Abstract
Human immunodeficiency virus (HIV) protease inhibitors (PIs) are important components of many highly active antiretroviral therapy regimens. However, development of phenotypic and/or genotypic resistance can occur, including cross-resistance to other PIs. Development of resistance takes place because trough levels of free drug are inadequate to suppress preexisting resistant mutant variants and/or to inhibit de novo-generated resistant mutant variants. There is thus a need for new PIs, which are more potent against mutant variants of HIV and show higher levels of free drug at the trough. We have optimized a series of substituted sulfonamides and evaluated the inhibitors against laboratory strains and clinical isolates of HIV type 1 (HIV-1), including viruses with mutations in the protease gene. In addition, serum protein binding was determined to estimate total drug requirements for 90% suppression of virus replication (plasma IC90). Two compounds resulting from our studies, designated DPC 681 and DPC 684, are potent and selective inhibitors of HIV protease with IC90s for wild-type HIV-1 of 4 to 40 nM. DPC 681 and DPC 684 showed no loss in potency toward recombinant mutant HIVs with the D30N mutation and a fivefold or smaller loss in potency toward mutant variants with three to five amino acid substitutions. A panel of chimeric viruses constructed from clinical samples from patients who failed PI-containing regimens and containing 5 to 11 mutations, including positions 10, 32, 46, 47, 50, 54, 63, 71, 82, 84, and 90 had mean IC50 values of <20 nM for DPC 681 and DPC 681, respectively. In contrast, marketed PIs had mean IC50 values ranging from 200 nM (amprenavir) to >900 nM (nelfinavir).
New agents for the management of human immunodeficiency virus (HIV) infection are needed in order to provide long-term suppression of virus in infected individuals. The ongoing requirement for new therapeutics arises both from the emergence of drug-resistant strains resulting from continuing replication in the presence of regimens that do not completely suppress virus production and from acute HIV infection with drug-resistant strains (5, 6, 9, 12, 14, 25, 28, 30).
Current therapeutics are targeted to two essential enzymes of the virus: the aspartyl protease and the reverse transcriptase (RT). The viral protease of HIV is responsible for the specific cleavage of two viral polyproteins leading to production of a set of structural proteins and enzymes essential for the replication of HIV (18). The construction of infectious DNA clones of the virus with mutations in the protease gene established the essentiality of the viral protease for proper and timely processing of the viral polyproteins, leading to the production of infectious virus particles (19). The wealth of structural information and knowledge of substrate specificity and mechanism of action led to the discovery, development, clinical testing, and subsequent approval of several inhibitors within this class of HIV drugs.
As a class, the protease inhibitors (PIs) have substantial clinical efficacy (4, 10, 11, 21; B. C. King, S. Brun, T. Marsh, R. Murphy, C. Hicks, J. Eron, J. Thommes, R. Gulick, M. Glesby, M. Thompson, C. White, M. Albrecht, H. Kessler, A. Hsu, R. Bertz, D. Kempf, N. Travers, K. Real, A. Japour, and E. Sun, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 546, 2000). Indeed, their ability to mediate significant decreases in viral burden led to a revolution in the understanding of HIV replication dynamics (5, 12, 15, 27) and to the adoption of a triple-drug regimen, including a PI, as the optimal therapy in 1996. This standard has recently been modified to include nonnucleoside reverse transcriptase inhibitors (NNRTIs) as part of highly active antiretroviral therapy (HAART) (3, 23). Regardless of specific makeup of the regimen, the goal of HAART is twofold: (i) suppression of wild-type virus to prevent new mutations from arising and (ii) suppression of likely preexisting mutant variants to block the further, sequential accumulation of mutations. In general, successful HAART regimens contain members of at least two classes of agents from among the three currently available classes (nucleoside RT inhibitors, NNRTIs, and PIs). There are currently six approved inhibitors of the PI class (ritonavir, indinavir, nelfinavir, amprenavir, lopinavir [ABT 378], and saquinavir).
Despite the clinical benefit observed with HAART regimens (4, 10, 21; King et al., 40th ICAAC), inevitably, most regimens fail; failure rates of 20 to 40%/year for PI-containing regimens have been reported (4, 22). If failure occurs concomitantly with the generation of resistant virus variants, further therapeutic options are limited owing to the cross-resistance shown by many PI-resistant variants containing certain amino acid substitutions. Thus, there is a clear need for compounds with improved properties such that outgrowth and the spread of resistant virus variants would be decreased or prevented. These properties include the ability of the drug to bind to and inhibit the target enzyme (inhibitor potency), the ability of the drug to be absorbed and retained within the bloodstream (pharmacokinetics), and the relative level of the drug able to diffuse across infected cell membranes to approach the target enzyme (plasma-free fraction defined by the extent of protein binding). Compounds with an improved overall profile would be considered expanded-spectrum PIs, which are suitable for use in both treatment-naive and treatment-experienced individuals harboring genotypically and phenotypically resistant virus variants. We simultaneously considered potency, protein binding, and pharmacokinetics, while optimizing peptido-mimetic compounds containing the sulfonamide moiety. We describe here the structure and biological properties in vitro of DPC 681 and DPC 684, two expanded-spectrum inhibitors of the viral protease.
MATERIALS AND METHODS
Synthesis of DPC 681 and DPC 684.
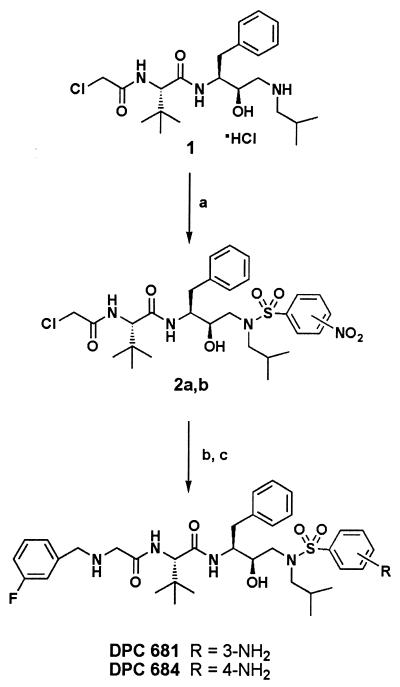
The synthesis of the substituted aminosulfonamide PIs DPC 681 and DPC 684 is depicted in Fig. 1. Treatment of N-[2R-hydroxy-3-[(2-methylpropyl)amino]-1S-(phenylmethyl)propyl]-2S-[(chloroacetyl)amino]-3,3-dimethylbutanamide hydrochloride 1 with 3- or 4-nitrobenzenesulfonylchloride and potassium carbonate in tetrahydrofuran (THF)-water gave the substituted sulfonamides 2a and 2b. The fluorinated benzyl residues were introduced by treating 2a or 2b with excess 3-fluorobenzylamine in refluxing THF. Reduction of the nitrosulfonamides by hydrogenation with palladium hydroxide-carbon gave the aminosulfonamides DPC 681 and DPC 684. Additional experimental details will be published elsewhere.
