Abstract
Introduction
Many clinical studies have identified significant predictors or risk factors for the severity or mortality of coronavirus disease 2019 (COVID‐19) cases. However, there are very limited reports on the risk factors for requiring oxygen therapy during hospitalization. In particular, we sought to investigate whether plasma glucose and HbA1c levels could be risk factors for oxygen therapy requirement.
Materials and Methods
A single‐center, retrospective study was conducted of 131 COVID‐19 patients hospitalized at Saitama Medical University Hospital between March 2020 and November 2020. To identify the risk factors for oxygen therapy requirement during hospitalization, a stepwise multivariate binary logistic regression analysis was performed using several clinical parameters commonly obtained on admission, including plasma glucose and HbA1c levels.
Results
Of the 131 patients with COVID‐19, 33.6% (44/131) received oxygen therapy during hospitalization. According to the logistic regression analysis, male sex (odds ratio [OR]: 8.76, 95% confidence interval [CI]: 1.65–46.5, P < 0.05), age (OR: 1.07, 95% CI: 1.02–1.12, P < 0.01), HbA1c levels (OR: 1.94, 95% CI: 1.09–3.44, P < 0.05), and serum C‐reactive protein (CRP) levels (OR: 2.22, 95% CI: 1.54–3.20, P < 0.01) emerged as independent variables associated with oxygen therapy requirement during hospitalization.
Conclusions
In addition to male sex, age, and serum CRP levels, HbA1c levels on admission may serve as a risk factor for oxygen therapy requirement during the clinical course of COVID‐19, irrespective of diabetes history and status. This may contribute to the efficient delegation of limited numbers of hospital beds to patients at risk for oxygen therapy requirement.
Keywords: coronavirus disease 2019, glycated hemoglobin, oxygen therapy, risk factor
To identify the risk factors for oxygen therapy requirement during the clinical course of coronavirus disease 2019 (COVID‐19), 131 patients diagnosed with COVID‐19 were identified and a multivariate binary logistic regression analysis was carried out using several clinical parameters commonly obtained on admission, including plasma glucose and glycated hemoglobin (HbA1c) levels. As a result, in addition to male sex, age, and serum C‐reactive protein (CRP) levels, it was successfully revealed that HbA1c levels may serve as a risk factor for oxygen therapy requirement, irrespective of diabetes history and status. Thus, we propose that evaluating the risk of hypoxia early in the disease process using the four aforementioned clinical parameters might contribute to efficient delegation of limited hospital beds to patients at risk for oxygen therapy requirement.

INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is now widespread globally. Although Japan has had significantly fewer COVID‐19 cases compared with Western countries, COVID‐19 patients have created a serious burden on the medical system throughout the past year, especially during the emergency period. Because the number of hospital beds dedicated to COVID‐19 patients is limited, it is very important to make efficient use of available beds for patients in need of hospitalization therapy.
Many clinical studies and meta‐analyses have identified significant predictors or risk factors for the severity or mortality of COVID‐19, including age, male sex, obesity, white blood cell (WBC) count, and serum levels of C‐reactive protein (CRP) and D‐dimer 1 , 2 , 3 , 4 . In addition, diabetes‐related parameters, including fasting plasma glucose and glycated hemoglobin (HbA1c) levels, and comorbidities including hypertension, respiratory diseases, chronic heart disease, liver disease, cardiovascular disease, and stroke, are also helpful in predicting the severity and mortality of COVID‐19 4 , 5 , 6 , 7 , 8 . However, these parameters are absolutely associated with the severity of COVID‐19, that is, the risk of ICU admission or death as the study outcome. Thus, they are unhelpful in predicting the need for hospitalization for COVID‐19, irrespective of the severity and mortality of the disease.
Therefore, the present study focused on identifying the risk factors for requiring oxygen therapy, a treatment that usually requires hospitalization, during the course of COVID‐19 treatment. We reasoned that identifying the risk factors early in the disease process, especially using simple clinical parameters (age, sex, and body mass index [BMI]) and common laboratory data collected on admission, could help in the efficient delegation of available beds by preferentially assigning them to patients at risk for oxygen therapy requirement, providing the best care to these patients with minimal burden on the medical system. Simultaneously, patients with mild COVID‐19, who are not expected to require oxygen therapy, could be treated in isolation at home or in hotels. However, given the fact that there have been some fatal cases due to COVID‐19 aggravation during recuperation at home or hotels in individuals who were initially diagnosed with mild COVID‐19, care should be taken to identify patients requiring early hospitalization who are at high risk for COVID‐19.
