Abstract
Aims/Introduction
Abdominal obesity is a risk factor for developing diabetes mellitus, but trajectories of abdominal obesity over time and incident diabetes mellitus have not been considered. We derived trajectories of abdominal volume index (AVI) over 16 years of follow up, and examined the associations between AVI trajectories and risk of diabetes mellitus.
Materials and Methods
Data were used from the China Health and Nutrition Survey, and 5,267 participants were enrolled to fit the trajectory of AVI by using latent class growth models. Multivariate logistic regression models explored the relationship between different AVI trajectories and risk of diabetes mellitus. In addition, we examined the slope of the AVI trajectories in relation to age to identify appropriate life course intervention opportunities for the prevention of diabetes mellitus.
Results
Three trajectories were derived reflecting graded categories in the speed and slope of increase in AVI over time: slow, intermediate and fast increase group, respectively. After multivariate adjustment, the odds ratios for diabetes mellitus among those in the intermediate and fast increase groups were 1.81 (95% confidence interval 1.37–2.38, P < 0.001) and 2.80 (95% confidence interval 1.85–4.24, P < 0.001) respectively, relative to the slow increase group. The distribution of AVI slope in the slow increase group showed an inverted "U" shape, whereas the fast increase group presented a "U" shape.
Conclusions
AVI trajectory is associated with an increased risk of diabetes mellitus. These results provide new insights on the relationship between abdominal adiposity and diabetes mellitus, which in turn can help improve clinical and public health intervention for diabetes mellitus prevention.
Keywords: Abdominal fat, Diabetes mellitus, Public health practice
Based on a 16‐year large‐scale cohort study, we identified three types of abdominal obesity trajectories with different incidences of diabetes mellitus in adults. Our research provides a new perspective on the management of abdominal obesity for the prevention of diabetes.

INTRODUCTION
According to the International Diabetes Federation, China had the largest number of diagnosed and undiagnosed adult diabetes mellitus patients worldwide, reaching 116 million in 2019, and the number is expected to grow to 140 million by 2030 1 . One of the most pressing issues in public health is to identify people at high risk of developing diabetes mellitus and to take appropriate preventive measures, and in turn, reduce the diabetes mellitus burden in the population. Insulin resistance caused by obesity is closely related to the occurrence of diabetes mellitus 2 , 3 . Traditionally in research, body mass index (BMI) has been used to define obesity, although some recent studies have focused on abdominal obesity, a specific phenotype of ectopic fat distribution 4 , 5 , 6 . Mendelian randomization studies and population‐based cohort studies suggest that after adjusting for BMI, there is a causal relationship between abdominal obesity and diabetes mellitus 7 , 8 , 9 . Therefore, monitoring abdominal obesity in the population is critically important in better preventing and managing diabetes mellitus.
Many anthropometric measurements utilize height, waist and hip circumference to reflect abdominal obesity (also known as central obesity) 10 , 11 , 12 , 13 . Of these, the abdominal volume index (AVI), a reliable and easy to calculate anthropometric tool, has been found to be highly correlated with diabetes mellitus in many studies that involved different populations 11 , 14 , 15 , 16 , 17 . Compared with studies that relied on one static baseline measurement, prospective studies have shown that the dynamic change of anthropometric indicators over time have enhanced precision in predicting diseases 18 , 19 , 20 . Thus, examination of AVI trajectories that reflect the accumulation of fat and changes in metabolism over time might yield new insights into diabetes mellitus etiology. However, despite being more informative than traditional obesity measures, such as BMI, the trajectory of AVI over time in relation to the risk of diabetes mellitus has not been considered. As adiposity and body mass tend to change with age at varying rates over time, considering multiple trajectories of AVI is warranted.
In this regard, the current study derived AVI trajectories, and analyzed the relationship between different AVI trajectories and risk of diabetes mellitus among participants of the China Health and Nutrition Survey (CHNS), a large, prospective population‐based cohort of Chinese adults. The present study also examined associations between AVI trajectories and diabetes mellitus according to sex, and across early, middle and late adulthood. Our research will improve the understanding of the longitudinal relationship between abdominal obesity and diabetes mellitus.
MATERIALS AND METHODS
Study population
The CHNS, an ongoing longitudinal community‐based cohort study carried out by the national and local government, was designed to evaluate key indicators of health and the nutritional status of populations 21 . Using a multistage, random cluster sampling process, the project completed six surveys in nine provinces (including Jiangsu, Hubei, Hunan, Guangxi, Guizhou, Liaoning, Shandong and Henan) from 1993 to 2009 with >12,000 individuals. The data collected from these provinces vary in geography, economic development, public resources and health indicators, which can be used as a representative dataset for all provinces in China. Study protocols were approved by the Institutional Review Committees of the University of North Carolina at Chapel Hill, USA, and the National Institute for Nutrition and Health (NINH, former National Institute of Nutrition and Food Safety) at the Chinese Center for Disease Control and Prevention (CCDC) at Beijing, China. The study was carried out under the guiding principles of the Declaration of Helsinki (as revised in Fortaleza, Brazil in October 2013). Each participant assigned the informed consent, and the relevant protocol of the present study has been published elsewhere 22 .
