with and without SARS-CoV-2 infection prior to vaccination (excluding 7 patients with breakthrough SARS-CoV-2 infection after the second vaccine dose and before the first serological testing) and found higher titers in those with (median 2550 BAU/mL, range 0–10,400) vs those without (median 493.6 BAU/mL, range 0–6338.6) (p < 0.0001) infection (Fig. 1).
Fig. 1.
Median anti-SARS-CoV-2 IgG-reactive antibodies titers measured in binding antibody units/mL (BAU/mL) at 3–6 weeks after the 2nd dose according to pre-vaccination SARS-CoV-2 infection. Patients with SARS-CoV-2 infection prior to vaccination (n = 109) had a median of 2550 BAU/mL (range 0–10,400) vs those without prior history of SARS-CoV-2 infection (n = 1118) median 493.6 BAU/mL (range 0–6338.6) (p < 0.0001)
Breakthrough SARS-CoV-2 infection
We identified 37 patients (2.6%) with breakthrough SARS-CoV-2 infection at median of 77 days (range 7–195) after the second vaccine dose. The overall incidence rate of breakthrough SARS-CoV-2 infection was 6.39 per 100 persons-year. The main clinical and SARS-CoV-2 breakthrough infection characteristics are detailed in Table 2. Most patients were diagnosed through molecular PCR testing (n = 22, 60%), whereas those remaining (n = 15, 40%) were diagnosed by seroconversion of anti-N between two consecutive serological tests. Seven patients (19%) developed SARS-CoV-2 infection between the second vaccine dose and SCoV2-R-A testing at 3–6 weeks, with an incidence rate of 6.14 per 100 persons-year. Twelve patients (32%) developed the infection between 3–6 weeks and 3 months after the complete vaccination with an estimated incidence rate of 6.9 per 100 persons-year. Finally, 18 patients had the infection between 3 and 7 months after the complete vaccination with an estimated incidence rate of 14.09 per 100 persons-year. Eighteen patients had COVID-19 (48.6%), whereas 19 (51.4%) were asymptomatic. SARS-CoV-2 was detected by PCR in 4 asymptomatic patients during screening performed before hospital admission for scheduled procedures/treatments. Pneumonia was documented in 7 cases (19%), whereas the SARS-CoV-2 infection-related hospital admission rate was 32% (n = 12). There were 3 COVID-19-related deaths (8%) at a median of 26 days (range 7–67) after SARS-CoV-2 detection.
Table 2.
Characteristics of patients with breakthrough SARS-CoV-2 infection
| Characteristics | SARS-CoV-2 infection (n = 37) |
|---|---|
| Prior COVID-19, n /n evaluable (%) | 0/109 |
| Type of vaccine, n/n evaluable (%) | |
| Moderna mRNA-1273 | 24/982 (2.4) |
| Pfizer-BioNTech BNT162b2 | 11/362 (3) |
| Adenoviral vector-based | 2/50 (4) |
| Age (years), n/n evaluable (%) | |
| 18–40 years | 6/144 (4.2) |
| 41–60 years | 17/495 (3.4) |
| 61–70 years | 6/373 (1.6) |
| > 71 years | 8/382 (2) |
| Male, n (%)/n evaluable (%) | 25/784 (3.2) |
| Baseline disease, n/n evaluable (%) | |
| AML | 5/180 (2.7) |
| ALL | 1/46 (2.1) |
| MDS | 5/158 (3.1) |
| B-cell NHL | 5/301 (1.7) |
| T cell NHL | 3/38 (8) |
| Plasma cell disorders | 5/236 (2.1) |
| CLL | 4/158 (2.5) |
| HD | 6/103 (5.8) |
| cMPN | 2/139 (1.4) |
| Aplastic anemia | 0/16 |
| Non-malignant disorders | 1/17 (5.5) |
| Cell therapy, n /n evaluable (%) | 18/501 (3.6) |
| Type of cell therapy, n /n evaluable (%) | |
| Allo-HSCT | 13/370 (3.5) |
| ASCT | 5/110 (4.7) |
