Abstract
Aims/Introduction
We investigated the association between gestational diabetes mellitus (GDM) and perinatal outcomes stratified by pre‐pregnancy body mass index (BMI) and/or gestational weight gain (GWG).
Materials and Methods
Data from the national birth cohort in the Japan Environment and Children's Study from 2011 to 2014 (n = 85,228) were used. Japan uses the GDM guidelines of the International Association of Diabetes and Pregnancy Study Groups. The odds ratios (ORs) of perinatal outcomes were compared between women with and those without GDM.
Results
The OR (95% confidence interval) of having a small for gestational age infant in the GDM group with a pre‐pregnancy BMI of ≥25.0 kg/m2 and insufficient GWG (<2.75 kg) was 1.78 (1.02–3.12). The OR of having a large for gestational age infant of the same BMI group with excessive GWG (>7.25 kg) was 2.04 (1.56–2.67). The OR of hypertensive disorders of pregnancy was higher in women with a BMI ≥18.5 kg/m2 in the GDM group than in the non‐GDM group.
Conclusions
Large for gestational age and hypertensive disorders of pregnancy were associated with pre‐pregnancy BMI and GWG in either normal weight or overweight/obese women, and the relationship was strengthened when GDM was present. Women with GDM and a BMI of ≥25.0 kg/m2 are at risk of having small for gestational age and large for gestational age infants depending on GWG.
Keywords: Body mass index, Gestational diabetes mellitus, Gestational weight gain
We examined maternal characteristics and perinatal outcomes of women with gestational diabetes mellitus categorized by pre‐pregnant body mass index and gestational weight gain for the 85,228 participants of a national birth cohort, the Japan Environment and Children's Study, collected from 2011 to 2014. Women with gestational diabetes mellitus and a body mass index of ≥25.0 kg/m2 are at risk of having small for gestational age and LGA infants depending on gestational weight gain.
INTRODUCTION
Gestational diabetes mellitus (GDM) is a common obstetric complication that can cause macrosomia, large for gestational age (LGA) infants, hypertensive disorders of pregnancy (HDP) and preterm birth 1 . Women diagnosed with GDM are more likely to develop obesity 2 and diabetes later in life. The increasing incidence of GDM in developing countries has a role in the global diabetes epidemic 3 . The 2008 Hyperglycemia and Adverse Pregnancy Outcomes 1 study resulted in adjustments to the strict GDM diagnostic criteria that are used in several countries, including Japan, since 2010. Japan, however, has witnessed increasing numbers of underweight women (body mass index [BMI] <18.5 kg/m2) becoming pregnant 4 , 5 , along with decreasing birthweights 5 , 6 .
Nutritional therapy in pregnant women with hyperglycemic disorders provides appropriate nutrition for healthy fetal development, and ensures strict glycemic control and appropriate weight gain 7 , 8 . The goal of glycemic control and nutritional therapy for all women with GDM is the same regardless of pre‐pregnancy BMI and weight gain during pregnancy 9 . In addition, a weight gain less than the recommended weight might be beneficial to prevent excessive birthweight in GDM pregnancies, particularly among overweight and obese women 10 . The results of studies on the association between underweight women with GDM and birthweight have been inconclusive 10 .
Although there are different indicators of appropriate weight gain for each pre‐pregnancy BMI, it is possible to make a difference in perinatal outcomes, especially among underweight women, using identical glycemic control and nutritional therapies in all women with GDM.
The purpose of the present study was to investigate the effect of GDM on perinatal outcomes, including fetal growth and the onset of HDP, compared with that of non‐GDM in the same pre‐pregnancy BMI and gestational weight gain (GWG) categories. The two classification systems that are most often used in Japan 11 for GWG determination were used in the present study, one from the Japanese Ministry of Health, Labor and Welfare (MHLW) 12 and the other, which is used worldwide, from the Institute of Medicine (IOM) in the USA 2 .
MATERIALS AND METHODS
Data collection
The study data were collected from the national birth cohort in the Japan Environment and Children's Study (JECS). The purpose of the JECS was to investigate environmental factors that might affect children's health and development 13 , 14 . Workers with 15 regional centers throughout Japan were responsible for recruiting women in early pregnancy who were living in the respective recruitment areas. The study gathered data from 45% of the births study area from January 2011 through March 2014 13 , 14 . The eligibility criteria for the participants (expectant mothers) were as follows: (i) they should reside in the study area at the time of recruitment and expect to reside continually in Japan for the foreseeable future; (ii) the expected date of delivery should be between 1 August 2011 and mid‐2014; and (iii) they should be capable of participating in the study without difficulty. The participants should be able to comprehend the Japanese language and complete the self‐administered questionnaire 13 . Women residing outside the study areas, even if they were receiving care from cooperating healthcare providers within the study areas, were excluded 13 . The women's medical records and a questionnaire given to mothers in the first trimester (16.4 ± 8.0 gestational weeks) and second or third trimester (27.9 ± 6.5 gestational weeks) were abstracted 13 . The dataset jecs‐ag‐20160424 was used in the present study.
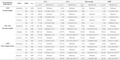
From a potential pool of 104,102 fetal records, 2,452 records were excluded because of uncollected data. An additional 16,422 records were excluded because they had incomplete data or did not meet the entry criteria for the following conditions: maternal height (65 records), maternal weight (2,876), gestational age at birth of <37 or ≥42 weeks (7,355), congenital anomaly or newborn disease (9,665), type 1 diabetes mellitus (76), type 2 diabetes mellitus (135) and others treated with insulin without GDM (51). Furthermore, duplicate records were excluded. A total of 85,228 participants were included in the final study group (Figure 1).
Figure 1.