This analysis included survey data from 1993 to 2009, as waist and hip circumference were not collected before the 1993 surveys. During the follow‐up period, participants underwent face‐to‐face surveys in 1997, 2000, 2004, 2006 and 2009 when waist and hip circumference were measured at each visit. In 2009, CHNS collected 9,549 fasting blood samples. Among them, we excluded individuals aged <18 years in 1993 (n = 2050) and >60 years in 1993 (n = 368), pregnant women (n = 13), diagnosed diabetes mellitus previously (n = 306), missing information in fasting blood glucose (FBG) or glycosylated hemoglobin (n = 71), and less than two measurements of waist and hip circumference (n = 1474). The analytic sample for the present study was n = 5267. The population screening process was shown in Figure S1.
Measures
Abdominal volume index
Waist circumference was measured with a tape measure positioned at the level of the umbilicus while the participants stood normally with their feet 25–30 cm apart and at the end of the exhalation. Hip circumference was measured at the widest part of the hip under the same conditions of waist circumference measurement. AVI was calculated using the waist and hip circumference, as directly assessed by study staff as 13 : [2 × (waist, cm)2 + 0.7 × (waist, cm − hip, cm)2] / 1,000.
Diabetes mellitus
According to the American Diabetes Association criteria, diabetes mellitus was defined as FBG ≥7.0 mmol/L and/or glycosylated hemoglobin ≥6.5% 23 . Homeostatic model assessment of insulin resistance was calculated by: FBG (mmol/L) × fasting insulin level (mIU/L) / 22.5.
Covariates
Participant sex, age, BMI, residence, education level, smoking status, alcohol consumption, hypertension, nutritional intake and blood‐based cardiometabolic indicators were controlled for in analysis. Height and weight were measured while the participants were wearing light clothing without shoes. Education level, health behavior (smoking and alcohol consumption) and history of hypertension were self‐reported. Smoking was defined as any previous smoking (yes/no). Alcohol consumption was defined as alcohol consumption greater than three times a week (yes/no). Nutrition intake was assessed through questionnaire that included three consecutive 24‐h diet recalls and one household food inventory, including the weighing and measuring of the product on the same 3 days (2 weekdays and 1 weekend day). By multiplying the intake of each food by the standard serving size (100 g), energy intake, carbohydrate intake, fat intake and protein intake were all calculated from the average dietary intake for 3 days 24 . Peripheral blood collection and blood sample processing were carried out by certified technicians at each study location, and quality control protocols were implemented to ensure high sample quality. Participants were asked to maintain a regular pattern of life, as well as emotional stability for at least 3 days and to fast for 8–12 h before blood sample collection. From these blood samples, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, total cholesterol, triglyceride, FBG and creatinine were measured by a Hitachi 7600 machine (Randox, Crumlin, UK and Kyowa, Tokyo, Japan). Glycosylated hemoglobin was measured by HLC‐723 G7, D10 and PDQ A1c (Tosoh, Tokyo, Japan; Bio‐Rad, Hercules, CA, USA; Primus, Kansas, MO, USA).
Statistical analysis
Descriptive statistics for study variables according to diabetes mellitus status collected at follow up in 2009 were calculated by t‐tests, the Mann–Whitney U‐test and χ2‐tests for continuous and dichotomous variables respectively. One‐way anova, Kruskal–Wallis H‐test and χ2‐tests for continuous and dichotomous variables were used to compare the study variables among different AVI trajectory classes. We explored the relationship between different AVI trajectories and diabetes mellitus through logistic regression; we estimated odds ratios (ORs) with 95% confidence intervals (CIs) for the associations, controlling for sex, age, BMI, residence, education level, smoking status, alcohol consumption, hypertension, nutritional intake and blood biochemical indicators.