| CAR-T | 0/21 |
| Status disease at vaccination, n /n evaluable (%) | |
| Complete remission | 21/825 (2.5) |
| Partial remission | 6/162 (3.7) |
| Active disease | 10/407 (2.4) |
| Time last treatment to COVID-19 vaccine, n /n evaluable (%) | |
| Untreated | 7/172 (4) |
| Active treatment | 10/509 (1.9) |
| ≥ 6 month to 1 year | 5/92 (5.4) |
| ≥ 1 year | 15/620 (2.4) |
| Immunosuppressant drugs at vaccination, n /n evaluable (%) | 13/300 (4.3) |
| Corticosteroids at vaccination, n /n evaluable (%) | 8/255 (3.1) |
| Daratumumab, n /n evaluable (%) | 1/46 (2.1) |
| Venetoclax, n /n evaluable (%) | 0/14 |
| Anti-CD-20 moAb, n /n evaluable (%) | 4/241 (1.6) |
| BTK inhibitor therapy, n /n evaluable (%) | 3/63 (4.7) |
| TKI therapy, n /n evaluable (%) | 1/40 (2.5) |
| Lenalidomide, n /n evaluable (%) | 2/120 (1.7) |
| Ruxolitinib therapy, n /n evaluable (%) | 0/14 |
| Absolute lymphocyte counts < 1 × 109/L, n /n evaluable (%) | 9/260 (3.4) |
| Intervals from 2nd dose to SARS-CoV-2 infection, n /n patients at risk (%) | |
| At 30 days after 2nd dose | 14/1361 (1) |
| At 60 days after 2nd dose | 3/1309 (0.2) |
| At 90 days after 2nd dose | 8/1227 (0.6) |
| At 180 days after 2nd dose | 12/518 (2.3) |
| SCoV2-R-A detection at 3–6 weeks, n /n evaluable (%) | 17/30 (57) |
| Median SCoV2-R-A titer at 3–6 weeks, BAU/mL (range) [27 evaluable patients] | 1.83 (0–4854.95) |
| SARS-CoV-2 infection after the third vaccine dose, n (%) | 2/541 (0.3) |
| SARS-CoV-2 diagnosis, n /n evaluable (%) | |
| PCR | 22/37 (60) |
| Seroconversion of anti-N antibodies | 15/37 (40) |
| Symptomatic SARS-CoV-2 infection, n /n evaluable (%) | 18/37 (48.6) |
| Pneumonia, n /n evaluable (%) | 7/37 (19) |
| Hospital admission, n /n evaluable (%) | 12/37 (32) |
| Oxygen requirement, n /n evaluable (%) | 10/37 (27) |
| ICU admission, n /n evaluable (%) | 3/37 (8) |
| Death, n /n evaluable (%) | 3/37 (8) |
| Median time to death from 2nd vaccine dose, days (range) | 82 (59–100) |
AML, Acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; B-cell NHL, B-cell non-Hodgkin lymphoma; T cell NHL, T cell non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; HD, Hodgkin disease; MPN, chronic myeloproliferative neoplasm; Allo-HSCT, allogeneic stem cell transplantation; ASCT, autologous stem cell transplantation; CAR-T, T cell chimeric antigen receptor; moAb, monoclonal antibody; BTK inhibitor, Bruton’s tyrosine kinase inhibitor; TKIs, tyrosine kinase inhibitors; SCoV2-R-A, SARS-CoV-2-reactive IgG antibodies; Anti-N, SARS-CoV-2 nucleocapsid antibodies; and ICU, intensive care unit
Risk factors (including antibody level titers) for breakthrough SARS-CoV-2 infection
After excluding 7 patients with breakthrough SARS-CoV-2 infection before the first serological testing, we performed a univariate and multivariate regression model to assess predictors of breakthrough SARS-CoV-2 infection (Table 3). None of the 109 patients who developed COVID-19 before vaccination developed breakthrough infection (p = 0.1 in univariate analysis). The only variable that was found to have an impact on the risk of breakthrough SARS-CoV-2 infection in univariate and multivariate analyses was absence of SCoV2-R-A detection at 3–6 weeks after the second vaccine dose [odds ratio (OR) 2.35 95% confidence interval (CI) 1.2–4.6, p = 0.012].