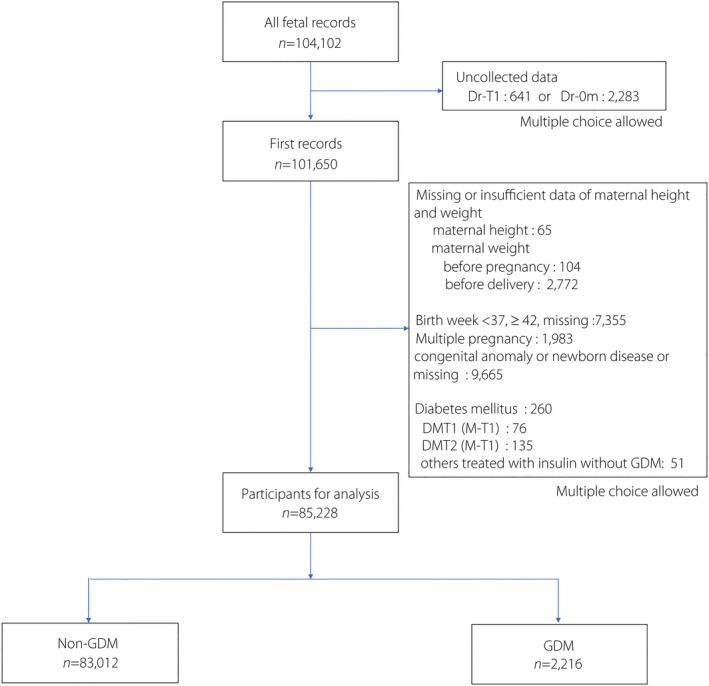
Flowchart of the selected participants. DMT1, type 1 diabetes; DMT2, type 2diabetes; Dr‐0m, medical records provided by a doctor or medical staff at birth; Dr‐T1, medical records provided by a doctor or medical staff in the first trimester; GDM, gestational diabetes mellitus; M‐T1, self‐administered questionnaires for mothers in the first trimester.
Definition of GDM
In Japan, GDM is diagnosed using the modified criteria of the International Association of Diabetes and Pregnancy Study Groups (Table S1) 11 , 15 . It is diagnosed when at least one value reaches or exceeds any of the following three thresholds in a 75‐g oral glucose tolerance test: 92–125 mg/dL for fasting plasma glucose (PG), 180 mg/dL for 1‐h PG and 153 mg/dL for 2‐h PG 11 . In the JECS, two items were related to GDM in the birth information recorded by physicians or medical staff. Women with GDM were defined as those who were indicated as having “GDM” in the record and those who were not indicated as having “GDM”, but provided the “gestational weeks at GDM diagnosis.”
Definition of pre‐pregnancy BMI
BMI was calculated using the maternal height and weight before pregnancy, and the participants were categorized into one of the three weight groups defined according to BMI, in accordance with the MHLW recommendations as follows: underweight, <18.5 kg/m2; normal weight, 18.5–24.9 kg/m2; and overweight/obese, ≥25.0 kg/m2 12 . The IOM divides women into four groups: underweight, <18.5 kg/m2; normal weight, 18.5–24.9 kg/m2; overweight, 25.0–29.9 kg/m2; and obese, ≥30.0 kg/m2 2 .
Definition of GWG
GWG was calculated by comparing prenatal maternal weight with weight measured at the time of labor. The recommended weight gains for the three MHLW groups were as follows: underweight, 9.0–12.0 kg; normal weight, 7.0–12.0 kg; and overweight/obesity, approximately 5.0 kg (Figure 2a) 13 . The recommended weight gain for the four IOM groups were as follows: underweight, 12.5–18.0 kg; normal weight, 11.5–16.0 kg; overweight, 7.0–11.5 kg; and obese, 5.0–9.0 kg (Figure 2b) 2 . Because the range for weight gain in the IOM overweight group was up to 4.5 kg, we chose “approximately 5.0 kg (2.75–7.25 kg)” as the metric for overweight/obese in the MHLW classification, which is consistent with the IOM range.
Figure 2.
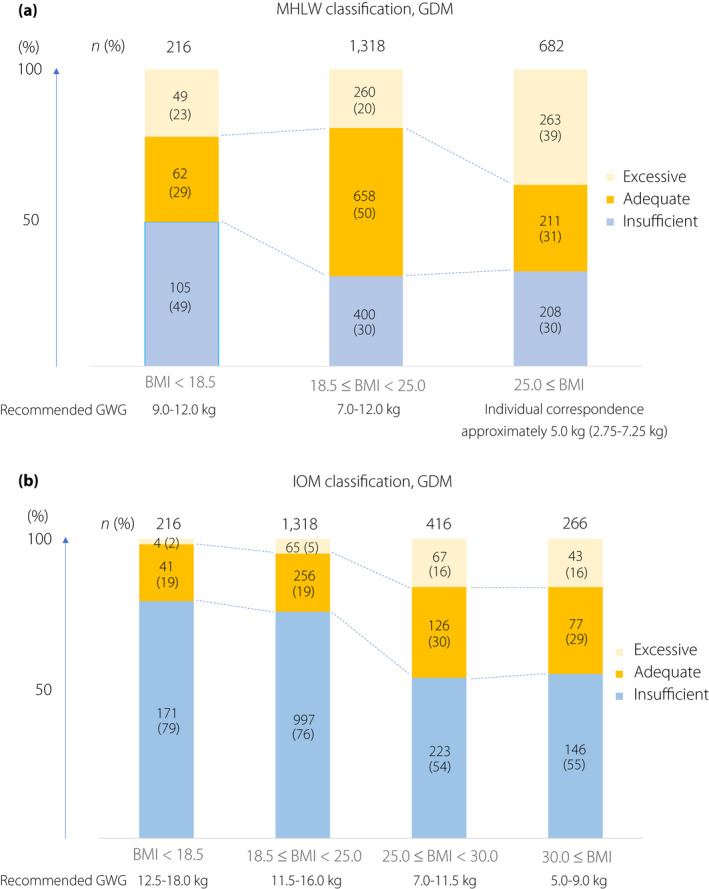
(a) Cumulative bar chart of the participants' gestational weight gain (GWG) stratified by pre‐pregnancy body mass index (BMI) in the Japanese Ministry of Health, Labor and Welfare (MHLW) classification. (b) Cumulative bar chart of the participants' GWGs stratified by pre‐pregnancy BMI in the Institute of Medicine (IOM) classification. GDM, gestational diabetes mellitus.
Perinatal outcomes
Low birthweight and macrosomia were defined as <2,500 g and ≥4,000 g, respectively. Small for gestational age (SGA) and LGA infants had birthweights of <10th or >90th percentile, respectively, according to the Japanese birthweight standard (2010) 16 . Polyhydramnios and HDP were collected from birth information recorded by doctors or medical staff. HDP, measured after 20 gestational weeks, was defined as a systolic blood pressure of ≥140 mmHg and diastolic blood pressure of ≥90 mmHg 17 . The short‐term prognoses of infants were assessed using the 5‐min Apgar score or umbilical arterial blood power of hydrogen.
