Abstract
SGLT2 inhibitors may have a potential to stop the decline of renal function independent of the stage of albuminuria in diabetic kidney disease, and can be a new light in the post‐RAS inhibitor era.
Sodium–glucose cotransporter‐2 (SGLT2) inhibitors were developed as a new class of antidiabetic agent that increase urinary glucose excretion. This increased glucose excretion is expected to lead to various metabolic improvements such as weight loss and reduction in blood glucose. Initially, there were several concerns about its potential adverse effects such as ketoacidosis, particularly in type 1 diabetes 1 , but their use has now become widespread as a relatively safe drug 2 . This is especially true among patients with type 2 diabetes, because of the strong cardio‐ and renoprotection of these inhibitors 3 , 4 . Growing evidence shows the renoprotective effect of SGLT2 inhibitors in diabetic kidney disease (DKD) 3 , 4 . In particular, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial 4 showed significant renoprotective effects of canagliflozin, an SGLT2 inhibitor, in DKD. The result has been published approximately 20 years after the Reduction of Endpoints in Non‐insulin Dependent Diabetes (NIDDM) with the Angiotensin II Antagonist Losartan (RENAAL) study showed the significant reduction of albuminuria by losartan, a renin–angiotensin system (RAS) inhibitor, in patients with type 2 diabetes 5 . Therefore, there are now great expectations for SGLT2 inhibitors for DKD.
It is difficult to make a direct comparison between the RENAAL and CREDENCE trials because of their different eras, follow‐up periods, and patient backgrounds. However, the results of the estimated glomerular filtration rate (eGFR) 30–45 mL/min/1.73 m2 group in the CREDENCE trial 4 , which is similar to the patient background of the RENAAL trial 5 , may be used to evaluate the 20 year evolution of renal prognosis in patients with type 2 diabetes and macroalbuminuria. In the RENAAL trial, progression to end‐stage kidney disease or a doubling of serum creatinine occurred in 34.5% of patients in the placebo group, and it was reduced to 30.1% with losartan treatment 5 . In the CREDENCE trial, when compared with the placebo group, canagliflozin treatment reduced the incidence of the same renal events from 17.5% to 12.9%, while almost all patients had been treated with RAS inhibitors 4 . These results suggest that we have reached a new era in which the decline in renal function in patients with type 2 diabetes and macroalbuminuria can be halved compared with 20 years ago, and that SGLT2 inhibitors can be a new light in the post‐RAS inhibitor era.
In addition to these large‐scale clinical trials, similar results have been reported in real‐world clinical evidence. Based on the results from the Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT‐2 Inhibitors 3 (CVD‐REAL 3), the initiation of SGLT2 inhibitor improved the annual eGFR decline rate by 1.53 mL/min/1.73 m2 per year and reduced the incidence of composite kidney outcomes by half compared with initiation of other glucose‐lowering drugs 6 . Similarly, a large database study conducted in Japan using the Japan CKD Database (J‐CKD‐DB), a nationwide multicenter CKD registry, also revealed that initiation of SGLT2 inhibitors slowed the eGFR decline rate by 0.75 mL/min/1.73 m2 per year in Japanese patients with DKD 7 . Thus, the benefits of SGLT2 inhibitors on DKD seem to be largely generalized to clinical practice.
Racial differences often affect drug efficacy. CVD‐REAL 3 suggests the efficacy of SGLT2 inhibitors across racial groups because this observational study included populations in many countries 6 . The efficacy of SGLT2 inhibitors on DKD in Asia has been confirmed. A post hoc analysis of the CREDENCE trial showed that the effects of canagliflozin on renal and safety outcomes in participants from eastern and southeastern Asian (EA) countries were generally similar to those of the non‐EA participants 8 . In addition, in Japanese patients with diabetes, ipragliflozin corrected hyperfiltration in the patients with high eGFR (eGFR ≥60), while it increased eGFR in patients with a low eGFR (30≤eGFR<60 mL/min/1.73 m2) 9 . Furthermore, an observational study provided real‐world evidence that SGLT2 inhibitors slowed the eGFR decline rate in Japanese patients with DKD 10 . Collectively, in Asians as well as other races, SGLT2 inhibitors show high promise for renoprotection in DKD.