To the best of our knowledge, there are very limited reports regarding the risk factors of oxygen therapy requirement in the COVID‐19 disease process 9 , 10 , 11 . Among these reports, no studies have employed multivariate logistic regression analysis using plasma glucose and HbA1c levels measured on admission as independent variables. HbA1c is usually measured only in patients suspected of having diabetes mellitus and is not routinely measured in every patient, possibly leading to a lack of HbA1c data on admission. Given that diabetes mellitus is reportedly associated with increased severity and mortality in patients with COVID‐19‐associated pneumonia 5 , 12 , 13 , 14 , 15 , we felt a deep need to clarify the association between these diabetes‐related parameters and oxygen therapy requirement. Thus, this study aimed to determine the clinical risk factors for oxygen therapy requirement during hospitalization in patients with COVID‐19, with an emphasis on clinical data collected on admission through routine laboratory tests, including plasma glucose and HbA1c levels.
MATERIALS AND METHODS
Research design and patients
This was a single‐center retrospective study design. We retrospectively identified 176 consecutive patients hospitalized for COVID‐19 treatment at Saitama Medical University Hospital, Saitama Prefecture, Japan, between March 2020 and November 2020. COVID‐19 was diagnosed during hospitalization based on the positive identification of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in nasopharyngeal swab specimens tested by real‐time polymerase chain reaction (RT‐PCR) assay. We obtained patient information and demographic characteristics of these patients, including sex, age, BMI, and laboratory data including WBC count, lymphocyte count, hemoglobin (Hb), platelet count, ad‐lib plasma glucose levels, HbA1c, D‐dimer, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), albumin, CRP, and creatine phosphokinase (CPK), and estimated glomerular filtration rate (eGFR) from medical records. In addition, information on the presence or absence of abnormalities on chest computed tomography (CT) findings was obtained from the medical records on admission. If the chest CT scan was not performed, information on the presence or absence of abnormalities on chest x‐ray findings was alternatively obtained on admission. All blood samples were collected on admission and analyzed using standard methods in the hospital laboratory. Additionally, we obtained information on the presence/absence of diabetes mellitus or hypertension as a comorbidity of interest. Hypertension and diabetes mellitus were defined according to the presence of antihypertensive and antidiabetic agents at baseline, respectively, or according to medical records. Furthermore, patients without a history of diabetes mellitus with HbA1c ≥ 6.5% in combination with fasting plasma glucose level ≥126 mg/dL and/or ad‐lib plasma glucose ≥200 mg/dL on admission were also defined as having diabetes mellitus. Of these patients, those aged <18 years (n = 4), those receiving dialysis (n = 6), and those with missing data (n = 35) were excluded. Thus, we identified and analyzed 131 patients with COVID‐19 (Figure 1).
Figure 1.
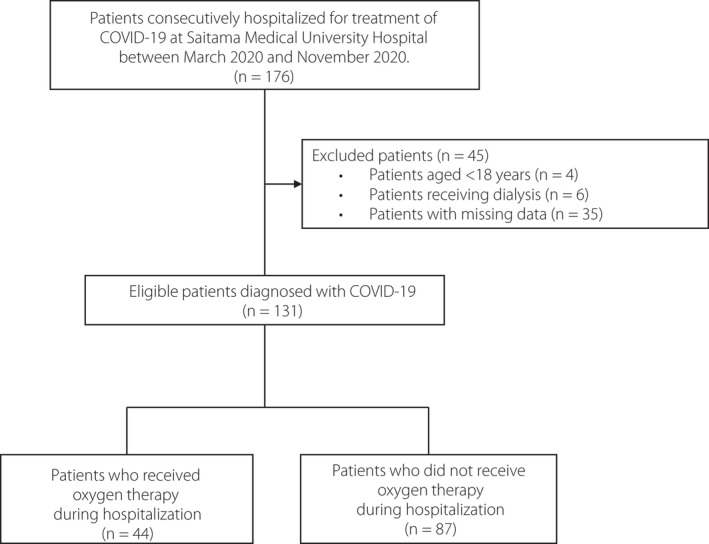
Patient characteristics. COVID‐19, coronavirus disease 2019.
Indication for starting oxygen therapy
Based on recommendations from the Surviving Sepsis Campaign guidelines for COVID‐19 16 , we initiated oxygen therapy for any COVID‐19 patient with a percutaneous oxygen saturation (SpO2) level below 92%, even if they showed no physical signs of low oxygen levels.
Statistical analysis
We confirmed that all continuous variables, excluding Hb, were not normally distributed using the Shapiro–Wilk test. Thus, all continuous variables are presented as medians (minimum–maximum). Comparisons of median values and proportions between the two groups were performed using the Mann–Whitney U‐test and chi‐square test, respectively. Univariate logistic regression analysis was performed with the presence/absence of oxygen therapy as a dependent variable and the variables listed in Table 1 as independent variables. Subsequently, stepwise multivariate binary logistic regression analysis was performed with the presence/absence of oxygen therapy as a dependent variable and the selected independent variables with a P‐value of <0.25 in the univariate logistic regression analysis. The cut‐off P‐value of 0.25 is supported by the literature 17 , 18 . Data are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). However, the presence/absence of diabetes mellitus was excluded from the multivariate logistic regression analysis to clarify the influence of plasma glucose and HbA1C levels on the requirement for oxygen therapy irrespective of diabetes history and status. Receiver operating characteristic (ROC) curve analysis was used to evaluate the accuracy of the obtained multivariate binary logistic regression model, using the predicted probability from the logistic regression. In addition, the optimal cut‐off value of HbA1c level associated with requiring oxygen therapy was determined by the highest Youden index (sensitivity + specificity – 1) in ROC curve analysis 19 . An ROC curve is a plot of the true positive rate (sensitivity) on the y‐axis against the false‐positive rate (1−specificity) on the x‐axis for all possible cut‐off values 20 . Each point on the ROC curve represents a different cut‐off value, and the ROC curve can be constructed by connecting all cut‐off points. Statistical significance was defined as P < 0.05. Statistical analyses were performed using BellCurve for Excel version 3.21 (Social Survey Research Information Co., Ltd, Tokyo, Japan) and SPSS (version 23.0; SPSS Inc., Chicago, IL, USA).