Variables
Multiple factors were abstracted from the mothers' or their partners' questionnaire for comparison between the GDM and non‐GDM groups. These included the maternal age, parity, gestational age (weeks) when GDM was diagnosed, insulin treatment, length of labor, cesarean delivery status, maternal smoking during pregnancy, partner smoking during pregnancy, drinking during pregnancy, annual household income, maternal educational background, nutritional support and hypertensive disorders before pregnancy. Smoking and drinking history during pregnancy were collected by a questionnaire survey, which asked about smoking and drinking history, spanning the period from the very beginning of pregnancy until before the pregnancy became apparent.
Statistical analysis
Maternal characteristics and perinatal outcomes for the GDM and non‐GDM groups were evaluated using the χ2‐test or analysis of variance. First, the participants were stratified by pre‐pregnancy BMI or GWG according to the MHLW and IOM classifications, respectively. For each of the same strata, the odds ratios (OR) of SGA, LGA, macrosomia and HDP in the GDM group compared with the non‐GDM group were calculated using a logistic regression model. Macrosomia and HDP were adjusted for maternal age, fetal sex, maternal smoking and GWG (stratified by pre‐pregnancy BMI) or pre‐pregnancy BMI (stratified by GWG). SGA was adjusted for maternal age, maternal smoking, HDP and pre‐pregnancy BMI or GWG, because SGA and LGA were defined using birthweight, gestational age and fetal sex 16 . LGA was adjusted for maternal age, maternal smoking and pre‐pregnancy BMI or GWG. Finally, pre‐pregnancy BMI and GWG were combined and stratified, and the relationship between GDM and each perinatal outcome was expressed as an OR using logistic regression analysis. All data were analyzed using JMP Pro 14.0 (SAS Institute, Cary, NC, USA), and missing data were excluded from the statistical analysis. A P‐value of <0.05 showed statistical significance.
Ethical approval
The JECS protocol was approved by the institutional review board of the Epidemiological Studies Ministry of the Environment and the ethics committees of all participating institutions. This study was carried out in accordance with the principles of the Declaration of Helsinki and its revisions. Written informed consent was obtained from all participants.
RESULTS
Of the 85,228 participants in the present study, 2,216 (2.6%) developed GDM. Their classifications according to the MHLW and IOM criteria are presented in Figure 2. Fewer women had excessive GWG according to the IOM classification, and the divisions of the women were more balanced across all categories in the MHLW classification than in the IOM classification.
Table 1 shows the maternal characteristics and perinatal outcomes of all participants according to the non‐GDM and GDM groups. Pre‐pregnancy BMI was significantly higher in the GDM group (23.7 ± 5.1 kg/m2) than in the non‐GDM group (21.1 ± 3.2 kg/m2, P < 0.001), but the GWG was 25% lower in the GDM group (7.9 ± 5.1 kg) than in the non‐GDM group (10.5 ± 4.3 kg, P < 0.001). Women with GDM were significantly older than those without GDM (33.3 ± 5.0 vs 31.0 ± 5.0 years, P < 0.001). Cesarean section rates were 10% higher in the GDM group (27.7%) than in the non‐GDM group (17.2%, P < 0.001). There were no significant differences in educational background, as well as prevalence rates of low birthweight, SGA, umbilical arterial blood power of hydrogen, and stillbirths between the two groups.
Table 1.
Maternal characteristics and perinatal outcomes: Gestational versus non‐gestational diabetes
| Non‐GDM | GDM | P‐value | |
|---|---|---|---|
| n = 83,012 (97.4%) | n = 2,216 (2.6%) | ||
| Maternal characteristics | |||
| Age (years) | 31.0 ± 5.0 | 33.3 ± 5.0 | <0.001*** |
| Primiparous, n (%) | 32,341 (39) | 857 (39) | 0.645 |
| Pre‐pregnancy BMI (kg/m2) | 21.1 ± 3.2 | 23.7 ± 5.1 | <0.001*** |
| Gestational weight gain (kg) | 10.5 ± 4.3 | 7.9 ± 5.1 | <0.001*** |
| Gestational week at GDM diagnosis (weeks) | (–) | 24.9 ± 7.7 | (–) |
| Insulin treatment, n (%) | (–) | 253 (11) | (–) |
| Labor duration (min) | 510 ± 437 | 488 ± 455 | 0.037* |
| Cesarean delivery, n (%) | 14,012 (17) | 599 (28) | <0.001*** |
| Smoking (mother) during pregnancy, n (%) | 14,837 (18) | 419 (19) | 0.246 |
| Smoking (partner) during pregnancy, n (%) | 40,057 (48) | 1,000 (45) | 0.001** |
| Drinking during pregnancy, n (%) | 40,161 (48) | 970 (44) | <0.001*** |
| Annual household income <4,000,000 JPY, n (%) | 30,635 (37) | 784 (35) | 0.043* |
| Maternal educational background, junior high school or high school, n (%) | 29,688 (36) | 817 (37) | 0.353 |
| Nutritional support, n (%) | 7,535 (9) | 1,567 (71) | <0.001*** |
| Hypertensive disorder before pregnancy, n (%) | 770 (1) | 70 (3) | <0.001*** |
| Hypertensive disorders of pregnancy, n (%) | 2,084 (3) | 151 (7) | <0.001*** |
| Perinatal outcomes | |||
| Infant sex, male, n (%) | 42,098 (51) | 1,141 (52) | 0.471 |
| Gestational age (days) | 276.3 ± 7.8 | 274.4 ± 8.2 | <0.001*** |
| Birthweight (g) | 3,064 ± 363 | 3,080 ± 400 | 0.031* |
| Low birthweight (<2,500 g), n (%) | 4,288 (5) | 129 (6) | 0.168 |
| Macrosomia (≥4,000 g), n (%) | 718 (1) | 36 (2) | <0.001*** |
| SGA (<10% percentile) | 5,241 (6) | 150 (7) | 0.4173 |
| LGA (>90% percentile) | 7,351 (9) | 311 (14) | <0.001*** |
| Polyhydramnios, n (%) | 241 (0) | 22 (1) | <0.001*** |
| Non‐reassuring fetal status, n (%) | 1,855 (2) | 71 (3) | 0.002** |
| Still birth, n (%) | 30 (0) | 1 (0) | 0.827 |
| Apgar score (5 min) <7, n (%) | 268 (0) | 14 (1) | 0.016* |
| UmA‐pH <7.0, n (%) | 141 (0) | 1 (0) | 0.139 |
Data are presented as the mean ± standard deviation or number (%). The non‐gestational diabetes mellitus (GDM) group was compared with the GDM group using the χ2‐test or independent t‐test. *P < 0.05; **P < 0.01; ***P < 0.001. BMI, body mass index; JPY, Japanese yen; LGA, large for gestational age; SGA, small for gestational age; UmA‐pH, umbilical cord artery pH.