To derive the AVI trajectories, a latent class growth model 25 (a group heterogeneity recognition tool) was used among participants who had three or more AVI measures available for analysis. Linear (primary growth curve) and non‐linear (quadratic growth curve) models were selected to show the latent class growth model with different trajectory shapes. Sex was adjusted in the growth modeling process. The model selection criterion was based on fit statistics, including the Bayesian information criterion, Vuong–Lo–Mendell–Rubin likelihood ratio test, efficiency of classification and posterior probabilities. After that, the estimated slope and variance of different trajectory classifications were obtained. Based on the predicted slope and age of each individual, we plotted their correlation to identify the distribution of the AVI slope with age to identify whether there was a particular rapid growth stage of AVI in adulthood. Io compare AVI trajectories with other traditional anthropometric indicators on the prediction of diabetes mellitus, receiver operating characteristic curves were carried out to calculate the area under the curves (AUCs) of the AVI slope (the basis for AVI trajectories classification), BMI and waist circumference. Furthermore, for clarifying the role of AVI trajectory in predicting diabetes mellitus independent of baseline AVI, we considered the impact of baseline AVI and further carried out a separate analysis with baseline AVI included in the multivariate adjustment models.
The proportion of missing data in the analytic sample was not more than 2%; missing data were interpolated using the method of last observation carried forward; if the last observation was unavailable, the mean value was used instead. Associations where P < 0.05 (two‐sided) were considered to be statistically significant. All of the analyses were carried out with Mplus 8 26 , Stata 15.0 (StataCorp, College Station, TX, USA) and R (version 3.6.1; The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Of the 4,786 people without diabetes mellitus at baseline, 481 patients (9.1%) developed diabetes mellitus during the 16‐year follow‐up period. The incidence of diabetes mellitus increases by baseline age, and is more common in men in young adulthood, whereas it is more common in women in late adulthood (Table S1). The geographic distribution of provinces where participants lived was roughly equivalent (Figure S2). Table 1 shows the characteristics of the population according to whether diabetes mellitus was diagnosed in 2009. Participants with diabetes mellitus had higher AVI, BMI, older age, and higher rates of hypertension and alcohol consumption than participants without diabetes mellitus.
Table 1.
Characteristics of study population in 2009
|
Non‐DM n = 4,786 |
DM n = 481 |
P‐value | |
|---|---|---|---|
| Age (years) | 53.1 ± 10.7 | 57.7 ± 11.0 | <0.001 |
| Male sex | 2,197 (45.9%) | 231 (48.0%) | 0.374 |
| Hip circumference (cm) | 94.4 ± 7.5 | 98.0 ± 8.3 | <0.001 |
| Waist circumference (cm) | 82.6 ± 9.8 | 89.0 ± 10.4 | <0.001 |
| AVI | 13.6 (11.6–16.0) | 15.9 (13.5–18.5) | <0.001 |
| BMI (kg/m2) † | 23.4 ± 3.3 | 25.2 ± 3.9 | <0.001 |
| Urban area † | 1,393 (29.1%) | 135 (28.1%) | 0.632 |
| Smoker | 1,541 (32.2%) | 146 (30.4%) | 0.409 |
| Alcohol consumption | 731 (15.3%) | 92 (19.1%) | 0.027 |
| Hypertension | 582 (12.2%) | 120 (24.9%) | <0.001 |
| FBG (mmol/L) | 5.1 ± 0.6 | 7.8 ± 2.6 | <0.001 |
| HbA1c (%) | 5.5 ± 0.4 | 7.1 ± 1.9 | <0.001 |
Data are expressed as the mean ± standard deviation, median (quartile 1– quartile 3) or n (%). AVI, abdominal volume index; BMI, body mass index; DM, diabetes mellitus; FBG: fasting blood glucose; HbA1c, glycosylated hemoglobin A1c.
The mean ± standard deviation or n (%) was calculated after filling in missing data.
According to the aforementioned model selection criteria, we sorted the AVI increasing change for each individual from slow to fast, compared the parameters of different class models (Table S2) and finally chose a three‐class model as the best fit. Class 1, class 2 and class 3, respectively, reflect those with slowly‐increasing AVI, those with intermediate‐increasing AVI and those with fast‐increasing AVI over time. Estimated and observed mean value of AVI were presented in Figure S3. The estimated parameters of each model, including intercept, slope and quadratic parameters, and the incidence of diabetes mellitus were listed in Table S3. The three latent variable parameters of the quadratic model were all positively correlated with the risk of diabetes mellitus. In other words, the increase in diabetes mellitus risk was related to the increase in intercept (initial AVI), slope and quadratic term. Figure 1 shows the longitudinal trajectory of the AVI of the participants aged 18–60 years, with slow increase (class 1, 49.6%, n = 2,614), intermediate increase (class 2, 40.1%, n = 2,114) and fast increase (class 3, 10.2%, n = 539), respectively. According to the mean value of AVI at baseline, class 3 had higher initial abdominal obesity compared with the other classes. Also, the slope of the curve for class 3 was the steepest of all classes, indicating that the changes of AVI with age in this group were the fastest. This pattern is the opposite of what was observed for class 1, which had the lowest baseline abdominal adiposity, and the slowest rate of increase of AVI. The pattern of class 2 was similar to class 1, but had a higher starting baseline adiposity. The change pattern of AVI in the young adulthood stage grew faster (positive slope) than in older adulthood, although later adulthood growth in class 1 and class 2 tended to be stable compared with class 3, which maintained a steeper and increasing slope with age. The trajectory of AVI with age by sex was also shown in Figure S4. The fast growth rate of class 3 was mainly contributed by women. The positive and significant associations between the linear slopes of AVI and diabetes mellitus grouped by age is shown in Table S4, showing that diabetes mellitus patients at all ages have higher AVI than those without diabetes mellitus. Next, we examined the association between age and the slope of AVI among the different classes (Figure 2). We found that in class 1 (slow increase), the maximum slope occurred at approximately 40 years‐of‐age, showing an inverted "U" shape. The maximum slope of class 2 was in late adulthood (intermediate increase), whereas the maximum slope of class 3 (fast increase) occurred in both young adulthood and late adulthood, showing a "U" shape.