Table 1.
Baseline clinical characteristics of the patients with COVID‐19
|
All patients (n = 131) |
Patients who received oxygen therapy (n = 44) | Patients who did not receive oxygen therapy (n = 87) |
P‐value (Patients who received vs who did not receive oxygen therapy) |
|
|---|---|---|---|---|
| Demographic characteristics | ||||
|
Male/Female (Male, %) |
76/55 (58.0) |
32/12 (72.7) |
44/43 (50.6) |
<0.05* |
| Age (years) | 51 (18–95) | 67 (27–95) | 34 (18–83) | <0.01** |
| BMI (kg/m2) | 22.6 (15.6–40.7) | 23.15 (16.2–35.6) | 22.1 (15.6–40.7) | N.S.** |
| Laboratory data | ||||
| White blood cell (×103/μL) | 5.07 (2.14–16.75) | 5.68 (2.14–16.75) | 4.98 (2.25–11.35) | <0.05** |
| Lymphocyte (×103/μL) | 1.28 (2.01–9.13) | 0.91 (2.01–9.13) | 1.46 (3.00–3.77) | <0.01** |
| Hemoglobin (g/dL) | 14.3 (9.5–19.5) | 13.75 (10.5–17.3) | 14.5 (9.5–19.5) | N.S.** |
| Platelet (×103/μL) | 199 (68–510) | 159.5 (77–510) | 209 (68–374) | <0.01** |
| D‐dimer (μg/mL) | 0.67 (0.50–58.8) | 1.28 (0.51–58.82) | 0.59 (0.50–2.83) | <0.01** |
| Plasma glucose (mg/dL) | 108 (73–396) | 129.5 (80–390) | 101 (73–396) | <0.01** |
| HbA1c (%) | 5.6 (3.5–11.3) | 6.15 (4.9–10.9) | 5.40 (3.50–11.3) | <0.01** |
| AST (U/L) | 26 (10–177) | 33.5 (10–132) | 23 (11–177) | <0.01** |
| ALT (U/L) | 22 (6–169) | 28.5 (9–169) | 19 (6–121) | <0.01** |
| LDH (U/L) | 220 (118–764) | 301.5 (171–764) | 203 (118–448) | <0.01** |
| Albumin (g/dL) | 4.1 (2.0–5.1) | 3.3 (2.0–4.4) | 4.3 (2.9–5.1) | <0.01** |
| eGFR (mL/min/1.73 m2) | 85.4 (31.4–194.8) | 70.8 (31.4–158.9) | 89.5 (47.2–194.8) | <0.01** |
| CPK (U/L) | 79 (9–3,182) | 105 (9–3,182) | 73 (19–2,659) | <0.05** |
| CRP (mg/dL) | 0.59 (0.10–21.79) | 4.915 (0.10–21.79) | 0.21 (0.10–5.26) | <0.01** |
| Chest imaging | ||||
|
Abnormal findings (yes/no) (Abnormalities, %) |
82/49 (62.6) |
40/4 (90.9) |
42/45 (48.3) |
<0.01* |
| Comorbidity | ||||
|
Hypertension (yes/no) (Hypertension, %) |
31/100 (23.7) |
19/25 (43.2) |
12/75 (13.8) |
<0.01* |
|
Diabetes (yes/no) (Diabetes, %) |
24/107 (18.3) |
20/24 (45.5) |
4/83 (4.6) |
<0.01* |
Data of continuous variables are presented as the median (minimum–maximum). *Chi‐square test. **Mann–Whitney U‐test. AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; COVID‐19, coronavirus disease 2019; CPK, creatine phosphokinase; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; LDH, lactate dehydrogenase.
RESULTS
Characteristics of hospitalized patients with COVID‐19
The baseline clinical characteristics of the patients on admission are presented in Table 1. A total of 76 men and 55 women, aged 51 (18–95) years, were included in the study. Regarding comorbidity, 23.7% (31/131) and 18.3% (24/131) of the patients were identified as having hypertension and diabetes mellitus, respectively. We identified 12 patients who were newly diagnosed with type 2 diabetes. All cases of diabetes mellitus were classified as type 2. Of the 131 patients, 33.6% (44/131) received oxygen therapy due to hypoxia associated with COVID‐19‐related lung involvement during hospitalization. Among them, nine patients subsequently received mechanical ventilation, and three patients ultimately died.