The typical clinical course of DKD is a decline in renal function with increased albuminuria, but it has been reported that the number of cases with decreased renal function without macroalbuminuria has been increasing gradually 11 . A sub‐analysis of the Canagliflozin Cardiovascular Assessment Study (CANVAS) program showed interesting results. In patients with type 2 diabetes, SGLT2 inhibitors not only halted the rapid eGFR decline in patients with macroalbuminuria, but they also significantly slowed the mild eGFR decline, even in patients without macroalbuminuria 12 (Figure 1). A similar effect of SGLT2 inhibitors was confirmed by the real‐world evidence from the J‐CKD‐DB. 7 Therefore, SGLT2 inhibitors may improve the rate of eGFR decline regardless of the stage of albuminuria in DKD, although there are no studies that have evaluated this effect as a primary endpoint so far. A study is currently underway, however, to determine the primary endpoint of renal prognosis in patients without macroalbuminuria with reduced eGFR. It is hoped that the results of this study will position SGLT2 inhibitors as therapeutic agents that can suppress the eGFR decline regardless of the stage of albuminuria in DKD.
Figure 1.
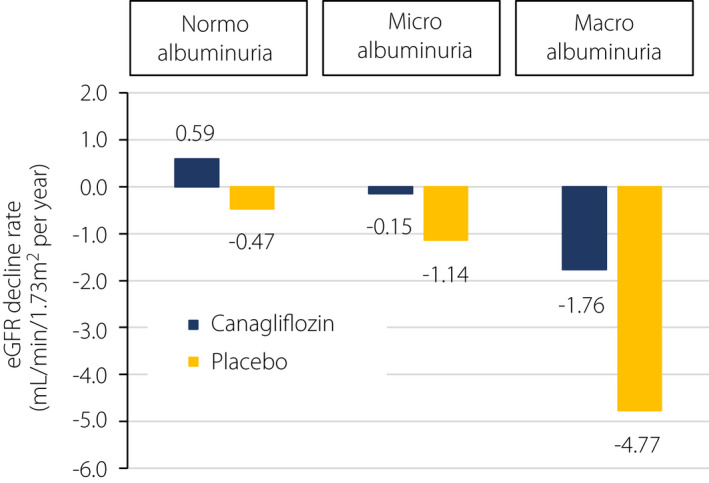
Effect of canagliflozin on annual eGFR decline rate in diabetic kidney disease. Sub‐analysis of the Canagliflozin Cardiovascular Assessment Study (CANVAS) program showed a significant improvement in the annual decline rate of estimated glomerular filtration rate (eGFR) in the canagliflozin‐treated group compared with the placebo group, regardless of stage of albuminuria at entry (modified from Neuen et al. 12 ).
Although the benefits of SGLT2 inhibitors have been increasingly demonstrated, there are still issues to be addressed. Overconfidence in SGLT2 inhibitors should be avoided; these are not magic pills, and their efficacy has been observed when added to conventional multifactorial intervention in all clinical trials and observational studies. Actually, the importance of blood pressure control in preventing the progression of DKD, even under SGLT2 inhibitor treatment, has been reported 13 . Next, there is no evidence that SGLT2 inhibitors decrease the new onset of microalbuminuria, and it is unclear whether they will be positioned as preventive agents for DKD defined by the appearance of albuminuria. Finally, it is unclear whether these inhibitors can significantly improve the renal prognosis of type 1 diabetes as well as type 2 diabetes.
Over the 20 years since the introduction of RAS inhibitors, the prognosis of DKD appears to have improved. Furthermore, SGLT2 inhibitors are beginning to show additional benefits on renal prognosis in DKD. It is hoped that renal prognosis in DKD over the next 20 years will be further improved by SGLT2 inhibitors, when used properly 14 , 15 . It is expected that DKD will be overcome in the future by practicing the improvement of DKD care with SGLT2 inhibitors and also by identifying issues for the post‐SGLT2 era.