Figure 1.
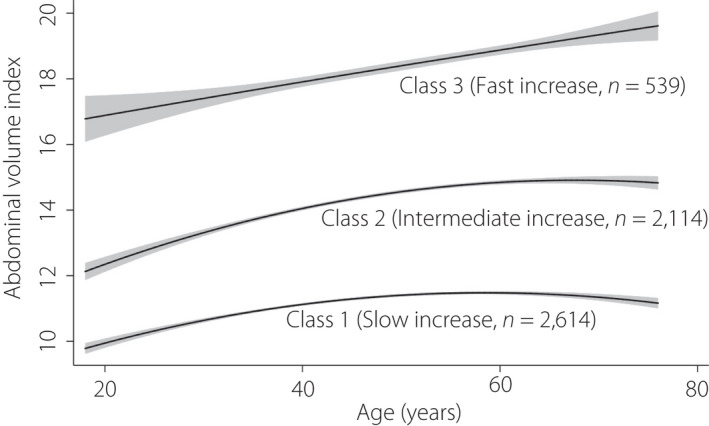
The predicted trajectory of the abdominal volume index with age presented by the solid line, and the 95% confidence interval is represented by shading.
Figure 2.
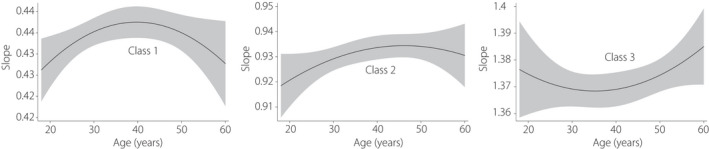
The association between age and the slope of the abdominal volume index among different classes, and the 95% confidence interval is represented by shading.
The characteristics of the research variables were summarized according to the three class model of AVI trajectories (Table 2). Compared with class 1, class 3 was mainly male, older, had a higher BMI, more energy intake, increased blood lipids and insulin resistance, and a higher proportion of smoking and alcohol consumption, taking high risk factors of diabetes mellitus. The incidence of diabetes mellitus was significantly different among the three AVI trajectory groups. Figure 3 shows the relationship between trajectory group and diabetes mellitus in logistic regression models for the total study population, and also separately for men and for women. Compared with class 1, the unadjusted ORs (aOR) of class 2 and class 3 were 2.73 (95% CI 2.18–3.43) and 6.12 (95% CI 4.66–8.05), respectively. After adjusting for baseline age, sex and BMI (model 1), the aOR of class 2 and class 3 were 1.96 (95% CI 1.50–2.56) and 3.12 (95% CI 2.09–4.66), respectively. Model 2 added the education level, smoking status and alcohol drinking, the aOR of class 2 and class 3 were 1.97 (95% CI 1.50–2.58) and 3.05 (95% CI 2.03–4.57), respectively. When we additionally considered energy intake (model 3), the aOR was 1.98 (95% CI 1.51–2.59) in class 2 and 3.09 (95% CI 2.06–4.64) in class 3. Model 4 included all covariates from prior models and the blood‐based cardiometabolic biomarkers, and the aOR was 1.81 (95% CI 1.37–2.38) in class 2 and 2.80 (95% CI 1.85–4.24) in class 3. Adjusted logistic regression results from the sex‐stratified models were similar, and also suggested that individuals in class 2 and class 3 had increased risk of diabetes mellitus compared with class 1. Odds ratios for women were slightly larger than for men in adjusted models.
Table 2.