FIG. 1.
General synthetic route. (a) 3- or 4-nitrobenzenesulfonyl chloride, THF-H2O, K2CO3. (b) 3-Fluorobenzylamine, THF, reflux. (c) 20% Pd(OH)2/C, H2, methanol.
Other PIs.
Nelfinavir (Viracept), indinavir (Crixivan), amprenavir (Agenerase), lopinavir (ABT 378; Kaletra), and the cyclic urea DMP 323 were prepared and/or chromatographically isolated at DuPont Pharmaceuticals Co. All inhibitors were stored as dimethyl sulfoxide solutions at 4°C.
Measurement of inhibition of viral and cellular proteases.
The ability of PIs to inhibit HIV type 1 (HIV-1) protease was assessed by using a fluorescent peptide substrate: aminobenzoyl-Ala-Thr-His-Gln-Val-Tyr-PheNO2-Val-Arg-Lys-Ala (the scissile bond is indicated in boldface) (8). Single-chain dimeric HIV protease was utilized to allow extremely low concentrations of the protease (50 pM) in reactions. Ki values were determined under conditions of substrate and inhibitor excess relative to the enzyme concentration (test compound concentration, 0.1 to 25 nM) by using the Michaelis-Menten equation for competitive inhibitors. Spectrophotometric and fluorometric assays were used to measure the inhibitory potency of DPC 681 and DPC 684 against cellular aspartyl proteases, chymotrypsin-like proteases, matrix metalloproteinases (MMPs), Factor X, and thrombin (8, 31).
Measurement of antiviral activity.
The ability of PIs to inhibit HIV replication in tissue culture was assessed by using five different assay systems. The yield of infectious virus produced in 3-day acute infections by the HIV-1 (RF) strain in MT-2 cells (multiplicity of infection [MOI] = 0.02 PFU/ml) or in a 7-day acute infection in peripheral blood mononuclear cells (PBMC) (MOI = 0.1 PFU/ml for IIIB virus) was measured by using a plaque assay of the culture fluid containing progeny virions as previously described (26). Virus from the yield reduction portion of the assay was serially diluted and added to MT-2 or MT-4 cells, and agar overlay was added 24 h later. After 7 days, the monolayer was stained and plaques were counted (26). The antiviral activity was also determined by measurement of viral RNA accumulation in HIV-1 (RF)-infected MT-2 cells (2). The titer of RF virus was established to determine the dilution producing 15 to 30 ng of RNA per well of HIV RNA after 3 days of infection. HIV-1 RNA was quantitated by using biotinylated capture and alkaline phosphatase-derivatized reporter oligonucleotides (2). In a third system, the effect of analogs on the replication of recombinant viruses in the HXB2 or NL4-3 background was determined as previously described (16). Recombinant viruses were recovered by transfecting ligated plasmids by lipofection. Titers of virus stocks recovered at 7 to 10 days posttransfection were determined on MT-4 cells to determine the dilution producing 1,000 to 3,000 ng of p24 in 4 days. This dilution was then used in drug susceptibility assays, wherein drug was added at 24 h postinfection of cells, and p24 was quantitated via enzyme-linked immunosorbent assay 3 days later. In a fourth system, recombinant viruses incorporating patient-derived protease and RT genes that had been PCR amplified from plasma virus were assayed in a reporter cell line as described by Hertogs et al.(13) and conducted at Virco Laboratories, Virco NV, Belgium. Viruses representing non-clade B isolates were subtyped by a combination of sequencing and heteroduplex mobility shift assay on regions of the Gag and Env genes (M.-P. de Bethune, K. Hertogs, L. Heyndrickx, J. Vingerhoets, K. Fransen, H. Azijn, L. Michiels, W. Janssens, A. Scholliers, B. Larder, S. Bloor, R. Pauwels, and G. Van der Groen, 3rd Int. Workshop HIV Drug Resist. Treatment Strategies, abstr. 49, 1999). The activity of the laboratory virus strain NL4-3 was assessed in PBMC by using the Department of Defense-ACTG protocol of Japour et al. (17), using an MOI of either 4,000 or 8,000 50% tissue culture infective doses (TCID50)/ml and 7-day incubations. PBMC from HIV-seronegative donors were obtained by Ficoll-Hypaque gradient centrifugation of heparinized blood and stimulated by incubation with phytohemagglutinin and interleukin-2 for 72 h. In all assays, the concentration of compound that reduced the measured parameter by 50 or 90% was designated the IC50 or the IC90, respectively.
Protein binding.
To estimate the effect of human plasma protein binding on antiviral efficacy, a functional assay and, in some cases, physical measurement of the extent of binding to serum proteins were used. In the functional assay, in vitro antiviral assays were conducted in the absence or presence of the two major components of human plasma, namely, human serum albumin (HSA) and alpha-1-acid glycoprotein (AAG) (antiviral shift assay). In the latter condition, the tissue culture medium contained a final concentration of 45 mg HSA and 1 mg of AAG per ml, concentrations of serum proteins likely found in the plasma of AIDS patients. The IC90 in the presence of these added components was then compared to the IC90 measured in the absence of components and reported as the fold increase in IC90 observed (i.e., the protein binding shift). Dialysis and/or ultrafiltration, followed by liquid chromatography-mass spectrometry (LC-MS) analysis of media, were used to determine the percent free drug present in human serum or in tissue culture medium containing 5% fetal bovine serum. Analyses were conducted for DPC 681, DPC 684, lopinavir, indinavir, amprenavir, and nelfinavir under identical conditions.