DISCLOSURE
Shinji Kume: Grants (Boehringer Ingelheim Co., Ltd), Lecture fees (Nippon Boehringer Ingelheim Co., Ltd, Eli Lilly Japan K.K.)
Hiroshi Maegawa: Lecture fees (Nippon Boehringer Ingelheim Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., daiichi Sankyo Co., Ltd, AstraZeneca, Eli Lilly Japan K.K.), Total amount of research fees (joint research, commissioned research, clinical trials, etc.) and grants (Nippon Boehringer Ingelheim Co., Ltd, Mitsubishi Tanabe Pharma Corporation), total amount of scholarships (incentives) (Nippon Boehringer Ingelheim Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co., Ltd, Kowa Company, Ltd).
J Diabetes Investig. 2022; 13: 765–767
REFERENCES
- 1. Horii T, Oikawa Y, Atsuda K, et al. On‐label use of sodium‐glucose cotransporter 2 inhibitors might increase the risk of diabetic ketoacidosis in patients with type 1 diabetes. J Diabetes Investig 2021; 12: 1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Utsunomiya K, Koshida R, Kakiuchi S, et al. Safety and effectiveness of tofogliflozin in Japanese patients with type 2 diabetes mellitus treated in real‐world clinical practice: results of a 36‐month post‐marketing surveillance study (J‐STEP/LT). J Diabetes Investig 2021; 12: 184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kashiwagi A, Araki S, Maegawa H. Sodium‐glucose cotransporter 2 inhibitors represent a paradigm shift in the prevention of heart failure in type 2 diabetes patients. J Diabetes Investig 2021; 12: 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 5. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 6. Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real‐world clinical practice (CVD‐REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol 2020; 8: 27–35. [DOI] [PubMed] [Google Scholar]
- 7. Nagasu H, Yano Y, Kanegae H, et al. Kidney outcomes associated with SGLT2 inhibitors versus other glucose‐lowering drugs in real‐world clinical practice: the Japan chronic kidney disease database. Diabetes Care 2021; 44: 2542–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wada T, Mori‐Anai K, Kawaguchi Y, et al. Renal, cardiovascular and safety outcomes of canagliflozin in patients with type 2 diabetes and nephropathy in East and South‐East Asian countries: Results from the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation Trial. J Diabetes Investig 2021. 10.1111/jdi.13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsuba I, Kawata T, Iemitsu K, et al. Effects of ipragliflozin on the development and progression of kidney disease in patients with type 2 diabetes: An analysis from a multicenter prospective intervention study. J Diabetes Investig 2020; 11: 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirai T, Kitada M, Monno I, et al. Sodium‐glucose cotransporter 2 inhibitors in type 2 diabetes patients with renal function impairment slow the annual renal function decline, in a real clinical practice. J Diabetes Investig 2021; 12: 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kume S, Araki SI, Ugi S, et al. Secular changes in clinical manifestations of kidney disease among Japanese adults with type 2 diabetes from 1996 to 2014. J Diabetes Investig 2019; 10: 1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neuen BL, Ohkuma T, Neal B, et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J Am Soc Nephrol 2019; 30: 2229–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobayashi K, Toyoda M, Hatori N, et al. Sodium‐glucose cotransporter 2 inhibitor‐induced reduction in the mean arterial pressure improved renal composite outcomes in type 2 diabetes mellitus patients with chronic kidney disease: a propensity score‐matched model analysis in Japan. J Diabetes Investig 2021; 12: 1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for Diabetes 2019. J Diabetes Investig 2020; 11: 1020–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Committee on the proper use of SGLT2 inhibitors. Recommendations on the Proper Use of SGLT2 inhibitors. J Diabetes Investig 2020; 11: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]