Table 3.
Logistic regression univariate and multivariate analyses of factors predicting SARS-CoV-2 breakthrough infection after full vaccination
| Characteristics | SARS-CoV-2 infection | p value | SARS-CoV-2 infection | p value |
|---|---|---|---|---|
| Univariate OR (95% CI) |
Multivariate OR (95% CI) |
|||
| Prior COVID-19 | 0.2 (0.02–1.2) | 0.1 | ns | |
| Type of vaccine | ||||
| Moderna mRNA-1273 | 1 | |||
| Pfizer-BionTech BNT162b2 | 0.6 (0.14–2.6) | 0.5 | ||
| Adenoviral vector-based | 0.75 (0.16–3.5) | 0.7 | ||
| Age (years) | ||||
| 18–40 years | 1 | |||
| 41–60 years | 0.8 (0.32–2.1) | 0.67 | ||
| 61–70 years | 0.37 (0.12–1.18) | 0.09 | ns | |
| > 71 years | 0.49 (0.16–1.44) | 0.19 | ||
| Male sex | 1.6 (0.8–3.2) | 0.166 | ||
| Baseline disease | ||||
| ALL | 1 | |||
| AML | 1.3 (0.14–11.2) | 0.8 | ||
| MDS | 1.47 (0.16–33) | 0.7 | ||
| B-cell NHL | 0.76 (0.08–6.6) | 0.8 | ||
| T cell NHL | 3.8 (0.38–38.4) | 0.25 | ||
| Plasma cell disorders | 0.97 (0.11–8.5) | 0.9 | ||
| CLL | 1.16 (0.12–10.7) | 0.9 | ||
| HD | 2.78 (0.32–23.8) | 0.35 | ||
| cMPN | 0.65 (0.05–7.4) | 0.7 | ||
| Aplastic anemia | 0.000 | 0.99 | ||
| Non-malignant disorders | 2.6 (0.15–44.7) | 0.5 | ||
| Status disease at vaccination | ||||
| Complete remission | 1 | |||
| Partial remission | 1.47 (0.58–3.7) | 0.4 | ||
| Active disease | 0.92 (0.45–2.06) | 0.9 | ||
| Time from last treatment to COVID-19 vaccine | ||||
| Untreated | 1 | |||
| Under treatment | 0.47 (0.17–1.26) | 0.13 | ||
| > 6 months to 1 year | 1.35 (0.41–4.39) | 0.6 | ||
| ≥ 1 year | 0.58 (0.23–1.45) | 0.24 | ||
| Cell therapy | ||||
| Yes | 0.58 (0.3–1.1) | 0.1 | ns | |
| No | 1 | |||
| Allo-HSCT | 1.6 (0.82–3.4) | 0.15 | ||
| ASCT | 2.19 (0.8–5.98) | 0.12 | ||
| CAR-T | 0.00 | 0.99 | ||
| Corticosteroids at vaccination | 1.2 (0.56–2.7) | 0.59 | ||
| Daratumumab | 0.8 (0.1–6) | 0.83 | ||
| Venetoclax | 0.00 | 0.99 | ||
| Anti-CD-20 moAb | 0.57 (0.2–1.6) | 0.29 | ||
| Bruton’s TKI therapy | 1.9 (0.57–6.4) | 0.29 | ||
| TKI therapy | 0.95 (0.12–7) | 0.9 | ||
| Lenalidomide | 0.6 (0.14–2.5) | 0.48 | ||
| Ruxolitinib therapy | 0.00 | 0.99 | ||
| SCoV2-R-A negative at 3–6 weeks after 2 doses | 2.5 (1.3–4.9) | 0.007 | 2.35 (1.2–4.6) | 0.012 |
| Lymphocyte count < 0.5 × 109/L | 0.75 (0.09–5.4) | 0.75 | ||
| Lymphocyte count < 1.0 × 109/L | 1.5 (0.7–3.3) | 0.27 | ||