Table 2 shows the association between GDM and perinatal outcomes stratified by pre‐pregnancy BMI using the MHLW classification. All ORs were compared between the GDM and the non‐GDM, same pre‐pregnancy BMI groups. The highest percentage of SGA was 12% in the underweight GDM group, but the OR of SGA was slightly elevated (1.30, 95% confidence interval [CI] 0.92–1.83] in the overweight/obese GDM group compared with the overweight/obese non‐GDM group. The incidence of LGA positively correlated with BMI, and the ORs of LGA in the normal weight and overweight/obese GDM groups were significantly higher than those in the normal weight and overweight/obese non‐GDM groups (1.56, 95% CI 1.30–1.87 and 1.67, 95% CI 1.37–2.04, respectively). The incidence of HDP was similar to that of LGA, with significantly higher ORs of HDP in the normal weight and overweight/obese participants in the GDM groups than in those in the non‐GDM groups (2.19, 95% CI 1.67–2.87 and 2.24, 95% CI 1.75–2.87, respectively). Notably, in all the stratified groups, GWG was much smaller among those with GDM than among those without GDM.
Table 2.
Association between gestational diabetes mellitus and perinatal outcomes stratified by pre‐pregnancy body mass index using the Ministry of Health, Labor and Welfare, Japan classification
| Pre‐pregnancy BMI (kg/m2) | GDM | n | GWG (kg) † | SGA | LGA | Macrosomia | HDP | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) ‡ |
OR (95% CI) |
n (%) ‡ |
OR (95% CI) |
n (%) ‡ |
OR (95% CI) |
n (%) ‡ |
OR (95% CI) |
||||
| <18.5 (Underweight) | No | 13,464 | 11.1 ± 5.6 | 1,292 (10) | Reference | 657 (5) | Reference | 40 (0) | Reference | 220 (2) | Reference |
| Yes | 216 | 9.6 ± 4.0 | 26 (12) |
1.00 (0.65–1.54) |
13 (6) |
1.46 (0.82–2.60) |
0 (0) | (–) | 4 (2) |
1.18 (0.90–1.54) |
|
| 18.5–24.9 (Normal weight) | No | 61,292 | 10.7 ± 3.7 | 3,624 (6) | Reference | 5,338 (9) | Reference | 493 (1) | Reference | 1,329 (2) | Reference |
| Yes | 1,318 | 8.9 ± 4.4 | 82 (6) |
0.82 (0.65–1.04) |
149 (11) |
1.56 (1.30–1.87)*** |
19 (1) |
2.14 (1.35–3.42)** |
59 (4) |
2.19 (1.67–2.87)*** |
|
| ≥25.0 (Overweight/obese) | No | 8,256 | 8.0 ± 5.1 | 325 (4) | Reference | 1,356 (16) | Reference | 185 (2) | Reference | 535 (6) | Reference |
| Yes | 682 | 5.5 ± 5.9 | 42 (6) |
1.30 (0.92–1.83) |
149 (22) |
1.67 (1.37–2.04)*** |
17 (2) |
1.30 (0.78–2.18) |
88 (13) |
2.24 (1.75–2.87)*** |
|
Logistic regression models are adjusted for maternal age, smoking, hypertensive disorders of pregnancy (HDP) and gestational weight gain (GWG) when the perinatal outcomes are small for gestational age (SGA). Logistic regression models are adjusted for maternal age, smoking and GWG when the perinatal outcomes are large for gestational age (LGA). Logistic regression models are adjusted for maternal age, smoking, infant sex and GWG when the perinatal outcomes are macrosomia and HDP. Odds ratio (OR) and 95% confidence interval (95% CI) is in comparison with the reference group. *P < 0.05; **P < 0.01; ***P < 0.001.
BMI, body mass index; GDM, gestational diabetes mellitus.
Mean ± standard deviation.
Percent (%) represents n case / n all × 100.
Table 3 shows the association between GDM and perinatal outcomes stratified by GWG using the MHLW classification. No significant difference in the incidence of SGA infants was observed between the GDM and non‐GDM groups, despite the weight gain during pregnancy. However, the OR of having a LGA infant significantly increased in the adequate and excessive weight gain GDM group compared with the adequate and excessive weight gain non‐GDM group (1.31, 95% CI 1.06–1.62 and 1.74, 95% CI 1.43–2.12, respectively). The OR of HDP was significantly increased in all the GDM groups compared with non‐GDM groups (1.68, 95% CI 1.16–2.43 for insufficient; 2.13, 95% CI 1.58–2.86 for adequate; and 1.54, 95% CI 1.13–2.11 for excessive GWG). The incidence of macrosomia was exceptionally low in the insufficient GWG group, and no significant difference in the OR of macrosomia was observed between the GDM and non‐GDM groups.
Table 3.
Association between gestational diabetes mellitus and perinatal outcomes stratified by gestational weight gain using the Ministry of Health, Labor and Welfare, Japan classification
| GWG | GDM | n |
Pre‐pregnancy BMI (kg/m2) † |
SGA | LGA | Macrosomia | HDP | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) ‡ |
OR (95% CI) |
n (%) ‡ |
OR (95% CI) |
n (%) ‡ |
OR (95% CI) |
n (%) ‡ |
OR (95% CI) |
||||
| Insufficient | No | 12,720 | 21.1 ± 3.8 | 1,427 (11) | Reference | 521 (4) | Reference | 41 (0) | Reference | 261 (2) | Reference |
| Yes | 713 | 23.9 ± 5.8 | 71 (10) |
1.07 (0.82–1.40) |
47 (7) |
0.99 (0.71–1.38) |
2 (0) |
0.50 (0.12–2.11) |
39 (5) |
1.68 (1.16–2.43)** |
|
| Adequate | No | 40,776 | 20.9 ± 2.8 | 2,688 (7) | Reference | 2,798 (7) | Reference | 199 (0) | Reference | 774 (2) | Reference |
| Yes | 931 | 22.9 ± 4.5 | 53 (6) |
0.93 (0.70–1.23) |
109 (12) |
1.31 (1.06–1.62)* |
14 (2) |
1.66 (0.93–2.96) |
57 (6) |
2.13 (1.58–2.86)*** |
|
| Excessive | No | 29,516 | 21.4 ± 3.4 | 1,126 (4) | Reference | 4,032 (14) | Reference | 478 (2) | Reference | 1,049 (4) | Reference |
| Yes | 572 | 24.6 ± 5.1 | 26 (5) |
1.41 (0.94–2.12) |
155 (28) |
1.74 (1.43–2.12)*** |
20 (4) |
1.33 (0.83–2.14) |
55 (10) |
1.54 (1.13–2.11)** |
|
Logistic regression models are adjusted for maternal age, smoking, hypertensive disorders of pregnancy (HDP) and pre‐pregnancy body mass index (BMI) when the perinatal outcomes are small for gestational age (SGA). Logistic regression models are adjusted for maternal age, smoking and pre‐pregnancy BMI when the perinatal outcomes are large for gestational age (LGA). Logistic regression models are adjusted for maternal age, smoking, infant sex and pre‐pregnancy BMI when the perinatal outcomes are macrosomia and HDP. Odds ratio (OR) and 95% confidence interval (95% CI) is in comparison with the reference group. *P < 0.05; **P < 0.01; ***P < 0.001.
