ABSTRACT
Although diabetic peripheral neuropathy is the most common diabetic microangiopathic complication, several other neuropathy syndromes can occur in the context of diabetes. We describe a rare case of polyneuropathy associated with diabetic ketoacidosis in a patient with new‐onset type 1 diabetes. A 42‐year‐old man with diabetic ketoacidosis was admitted to our hospital with complications of respiratory and renal failure requiring mechanical ventilation and hemodialysis, respectively. After diabetic ketoacidosis improved from the critical state, he developed upper‐ and lower‐limb paralysis with sensory disturbances and pain, as well as right facial paralysis, left recurrent nerve paralysis, and left hypoglossal nerve paralysis. Autonomic nerve function was also impaired. As the pathophysiology, prevention, and treatment of polyneuropathy associated with diabetic ketoacidosis are unclear, the neurologic function of patients with diabetic ketoacidosis should be closely monitored.
Keywords: Diabetic ketoacidosis, Polyneuropathy, Type 1 diabetes
A 42‐year‐old man with diabetic ketoacidosis was admitted to our hospital with complications of respiratory and renal failure requiring mechanical ventilation and hemodialysis, respectively. After diabetic ketoacidosis improved from the critical state, he developed upper‐ and lower‐limb paralysis with sensory disturbances and pain, as well as right facial paralysis, left recurrent nerve paralysis, and left hypoglossal nerve paralysis, autonomic neuropathy. As the pathophysiology, prevention, and treatment of polyneuropathy associated with diabetic ketoacidosis are unclear, the neurologic function of patients with diabetic ketoacidosis should be closely monitored.
INTRODUCTION
The prevalence of diabetes has increased worldwide, and 425 million adults had diabetes in 2017 1 . Diabetes frequently affects the peripheral nervous system and is currently the most common cause of neuropathy. Up to 50% of patients with diabetes will develop peripheral neuropathy 2 .
Typical diabetic peripheral neuropathy (DPN) is a chronic, symmetrical, nerve length‐dependent sensorimotor polyneuropathy. Although DPN is the most common diabetic microangiopathic complication 3 , several other neuropathy syndromes can occur in the context of diabetes. Acute neurologic complications of diabetic ketoacidosis (DKA) are very rare 4 . Here, we report a case of polyneuropathy that developed during treatment of diabetic ketoacidosis in a patient with new‐onset type 1 diabetes.
CASE REPORT
A 42‐year‐old man was brought to our hospital in a comatose state. He had no medical history of diabetes or episodes of neurologic deficits, but he had lost 10 kg in weight over the previous year. Table 1 summarizes his medical history and physical findings. Laboratory examinations revealed hyperglycemia, high HbA1c level, metabolic acidosis, and positive urinary ketone bodies (Table 1). Based on these findings, he was diagnosed with diabetic ketoacidosis and subsequently was diagnosed with type 1 diabetes. Although the hyperglycemia and metabolic acidosis steadily improved by intravenous insulin therapy, his respiratory and renal function worsened on hospital day (HD) 2. The patient was intubated and kept on mechanical ventilation; hemodialysis was initiated but discontinued on HD 4.
Table 1.
Patient characteristics and laboratory data on admission
| [Present symptoms] | [Urine testing] | [Blood chemistry] | [Immune‐related] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Height | 175 cm | pH | 5.0 | TP | 6.4 g/dL | ANA | <40 | ||
| Weight | 61.4 kg | Glucose | 4+ | Alb | 3.7 g/dL | MPO‐ANCA | <0.5 U/mL | ||
| BMI | 20 kg/m2 | Protein | 1+ | T‐bil | 0.5 mg/dL | PR3‐ANCA | <0.5 U/mL | ||
| Consciousness (GCS) | E4V3M5 | Ketone | 3+ | AST | 21 U/L | ||||
| Body temperature | Unrecordable | Occult blood | 1+ | ALT | 17 U/L | [Anti‐ganglioside antibodies] | |||
| Blood pressure | 65/44 mmHg | ALP | 142 U/L | IgM | IgG | ||||
| Pulse rate | 83 beats/min | [Complete blood count] | γ‐GTP | 48 U/L | GM1 | − | − | ||
| Respiratory rate | 19 breaths/min | WBC | 22.3 × 103/μL | LDH | 206 U/L | GM2 | − | − | |