When the patients were divided into two groups according to the presence or absence of oxygen therapy during hospitalization (n = 44 and 87, respectively), the proportion of males was significantly higher in the group that received oxygen therapy than in the group that did not receive oxygen therapy (72.7% vs 50.6%, P < 0.05). In addition, the median age was significantly higher in the group that received oxygen therapy than in those who did not (67 vs 34 years, P < 0.01). There was no significant difference in BMI between the two groups. All laboratory data examined, excluding Hb levels, were significantly different between the groups that received and did not receive oxygen therapy. The proportion of abnormalities in the chest imaging findings (patients who underwent chest CT scan, n = 128; patients who underwent chest radiography but not chest CT scan, n = 3) was significantly higher in the group that received oxygen therapy than in the group that did not (90.9% vs 48.3%, P < 0.01). With regard to comorbidity, the proportions of hypertension and diabetes mellitus (type 2 diabetes) were significantly higher in the group that received oxygen therapy than in those who did not (Table 1). As for the timing of initiation of oxygen therapy, patients who received oxygen therapy (n = 44) consisted of 31 individuals who initiated oxygen therapy on the day of admission, e.g., hospital day 0, and 13 individuals who initiated oxygen therapy 1 day or more following admission, e.g., hospital day ≥1. The median (minimum–maximum) hospital day on which oxygen therapy was initiated was day 4 (days 1–9).
Relationship between oxygen therapy requirement and baseline clinical characteristics in patients with COVID‐19
We evaluated the association between oxygen therapy requirement and baseline clinical parameters using univariate logistic regression analysis (Table 2). Consequently, we identified all independent variables, excluding BMI, Hb, platelet count, and CPK levels, as potential predictors of oxygen therapy requirement (P < 0.05). Subsequently, all independent variables (P < 0.25) in the univariate logistic regression analyses (i.e., male sex, age, WBC count, lymphocyte count, Hb, D‐dimer, plasma glucose, HbA1c, AST, ALT, LDH, albumin, eGFR, CPK, CRP, presence or absence of abnormal chest imaging findings, and presence or absence of hypertension), excluding the presence or absence of diabetes mellitus, were included in the stepwise multivariate binary logistic regression analysis. Finally, male sex (OR: 8.76, 95% CI: 1.65–46.5, P < 0.05), age (OR: 1.07, 95% CI: 1.02–1.12, P < 0.01), HbA1c levels (OR: 1.94, 95% CI: 1.09–3.44, P < 0.05), and serum CRP levels (OR: 2.22; 95% CI: 1.54–3.20, P < 0.01) emerged as independent variables associated with oxygen therapy requirement during hospitalization (Table 2). The percentage of correctly predicted values for oxygen therapy requirement based on the full model of multivariate binary logistic regression using the four selected significant predictors was 91.6%. Moreover, the ROC analysis based on the predicted probability obtained from the multivariate logistic regression for oxygen therapy requirement demonstrated that the area under the ROC curve (AUC) was 0.962 (95% CI: 0.927–0.997, P < 0.001) (Figure 2). In addition, the optimal cut‐off value of HbA1c level for oxygen therapy requirement was 5.9% (sensitivity, 0.682; specificity, 0.885) (Figure 3).
Table 2.
Results of binary logistic regression analyses of potential risk factor for oxygen therapy in patients with COVID‐19
| Univariate logistic regression | Multivariate logistic regression† | ||||||
|---|---|---|---|---|---|---|---|
| Wald χ2 | P | OR (95% CI) | Wald χ2 | P | OR (95% CI) | ||
| Male (vs female) | 5.714 | 0.017 | 2.606 (1.188–5.716) | 6.506 | 0.011 | 8.764 (1.653–46.464) | |
| Age | 31.797 | <0.001 | 1.084 (1.054–1.115) | 8.400 | 0.004 | 1.071 (1.022–1.122) | |
| BMI | 0.373 | 0.542 | 1.026 (0.944–1.115) | – | – | – | |
| White blood cell | 11.297 | <0.001 | 1.305 (1.117–1.525) | ||||
| Lymphocyte | 9.112 | 0.003 | 0.999 (0.998–1.000) | ||||
| Hemoglobin | 2.706 | 0.100 | 0.851 (0.702–1.031) | ||||
| Platelet | 1.162 | 0.281 | 0.997 (0.993–1.002) | – | – | – | |
| D‐dimer | 16.431 | <0.001 | 5.876 (2.496–13.833) | ||||
| Plasma glucose | 12.136 | <0.001 | 1.024 (1.010–1.037) | ||||
| HbA1c | 19.575 | <0.001 | 4.178 (2.218–7.872) | 5.132 | 0.024 | 1.939 (1.093–3.438) | |
| AST | 10.111 | 0.002 | 1.036 (1.014–1.058) | ||||
| ALT | 8.664 | 0.003 | 1.027 (1.009–1.045) | ||||
| LDH | 25.797 | <0.001 | 1.017 (1.010–1.024) | ||||
| Albumin | 32.215 | <0.001 | 0.030 (0.009–0.101) | ||||
| eGFR | 9.334 | 0.002 | 0.973 (0.956–0.990) | ||||
| CPK | 2.090 | 0.148 | 1.001 (1.000–1.002) | ||||
| CRP | 28.437 | <0.001 | 2.798 (1.917–4.084) | 18.280 | <0.001 | 2.219 (1.540–3.197) | |
| Abnormal chest imaging findings | 17.520 | <0.001 | 10.714 (3.529–32.528) | ||||
| Hypertension | 12.825 | <0.001 | 4.750 (2.025–11.144) | ||||
| Diabetes mellitus | 22.967 | <0.001 | 17.292 (5.390–55.473) | – | – | – | |
Factors with P < 0.25 on univariate logistic regression analysis excluding diabetes mellitus were included in the multivariate logistic regression analysis. AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; CI, confidence interval; COVID‐19, coronavirus disease 2019; CPK, creatine phosphokinase; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; LDH, lactate dehydrogenase; OR, odds ratio.