AL, Acute leukemia; MDS, myelodysplastic syndrome; B-cell NHL, B-cell non-Hodgkin lymphoma; MM, multiple myeloma; CLL, chronic lymphocytic leukemia; HD, Hodgkin disease; MPN, chronic myeloproliferative neoplasm; Allo-HSCT, allogeneic stem cell transplantation; ASCT, autologous stem cell transplantation; moAb, monoclonal antibody; TKIs, tyrosine kinase inhibitors; and SCoV2-R-A, SARS-CoV-2-reactive IgG antibodies
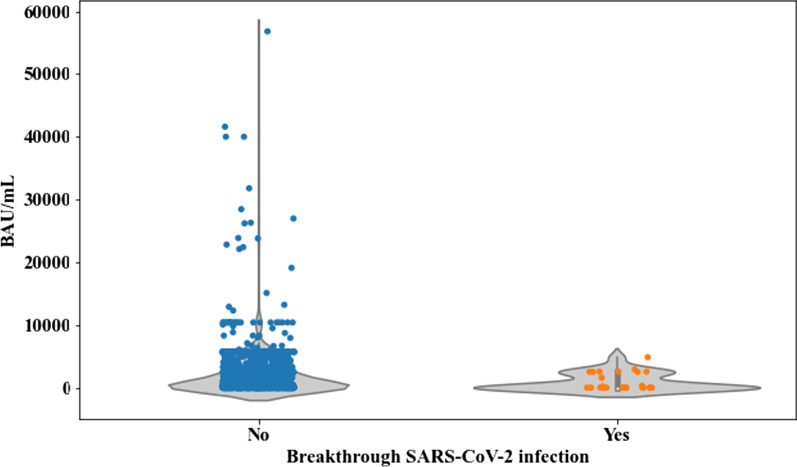
Regarding the impact of the magnitude of SCoV2-R-A load, we restricted the analysis to 27 patients, since 7 cases became infected before the first antibody determination after vaccination and another 3 cases only had qualitative SCoV2-R-A testing. Overall, 1234 patients were evaluable for antibody levels normalized as BAU/mL. The median SCoV2-R-A levels at 3–6 weeks after the full vaccination were significantly lower in the 27 patients with breakthrough SARS-CoV-2 infection as compared to those without [1.83 BAU/mL (range 0–4854.93) vs 730.81 BAU/mL (range 0–56,800), respectively (p = 0.007)] (Fig. 2). SCoV2-R-A levels were classified as “low” (< 250 BAU/mL) in 501 patients (41%) and as “high” (> 250 BAU/mL) in 542 (44%) or “very high” (> 4900 BAU/mL) in 191 (15%) cases.
Fig. 2.
Median anti-SARS-CoV-2 IgG-reactive antibody titers measured in binding antibody units/mL (BAU/mL) at 3–6 weeks after the 2nd dose according to SARS-CoV-2 breakthrough infection. Patients who did not developed SARS-CoV-2 infection (n = 1207) had a median of 730.81 BAU/mL (range 0–58,600) vs 1.83 BAU/mL (range 0–4854.93) in those who did develop breakthrough infection (n = 27) (p = 0.007)
Although the median SCoV2-R-A levels were higher in asymptomatic breakthrough SARS-CoV-2 infection (n = 13) than in those with COVID-19 (n = 14), the difference was not statistically significant [792.57 BAU/mL (range 0–2550) vs 0 BAU/mL (range 0–4854.93), p = 0.44]. Additionally, median SCoV2-R-A levels were lower, although not statistically significant, in those requiring hospital admission vs those who did not [0 BAU/mL (range 0–195.15) vs 309.57 BAU/mL (range 0–4854.93), respectively, p = 0.2]. However, breakthrough SARS-CoV-2 infection was more common in the group with “low” SCoV2-R-A levels, as was COVID-19, pneumonia, hospital admission and oxygen requirement (p ≤ 0.05 for all comparisons) (Table 4). Breakthrough SARS-CoV-2 infection severity did not show significant differences according to the timing after the second vaccine dose (after 2nd dose and first serological testing vs the first testing and 3rd months vs after the 3rd months).