GDM, gestational diabetes mellitus; GWG, gestational weight gain.
Mean ± standard deviation.
Percent (%) represents n case / n all × 100.
In Table 4, pre‐pregnancy BMI and GWG were combined and subdivided into nine groups. Each group was further stratified into GDM and non‐GDM groups, and the number (%) and OR (95% CI) of each outcome for GDM compared with non‐GDM were calculated for each group. In the pre‐pregnancy underweight and insufficient GWG group, the incidence of SGA infants was significantly higher (16% in the non‐GDM group and 17% in the GDM group) than in any of the other subgroups. However, in the overweight/obese and insufficient GWG group, the OR of having a SGA infant was 1.78 (95% CI 1.02–3.12) in the GDM group compared with that in the non‐GDM group. In the pre‐pregnancy underweight group, the incidence rates of LGA, macrosomia and HDP were low in all GWG subgroups, and no significant difference was observed in the relationship between GDM and non‐GDM. The incidence rate of LGA infants significantly increased in the normal pre‐pregnancy weight and excessive GWG with GDM (24%), and pre‐pregnancy overweight/obese and excessive GWG with GDM (33%) groups. The OR of having a LGA infant in the GDM group compared with the non‐GDM group was 1.91 (95% CI 1.42–2.56) for the normal weight and excessive GWG group, and 2.04 (95% CI 1.56–2.67) for the overweight/obesity and excessive GWG group. The incidence of HDP in the GDM group was increased to 14% in the pre‐pregnancy overweight/obese subgroups with adequate and excessive GWG. The ORs of HDP in the pre‐pregnancy overweight/obese with adequate and excessive GWG subgroups with GDM were higher at 2.66 (95% CI 1.73–4.10) and 1.84 (95% CI 1.26–2.68), respectively, than those in the same subgroups without GDM.
Table 4.
Association between gestational diabetes mellitus and perinatal outcomes stratified by both pre‐pregnancy body mass index and gestational weight gain using the Ministry of Health, Labor and Welfare, Japan classification
| Pre‐pregnancy BMI (kg/m2) | GWG | GDM | n all | SGA | LGA | Macrosomia | HDP | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n case (%) † | OR (95% CI) | n case (%) † | OR (95% CI) | n case (%) † | OR (95% CI) | n case (%) † | OR (95% CI) | ||||
| <18.5 (Underweight) | Insufficient | No | 3,499 | 546 (16) | Reference | 68 (2) | Reference | 6 (0) | Reference | 51 (1) | Reference |
| Yes | 105 | 18 (17) | 1.07 (0.63–1.82) | 5 (5) | 2.38 (0.94–6.04) | 0 (0) | (–) | 2 (2) | 1.21 (0.29–5.05) | ||
| Adequate | No | 5,192 | 486 (9) | Reference | 207 (4) | Reference | 8 (0) | Reference | 71 (1) | Reference | |
| Yes | 62 | 6 (10) | 1.07 (0.46–2.53) | 3 (5) | 1.32 (0.41–4.26) | 0 (0) | (–) | 1 (2) | 1.11 (0.15–8.12) | ||
| Excessive | No | 4,773 | 260 (5) | Reference | 382 (8) | Reference | 26 (1) | Reference | 98 (2) | Reference | |
| Yes | 49 | 2 (4) | 0.73 (0.18–3.04) | 5 (10) | 1.32 (0.52–3.37) | 0 (0) | (–) | 1 (2) | 0.99 (0.14–7.26) | ||
| 18.5–24.9 (Normal weight) | Insufficient | No | 8,149 | 828 (10) | Reference | 332 (4) | Reference | 26 (0) | Reference | 151 (2) | Reference |
| Yes | 400 | 34 (9) | 0.80 (0.56–1.15) | 16 (4) | 0.89 (0.53–1.52) | 1 (0) | 0.72 (0.10–5.31) | 16 (4) | 2.05 (1.21–3.46)** | ||
| Adequate | No | 33,096 | 2,063 (6) | Reference | 2,275 (7) | Reference | 156 (0) | Reference | 568 (2) | Reference | |
| Yes | 658 | 35 (5) | 0.80 (0.56–1.13) | 71 (11) | 1.58 (1.22–2.04)*** | 12 (2) | 3.71 (2.05–6.72)*** | 27 (4) | 2.27 (1.52–3.40)*** | ||
| Excessive | No | 20,047 | 733 (4) | Reference | 2,731 (14) | Reference | 311 (2) | Reference | 610 (3) | Reference | |
| Yes | 260 | 13 (5) | 1.33 (0.75–2.33) | 62 (24) | 1.91 (1.42–2.56)*** | 6 (2) | 1.50 (0.66–3.40) | 16 (6) | 1.96 (1.17–3.28)* | ||
| ≥25.0 (Overweight/obese) | Insufficient | No | 1,072 | 53 (5) | Reference | 121 (11) | Reference | 9 (1) | Reference | 59 (6) | Reference |
| Yes | 208 | 19 (9) | 1.78 (1.02–3.12)* | 26 (13) | 1.13 (0.72–1.78) | 1 (0) | 0.54 (0.07–4.32) | 21 (10) | 1.81 (1.06–3.09)* | ||
| Adequate | No | 2,488 | 139 (6) | Reference | 316 (13) | Reference | 35 (1) | Reference | 135 (5) | Reference | |
| Yes | 211 | 12 (6) | 0.93 (0.51–1.72) | 35 (17) | 1.34 (0.91–1.98) | 2 (1) | 0.61 (0.15–2.57) | 29 (14) | 2.66 (1.73–4.10)*** | ||
| Excessive | No | 4,696 | 133 (3) | Reference | 919 (20) | Reference | 141 (3) | Reference | 341 (7) | Reference | |
| Yes | 263 | 11 (4) | 1.44 (0.76–2.70) | 88 (34) | 2.04 (1.56–2.67)*** | 14 (5) | 1.70 (0.96–3.00) | 38 (14) | 1.84 (1.26–2.68)** | ||
Logistic regression models are adjusted for maternal age, smoking and hypertensive disorders of pregnancy (HDP) when the perinatal outcomes are small for gestational age (SGA). Logistic regression models are adjusted for maternal age and smoking when the perinatal outcomes are large for gestational age (LGA). Logistic regression models are adjusted for maternal age, smoking and infant sex when the perinatal outcomes are macrosomia and HDP. Odds ratio (OR) and 95% confidence interval (95% CI) is in comparison with the reference group. *P < 0.05; **P < 0.01; ***P < 0.001.