Figure 2.
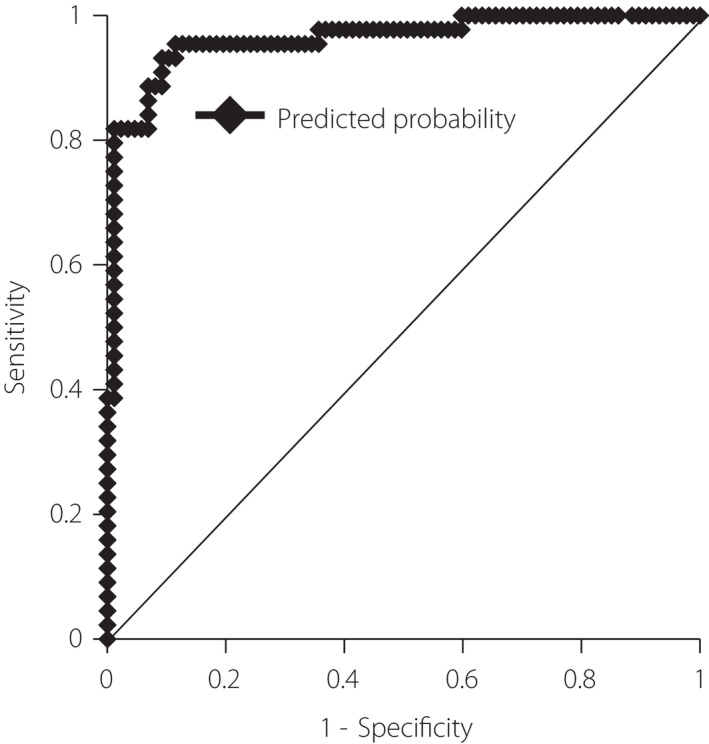
The receiver operating characteristic (ROC) curve for the multivariate binary logistic regression model. The ROC curve was used to assess the accuracy of the obtained multivariate binary logistic regression model to predict the requirement for oxygen therapy. The area under the ROC curve (AUC) was 0.962 (95% CI: 0.927–0.997, P < 0.001). AUC, area under the receiver operating characteristic curve; ROC, receiver operating characteristic.
Figure 3.

The receiver operating characteristic (ROC) curve of HbA1c for predicting oxygen therapy requirement. The optimal cut‐off value of HbA1c level for oxygen therapy requirement was 5.9% (AUC 0.850, 95% CI: 0.781–0.921, P < 0.001, sensitivity 0.682, specificity 0.885). AUC, area under the receiver operating characteristic curve; ROC, receiver operating characteristic.
Meanwhile, when the presence/absence of diabetes mellitus was included as an independent factor in the logistic regression analysis, male sex (OR: 6.388, 95% CI: 1.234–33.068, P = 0.027), age (OR, 1.074; 95% CI, 1.025–1.127; P = 0.003), serum CRP levels (OR, 2.196; 95% CI, 1.507–3.201; P < 0.001), and diabetes mellitus status (OR, 14.474; 95% CI, 2.218–94.468; P = 0.005), but not plasma glucose and HbA1c levels, emerged as risk factors for oxygen therapy requirement during hospitalization.
DISCUSSION
To the best of our knowledge, only a few studies have focused on the risk factors of oxygen therapy requirement during the clinical course of COVID‐19. Lee et al. 11 demonstrated that CRP levels, hypertension, age, neutrophils, and lymphocytes are useful predictors of oxygen therapy requirement. Kitajima et al. 10 demonstrated that age, hemodialysis, and CRP levels are candidate predictors of the need for oxygen supply. Noh et al. 9 revealed that current smoking status, higher heart rate, AST levels, blood urea nitrogen levels, and chest radiographic findings were associated with oxygen therapy requirements. However, no previous studies have focused on the association between oxygen therapy requirement and plasma glucose/HbA1c levels on admission.