Table 4.
SARS-CoV-2 infection severity according to anti-SARS-CoV-2 IgG-reactive antibody cutoffs in the 27 evaluable patients
| Variable | < 250 BAU/mL (n = 501) | 250 to 4900 BAU/mL (n = 542) | > 4900 BAU/mL (n = 191) | p value |
|---|---|---|---|---|
| SARS-CoV-2 infection | 17 (3.4%) | 10 (1.8%) | 0 | 0.018 |
| Symptomatic SARS-CoV-2 | 10 (2%) | 3 (0.5%) | 0 | 0.035 |
| Pneumonia | 4 (0.7%) | 0 | 0 | 0.05 |
| Hospital admission | 8 (1.5%) | 0 | 0 | 0.012 |
| Oxygen requirement | 7 (1.3%) | 0 | 0 | 0.006 |
| ICU admission | 2 (0.35%) | 0 | 0 | 0.2 |
| Death | 2 (0.35%) | 0 | 0 | 0.2 |
Discussion
The current study highlights the influence of qualitative and quantitative humoral response monitoring early after full SARS-CoV-2 vaccination in predicting the risk of breakthrough SARS-CoV-2 infection in hematological patients. Patients lacking SCoV2-R-A at 3–6 weeks after vaccination had an increased risk of breakthrough SARS-CoV-2 infection. In addition, higher levels of SCoV2-R-A early after complete vaccination were linked to a lower risk of breakthrough SARS-CoV-2 infection and lower disease severity.
Hematological patients are historically characterized by a low humoral response rate with any vaccine-preventable disease [22, 23]. However, development of mRNA vaccines during the SARS-CoV-2 pandemic has overcome the poor serological response rates in this particular population (> 70%) [14, 19, 20, 24, 25]. The median 720.26 BAU/mL found in our cohort was similar to the results reported in other large series of patients with diverse hematological conditions (median values < 1 × 103 BAU/mL) and significantly lower than those found in healthy individuals (> 1 × 103 BAU/m) [26]. Although the clinical benefit of mounting a serological response is currently lacking in this scenario, to the best of our knowledge this is the first report providing evidence of its link with clinical efficacy. Notably, although prior SARS-CoV-2 infection was not significantly associated with lower risk of breakthrough SARS-CoV-2 infection in our series, none of these patients were infected after vaccination. We previously reported a higher rate of detectable SCoV2-R-A in this patient subset [21], whereas in the current study we were able to demonstrate higher SCoV2-R-A titers compared to SARS-CoV-2-naïve patients. It is likely that the higher antibody titers along with natural immunity may confer strong protection in these patients. The design of our registry (prospective longitudinal with several SCoV2-R-A determinations over time) enabled us to capture occurring breakthrough SARS-COV-2 infections through PCR screening in symptomatic patients or in asymptomatic patients before planned treatments but also by monitoring anti-N seroconversion in asymptomatic patients at different pre-specified time points.