Baseline characteristics of study population at follow up by different classes
| Characteristic |
Class 1 n = 2,614 |
Class 2 n = 2,114 |
Class 3 n = 539 |
P |
|---|---|---|---|---|
| Age (years) | 51.9 ± 11.0 | 54.7 ± 10.3 | 56.7 ± 10.7 | <0.001 |
| Male sex | 1,033 (39.5%) | 1,084 (51.3%) | 311 (57.7%) | <0.001 |
| Hip circumference (cm) | 90.3 ± 5.8 | 97.5 ± 5.9 | 105.2 ± 6.5 | <0.001 |
| Waist circumference (cm) | 76.4 ± 6.8 | 87.6 ± 6.4 | 99.0 ± 6.9 | <0.001 |
| AVI | 11.8 (10.5–13.2) | 15.5 (14.1–17.0) | 19.6 (18.1–21.6) | <0.001 |
| BMI (kg/m2) † | 21.5 ± 2.3 | 24.8 ± 2.5 | 28.5 ± 3.0 | <0.001 |
| Urban area † | 701 (26.8%) | 646 (30.6%) | 181 (33.6%) | <0.001 |
| Smoker | 742 (28.4%) | 757 (35.8%) | 188 (34.9%) | <0.001 |
| Alcohol consumption | 350 (13.4%) | 359 (17.0%) | 114 (21.2%) | <0.001 |
| Hypertension | 183 (7.0%) | 357 (16.9%) | 162 (30.1%) | <0.001 |
| Average energy intake (kcal/day) † | 2,084.1 (1,697.0–2,513.6) | 2,140.2 (1,746.4–2,587.2) | 2,141.0 (1,746.4–2,622.1) | 0.016 |
| Average carbohydrate intake (g/day) † | 289.2 (231.6–361.1) | 289.1 (229.8–359.0) | 288.5 (229.2–363.1) | 0.754 |
| Average fat intake (g/day) † | 67.6 (47.6–91.9) | 72.4 (51.2–98.3) | 72.4 (50.9–97.2) | <0.001 |
| Average protein intake (g/day) † | 61.7 (49.8–76.9) | 63.2 (50.4–79.7) | 65.3 (52.5–82.1) | <0.001 |
| Level of education † | 0.044 | |||
| Primary school or below | 725 (27.7%) | 579 (27.4%) | 148 (27.5%) | |
| Graduated from primary school | 598 (22.9%) | 463 (21.9%) | 121 (22.4%) | |
| Lower middle‐school degree | 827 (31.6%) | 652 (30.8%) | 153 (28.4%) | |
| Upper middle‐school degree | 281 (10.7%) | 219 (10.4%) | 57 (10.6%) | |
| Technical or vocational degree | 117 (4.5%) | 121 (5.7%) | 32 (5.9%) | |
| University or college degree | 66 (2.5%) | 80 (3.8%) | 28 (5.2%) | |
| FBG (mmol/L) | 5.1 ± 0.9 | 5.5 ± 1.4 | 6.0 ± 1.9 | <0.001 |
| HbA1c (%) | 5.5 ± 0.7 | 5.7 ± 0.8 | 6.1 ± 1.1 | <0.001 |
| HOMA‐IR † | 2.0 (1.3–2.9) | 2.6 (1.8–3.9) | 3.6 (2.3–5.9) | <0.001 |
| Serum creatinine (μmol/L) † | 86.6 ± 31.1 | 87.7 ± 16.4 | 89.3 ± 16.4 | 0.047 |
| HDL‐C (mmol/L) | 1.5 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.5 | <0.001 |
| LDL‐C (mmol/L) † | 2.9 ± 1.0 | 3.2 ± 1.0 | 3.2 ± 1.0 | <0.001 |
| Triglyceride (mmol/L) | 4.8 ± 0.9 | 5.1 ± 1.0 | 5.2 ± 1.0 | <0.001 |
| Total cholesterol (mmol/L) | 1.4 ± 1.1 | 1.9 ± 1.6 | 2.4 ± 1.9 | <0.001 |
Data are expressed as the mean ± standard deviation, median (quartile 1– quartile 3) or n (%). AVI, abdominal volume index; BMI, body mass index; DM, diabetes mellitus; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment of insulin resistance; LDL‐C, low‐density lipoprotein cholesterol.
The mean ± standard deviation, median (quartile 1– quartile 3) or n (%) was calculated after filling in missing data.
Figure 3.