The present study successfully revealed that, in addition to male sex, greater age, and higher serum CRP levels, higher HbA1c levels might be a risk factor for oxygen therapy requirement during hospitalization, irrespective of diabetes history or status. These findings suggest that impaired glucose tolerance or hyperglycemia prior to hospitalization for COVID‐19 might be associated with the development of COVID‐19‐related respiratory disorders requiring oxygen therapy shortly afterward.
In the present study, in order to clarify the association between glycemic control and oxygen therapy requirement, we did not include the presence/absence of diabetes mellitus as an independent factor in the logistic regression analysis, because the clinical information does not accurately reflect the state of glycemic control on admission. In fact, when the presence/absence of diabetes mellitus was included as an independent factor in the logistic regression analysis, diabetes mellitus status, but not plasma glucose and HbA1c levels, emerged as a significant variable associated with oxygen therapy requirement during hospitalization. However, this analysis revealed an extremely wide 95% CI (2.218–94.468) for diabetes mellitus, suggesting the possible uncertainty of the result due to the small sample size. Moreover, it is often difficult for physicians to know whether a medical interview regarding diabetes mellitus is accurate, especially in new patients. In addition, it is likely that grouping diabetics with well‐controlled glycemia with those with poorly controlled glycemia might prevent an accurate analysis. Thus, we believe that it is reasonable to exclude the presence/absence of diabetes mellitus from the logistic regression analysis and instead focus on plasma glucose and HbA1c levels as diabetes‐related clinical parameters.
The reason why HbA1c levels on admission could be a risk factor for requiring oxygen therapy remains to be determined in future studies. A possible reason for this phenomenon is that hyperglycemia can compromise innate immune responses to infection 21 , 22 , contributing to the development of COVID‐19‐related lung involvement and leading to oxygen therapy requirements. Additionally, it was recently reported that glycosylation of angiotensin‐converting enzyme 2 (ACE2), which is a receptor for SARS‐CoV‐2 and expressed in lung epithelial cells 23 , facilitates viral binding to the receptors, enabling viral entry and leading to inflammatory processes in the lungs 22 , 24 . For these reasons, the HbA1c level reflecting chronic hyperglycemia might be associated with the requirement of oxygen therapy early in the clinical course of COVID‐19.
HbA1c levels are reportedly affected by certain conditions, such as chronic anemia, chronic kidney disease (CKD) with eGFR <30 mL/min/1.73 m2, and erythropoietin administration, which can lead to falsely low HbA1c results 25 , potentially influencing the outcome of the present study. In the present study, dialysis patients, who might usually and periodically receive erythropoietin, were excluded from the analysis, and patients with eGFR <30 mL/min/1.73 m2 were not originally included (Figure 1 and Table 1). Thus, the effect of CKD or erythropoietin administration on HbA1c levels was not considered in the present study. Meanwhile, a portion of our subjects had mild anemia with a minimum Hb level of 9.5 g/dL (Table 1). However, given that Hb level was not selected as an independent variable associated with oxygen therapy requirement in the multivariate logistic regression analysis, mild anemia with Hb level >9.5 g/dL might not influence the relationship between HbA1c level and oxygen therapy requirement observed in the present study. In contrast, clinical conditions, including severe anemia and severe CKD requiring erythropoietin, may affect the outcome.
Male sex has been identified as a risk factor for severity or mortality in patients with COVID‐19 26 , 27 . The present study confirmed that male sex might also be a risk factor for requiring oxygen therapy. Some potential confounding factors linked to male sex, such as history of smoking and cardiovascular disease, might underlie this association, although the details remain to be elucidated in future studies. Additionally, as reviewed elsewhere 28 , male patients are more likely to have a higher expression of ACE2 than female patients, contributing to the development and aggravation of COVID‐19‐related lung involvement requiring oxygen therapy.
Several studies have demonstrated that a higher fasting plasma glucose level on admission for COVID‐19 is an independent predictor of severity and mortality in patients with COVID‐19 4 , 29 , 30 . In addition, a higher HbA1c level is reportedly associated with a risk of COVID‐19‐related mortality 5 , 7 . In general, plasma glucose level on admission is interpreted as a biomarker of systemic inflammation at the time, whereas the HbA1c level reflects chronic hyperglycemia for the past 3 months 31 . Considering that increased HbA1c levels might be associated with dysregulated immune cell function 32 , 33 , fasting plasma glucose and HbA1c levels already increased on admission may reflect the aggravated inflammation of COVID‐19 and the vulnerability to the inflammation, possibly leading to an increased risk of severity and mortality in patients with COVID‐19. On the other hand, tight glycemic control using intravenous insulin infusion was shown to reduce disease severity and mortality in patients with COVID‐19 and hyperglycemia 34 , 35 . These findings suggest that poor glycemic control on admission might lead to the severity of COVID‐19‐related pneumonia, and that early recognition and treatment of hyperglycemia may greatly benefit these patients.