| Skin | Dry | RBC | 461 × 104/μL | CK | 788 U/L | GM3 | − | − | |
| Mouth | Dry | Hb | 15.0 g/dL | AMY | 84 U/L | GD1a | − | − | |
| Thyroid | No goiter | Ht | 45.9% | BUN | 52 mg/dL | GD1b | − | − | |
| Heart sounds | No murmur | Plt | 34.5 × 104/μL | Cr | 2.4 mg/dL | GD3 | − | − | |
| Respiration | Kussmaul's breathing | eGFR | 25.5 mL/min/1.73 m2 | GT1b | − | − | |||
| Respiration sounds | Clear to auscultation bilaterally, no rales | [Arterial blood gas analysis (O2 1 L/min)] | HDL‐C | 57 mg/dL | GQ1b | − | − | ||
| Abdomen | Soft and flat, no tenderness | pH | 6.854 | LDL‐C | 115 mg/dL | Gal‐C | − | − | |
| Bowel sounds | Normal | PaO2 | 201 mmHg | TG | 195 mg/dL | GalNAc‐GD1a | + | − | |
| Extremities | No edema | PaCO2 | 13.1 mmHg | UA | 9.2 mg/dL | GD1a/GD1b | − | − | |
| Perspiration |
|
2.2 mmol/L | Na | 124 mEq/L | |||||
| BE | −34.3 mmol/L | K | 4.5 mEq/L | [Cerebrospinal fluid analysis] | (HD 27) | ||||
| [Medical history] | No special findings | Cl | 96 mEq/L | Color | Colorless | ||||
| [Life history] | No smoking, no drinking, no allergies | [Diabetes‐related] | Ca | 8.4 mg/dL | Turbidity | Clear | |||
| [Family history] | Father and grandmother: type 2 diabetes | Plasma glucose | 1188 mg/dL | Mg | 2.9 mg/dL | Cell | 1/μL | ||
| HbA1c | 14.5% | P | 3.2 mg/dL | Neutrophils | 0% | ||||
| [Chest x‐ray] | CTR 42%, CP‐A sharp/sharp | IRI | 2.9 μU/mL | CRP | 5.5 mg/dL | Lymphocytes | 0% | ||
| [Electrocardiogram] | 83 bpm, sinus rhythm | CPR | 1.8 ng/mL | Endotoxin | <0.8 pg/mL | Monocytes | 100% | ||
| QT/QTc interval: 448/488 ms, J‐wave | CPR (HD 31) | 0.5 ng/mL | Protein | 132 mg/dL | |||||
| CVR‐R 1.63/4.06% | 24‐h urine CPR | 6.7 μg/day | [HLA haplotype] | Glucose | 45 mg/dL | ||||
| [Echocardiography] | EF:72.1%, wall motion good | Anti‐GAD antibody | 2,000 U/mL | DRB1*04:05‐DQB1*04:01 | Peripheral blood glucose | 110 mg/dL | |||
| [Funduscopic findings] | No retinopathy | DRB1*15:02‐DQB1*06:01 |
γ‐GTP, γ‐glutamyl transpeptidase; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMY, amylase; ANA, antinuclear antibody; AST, aspartate aminotransferase; BE, base excess; BMI, body mass index; BUN, blood urea nitrogen; Ca, calcium; CK, creatine kinase; Cl, chloride; CPR, C‐peptide immunoreactivity; CP‐A, cardio‐phrenic angle; Cr, creatinine; CRP, C‐reactive protein; CTR, cardiothoracic ratio; CVR‐R, coefficient of variation of R‐R interval; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GAD, glutamic acid decarboxylase; GCS, Glasgow coma scale; Hb, hemoglobin; HbA1c, hemoglobin A1c; , bicarbonate; HD, hospital day; HDL‐C, high‐density lipoprotein cholesterol; HLA, human leukocyte antigen; Ht, hematocrit; IRI, immunoreactive insulin; K, potassium; LDH, lactate dehydrogenase; LDL‐C, low‐density lipoprotein cholesterol; Mg, magnesium; MPO‐ANCA, myeloperoxidase‐anti‐neutrophil cytoplasmic antibodies; Na, sodium; P, phosphorus; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; pH, power of hydrogen; Plt, platelets; PR3‐ANCA, proteinase‐3‐anti‐neutrophil cytoplasmic antibodies; RBC, red blood cells; T‐bil, total bilirubin; QTc, corrected QT interval; TG, triglyceride; TP, total protein; UA, uric acid; WBC, white blood cells.
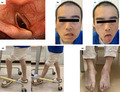
After extubation on HD 5, the patient reported hoarseness and paralysis of the upper and lower limbs. Sensory disturbances and pain were also observed in the upper extremities with ulnar predominance distal to the forearm and in the lower extremities distal to the lateral side below the knee. On HD 12, right facial paralysis was diagnosed, and tongue deviation to the left was observed (Figures 1 and S1, Video S1). Idiopathic facial nerve palsy was suspected and treatment with prednisolone and valacyclovir was started. Manual muscle testing (MMT) was conducted on HD 21. In the upper extremities, mild weakness was observed in the extensor digitorum muscle. The lower‐limb bilateral tibialis anterior muscles exhibited severe weakness (MMT level 0‐1), and the gastrocnemius exhibited mild weakness (MMT level 3‐4). Proximal muscle weakness was unremarkable (Table S1). No specific findings were observed on brain magnetic resonance imaging.