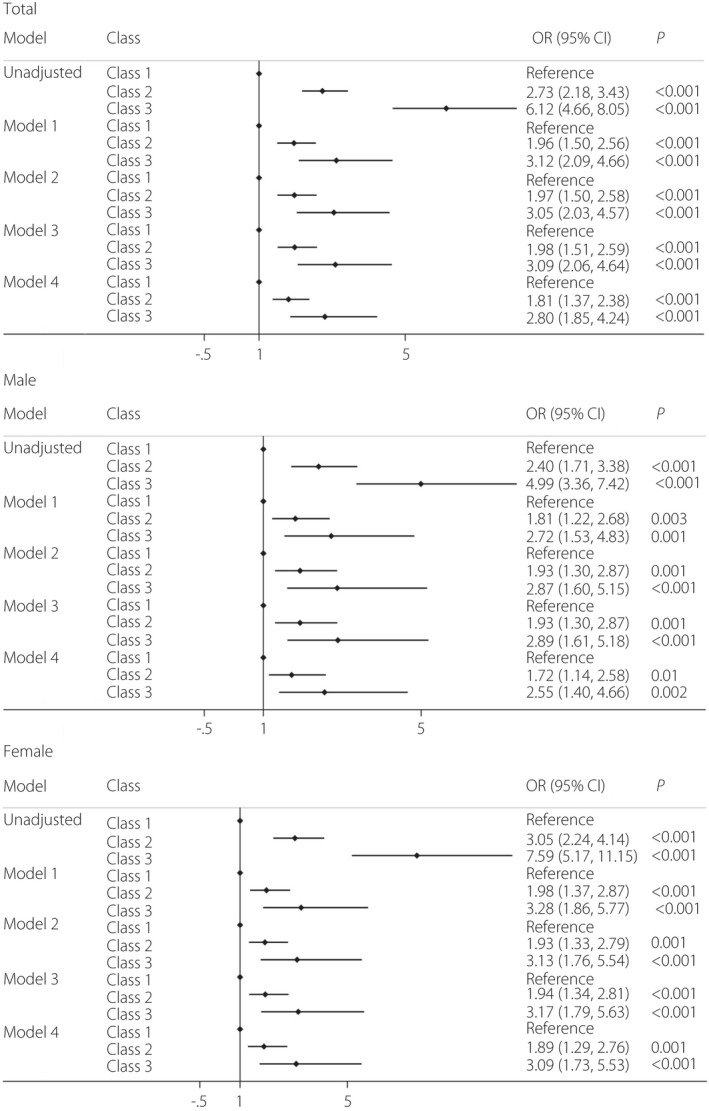
Forest plot of the relationship between trajectory group and incidence of diabetes mellitus in total, and the male and female group. Model 1 was adjusted for age, sex, residence and body mass index (BMI) Model 2 was adjusted for age, sex, residence, BMI, education level, hypertension, smoking status and alcohol consumption. Model 3 was adjusted for age, sex, residence, BMI, education level, hypertension, smoking status, alcohol consumption, average energy intake, average carbohydrate intake, average fat intake and average protein intake. Model 4 was adjusted for age, sex, residence, BMI, education level, hypertension, smoking status, alcohol consumption, average energy intake, average carbohydrate intake, average fat intake, average protein intake, creatinine, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglyceride and total cholesterol.
In analyzing whether the baseline AVI could affect the role of AVI trajectory on incident diabetes mellitus, we found that although the relationship between baseline AVI (tertile) and incident diabetes mellitus was statistically significant, the effect size was modest relative to the AVI trajectory (Table S5). Also, when we included baseline AVI in the multivariate adjusted models to consider the associations among them, the relationship between AVI trajectory and incident diabetes mellitus was still prominent (Table S6).
Receiver operating characteristic analyses that evaluated the utility of AVI trajectories versus traditional anthropometric parameters in predicting diabetes mellitus found that the AVI slope (AUC 0.693) better predicted diabetes mellitus compared with BMI (AUC 0.644) and waist circumference (AUC 0.675; Figure S5).
To assess potential selection effects due to the limitation of the analysis based on available data, we compared all enrolled participants with those excluded for having less than three AVI measurements (Table S7). The two groups had similar demographic characteristics, AVI at follow up and incidence of diabetes mellitus, suggesting selection bias is unlikely.
DISCUSSION
After analyzing data from a large, population‐based longitudinal survey in China extending over a period of 16 years, we identified three different AVI trajectories. Our data showed that there are different trajectories of AVI that emerge over time, and that these different trajectories played an important role in the risk of developing diabetes mellitus. After multivariate adjustment and when compared with those in the slowest AVI class, we found nearly a threefold elevated risk for incident diabetes mellitus for the fastest AVI increasing trajectory, and nearly a twofold elevated risk for diabetes mellitus among those with moderately increasing AVI over time. We also found that diabetes mellitus risk associated with trajectory group varied according to age and sex. These findings are particularly noteworthy, as we adjusted for several key sociodemographic, behavioral and biological covariates. In sum, the present results are the first to represent the relationship between change in abdominal obesity over time in relation to incident diabetes mellitus in China, and to underscore the importance of considering the development of abdominal obesity trajectories in the prevention and control of diabetes mellitus across adult life.