In this study, nine patients eventually received mechanical ventilation due to exacerbation of COVID‐19‐related pneumonia; since the number of these patients was very small 36 , multivariate logistic analysis of risk factors for severity of COVID‐19 could not be performed. Furthermore, at our institution, all patients who initiate oxygen therapy simultaneously begin intravenous steroid administration. Because steroid therapy might influence the clinical course of COVID‐19‐related pulmonary involvement, we believe it might be difficult to clarify the association between glycemic control on admission and severity of COVID‐19‐related pneumonia after oxygen therapy in the present study.
In the present study, we did not conduct a logistic regression analysis using the SpO2 levels on admission, which is a simple clinical parameter. As described above, 70.5% (31/44) of patients who received oxygen therapy during hospitalization required its initiation on the day of admission. Moreover, 96.8% (30/31) of the patients required initiation of oxygen therapy within 4 h of admission. Considering that an SpO2 level below 92% was used as the basis for initiation of oxygen therapy in the present study, we believe that SpO2 level on admission should be treated as a factor determining the need for oxygen therapy rather than a risk factor for oxygen therapy for the 31 patients and that the logistic regression analysis using SpO2 levels might be unsuitable for identifying risk factors associated with oxygen therapy. Therefore, to evaluate the influence of SpO2 level on oxygen therapy as a risk factor, a similar analysis using more patients that required initiation of oxygen therapy sometime after admission, e.g., after the next day of admission, should be conducted. Because we could include only 13 patients requiring initiation of oxygen therapy after the next day of admission, the influence of SpO2 level on oxygen therapy could not be evaluated through the logistic regression analysis because of the small sample size.
This study has some limitations. First, this was a single‐hospital study with a relatively small sample size, which prevented accurate analysis. Thus, it may be difficult to generalize our findings. Second, due to the retrospective nature of the study design, the causal correlation between the selected clinical parameters and the requirement for oxygen therapy remains unknown. To clarify this, a longitudinal prospective study is required. Furthermore, the present study included a limited number of patients who initiated oxygen therapy the day after admission, which prevented an accurate logistic analysis on whether laboratory data obtained on admission could serve as useful predictive markers for future oxygen therapy after admission or not 36 . Third, we could not obtain certain information, including smoking history and the presence/absence of respiratory and cardiovascular diseases, which were previously reported as predictors for severity of COVID‐19 5 and may have some influence on the findings in the present study. Finally, our findings may be affected by differences in SARS‐CoV‐2 strains or SARS‐CoV‐2 vaccination status.
In conclusion, in addition to male sex, age, and serum CRP levels, HbA1c levels on admission may be a risk factor for the need for oxygen therapy during the clinical course of COVID‐19, irrespective of diabetes history and status. Evaluating the risk of hypoxia early in the disease process by using the four simple clinical parameters indicated in this study may contribute to the efficient delegation of limited numbers of hospital beds to patients at risk for oxygen therapy requirement.
DISCLOSURE
A. Sh. received lecture fees from Novo Nordisk Pharma Ltd and Sanofi KK, and scholarship grants from Novo Nordisk Pharma Ltd and Ono Pharmaceutical Co., Ltd. The other authors declare that they have no conflicts of interest.
This study was conducted in accordance with the Declaration of Helsinki.
Approval of the research protocol: This research protocol was approved by the Institutional Review Board of Saitama Medical University Hospital (No. 20143.02, dated June 7, 2021).
Informed consent: This study was conducted without informed consent, but patients were given the opportunity to actively opt out of participation.