SARS-CoV-2 infection in hematological patients mirrored national epidemiological data [1, 27]. The monitoring period during which our study was conducted (mostly from March 2021 to early December 2021) spanned the period between the fifth and sixth COVID-19 wave when the Delta variant was dominant in Spain. Thus, it is likely that our findings do not apply to the Omicron SARS-CoV-2 variant. In that period, the incidence of COVID-19 was relatively low [28]. This fact may explain in part the somewhat low incidence rate of SARS-CoV-2 infection (overall 6.39 per 100 persons-year) in the current study. Although hematological malignancies showed a higher risk of breakthrough infection compared to solid tumors [29], the rate observed in our cohort (2.6%) was similar to other series with comparable median follow-ups which included hematological patients (2.3%) [26] or health care workers (2.3%) [30]. However, we observed that the risk of breakthrough infection increased with longer follow-up. This fact could suggest either that a decrease in antibody titters over time may reduce protection (which formed the rationale behind boosters), or that the risk of being infected increases with longer exposure time in an ongoing pandemic.
The protective threshold levels of anti-SARS-CoV-2 antibodies below which the humoral defense against different SARS-CoV-2 variants is suboptimal have not yet been established. However, both binding and neutralizing antibodies are thought to be potential correlates of protection against COVID-19 and are correlated with each other [31, 32]. In fact, recent data in the general population suggest that higher levels of binding and neutralizing antibodies after the second dose correlate with a reduced risk of symptomatic infection [33, 34]. In line with these observations, our findings support that SCoV2-R-A titration early after vaccination could be an accurate strategy to predict breakthrough infection risk and could be useful in counseling additional vaccine doses or anti-SARS-CoV-2 monoclonal antibodies in this immunosuppressed population. This assumption is supported by the fact that patients without detectable antibodies had higher risk of breakthrough infection, patients with breakthrough infection had lower SCoV2-R-A titers and those with higher antibody titers showed lower disease severity.
A vaccine efficacy of 80% against symptomatic infection in the general population was achieved with 264 BAU/ml [33]. In the COVE trial, this threshold shows > 90% vaccine efficacy [34]. Based on these data, we empirically selected the cutoff of 250 BAU/mL to segregate our cohort into poor and good responders. The cutoff of 250 BAU/mL in our series predicted risk of breakthrough infection and disease severity. No patients with SCoV2-R-A > 250 BAU/mL were admitted to hospital due to SARS-CoV-2 in our cohort. Nevertheless, the kinetics wane of SCoV2-R-A waning after vaccination in this scenario remains to be determined in order to establish the best time points for serological monitoring and the optimal moment to administer further vaccine doses.
Finally, we observed an important reduction in COVID-19 severity, exemplified by reduced overall pneumonia (19%), symptomatic SARS-CoV-2 infection (48.6%) and mortality (8%) rates. Rates of these outcomes in hematological patients with COVID-19 during the first and second COVID-19 waves were as high as > 70%, > 90% and > 25%, respectively [1]. Our findings are in line with real world data, where both mRNA vaccines were shown to reduce symptomatic SARS-CoV-2 infection, COVID-19-related symptoms, hospital admissions and mortality in adults [35–38]. Although the severity of COVID-19 seems lower after vaccination, severely immunosuppressed patients still develop life-threatening disease. In these vulnerable patients, preventive transmission measures (hand washing, social distancing, wearing mask, etc.) are still highly recommended. However, the largest reduction in the incidence of respiratory virus infections in immunocompromised patients has been observed when preventive transmission measures have been instituted globally [39] as compared to when such measures are applied only to immunosuppressed patients and their caregivers; in fact, in these latter scenarios the time of onset and incidence of different respiratory virus infections (including SARS-CoV-2) in HSCT recipients strongly mirror those in the patients’ communities [1, 19].
The limitations of this study comprise the use of different serological tests, absence of neutralizing antibody testing, absence of cellular immune response analyses and the lack of molecular data regarding the SARS-CoV-2 variants in patients with breakthrough infections. However, most SARS-CoV-2 infections reported in our cohort occurred when the Delta variant was dominant in Spain. The performance of antibody titration with the omicron variant remains to be evaluated.
Conclusion
We provide evidence that serological monitoring after SARS-CoV-2 vaccination could be useful in identifying hematological patients at high risk of breakthrough SARS-CoV-2 infection. SCoV2-R-A levels link with protection in this vulnerable population being 250 BAU/mL a potentially discriminative cutoff for non-Omicron SARS-CoV-2 variants. Finally, severity of SARS-CoV-2 infection in hematological patients has experienced an encouraging improvement in the post-vaccine period.