Pharmacokinetic studies.
The pharmacokinetics of DPC 681 and DPC 684 in beagle dogs was determined after oral and intravenous (i.v.) dosing. Groups of dogs (one male and two female) were given either a single oral 10-, 30-, or 100-mg/kg dose of DPC 681 or DPC 684 in methanesulfonic acid solution. In addition, two groups of male dogs (n = 3) were given a single 1-mg/kg dose of either DPC 681 or DPC 684 in a solution of N,N-dimethylacetamide, propylene glycol, and water (10/40/50 [vol/vol/vol] by i.v. infusion. Plasma was extracted by solid-phase extraction and analyzed by LC-MS. Pharmacokinetic parameters were calculated by using noncompartmental methods.
RESULTS AND DISCUSSION
DPC 681 and DPC 684 are potent, selective inhibitors of the HIV-1 protease.
The concentration of compound required to inhibit cleavage of the substrate by 50% was designated the IC50. The Ki values for the PIs are shown in Table 1 for wild-type enzyme. For reference, assay values for indinavir, saquinavir, nelfinavir, ritonavir, amprenavir, and lopinavir are also included. DPC 681 and DPC 684 have ca. 10-fold-higher potency relative to the marketed PIs against peptide substrate as determined by a sensitive fluorescence-based assay using subnanomolar levels of tethered HIV protease dimer.
TABLE 1.
Potency of PIs as determined by using wild-type enzyme
| Inhibitor | Ki (nM) ± SDa (n) |
|---|---|
| DPC 681 | 0.012 ± 0.001 (4) |
| DPC 684 | 0.021 ± 0.002 (3) |
| Indinavir | 0.37 ± 0.04 (3) |
| Saquinavir | 0.27 ± 0.21 (11) |
| Nelfinavir | 0.53 ± 0.18 (2) |
| Amprenavir | 0.17 ± 0.06 (3) |
| Ritonavir | 0.37 ± 0.06 (2) |
| Lopinavir | 0.019 ± 0.009 (5) |
Determined by using fluorescent peptide substrate at pH 5.5.
The potential for inhibition of cellular aspartyl protease, chymotrypsin-like protease or matrix metalloprotease was examined. The initial test concentrations of DPC 681 and DPC 684 were selected to reflect levels at least 750 times that required to inhibit HIV protease by 50% (IC50) as determined against the viral polyprotein GAG substrate and >5 × 105 times the apparent Ki value for the viral protease. Less than 50% inhibition was observed at concentrations of >13 μM against renin, pepsin, cathepsin D, cathepsin G, chymotrypsin, MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-13, MMP-14, MMP-15, thrombin, Factor Xa, trypsin, or plasmin. Thus, both DPC 681 and DPC 684 are highly selective for the retroviral protease relative to the cellular proteases. In contrast, ritonavir showed inhibition of the three mammalian aspartic acid proteases tested, including moderately potent inhibition of renin (IC50 = 1.7 μM). The clinical relevance of such inhibition is unknown.
Antiviral activity against laboratory strains and clinical isolates of HIV-1.
The ability of DPC 681 and DPC 684 to inhibit the replication of HIV-1 was measured by several techniques for several laboratory strains and clinical isolates. Thai isolate H9466 was originally identified in Chiang Mai, Thailand, whereas isolate E is a clinical isolate obtained from an individual resistant to zidovudine (29). A panel of recombinant, chimeric isolates constructed by de Bethune et al. (3rd Int. Workshop HIV Drug Resist. Treatment Strategies, abstr. 49) was used to assess the inhibitory potency toward non-clade B isolates. The antiviral activity of DPC 681 and DPC 684 against laboratory strains and clinical isolates is summarized in Tables 2 and 3. The mean and standard deviation values are shown. DPC 681 and DPC 684 are extremely potent inhibitors of wild-type HIV-1. When all of the HIV-1 strains tested are considered, the average concentrations required for 90% inhibition of replication were 7.3 ± 3.4 and 14.5 ± 11.1 nM for DPC 681 and DPC 684, respectively. Slightly higher IC90 values were observed in PBMC or with HIV-2, but the IC90 values for these viruses still fall within the threefold range typical for antiviral assays (2, 17, 26). Both inhibitors are equally potent against clades A, B, C, D, E, F, G, and H of the main group M viruses. A single isolate of group O showed lowered ability to be inhibited by DPC 684, but more isolates must be examined prior to concluding that there is a general loss of sensitivity of group O isolates to this protease inhibitor.
TABLE 2.
Antiviral activities of DPC 681 and DPC 684 against laboratory and clinical isolates
| Virus | Cell type | Assaya | DPC 681
|
DPC 684
|
||
|---|---|---|---|---|---|---|
| IC90 (nM)b | n | IC90 (nM)b | n | |||
| RF | MT-2 | RNA | 3.9 ± 0.7 | 3 | 5.7 | 1 |
| RF | MT-2 | Yield | 4.1 ± 1.2 | 6 | 7.8 ± 3.6 | 5 |
| HXB2 | MT-4 | p24 | 5.3 ± 2.5 | 5 | 12 ± 6 | 7 |
| NL4-3 | PBMC | p24 | 6.5 | 2 | 20 | 2 |
| IIIB | MT-2 | Yield | 4.6 ± 1.4 | 3 | 8.8 | 2 |
| IIIB | PBMC | Yield | 12 | 1 | 40 | 1 |
| Thai 9466 | MT-2 | Yield | 11 | 1 | 11 | 1 |
| Patient E | MT-2 | Yield | 11 | 1 | 11 | 1 |
| HIV-2 | MT-2 | Yield | 10 | 1 | 32 | 1 |
Methods are described in the text.
Mean (± the standard deviation).
TABLE 3.