Figure 1.
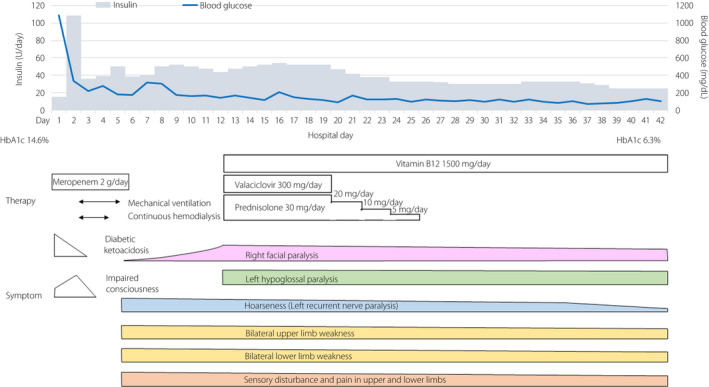
Clinical course and treatment of the patient in the present case. HbA1c, hemoglobin A1c.
Tachycardia persisted even after the hyperglycemia improved, the R‐R interval coefficient of variation decreased, and Schellong testing was positive, suggesting autonomic neuropathy. Nerve conduction studies (NCS) showed markedly decreased compound muscle action potentials (CMAPs) in the median and ulnar nerves but only a slight decrease in motor nerve conduction velocity, which was considered axonal damage. Sensory nerve action potentials and CMAPs of the lower extremities could not be evoked (Table 2, Figure S2). Albuminocytologic dissociation was evident in the cerebrospinal fluid on HD 27 (Table 1). Except for the recurrent nerve paralysis, no significant improvement in paralysis occurred, so he was transferred to a rehabilitation hospital on HD 42. Six months later, the right facial nerve and left hypoglossal palsy had improved. The upper‐ and lower‐limb paralysis also improved, but the paralysis in the ulnar and peroneal nerve regions continued.
Table 2.
Nerve conduction study (on hospital day 21)
| Motor | |||||
|---|---|---|---|---|---|
| Site | DL (ms) | CMAP (mV) | MCV (m/s) | F‐latency (ms) | FWCV (m/s) |
| Distal/proximal | |||||
| Right median | 4.2 | 0.82/0.83 | 41.2 | 39.6 | 53.6 |
| Right ulnar | 3.1 | 2.2/0.82 | 41.7 | 33.9 | 52.2 |
| Right tibial | NE | ||||
| Right peroneal | NE | ||||
| Sensory | ||
|---|---|---|
| Site | SNAP (μV) | SCV (m/s) |
| Right median | NE | |
| Right ulnar | NE | |
| Right sural | NE |
CMAP, compound muscle action potential; DL, distal latency; FWCV, F‐wave conduction velocity; MCV, motor nerve conduction velocity; NE, not evoked; SCV, sensory nerve conduction velocity; SNAP, sensory nerve action potential.
DISCUSSION
We report a rare case of polyneuropathy with a variety of symptoms that developed in a 42‐year‐old man with acute‐onset type 1 diabetes. To clarify the clinical features of polyneuropathy associated with diabetic ketoacidosis, we searched the literature for articles regarding DKA‐related motor‐dominant polyneuropathy and found 45 cases (Table S2). There were 11 cases diagnosed as Guillain‐Barré syndrome (GBS), 10 cases diagnosed as mononeuropathy (including multiple mononeuropathies) and 5 cases diagnosed as critical illness polyneuropathy (CIP) among the cases who developed paralysis associated with diabetic ketoacidosis. These results suggest that this rare polyneuropathy may simply be a combination of diabetic ketoacidosis and Guillain‐Barré syndrome, or it may be diabetic polyneuropathy or CIP caused by diabetic ketoacidosis.
First, we discuss the combination of diabetic ketoacidosis and Guillain‐Barré syndrome. This case fulfilled Asbury's diagnostic criteria for Guillain‐Barré syndrome, ‘features necessary for diagnosis’, and fulfilled most of the ‘features that strongly support the diagnosis’ 5 . Nerve conduction studies showed predominantly reduced amplitude in both motor and sensory nerves, consistent with acute motor‐ and sensory‐axonal neuropathy (AMSAN). Only anti‐GalNAc‐GD1a IgM anti‐ganglioside antibody was positive, with a low titer. These results suggest that Guillain‐Barré syndrome of the AMSAN type can be diagnosed, and the combination of diabetic ketoacidosis and Guillain‐Barré syndrome is one possible explanation for the polyneuropathy in this case.