Supplementary Information
Additional file 1: Table S1. Characteristics of serological assays used in the study. Table S2. Commercial PCR test available in participating centers.
Acknowledgements
REDCap is developed and supported by the Vanderbilt Institute for Clinical and Translational Research. We thank the Spanish Society of Hematology (SEHH) for its support on the study diffusion. We also offer our sincere thanks to the invaluable microbiology services for their commitment in SARS-CoV-2-reactive IgG antibody monitoring in these highly immunosuppressed patients from all participating centers, in particular to Santiago Muñoz Criado from Microbiological Division of Hospital Clínico Universitario of Salamanca and to Tamar Talaván from microbiological Division of Hospital Infanta Leonor of Madrid. Special thanks to all hematology units from participating centers for their commitment in the current study. Finally, we also want to thank patients, nurses and study coordinators for their foremost contributions in this study.
Author contributions
JLP, RM, DN and AC were responsible for the conception and the design of the study. JLP, RM, PR-B and DN performed the data analysis and generated the tables and figures. JLP, LL-C, RM, LV, AP, GM-M, AF-M, BG, GS-L, AS-S, LV, VC-G, MTO, JL-J, SM-C, MT, MG-B, JÁH-R, JM, IE, CA, JCH-B, MR-G, JLM-B, CS, AS and DN were responsible for patient recruitment. JLP, RM and DN were responsible for writing and supervising the manuscript. All co-authors were responsible for reviewing the analysis interpretation, suggesting modifications to the text, critically reviewing the manuscript and for final approval of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data are available upon request by email to the Spanish hematopoietic transplant and cell therapy group (GETH-TC).
Declarations
Ethics approval and consent to participate
The local research ethics committee of the Hospital Clínico Universitario of Valencia approved the registry and study protocol (reference code 35.21). All patients included in this registry gave their sign informed consent in accordance with the declaration of Helsinki.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Piñana JL, Martino R, García-García I, Parody R, Morales MD, Benzo G, et al. Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH). Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, Asociación Madrileña de Hematología y Hemoterapia (AMHH) et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13(1):133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M, EPICOVIDEHA Working Group et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA) J Hematol Oncol. 2021;14(1):168. doi: 10.1186/s13045-021-01177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;S2352–3026(20):30429–30434. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungman P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani MA, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;2:1–10. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribera JM, Morgades M, Coll R, Barba P, López-Lorenzo JL, Montesinos P, et al. Frequency, clinical characteristics and outcome of adults with acute lymphoblastic leukemia and COVID 19 infection in the first vs. second pandemic wave in Spain. Clin Lymphoma Myeloma Leuk. 2021;21(10):e801–e809. doi: 10.1016/j.clml.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piñana JL, Vázquez L, Martino R, de la Cámara R, Sureda A, Rodríguez-Veiga R, et al. Spanish Society of Hematology and Hemotherapy expert consensus opinion for SARS-CoV-2 vaccination in onco-hematological patients. Leuk Lymphoma. 2021;63:1–13. doi: 10.1080/10428194.2021.1992619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Society for Medical Oncology. ESMO statements for vaccination against COVID-19 in patients with cancer. https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination. Accessed 7 Jan 2021.
- 9.Auletta J, Chemaly R, Khawaja F, Papanicolau G, Hill J, Kanter J. ASH-ASTCT COVID-19 and vaccines: frequently asked questions. 2021. p. 2–5.
- 10.https://ehaweb.org/covid-19/eha-statement-on-covid-19-vaccines/recommendations-for-covid-19-vaccination-in-patients-with-hematologic-cancer/.
- 11.https://www.prnewswire.com/news-releases/nccn-shares-new-guidance-principles-for-vaccinating-people-with-cancer-against-covid-19-301213154.html.