Activity against non-clade B isolates
| Subtypea | No. of isolates | Avg IC50 (range)
|
|
|---|---|---|---|
| DPC 681 | DPC 684 | ||
| A | 2 | 1.7 (1.2–2.2) | 2.7 (2.1–3.2) |
| B | 6 | 2.0 (1.3–3.1) | 2.3 (1.9–3.5) |
| C | 5 | 1.0 (0.6–2.4) | 2.1 (1.2–3.3) |
| D | 4 | 1.2 (1.0–1.4) | 2.1 (1.4–2.6) |
| E | 3 | 1.2 (0.7–1.8) | 2.6 (1.9–3.6) |
| F | 4 | 1.9 (0.9–2.9) | 1.9 (1.5–2.3) |
| G | 2 | 0.9 (0.3–1.4) | <1.2 |
| H | 3 | 1.5 (1.2–1.9) | 2.2 (1.8–2.7) |
| Group O | 1 | 3.0 | 6.3 |
Clade subtyping according to the Env gene.
Antiviral potency against recombinant mutant HIVs.
To characterize the antiviral potency of DPC 681 and DPC 684 further, a panel of recombinant viruses with selected mutations in the protease gene, as well as viruses that appeared in tissue culture in the presence of suboptimal levels of PI, was utilized. The recombinant mutant HIVs were constructed by site-directed mutagenesis techniques by using the infectious proviral clone HXB2 (16). Viruses constructed corresponded to those which are known to cause resistance to members of the PI class, including D30N (identified in nelfinavir-exposed treatment failures), M46I/I47V/I50V (identified in amprenavir selection experiments [24]), a multiply substituted variant corresponding to virus isolated from indinavir failures (L10R, M46I, L63P, V82T, and I84V [6]), and a multiply substituted variant described as highly resistant to ritonavir (M46I, L63P, A71V, V82F, and I84V [21]) Note that several of the amino acid changes present in these recombinant viruses (e.g., L63P, A71V, and L10R) are likely compensatory in nature (6; E. D. Anton, L. Bacheler, S. Garber, C. Reid, R. Buckery, H. Scarnati, B. Korant, and D. L. Winslow, 4th Int. Workshop HIV Drug Resist., abstr. 60, 1995). The viruses sI84V and sV82F/I84V/Gag p17, designated with the “s” prefix, arose in tissue culture experiments with the cyclic urea PI DMP 323. Although cyclic ureas such as DMP 323 are structurally distinct from peptidic inhibitors such as indinavir and nelfinavir, they also occupy the S3-S3′ subsites within the protease dimer and have somewhat overlapping resistance profiles (8, 16). The V82F/I84V double protease mutant was subsequently found to require compensatory mutations in the substrate Gag within the matrix (p17) region and is thus designated as a triple mutant (Anton et al., 4th Int. Workshop HIV Drug Resist., abstr. 60). Potency against these selected viruses in MT-2 cells was determined by measuring yield reduction. As seen in Table 4, DPC 681 and DPC 684 show potency losses of 5.3-fold or less against these site-directed recombinant and in vitro-selected viruses. The low absolute concentrations required for 90% inhibition of these mutant viruses should translate to a need for relatively low levels in plasma to maintain suppression. Provided sufficient levels of drug are maintained at the trough to cause 90% suppression of the wild-type virus, substantial inhibition of mutants associated with nelfinavir, indinavir, ritonavir, and amprenavir failure will also occur, including variants with multiple substitutions that are the hallmark of highly PI-experienced patients.
TABLE 4.
Antiviral potencies of DPC 681 and DPC 684 against mutant HIV-1 variants
| Virus | DPC 681
|
DPC 684
|
||||
|---|---|---|---|---|---|---|
| IC90 (nM) ± SD | n | Fold increased | IC90 (nM) ± SD | n | Fold increased | |
| HXB2 | 5.3 ± 2.5 | 5 | WT | 13 ± 5.7 | 7 | WT |
| D30N | 3.8 ± 3.0 | 2 | 0.7 | 8.8 ± 8.0 | 2 | 0.7 |
| Ritonavir resistanta | 28 ± 13 | 4 | 5.3 | 40 ± 14 | 5 | 3.1 |
| Indinavir resistantb | 25 ± 9.6 | 3 | 4.4 | 26 ± 3.4 | 3 | 2.0 |
| Amprenavir resistantc | 17 ± 1.0 | 3 | 3.0 | 38 ± 23 | 4 | 3.0 |
| RF | 4.1 ± 1.2 | 6 | WT | 7.8 ± 3.6 | 5 | WT |
| sI84V | 7.9 ± 2.3 | 5 | 1.9 | 12 ± 1.4 | 4 | 1.5 |
| sV82F/I84V/Gag | 9.1 ± 1.3 | 5 | 2.2 | 14 ± 3.7 | 4 | 1.8 |
Site-directed mutant virus containing five mutations (M46I, L63P, A71V, V82F, and I84V) and resistant to ritonavir as described by Markowitz et al. (21).
Site-directed mutant virus containing five mutations (21). (L10R, M46I, L63P, V82T, and I84V) and resistant to indinavir as described by Condra et al. (6).
Site-directed mutant virus containing three mutations (M46I, I47V, and I50V) and resistant to amprenavir as described by Pazhanisamy et al. (24).
Fold increase, fold increase over the wild type (WT).
Antiviral potency against PI-resistant clinical isolates.