The representativeness of geographical distribution of study populations across provinces and the similarity of diabetes mellitus prevalence with previous research ensured the quality of the analysis 27 . As many factors could affect the incidence of diabetes mellitus, such as individual characteristics, residence, education levels, risk behaviors and physical fitness, multivariate logistic regression models were used to adjust for confounding factors. Compared with the unadjusted model, the adjusted OR was more objectively and conservatively reflective of the significant association between the trajectory of AVI and diabetes mellitus. Similar findings were observed in the adjusted sex‐specific models. Furthermore, we studied the trend of AVI change rate with age. The slow increase group showed as an inverted "U" shaped curve, indicating that AVI has the largest increase rate in middle‐adulthood. This corresponds to middle age‐related decline in metabolic rate, changes in eating habits, decreased physical activity and muscle atrophy, all of which are common in the general population in mid‐life. On the contrary, the fast increase group showed a "U" shaped curve. The first peak appeared in young adulthood, which suggests adiposity and concomitant diabetes mellitus risk for this group originated in adolescence or childhood, indicating that early life environments and the development of health habits in childhood are important considerations for AVI and diabetes mellitus. The second peak occurred in late adulthood, which might be related to challenges with weight management and the uptake of poor health behaviors. We also compared AVI trajectories with other traditional anthropometric parameters, and clarified its superiority as a monitoring indicator for the occurrence and development of diabetes mellitus. In sum, these findings not only indicate that distinct trajectories of AVI exist in the general population, but also that age and life stage are critically important to consider for the prevention of abdominal obesity and diabetes mellitus onset.
AVI has been used as a basic anthropological parameter to predict metabolic disorder in many studies 14 , 28 , 29 , 30 . Referring to cylindrical and vertical cone volume formulas, Guerrero‐Romero originally used the geometric data of the human model including waist and hip circumference to simulate and calculate AVI to predict impaired glucose tolerance and diabetes mellitus 11 . This index mainly reflected the total volume of the abdomen, indirectly estimated the visceral fat volume and represented abdominal obesity. Chin applied AVI in adolescents as a screening tool to test for metabolic syndrome in adulthood, which has been verified across populations in different countries 28 . Wu carried out a 20‐year follow‐up study to explore the effects of anthropometric indexes other than BMI on adult glucose homeostasis, and suggested that AVI had the highest predictive value in insulin resistance, β‐cell dysfunction, elevated fasting insulin and impaired glucose tolerance 29 . All of these prior studies showed that AVI is a sensitive indicator of abdominal obesity‐related metabolic abnormalities and an important factor in diabetes mellitus study. The present study is congruent with this existing evidence, and shows that different shapes and rates of prospectively assessed AVI change over time (slow, intermediate, fast) have a graded association with diabetes mellitus risk, independent of baseline AVI.
When it comes to chronic diseases, such as diabetes mellitus, and related cardiometabolic conditions, such as hypertension and cardiovascular disease, the long‐term accumulation of risk factors are known to contribute to disease, and as such, the need to evaluate the life course trajectory of risk factors is well understood 31 , 32 . However, just a few prior studies have actually focused on changes in abdominal obesity and the incidence of diabetes mellitus. For example, both Liu et al. 33 and Wander et al. 34 used computed tomography to assess the changes in abdominal fat during a 10‐year follow‐up database, and proposed that the increase in abdominal fat showed a decrease in insulin sensitivity and development of type 2 diabetes mellitus. Zhang concluded that the trajectory of visceral adiposity index can identify the occurrence of diabetes mellitus in Chinese women, but also pointed out that the visceral adiposity index was not better than simple anthropometric measurement, such as waist circumference 35 . The present study chose a simple measurement of AVI to better understand the relationship between abdominal obesity and diabetes mellitus in adulthood, and emphasized the importance of different baseline AVI and trajectories in predicting the risk of diabetes mellitus.