- 12.Maneikis K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R, Kryžauskaitė L, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021 doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzog Tzarfati K, Gutwein O, Apel A, Rahimi-Levene N, Sadovnik M, Harel L, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021 doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298–299. doi: 10.1016/S0140-6736(21)01594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali H, Ngo D, Aribi A, Arslan S, Dadwal S, Marcucci G, et al. Safety and tolerability of SARS-CoV-2 emergency-use authorized vaccines allogeneic hematopoietic stem cell transplant recipients. Transplant Cell Ther. 2021 doi: 10.1016/j.jtct.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–1033. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avivi I, Balaban R, Shragai T, Sheffer G, Morales M, Aharon A, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021 doi: 10.1111/bjh.17608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piñana JL, Pérez A, Montoro J, Giménez E, Gómez MD, Lorenzo I, et al. Clinical effectiveness of influenza vaccination after allogeneic hematopoietic stem cell transplantation: a cross-sectional, prospective, observational study. Clin Infect Dis. 2019;68(11):1894–1903. doi: 10.1093/cid/ciy792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piñana JL, López-Corral L, Martino R, Montoro J, Vazquez L, Pérez A, et al. Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC). SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am J Hematol. 2022;97(1):30–42. doi: 10.1002/ajh.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piñana JL, Garcia-Sanz R, Martino R, Garcia-Roa M, Martin-Martin GA, Risco-Gálvez I, et al. Booster effect after SARS-CoV-2 vaccination in immunocompromised hematology patients with prior COVID-19. Blood Adv. 2022;6(3):848–853. doi: 10.1182/bloodadvances.2021006326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikulska M, Cesaro S, de Lavallade H, et al. European Conference on Infections in Leukaemia Group. Vaccination of patients with hematological malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7) Lancet Infect Dis. 2019;19(6):e188–e199. doi: 10.1016/S1473-3099(18)30601-7. [DOI] [PubMed] [Google Scholar]
- 23.Cordonnier C, Einarsdottir S, Cesaro S, et al. European Conference on Infections in Leukaemia Group. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7) Lancet Infect Dis. 2019;19(6):e200–e212. doi: 10.1016/S1473-3099(18)30600-5. [DOI] [PubMed] [Google Scholar]
- 24.Dhakal B, Abedin SM, Fenske TS, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR-T cell therapy. Blood. 2021;138:1278–1281. doi: 10.1182/blood.2021012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a singlecenter prospective cohort study. Transplant Cell Ther. 2021;27(9):788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haggenburg S, Lissenberg-Witte BI, van Binnendijk RS, et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022;6(5):1537–1546. doi: 10.1182/bloodadvances.2021006917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Actualización nº 130. Enfermedad por el coronavirus (COVID-19). 08.06.2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_130_COVID-19.pdf.
- 28.https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_525_COVID-19.pdf.
- 29.Song Q, Bates B, Shao YR, et al. National COVID Cohort Collaborative Consortium. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-World evidence from the National COVID cohort collaborative. J Clin Oncol. 2022 doi: 10.1200/JCO.21.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folegatti PM, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emary KRW, et al. Efcacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al; Immune Assays Team§; Moderna, Inc. Team§; Coronavirus Vaccine Prevention Network (CoVPN)/Coronavirus Efficacy (COVE) Team§; United States Government (USG)/CoVPN Biostatistics Team§. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed]
- 35.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case–control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged >/=65 years United States, January–March 2021. Morb Mortal Wkly Rep. 2021;70(18):674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De la Puerta R, Montoro J, Aznar C, et al. Common seasonal respiratory virus infections in allogeneic stem cell transplant recipients during the SARS-COV-2 pandemic. Bone Marrow Transplant. 2021;56(9):2212–2220. doi: 10.1038/s41409-021-01319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Characteristics of serological assays used in the study. Table S2. Commercial PCR test available in participating centers.
Data Availability Statement
Data are available upon request by email to the Spanish hematopoietic transplant and cell therapy group (GETH-TC).