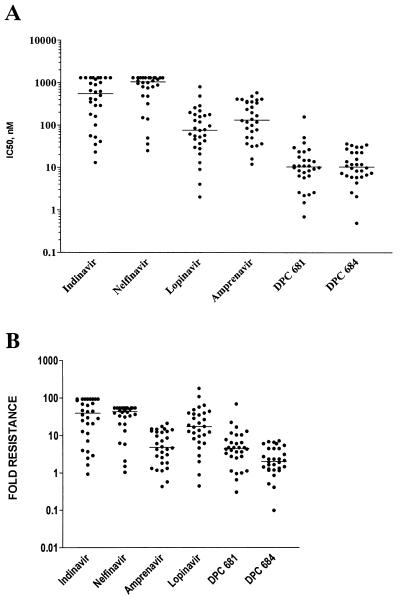
A panel of 30 isolates was selected from the Virco collection to approximate the prevalence of phenotypic resistance to various PIs observed among samples submitted for routine clinical testing. The isolates included in the panel included 20 isolates with phenotypic resistance to 4 or more PIs (containing 6 to 10 mutations), 7 isolates resistant to either amprenavir, nelfinavir, or ritonavir, and 3 isolates resistant either to indinavir and ritonavir or to nelfinavir and ritonavir. The complete genotypes for these viruses are provided in Table 5. These isolates were examined for their sensitivity to marketed PIs, as well as DPC 681 and DPC 684; the data are shown as absolute measurements and the fold increase relative to the control (HXB2) strain used (Fig. 2). An expanded concentration range was utilized for lopinavir, DPC 681, and DPC 684 to obtain IC50 values for all isolates. In contrast, data for nelfinavir and indinavir were truncated at 1.3 μM, the highest concentration tested. DPC 681 and DPC 684 are significantly more potent against these clinical chimeras than any of the marketed inhibitors. For comparison purposes, the concentration corresponding to the average IC50 was determined. For lopinavir this value was 130 nM, while for DPC 681 and DPC 684 the average IC50s were 18 and 14 nM, respectively.
TABLE 5.
Genotypes of PI-resistant isolates
| Protease phenotype and Virco ID | Protease genotype |
|---|---|
| Ritonavir resistant | |
| 1 | L10I, K20R/K, M36I, I54V, L63P, A71V, V82A, I84V/I |
| Nelfinavir resistant | |
| 2 | D30N, M36I, L63P, A71V, N88D |
| 3 | L10I, D30N, M36V, L63P, N88D, I93L |
| Ritonavir-nelfinavir resistant | |
| 4 | L10I, K20V, I54L, L63P, A71V, G73S, L90M |
| 5 | K20R, M36I, M46L, I54V, L63H, V82A, L90M |
| Indinavir-ritonavir resistant | |
| 6 | L10I, L24I, M46I, I54V, L63P, V82A |
| Amprenavir resistant | |
| 7 | L10L/I, I50V, A71V |
| 8 | L10I, K20T/K, M36I, M46M/I, I50V/I, I54V/I, L63P, V77V/I, V82A/V, I93L |
| 9 | L10F, V32I, M46I, I47V |
| 10 | L10F, M46M/I, I50V, L63A, V77I |
| Cross-resistant (>10 fold) to multiple (four or more) PIs | |
| 11 | L10I, L24I, M36I, M46I, I54V, L63P, A71V, V82A, I93L |
| 12 | L10M, K20R, M36I, I54V, L63P, A71V, G73S, V82A, L90M |
| 13 | L10I, K20R, M36I, I54V, L63P, A71V, V82A, L90M |
| 14 | L10I, V32I, I54V, L63P, A71V, V77I, G73C, V82A, L90M |
| 15 | L10I, I54V, D60E, L63P, A71V, V82A, L90M |
| 16 | L10I, L24I, M36I, I54V, L63P, A71V, G73G/S, I84V |
| 17 | L10I, L24I, M36I, I54V, L63P, A71V, I84V |
| 18 | L10I, K20I, M46I, D60E, L63P, H69HNI, G73C, V77I, I84V, L90M |
| 19 | L10R/C, K20R, M36I, M46M/I, I54V, D60E, L63P, A71V/I, V82T, L90M |
| 20 | L10I, V32T, M46I, L63P, A71V, I84V |
| 21 | L10I, V32T, M46I, L63P, A71V, I84V, |
| 22 | L10I, M36I, M46L, L63P, A71V, I84V, N88D, L90M |
| 23 | L10I, K20R, M36I, I54V, A71V, V82T, I84V |
| 24 | L10I, K20R, L24I, M36I, I54V, L63P, A71V, V82T, I84V |
| 25 | L10I, K20M, I54V, L63P, A71V, I84V, L90M, I93L |
| 26 | L10I, K20I, D60E, L63P, 69HNI, G73C, V77I, I84V, L90M |
| 27 | L10I, K20I, M46I, D60E, L63P, A71T, G73C, V77I, I84V, L90M, I93L |
| 28 | L24I, M36L, M46L, I54V, L63P, A71V, I84V |
| 29 | L10F, K20I, L63P, A71V, G73S, V77I, I84V, L90M |
| 30 | L10I, I54V, L63P, A71V, G73S, V82A,L90M |
FIG. 2.
A panel of 30 isolates with resistance to one or more PIs was assessed for sensitivity of DPC 681, DPC 684, lopinavir, indinavir, nelfinavir, and amprenavir. The protease and RT genes from plasma virus were PCR amplified and installed in an HXB2 genetic background, and the method of Hertogs et al. (13) was utilized to measure the concentration of added compound to cause 50% inhibition of assay signal. Note that the data for indinavir and nelfinavir are truncated at 1.3 μM, the highest concentration tested. (A) Measured IC50 values for 30 isolates. Each symbol corresponds to the IC50 for a recombinant isolate; the horizontal line indicates the median IC50 for each inhibitor. (B) Fold resistance for each inhibitor calculated by comparison of the measured IC50 with the IC50 for the isogenic wild-type strain, HXB2. Each symbol corresponds to the fold resistance for a recombinant isolate; the horizontal line indicates the median fold resistance for each inhibitor.
Antiviral activity in the presence of human serum components.
Many drugs bind to plasma proteins. The effect of such binding is to decrease the concentration of free drug available to interact with the target. For antiretrovirals, clinical failure has been associated with significant and unexpected protein binding. Protein binding was estimated in a functional assay in which the effect of added human serum (50%) or purified components of human serum (HSA and AAG) on the measured IC90 was determined. This analysis is designated the protein binding shift assay, and results are expressed as a fold increase in measured IC90. The data shown in Table 6 for protein binding shift indicate that nelfinavir, amprenavir, lopinavir, DPC 681, and DPC 684 are subject to a much larger impact of added serum proteins than is indinavir. Similar shift assays using tissue culture experiments in 50% human serum have been recently reported for the approved inhibitors (7); nelfinavir showed the largest degree of impact of added serum, while indinavir showed only a small shift. Shift assays of these types can underestimate the impact of protein binding to the extent that serum factors other than HSA or AAG may bind drugs and because of assumptions of linearity in extrapolating from data derived in tissue culture experiments carried out in 50% human serum. These features of protein binding may or may not be equivalent for different inhibitors. Since we wished to directly compare the new PIs with approved agents, we determine the impact of serum proteins by determining the serum-free fraction by physical separation and detection. Recent studies designed to assess the impact of PIs on human umbilical venous endothelial cells have suggested that intracellular levels of PIs correlate well with the extracellular level of unbound drug (1). The fractional binding of DPC 681; DPC 684, and marketed PIs to tissue culture media and to human serum was determined by using dialysis or ultrafiltration and LC-MS detection (Table 6). We then calculated the total drug required for suppression of virus by adjusting the measured antiviral potency by the protein binding to tissue culture medium and then calculating the corresponding total drug equivalents by adjusting for the serum protein binding. This total drug quantity, defined as the plasma IC90, is determined as the measured IC90 × free fraction in tissue culture medium/free fraction in human serum.