Second, if neuropathy is considered to be secondary to diabetic ketoacidosis, DKA‐associated polyneuropathy is characterized by motor‐dominant polyneuropathy involving lower motor neurons and cranial nerves 4 , which can be considered as a differential diagnosis. Recently, Hamada et al. also reported severe sensory‐motor axonal neuropathy of the lower extremities associated with diabetic ketoacidosis 6 . They concluded that the neuropathy was triggered by rapid correction of hyperglycemia, and that both metabolic factors and immunological mechanisms were involved in the pathogenesis of the neuropathy. However, the clinical picture of DKA‐associated polyneuropathy remains ambiguous. The paucity of reports on DKA‐associated polyneuropathy and the lack of clear diagnostic criteria hinder making a definitive diagnosis, but DKA‐associated polyneuropathy should not be overlooked as a possible cause of the neuropathy in the present case. Accumulation of cases of polyneuropathy with diabetic ketoacidosis is awaited not only to establish polyneuropathy with diabetic ketoacidosis as a distinct disease entity, but also to establish diagnostic criteria.
Third, critical illness polyneuropathy should also be considered as a differential diagnosis. Critical illness polyneuropathy is a distal axonal sensory‐motor polyneuropathy affecting limb and respiratory muscles. This case fulfilled some of Bolton’s diagnostic criteria for CIP, which include critical illness with multiorgan dysfunction and axonal motor‐ and sensory‐polyneuropathy on electrophysiological examination 7 . However, contrary to CIP diagnostic criteria, the patient was easily weaned from the ventilator, had facial paralysis, severe autonomic neuropathy, and albuminocytologic dissociation. Thus, the possibility of CIP is low.
Finally, nerves susceptible to compression or cumulative trauma, including the median, fibular, and plantar nerves, are frequently injured in patients with diabetes 8 . As there was no evidence of compression and/or trauma in our patient, this was also unlikely the cause of polyneuropathy in the upper and lower limbs. However, the possibility of Tapia syndrome 9 associated with tracheal intubation could not be ruled out for Xth and XIIth cranial nerve palsy.
In summary, we present a rare case of polyneuropathy that developed in a 42‐year‐old man with acute‐onset type 1 diabetes after achieving steady control of his blood glucose levels. The pathophysiology of the complicated polyneuropathy remains unknown, but Guillain‐Barré syndrome or polyneuropathy associated with diabetic ketoacidosis, Tapia syndrome, or a combination thereof were considered. Patients with diabetic ketoacidosis thus require careful monitoring of neurologic function.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: Informed consent was obtained from the patient.
Approval date of registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Figure S1 | Photograps of paralyses.
Figure S2 | Results of motor nerve conduction study and deep tendon and pathological reflex tests.
Table S1 | Manual muscle test (on hospital day 21)
Table S2 | Clinical characteristics of the present case and previously reported cases of neuropathy associated with diabetic ketoacidosis
Video S1 | Video of paralyses.
ACKNOWLEDGMENTS
We thank Prof. Susumu Kusunoki (Department of Neurology, Kinki University Medical School) for conducting the analysis of anti‐ganglioside antibodies.
J Diabetes Investig. 2022; 13: 918–922
REFERENCES
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018; 138: 271–281. [DOI] [PubMed] [Google Scholar]
- 2. Feldman EL, Callaghan BC, Pop‐Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers 2019; 5: 41. [DOI] [PubMed] [Google Scholar]
- 3. Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sinnreich M, Taylor BV, Dyck PJ, et al. Diabetic neuropathies. classification, clinical features, and pathophysiological basis. Neurologist 2005; 11: 63–79. [DOI] [PubMed] [Google Scholar]
- 5. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barré syndrome. Ann Neurol 1990; 27: S21–24. [DOI] [PubMed] [Google Scholar]
- 6. Hamada Y, Takahashi K, Hokkoku K, et al. Severe sensory‐motor axonal neuropathy following diabetic ketoacidosis. Rinsho Shinkeigaku (Clin Neurol) 2020; 60: 614–619. (Japanese). [DOI] [PubMed] [Google Scholar]
- 7. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011; 10: 931–941. [DOI] [PubMed] [Google Scholar]
- 8. Albers JW, Pop‐Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep 2014; 14: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coninckx M, Cardoen S, Hamelsoet D, et al. Tapia's syndrome in the intensive care unit: a rare cause of combined cranial nerve palsy following intubation. Acta Neurol Belg 2015; 115: 533–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Photograps of paralyses.
Figure S2 | Results of motor nerve conduction study and deep tendon and pathological reflex tests.
Table S1 | Manual muscle test (on hospital day 21)
Table S2 | Clinical characteristics of the present case and previously reported cases of neuropathy associated with diabetic ketoacidosis
Video S1 | Video of paralyses.