Approval date of registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
J Diabetes Investig. 2022; 13: 909–917
References
- 1. Mudatsir M, Fajar JK, Wulandari L, et al. Predictors of COVID‐19 severity: a systematic review and meta‐analysis. F1000Research 2020; 9: 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panda S, Nanda R, Tripathy PK, et al. Immuno‐inflammatory predictors of disease severity in COVID‐19: a systematic review and meta‐analysis. J Family Med Prim Care 2021; 10: 1102–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao R, Su Z, Komissarov AA, et al. Associations of D‐Dimer on admission and clinical features of COVID‐19 patients: a systematic review, meta‐analysis, and meta‐regression. Front Immunol 2021; 12: 691249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang S, Ma P, Zhang S, et al. Fasting blood glucose at admission is an independent predictor for 28‐day mortality in patients with COVID‐19 without previous diagnosis of diabetes: a multi‐centre retrospective study. Diabetologia 2020; 63: 2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature 2020; 584: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Unluguzel Ustun G, Keskin A, Aci R, et al. Association between Hb A1c and severity of COVID‐19 patients. Hemoglobin 2021; 45: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prattichizzo F, de Candia P, Nicolucci A, et al. Elevated HbA1c levels in pre‐Covid‐19 infection increases the risk of mortality: a systematic review and meta‐analysis. Diabetes Metab Res Rev 2021: e3476. 10.1002/dmrr.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsushita K, Ding N, Kou M, et al. The relationship of COVID‐19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta‐analysis. Glob Heart 2020; 15: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noh CS, Kim WY, Baek MS. Risk factors associated with the need for oxygen therapy in patients with COVID‐19. Medicine (Baltimore) 2021; 100: e25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitajima H, Hirashima T, Suzuki H, et al. Scoring system for identifying Japanese patients with COVID‐19 at risk of requiring oxygen supply: a retrospective single‐center study. J Infect Chemother 2021; 27: 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee EE, Hwang W, Song KH, et al. Predication of oxygen requirement in COVID‐19 patients using dynamic change of inflammatory markers: CRP, hypertension, age, neutrophil and lymphocyte (CHANeL). Sci Rep 2021; 11: 13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. Lancet Diabetes Endocrinol 2020; 8: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia – A systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr 2020; 14: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dennis JM, Mateen BA, Sonabend R, et al. Type 2 diabetes and COVID‐19‐related mortality in the critical care setting: a national Cohort Study in England, March‐July 2020. Diabetes Care 2021; 44: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGurnaghan SJ, Weir A, Bishop J, et al. Risks of and risk factors for COVID‐19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol 2021; 9: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alhazzani W, Moller MH, Arabi YM, et al. Surviving sepsis Campaign: guidelines on the management of critically Ill adults with Coronavirus Disease 2019 (COVID‐19). Crit Care Med 2020; 48: e440–e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grant SW, Hickey GL, Head SJ. Statistical primer: multivariable regression considerations and pitfalls. Eur J Cardiothorac Surg 2019; 55: 179–185. [DOI] [PubMed] [Google Scholar]
- 18. Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Community Health 2020; 8: e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez‐Camblor P, Pardo‐Fernandez JC. The Youden Index in the generalized receiver operating characteristic curve context. Int J Biostat 2019; 15. 10.1515/ijb-2018-0060 [DOI] [PubMed] [Google Scholar]
- 20. Zou KH, O'Malley AJ, Mauri L. Receiver‐operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007; 115: 654–657. [DOI] [PubMed] [Google Scholar]
- 21. Jafar N, Edriss H, Nugent K. The effect of short‐term hyperglycemia on the innate immune system. Am J Med Sci 2016; 351: 201–211. [DOI] [PubMed] [Google Scholar]
- 22. Liao YH, Zheng JQ, Zheng CM, et al. Novel molecular evidence related to COVID‐19 in patients with diabetes mellitus. J Clin Med 2020; 9: 3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu J, Xu X, Jiang L, et al. SARS‐CoV‐2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res 2020; 21: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehdipour AR, Hummer G. Dual nature of human ACE2 glycosylation in binding to SARS‐CoV‐2 spike. Proc Natl Acad Sci USA 2021; 118: e2100425118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu NA, Reichert S, Harris SB. Limitations of hemoglobin A1c in the management of type 2 diabetes mellitus. Can Fam Physician 2020; 66: 112–114. [PMC free article] [PubMed] [Google Scholar]
- 26. Biswas M, Rahaman S, Biswas TK, et al. Association of sex, age, and comorbidities with mortality in COVID‐19 patients: a systematic review and meta‐analysis. Intervirology 2021; 64: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis 2021; 73: e4208–e4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. La Vignera S, Cannarella R, Condorelli RA, et al. Sex‐specific SARS‐CoV‐2 mortality: among hormone‐modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D. Int J Mol Sci 2020; 21: 2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lazarus G, Audrey J, Wangsaputra VK, et al. High admission blood glucose independently predicts poor prognosis in COVID‐19 patients: a systematic review and dose‐response meta‐analysis. Diabetes Res Clin Pract 2021; 171: 108561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee MH, Wong C, Ng CH, et al. Effects of hyperglycaemia on complications of COVID‐19: a meta‐analysis of observational studies. Diabetes Obes Metab 2021; 23: 287–289. [DOI] [PubMed] [Google Scholar]
- 31. Klein SJ, Mayerhofer T, Fries D, et al. Elevated HbA1c remains a predominant finding in severe COVID‐19 and may be associated with increased mortality in patients requiring mechanical ventilation. Crit Care 2021; 25: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kulcsar KA, Coleman CM, Beck SE, et al. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS‐CoV infection. JCI Insight 2019; 4: e131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lecube A, Pachon G, Petriz J, et al. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6: e23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sardu C, D'Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID‐19: can we do more on glycemic control? Diabetes Care 2020; 43: 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Satomura A, Oikawa Y, Nakanishi S, et al. Clinical features resembling subcutaneous insulin resistance observed in a patient with type 2 diabetes and severe COVID‐19‐associated pneumonia: a case report. Diabetol Int 2021; 12: 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379. [DOI] [PubMed] [Google Scholar]