TABLE 6.
Plasma IC values for HIV PIs
| Propertya | DPC 681 | DPC 684 | Lopinavir | Indinavir | Amprenavir | Nelfinavir |
|---|---|---|---|---|---|---|
| IC90 (nM ± SD) | ||||||
| Wild-type HIVa | 6.3 ± 3.0 | 9.3 ± 3.9 | 17 ± 4.6 | 35 ± 5.8 | 71 ± 27 | 28 ± 8.0 |
| Ritonavir-resistant HIV | 28 ± 13 | 40 ± 14 | 540 ± 160 | 370 ± 190 | 1,330 ± 385 | 450 ± 310 |
| Amprenavir-resistant HIV | 17 ± 1.0 | 38 ± 23 | 255 ± 170 | 45 ± 21 | 1,940 ± 910 | 35 ± 9 |
| Indinavir-resistant HIV | 25 ± 9.6 | 26 ± 3.4 | 330 ± 110 | 470 ± 250 | 550 ± 310 | 250 ± 76 |
| Mean IC50 (nM) for 30 clinical isolatesb | 18 | 14 | 130 | >640 | 200 | >900 |
| Median IC50 (nM [range]) for 30 clinical isolates | 11 (0.7–160) | 11 (0.5–37) | 78 (2.0–810) | 550 (13–>1,300) | 140 (12–590) | 1,100 (25–>1,300) |
| PB Shift HSA + AAGc | 22 | 12.8 | 20 | 2 | 29 | 32 |
| % Free in tissue culture medium | 20.8 | 15.2 | 30.1 | 95.5 | 64 | 27.4 |
| % Free in human serum | 1.6 | 1.9 | 1.4 | 34.3 | 6.0 | 0.24 |
| Plasma IC90 (nM) | ||||||
| Wild-type HIV | 82 | 74 | 370 | 97 | 760 | 3,200 |
| Site-directed mutant virusesd | 360 | 320 | 11,600 | 1,030 | 14,200 | 51,400 |
| Plasma IC50 (nM) for 30 clinical isolates | 230 | 110 | 2,900 | 1,800 | 2,200 | 100,000 |
| Plasma IC90 (nM) for 90% of PI-resistant clinical isolates | 1,180 | 720 | 12,900 | >10,000 | 13,400 | >100,000 |
Average of values obtained using HIV-1 (RF), HXB2, NL4-3, Thai H9566, E, and IIIB viruses using RNA capture, yield reduction, or p24 measurement. The total number of values for each inhibitor range from 10 to 29.
IC50 values reported by Virco using clinical isolate chimeric virus.
Ratio of measured 90% inhibition values in tissue culture in the presence versus the absence of added HSA and AAG (see Materials and Methods).
Protein binding-adjusted IC90 for ritonavir-resistant virus.
Table 6 compares the measured potency and the calculated plasma IC90 for wild-type and mutant viruses for DPC 681, DPC 684, and several marketed PIs. In order to compare the data obtained with site-directed recombinant viruses (IC90 measured) with that from clinical chimeras (IC50 measured), a IC50-to-IC90 conversion factor was needed. The IC50 and IC90 values were compared in in vitro assays performed using the sensitive plaque assay, and the following IC90/IC50 ratios were observed: DPC 681, 2.3 ± 0.8; DPC 684, 2.7 ± 1.1; lopinavir, 3.2 ± 0.8; indinavir, 3.6 ± 1.1; nelfinavir, 3.4 ± 1.9; and amprenavir, 2.9 ± 0.3. Therefore, a conversion factor of 3.0 was applied. Further, the corresponding plasma IC90 for 90% of the clinical chimeras was determined (i.e., the concentration required for 90% suppression of 27 of 30 viruses in the Virco PI-resistant virus panel). The 90% cutoff was chosen to reflect an ambitious goal: suppression of the majority of highly mutant viral variants. These data suggest that if plasma levels can be maintained in excess of ∼1 μM, either DPC 681 or DPC 684 should provide potent suppression of even highly mutated viral variants. In contrast, indinavir, nelfinavir, amprenavir, or lopinavir would require blood levels at trough of at least 10 to 100 μM, concentrations unlikely to be achieved or adequately tolerated with twice-daily or three-times-daily dosing regimens, even with pharmacologic modulators such as cytochrome P450 inhibitors.
Pharmacokinetics of DPC 681 and DPC 684.
Table 7 shows the pharmacokinetic parameters for DPC 681 and DPC 684 in dogs. The total body clearance (CL) of DPC 681 in dogs was high (1.8 liter/h/kg) equaling hepatic blood flow for this species (1.8 liter/h/kg). After an oral dosing, the Cmax increased ninefold between the 10- and 30-mg/kg DPC 681 dose groups. Bioavailability also increased between the 10- and 30-mg/kg dose groups (18.3 and 78.1%, respectively). These data suggest that hepatic extraction (first-pass effect) can be saturated in the dog. The CL of DPC 684 was 0.94 liter/h/kg, about half of the hepatic blood flow for dogs. The Cmax increased 14-fold between the 10- and 30-mg/kg DPC 684 oral-dose groups. DPC 684 bioavailability also increased with dose, again suggesting saturable hepatic extraction. The apparent half-life for both DPC 681 and DPC 684 in dogs was between 1 and 2 h. DPC 681 and DPC 684 levels in plasma at ca. 3 to 6 h after a 30-mg/kg dose were sufficient to inhibit 90% of the wild-type and mutant viruses.