BMI, body mass index; CI, GDM, gestational diabetes mellitus; GWG, gestational weight gain.
Percent (%) represents n case / n all × 100.
In the present study, identical analyses were carried out using the IOM classification, and the association between GDM and perinatal outcomes was similar to the outcome obtained using the MHLW classification (Tables S2‐S4).
DISCUSSION
The present study had the following main findings: (i) the strongest relationship between GDM and SGA was found in the subgroup of participants who were overweight/obese pre‐pregnancy and had insufficient GWG (OR 1.78, 95% CI 1.02–3.12; Table 4); and (ii) LGA and HDP were strongly related to pre‐pregnancy BMI and GWG in women with either pre‐pregnancy normal weight or overweight/obese, and GDM further strengthened the relationship (Tables 2, 3, 4).
The incidence of SGA infants has been reported to increase in women with GDM when strict glycemic control is applied 18 , 19 , 20 . Similarly, as shown in Table 4, a significantly increased OR of having a SGA infant was observed in overweight/obese women with GDM and insufficient GWG compared with those without GDM. The high OR of having a SGA infant in the overweight/obese and insufficient GWG GDM group might be caused by their much lower average GWG (−1.0 ± 3.5 kg). In all the groups stratified by pre‐pregnancy BMI, the smaller GWG in the GDM group might be owing to dietary restrictions for patients with GDM. In particular, the incidence of SGA infants in the GDM subgroup with a BMI of ≥25.0 kg/m2 and insufficient GWG should be noted. However, the highest incidence of SGA infants was observed in the pre‐pregnancy underweight group with insufficient GWG, consistent with SGA being more affected by underweight and insufficient GWG 21 .
It might be beneficial for underweight women diagnosed with GDM to increase their GWG to reduce their risk of having a SGA infant. Infants born with SGA are considered as having a high risk for future cardiovascular events and other health risks 22 , 23 . Using the MHLW classification, we found that the incidence of SGA infants in the present study was 17% (18/105) among the pre‐pregnancy underweight women with insufficient GWG who developed GDM, and 10% (6/62) for this subgroup with adequate weight gain. Therefore, an adequate GWG of ≥9.0 kg is important to reduce the incidence of SGA infants among underweight women with GDM.
In the present study, we found that the incidence rates of LGA, macrosomia and HDP were lower in the pre‐pregnancy underweight group, and observed no relationship between GDM and these outcomes in that group. This emphasizes the need for guidance on appropriate weight gain to prevent the occurrence of SGA infants in the underweight group. However, LGA and HDP strongly correlated with GDM in the pre‐pregnancy overweight/obese group, and special attention should be paid to the occurrence of LGA (33%) and HDP (14%) in this group with excessive GWG. Previous studies reported that for LGA and HDP, pre‐pregnancy BMI and GWG strongly correlated 24 , 25 , but GDM further strengthened the relationship 24 . Table 3 shows that HDP is affected by GDM, even among those with insufficient GWG, which suggests that GDM is a strong risk factor of HDP.
Among overweight/obese women, adequate weight control is particularly important, because an increased OR of having a SGA infant was observed in the GDM group when GWG was insufficient compared with the non‐GDM group, and the ORs of LGA infants and HDP increased with excessive GWG. The OR of macrosomia in the GDM group significantly increased among those with normal weight and adequate GWG compared with that in the non‐GDM group. However, the incidence of macrosomia was low in other subgroups, and the relationship among BMI, GWG and macrosomia was unclear.
The present study showed a lower prevalence of GDM (3.9–7.0%) than those reported in previous studies 26 , 27 , 28 , 29 , 30 . Those studies might have overestimated the prevalence, because Morikawa et al. 26 , 27 included women with diabetes mellitus, and the study by Iwama et al. 28 was a multicenter study involving relatively large hospitals that treated high‐risk pregnancies. In a large previous study of secondary and tertiary centers in Japan, 13,037 (5.5%) of 237,941 women were diagnosed with GDM, including 13.3% of preterm births 29 . In a population‐based Japanese study of 46,365 women, the prevalence of GDM, including preterm birth, was reported to be 3.9%, which is closer to the present study 30 . However, the present study found a 2.6% prevalence of GDM among those with only term births. Preterm births and miscarriages were excluded from this study, and GDM is considered a risk factor of preterm birth 1 . A meta‐analysis of 5,349,476 pregnant women worldwide using the International Association of Diabetes and Pregnancy Study Groups diagnostic criteria found a 10.6% prevalence of GDM (95% CI 10.5–10.6) 31 , whereas it was 11.5% (95% CI 10.9–12.1) in 20 countries across Asia 32 . One of the reasons for the low prevalence of GDM in Japan is that only few pregnant women are overweight/obese. Another reason for the low prevalence might be that the participants in the present study included relatively healthy women. In the present study, the prevalence of need for insulin therapy in participants was 11% (Table 1), whereas previous studies, including studies carried out in Japan, have reported between 9 and 59% of women with GDM required insulin therapy 33 , 34 , 35 , 36 , 37 . In general, high‐risk GDM women are referred to high‐risk perinatal centers. Thus, high‐risk GDM women might be lost to follow up, resulting in the possibility of fewer high‐risk GDM women with low need for insulin therapy. The JECS is reportedly a very similar population to Japan's 2013 Vital Statistics Survey 14 . However, the GDM subpopulation might be controversial in the JECS, and further research with an appropriate study design is required to clarify the prevalence of GDM.