TABLE 7.
Pharmacokinetics of DPC 681 and DPC 684 in dog plasma after single oral or i.v. dosesa
| Compound and dose (mg/kg) | Route | Concn (μM)
|
t1/2 (h) | CL (liter/h/kg) | F (%) | ||
|---|---|---|---|---|---|---|---|
| Cmax | C4 h | C6 h | |||||
| DPC 681 | |||||||
| 10 | Oral | 0.9 ± 0.7 | 0.04 ± 0.01 | 0.012 ± 0.01 | 1.0 ± 0.3 | 18.3 | |
| 30 | Oral | 8.5 ± 2.2 | 1.2 ± 0.19 | 0.2 ± 0.04 | 1.7 ± 0.6 | 78.1 | |
| 100 | Oral | 22.4 ± 7.0 | 9.82 ± 5.91 | 3.59 ± 2.54 | 1.2 ± 0.3 | 91.0 | |
| 1 | i.v. | 1.0 ± 0.3 | 1.8 ± 0.3 | ||||
| DPC 684 | |||||||
| 10 | Oral | 0.3 ± 0.1 | 0.01 ± 0.002 | BQL | NC | 1.9 | |
| 30 | Oral | 4.3 ± 2.3 | 0.190 ± 0.153 | 0.046 ± 0.03 | 1.7 ± 1.0 | 10.5 | |
| 100 | Oral | 15.0 ± 9.1 | 4.21 ± 6.16 | 0.74 ± 1.11 | 1.1 ± 0.3 | 19.0 | |
| 1 | i.v. | 0.89 ± 0.14 | 0.94 ± 0.68 | ||||
BQL, below quantifiable limit; NC, not calculated; F, fraction orally biovailable.
These DPC 681 and DPC 684 pharmacokinetic parameters were used to compare analogs and were not used to predict human trough levels. The human pharmacokinetics of another sulfonamide-containing PI, amprenavir, were not predicted by those observed in dogs. Amprenavir was not detected 8 h postdose after oral administration in the dog (data not shown), whereas ∼1 μM levels have been reported in humans given a similar dose (Agenerase package insert). Moreover, the half-life of amprenavir in the dog was ca. 0.3 h, much shorter than the reported clinical half-life (3 to 9 h).
Summary.
The potent PIs DPC 681 and DPC 684 described here show substantial improvements in their protein binding-adjusted resistance profile compared to currently available PIs. The potential ability of these compounds to inhibit multi-PI-resistant isolates is attributable to a combination of improved binding to the target enzyme and the projected adequacy of the plasma-free fraction.
The major findings for DPC 681 and DPC 684 in 2-week safety assessment studies in rats and dogs were histologic changes in the livers of rats given DPC 681 or DPC 684 and electrocardiographic findings in dogs given DPC 684. Multinucleation, increased mitoses, and single-cell necrosis of hepatocytes characterized the histologic changes in the livers of rats. This liver histology in rats occurred at concentrations of DPC 681 or DPC 684 in plasma that were severalfold higher than that anticipated in humans. The electrocardiographic finding in dogs given DPC 684 was mild 1° atrioventricular (AV) block. The 1° AV block, largely characterized by prolonged PR duration, did not progress to 2° AV block and was completely reversible. QT duration was not prolonged, and no abnormal arrhythmias were observed in any dogs given DPC 684. In humans given DPC 684, potential electrocardiographic changes can be directly monitored.
Because of the poor predictability of preclinical pharmacokinetics of the available PIs to mimic the trough levels in humans, the final assessment of the potential of DPC 681 and DPC 684 to extend treatment options for HIV-infected patients who have failed PI-containing regimens awaits confirmation in phase I clinical trials. Such studies are now in progress.
ACKNOWLEDGMENTS
We thank John Giannaras for the MMP assays and Irene Feingold and Steven Wu for their support of protein binding experiments, pharmacokinetic studies, and LC-MS analysis. We are grateful to Chris Teleha, Jan Hytrek, and Al Mical for purification of the marketed PIs from clinical formulations. We thank Kurt Hertogs and Brendan Larder (Virco) for Antivirogram susceptibility testing.
REFERENCES
- 1.Armbruster C, Vorbach H, Steindl F, El Menyawi I. Intracellular concentration of the HIV protease inhibitors indinavir and saquinavir in human endothelial cells. J Antimicrob Chemother. 2001;47:487–490. doi: 10.1093/jac/47.4.487. [DOI] [PubMed] [Google Scholar]
- 2.Bacheler L T, Paul M, Otto M J, Jadhav P K, Stone B A, Miller J A. An assay for HIV RNA in infected cell lysates, and its use for rapid evaluation of antiviral efficacy. Antiviral Chem Chemother. 1994;5:111–121. [Google Scholar]
- 3.Carpenter C C, Cooper D A, Fischl M A, Gatell J M, Gazzard B G, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman DD, Saag M S, Schecter M, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society—USA panel. JAMA. 2000;283:381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Chuah J, Hudson J, French M, Hoy J, Law M, Sayer D, Emery S, Cooper D. A randomized, open-label comparison of three highly active antiretroviral therapy regimens including two nucleoside analogues and indinavir for previously untreated HIV-1 infection: the OzCombo 1 study. AIDS. 2000;14:1171–1180. doi: 10.1097/00002030-200006160-00014. [DOI] [PubMed] [Google Scholar]
- 5.Coffin J. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 6.Condra J H, Holder D J, Schleif W A, Blahy O M, Danovich R M, Gabryelski L J, Graham D J, Laird D, Quintero J C, Hrodes A, Robbins H L, Roth E, Shivaprakash M, Yang T, Chodakewitz J A, Deutsch P J, Leavitt R Y, Massari F E, Mellors J W, Squires K E, Steigbigel R T, Teppler H, Emini E A. Genetic correlates of in vivo viral resistance to indinavir, a human immunodeficiency virus type 1 protease inhibitor. J Virol. 1996;70:8270–8276. doi: 10.1128/jvi.70.12.8270-8276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condra J H, Petropoulos C J, Ziermann R, Schleif W A, Shivaprakash M, Emini E A. Drug resistance and predicted virologic response to human immunodeficiency virus type 1 protease inhibitor therapy. J Infect Dis. 2000;182:758–765. doi: 10.1086/315782. [DOI] [PubMed] [Google Scholar]
- 8.Erickson-Viitanen S K, Klabe R M, Cawood P G, O'Neal P L, Meek J L. Potency and selectivity of inhibition of human immunodeficiency virus protease by a small nonpeptide cyclic urea, DMP 323. Antimicrob Agents Chemother. 1994;38:1628–1635. doi: 10.1128/aac.38.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flexner C. HIV genotype and phenotype—arresting resistance? JAMA. 2000;283:2442–2445. doi: 10.1001/jama.283.18.2442. [DOI] [PubMed] [Google Scholar]
- 10.Grabar S, Le Moing V, Goujard C, Leport C, Kazatchkine M D, Costagliola D, Weiss L. Clinical outcome of patients with HIV-1 infection acording to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Feinberg J E, Balfour H H, Jr, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeters or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 12.Havlir D V, Eastmen S, Garnst A, Richman D D. Kinetics of replication and estimated prevalence in untreated patients. J Virol. 1996;70:7894–7899. doi: 10.1128/jvi.70.11.7894-7899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertogs K, de Bethune M-P, Miller V, Ivens T, Schel P, Van Cauwenberge A, Van Den Eynde C, Van Gerwen V, Aziji H, Van Houtte M, Peeters F, Staszewski S, Conant M, Bloor S, Kemp S, Larder B, Pauwels R. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Chemother. 1998;42:269–276. doi: 10.1128/aac.42.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch M S. Resistance to antiretroviral drugs—an emerging problem. AIDS. 1998;12:6–7. [Google Scholar]
- 15.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 16.Jadhav P K, Ala P, Woerner F J, Chang C-H, Garber S S, Anton E D, Bacheler L T. Cyclic urea amides: HIV-1 protease inhibitors with low nanomolar potency against both wild types and protease inhibitor resistant mutants of HIV. J Med Chem. 1997;40:181–190. doi: 10.1021/jm960586t. [DOI] [PubMed] [Google Scholar]
- 17.Japour A J, Mayers D L, Johnson V A, Kuritzkes D R, Beckett L A, Lane J-M J, Black R J, Reichelderfer P S, D'Aquilla R T, Crumpacker C S the RV-43 Study Group; the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. Antimicrob Agents Chemother. 1993;34:1095–1101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz R A, Skalka A M. The retroviral enzymes. Annu Rev Biochem. 1994;63:133–173. doi: 10.1146/annurev.bi.63.070194.001025. [DOI] [PubMed] [Google Scholar]
- 19.Kohl N E, Emini E A, Schleif W A, Davis L J, Heimbach J C, Dixon R A F, Scolnick E M, Sigal I S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci USA. 1988;85:4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz M, Conant M, Hurley A, Schluger R, Duran M, Perterkin J, Chapman S, Patick A, Hendricks A, Yuen G J, Hoskins W, Clendeninn N, Ho D D. A preliminary evaluation of nelfinavir mesylate, an inhibitor of human immunodeficiency virus (HIV-1) protease, to treat HIV infection. J Infect Dis. 1998;177:1533–1540. doi: 10.1086/515312. [DOI] [PubMed] [Google Scholar]
- 21.Markowitz M, Mo H, Kempf D J, Norbeck D W, Bhat T N, Erickson J W, Ho D D. Selection and analysis of human immunodeficiency virus type I vairants with increased resistanct to ABT 538, a novel protease inhibitor. J Virol. 1995;69:701–706. doi: 10.1128/jvi.69.2.701-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mocroft A, Miller V, Chiesi A, Blaxhult A, Katlama C, Clotet B, Barton S, Lundgren J D. Virological failure among patients on HAART from across Europe: results from the EuroSIDA study. Antivir Ther. 2000;5:107–112. [PubMed] [Google Scholar]
- 23.Moyle G J. Efavirenz: shifting the HAART paradigm in adult HIV-1 infection. Exp Opin Investig Drugs. 1999;8:473–486. doi: 10.1517/13543784.8.4.473. [DOI] [PubMed] [Google Scholar]
- 24.Pazhanisamy S, Partaledis J A, Rao B G, Livingston D J. In vitro selection and characterization of VX-478 resistant HIV-1 variants. Adv Exp Med Biol. 1998;436:75–83. doi: 10.1007/978-1-4615-5373-1_10. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro R M, Bonhoeffer S. Production of resistant HIV mutants during antiretroviral therapy. Proc Natl Acad Sci USA. 2000;97:7681–7686. doi: 10.1073/pnas.97.14.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smallheer J M, Otto M J, Amarl-Ly C A, Earl R A, Myers M J, Pennev P, Montefiori D C, Wuonola M A. Synthesis and anti-HIV activity of a series of 2-indolinones and related analogs. Antiviral Chem Chemother. 1993;4:27–39. [Google Scholar]
- 27.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type I infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 28.Wodarz D, Nowak M A. HIV therapy: managing resistance. Proc Natl Acad Sci USA. 2000;97:8193–8195. doi: 10.1073/pnas.97.15.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winslow D L, Stack S, King R, Scarnati H, Bincsik A, Otto M J. Limited sequence diversity of the HIV type 1 protease gene from clinical isolates and in vitro susceptibility to HIV protease inhibitors. AIDS Res Hum Retrovir. 1995;11:107–113. doi: 10.1089/aid.1995.11.107. [DOI] [PubMed] [Google Scholar]
- 30.Wong J K, Hezarch M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 31.Xue C B, He X, Roderick J, Degrado W F, Cherney R J, Hardman K D, Nelson D J, Copeland R A, Jaffe B D, Decicco C P. Design and synthesis of cyclic inhibitors of matrix metalloproteinases and TNF-alpha production. J Med Chem. 1998;41:1745–1748. doi: 10.1021/jm970849z. [DOI] [PubMed] [Google Scholar]