The IOM classification system, which was designed on the basis of the body size of American women, appears to be inappropriate for the physique of Japanese women. When establishing an optimal GWG for Japanese women using the IOM classification, the lower end of the range for their pre‐pregnant BMI values should be used 6 , 38 . The optimal GWG in the IOM classification is based not only on BMI, but also on height. The mean height in the JECS was 158.1 ± 5.4 cm, which is similar to the IOM's definition of short height, which is 157 cm 2 , 38 . Therefore, on the basis of these findings, the weight gain index for pregnant Japanese women with GDM should be based on the MHLW classification.
The present study had the following strengths. First, data from a Japanese national study that included 13,680 pregnant women with a low pre‐pregnancy BMI (<18.5 kg/m2) can be valuable for other populations worldwide. The 15 regional Centers in the JECS birth cohort provide uniform care standards for obstetric patients based on Japan's standard obstetric practice guidelines established in 2008 11 and the Japanese Clinical Practice Guideline for Diabetes 8 . Second, the present study used both the MHLW and IOM classification systems. Because similar results were obtained using both classification systems, the findings relative to GDM in this study should be reliable.
However, the present study had several limitations as well. First, the appropriate GWG for the overweight/obese group of the MHLW classification, approximately 5 kg, was applied to the IOM recommended range of 4.5 kg for overweight women. As the overweight/obese group accounted for two‐thirds of the overweight women in this study, the 4.5 kg range was preferred to the 4 kg range for the obese group in the IOM recommendation. No significant change was observed in the results when the range was set at 4 kg. However, the definition of this range is temporary and illogical, so it is not universal. Second, “overt diabetes 39 ” might have been counted as GDM in the JECS. Its prevalence in Japan is not yet known, but it is expected to be low and should not affect these results. Third, the present study did not include data for 75‐g oral glucose tolerance test PG levels; therefore, assessing perinatal outcomes related to PG levels is difficult. Fourth, this study has limited generalizability beyond Japanese women; however, the present findings might be useful in studying other groups with statures similar to those of Japanese women. Fifth, perinatal outcomes were studied only among term women with GDM. Because insufficient GWG among women with GDM increased the incidence of preterm birth by 3.5‐fold compared with sufficient GWG 40 , the impact of insufficient GWG on pre‐pregnancy underweight women with GDM and preterm birth needs to be determined. Finally, as the prevalence of GDM is lower than previously reported, the results need to be interpreted in the light of the possibility that participants in JECS are a more environmentally and health‐conscious population, and should be investigated further.
LGA and HDP were strongly associated with pre‐pregnancy BMI and GWG in the pre‐pregnancy normal weight and overweight/obese women, and the relationship was further strengthened by GDM. In GDM patients with a pre‐pregnancy BMI of ≥25.0 kg/m2, the incidence rates of SGA and LGA infants relied on GWG. This result was similar regardless of whether the MHLW or IOM classification system was used.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The JECS protocol was approved by the institutional review board of the Japan National Institute for Environmental Studies (registration number: 2017‐002) and by the Ethics Committees of all participating institutions. This study was also approved by the Ethics Committee of the Hokkaido University Center for Environmental Health Sciences (Registration number: 21‐130; Approval date of registry: August 26, 2021).
Informed consent: The JECS was carried out in accordance with the Declaration of Helsinki, and other internationally valid regulations and guidelines for research on human subjects, and with written informed consent from all participants.
Registry and the registration no. of the study/trial: Not applicable because JECS is not a clinical trial.
Animal studies: Not applicable.
Supporting information
Table S1 | Methods for diagnosing gestational diabetes mellitus in Japan.
Table S2 | Association between gestational diabetes mellitus and perinatal outcomes stratified by pre‐pregnancy body mass index using the Institute of Medicine classification.
Table S3 | Association between gestational diabetes mellitus and perinatal outcomes stratified by gestational weight gain using the Institute of Medicine classification.
Table S4 | Association between gestational diabetes mellitus and perinatal outcomes stratified by both pre‐pregnancy body mass index and gestational weight gain using the Institute of Medicine classification.
ACKNOWLEDGMENTS
This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the government. We also thank all the families who participated in this research and all the staff who cooperated.
Appendix 1.
JAPAN ENVIRONMENT AND CHILDREN'S STUDY (JECS) GROUP
Members of the JECS Group as of 2021: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Youichi Kurozawa (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan) and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
J Diabetes Investig.2022; 13: 889–899
Contributor Information
Sumitaka Kobayashi, Email: sukobayashi@cehs.hokudai.ac.jp.
the Japan Environment and Children's Study (JECS) group:
Michihiro Kamijima, Shin Yamazaki, Yukihiro Ohya, Reiko Kishi, Nobuo Yaegashi, Koichi Hashimoto, Chisato Mori, Shuichi Ito, Zentaro Yamagata, Hidekuni Inadera, Takeo Nakayama, Hiroyasu Iso, Masayuki Shima, Youichi Kurozawa, Narufumi Suganuma, Koichi Kusuhara, and Takahiko Katoh
REFERENCES
- 1. Yogev C, Hod C, Oats M, et al. Hyperglycemia and adverse pregnancy outcome (HAPO) study: preeclampsia. Am J Obstet Gynecol 2008; 202: e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rasmussen K, Yaktine AL. Weight Gain During Pregnancy: Reexamining The Guidelines. Washington, DC: National Academies Press, 2009. [PubMed] [Google Scholar]
- 3. Damm P, Houshmand‐Oeregaard A, Kelstrup L, et al. Gestational diabetes mellitus and long‐term consequences for mother and offspring: a view from Denmark. Diabetologia 2016; 59: 1396–1399. [DOI] [PubMed] [Google Scholar]
- 4. Japanese Ministry of Health, Labour and Welfare National nutrition survey in Japan. 2011.
- 5. Takemoto YO, Ota E, Yoneoka D, et al. Japanese secular trends in birthweight and the prevalence of low birthweight infants during the last three decades: a population‐based study. Sci Rep 2016; 6: 31396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kubota K, Itoh H, Tasaka M, et al. Changes of maternal dietary intake, bodyweight and fetal growth throughout pregnancy in pregnant Japanese women. J Obstet Gynaecol Res 2013; 39: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 7. Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within‐family comparison. Lancet 2010; 376: 984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. Diabetol Int 2018; 9: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig 2020; 11: 1020–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viecceli C, Remonti LR, Hirakata VN, et al. Weight gain adequacy and pregnancy outcomes in gestational diabetes: a meta‐analysis. Obes Rev 2017; 18: 567–580. [DOI] [PubMed] [Google Scholar]
- 11. Minakami H, Hiramatsu Y, Koresawa M, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2011 edition. J Obstet Gynaecol Res 2011; 37: 1174–1197. [DOI] [PubMed] [Google Scholar]
- 12. The Ministry of Health Labour and Welfare, Japan . Promotion Committee of the “Healthy Parents and Children 21”. 2006. http://rhino.med.yamanashi.ac.jp/sukoyaka/ninpu_syoku.html. (Japanese)
- 13. Kawamoto T, Nitta H, Murata K, et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014; 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michikawa T, Nitta H, Nakayama SF, et al. Baseline Profile of Participants in the Japan Environment and Children's Study (JECS). J Epidemiol 2018; 28: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Itabashi K, Fujimura M, Kusuda S, et al. Introduction of new standard value of physique at birth according to gestational age. J Japan Pediatr Soc 2010; 114: 1271–1293. (Japanese). [Google Scholar]
- 17. Watanabe K, Naruse K, Tanaka K, et al. Outline of definition and classification of “pregnancy induced hypertension (PIH)”. Hypertension Res Preg 2013; 1: 3–4. [Google Scholar]
- 18. Langer O, Levy J, Brustman L, et al. Glycemic control in gestational diabetes mellitus–how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol 1989; 161: 646–653. [DOI] [PubMed] [Google Scholar]
- 19. Silva ALD, Amaral ARD, Oliveira DSD, et al. Neonatal outcomes according to different therapies for gestational diabetes mellitus. J Pediatr 2017; 93: 87–93. [DOI] [PubMed] [Google Scholar]
- 20. Bonomo M, Cetin I, Pisoni MP, et al. Flexible treatment of gestational diabetes modulated on ultrasound evaluation of intrauterine growth: a controlled randomized clinical trial. Diabetes Metab 2004; 30: 237–244. [DOI] [PubMed] [Google Scholar]
- 21. Enomoto K, Aoki S, Toma R, et al. Pregnancy outcomes based on pre‐pregnancy body mass index in Japanese women. PLoS One 2016; 11: e0157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varvarigou AA. Intrauterine growth restriction as a potential risk factor for disease onset in adulthood. J Pediatr Endocrinol Metab 2010; 23: 215–224. [DOI] [PubMed] [Google Scholar]
- 23. Hong Y, Chung S. Small for gestational age and obesity related comorbidities. Ann Pediatr Endocrinol Metab 2017; 23: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yasuda S, Iuchi T, Goto A, et al. Weight control before and during pregnancy for patients with gestational diabetes mellitus. J Diabetes Investig 2019; 10: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macrì F, Pitocco D, di Pasquo E, et al. Gestational weight gain as an independent risk factor for adverse pregnancy outcomes in women with gestational diabetes. Eur Rev Med Pharmacol Sci 2018; 22: 4403–4410. [DOI] [PubMed] [Google Scholar]
- 26. Morikawa M, Yamada T, Yamada T, et al. Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Res Clin Pract 2010; 90: 339–342. [DOI] [PubMed] [Google Scholar]
- 27. Morikawa M, Yamada T, Yamada T, et al. Prevalence of hyperglycemia during pregnancy according to maternal age and pre‐pregnancy body mass index in Japan, 2007–2009. Int J Gynaecol Obstet 2012; 118: 198–201. [DOI] [PubMed] [Google Scholar]
- 28. Iwama N, Sugiyama T, Metoki H, et al. Difference in the prevalence of gestational diabetes mellitus according to gestational age at 75‐g oral glucose tolerance test in Japan: The Japan Assessment of Gestational Diabetes Mellitus Screening trial. J Diabetes Investig 2019; 10: 1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morikawa M, Sugiyama T, Sagawa N, et al. Perinatal mortality in Japanese women diagnosed with gestational diabetes mellitus and diabetes mellitus. J Obstet Gynaecol Res 2017; 43: 1700–1707. [DOI] [PubMed] [Google Scholar]
- 30. Ogawa K, Morisaki N, Piedvache A, et al. Association between birth weight and risk of pregnancy‐induced hypertension and gestational diabetes in Japanese women: JPHC‐NEXT study. J Epidemiol 2021; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Behboudi‐Gandevani S, Amiri M, Bidhendi Yarandi R, et al. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta‐analysis. Diabetol Metab Syndr 2019; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta‐analysis. BMC Pregnancy Childbirth 2018; 18: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakanishi S, Aoki S, Kasai J, et al. Have pregnancy outcomes improved with the introduction of the International Association of Diabetes and Pregnancy Study Groups criteria in Japan? J Diabetes Investig 2020; 11: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Eng J Med 2005; 352: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 35. Schaefer‐Graf UM, Kleinwechter H. Diagnosis and new approaches in the therapy of gestational diabetes mellitus. Curr Diabet Rev 2006; 2: 343–352. [DOI] [PubMed] [Google Scholar]
- 36. Yamashita H, Yasuhi I, Kugishima Y, et al. Factors associated with patients with gestational diabetes in Japan being at increased risk of requiring intensive care. Int J Gynecol Obstet 2018; 140: 170–174. [DOI] [PubMed] [Google Scholar]
- 37. Yasuhi I, Yamashita H, Maeda K, et al. High‐intensity breastfeeding improves insulin sensitivity during early postpartum period in obese women with gestational diabetes. Diab Metab Res Rev 2019; 35: e3127. [DOI] [PubMed] [Google Scholar]
- 38. Institute of Medicine, Subcommittee on Nutritional Status and Weight Gain during Pregnancy . Nutrition During Pregnancy. Washington DC: National Academy Press, 1990. [Google Scholar]
- 39. Minakami H, Maeda T, Fujii T, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res 2014; 40: 1469‐1499. [DOI] [PubMed] [Google Scholar]
- 40. Gou B‐H, Guan H‐M, Bi Y‐X, et al. Gestational diabetes: weight gain during pregnancy and its relationship to pregnancy outcomes. Chin Med J 2019; 132: 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Methods for diagnosing gestational diabetes mellitus in Japan.
Table S2 | Association between gestational diabetes mellitus and perinatal outcomes stratified by pre‐pregnancy body mass index using the Institute of Medicine classification.
Table S3 | Association between gestational diabetes mellitus and perinatal outcomes stratified by gestational weight gain using the Institute of Medicine classification.
Table S4 | Association between gestational diabetes mellitus and perinatal outcomes stratified by both pre‐pregnancy body mass index and gestational weight gain using the Institute of Medicine classification.