The pattern of the associations observed for the AVI trajectory groups and diabetes mellitus according to age and sex suggest different mechanisms could be involved in the associations. In the present study, we found that the fast increase trajectory group (which had the highest baseline AVI) had significantly increased the risk of developing diabetes mellitus in the future compared with other groups. One possible mechanism to explain this association could be that the increased AVI might represent a change in the distribution of adipose tissue, which will produce harmful metabolic effects (such as increased insulin resistance or pro‐inflammatory cytokines), thereby increasing the risk of developing diabetes mellitus 36 . This finding suggests that in adulthood, postponing the onset of abdominal obesity will help to prevent diabetes mellitus. Additionally, the high value of baseline AVI observed for the fast increasing trajectory group could reflect adiposity risk originating in childhood or adolescence. Some prior studies have shown that adolescents had a particularly high risk of diabetes mellitus when combined with obesity, suggesting that weight control from adolescence and perhaps earlier is critical to prevent diabetes mellitus from emerging later in life 37 , 38 . In addition, we found that the diabetes mellitus risk in the fast increase trajectory group was mainly contributed by women aged >45 years. One possible explanation for this finding could be related to metabolism changes experienced by postmenopausal women. Hormonal changes after menopause can cause a decrease in women's overall metabolic rate and increase in fat, especially abdominal fat 39 . Therefore, the postmenopausal time in the life course represents a time of increased risk for women; clinicians and individual patients should monitor AVI closely during this life stage and to avoid rapid growth. As the present study did not assess adiposity before adulthood, or consider hormonal contributors to the AVI and diabetes mellitus associations, we encourage future work to explore these possibilities explicitly.
The present research had several strengths. First, the large‐scale CHNS cohort included Chinese participants from different provinces across China, showing that our work is highly generalizable to the general population in China. Second, the 16‐year follow‐up period was an ideal time period for studying the longitudinal development of chronic diseases, such as diabetes mellitus. Furthermore, our multimodal data collection protocol, which included directly assessed anthropomorphic measurements, lifestyle–behavioral assessments, sociodemographic information and biomarker acquisition, were all implemented in accordance with established and standardized operating procedures, making the data valid and reliable. In addition, we excluded patients who suffered from diabetes mellitus at baseline or onset of diabetes mellitus during the study period, mainly to reduce the bias in simulating the trajectory of AVI, because individuals will change their behaviors, such as weight loss, after being informed that they have diabetes mellitus.
However, several limitations of the present study need to be considered. First, CHNS did not distinguish the type of diabetes mellitus in adult participants. Although most diabetes mellitus diagnoses were made in adulthood and thus probably reflect type 2 diabetes mellitus, we acknowledge type 1 diabetes mellitus might be included here as well. Second, due to the missing data of AVI, we excluded 1,474 people, which might lead to a biased model parameter estimate. However, baseline characteristics were highly comparable between included participants and those excluded for having less than three AVI measurements, which helps to mitigate this concern. Third, as the study data used here were obtained from Chinese people, the present findings might not be generalized to people in other countries.
Based on 16‐year follow‐up data of the CHNS cohort, we identified three different AVI trajectories that carried differential risk of developing diabetes mellitus over time. People with the fastest increase in AVI over time had the highest risk of incident diabetes mellitus compared with those with slower and more moderately increasing AVI. We also found the rate of AVI change within trajectories varied by age and sex. The results from the present study can help inform guidelines for diabetes mellitus prevention and control. In particular, the present results show that clinical emphasis on the control of AVI in young adulthood and effective management of AVI in older adulthood, especially in menopausal woman, might contribute substantially to reducing the incidence of diabetes mellitus in the population.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The study protocols were approved by the Institutional Review Committees of the University of North Carolina at Chapel Hill, USA, and the National Institute for Nutrition and Health (NINH, former National Institute of Nutrition and Food Safety) at the Chinese Center for Disease Control and Prevention (CCDC) at Beijing, China.
Informed consent: Each participant gave informed consent.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Figure S1 | Flowchart of screening process of the study population.
Figure S2 | The geographical distribution of the population included in the study.
Figure S3 | Estimated and observed mean value of abdominal volume index.
Figure S4 | The predicted trajectory of abdominal volume index with age in different sex presented by the solid line, and 95% of the confidence interval were represented by shading.
Figure S5 | Area under the curve of receiver operating characteristic for the comparison among abdominal volume index slope, body mass index and waist circumference in the prediction of diabetes mellitus.
Table S1 | Incidence of diabetes mellitus according to different sex and age at enrollment.
Table S2 | Fitting parameters and results of different classifications in latent class growth mixed model.
Table S3 | Estimated mean of slope, intercept and quadratic parameters for each class, and the odds ratio of the incidence of diabetes mellitus.
Table S4 | Linear slope of abdominal volume index by incidence of diabetes mellitus with age.
Table S5 | Odds ratio and 95% confidence interval of incident diabetes mellitus according to tertiles of baseline abdominal volume index.
Table S6 | Odds ratio and 95% confidence interval of incident diabetes mellitus adjusting for covariate and baseline abdominal volume index.
Table S7 | Comparison between enrolled and excluded participants.
ACKNOWLEDGMENTS
We thank the investigators who worked hard for the China Health and Nutrition Survey, and the CHNS participants and their families for the data provided. Our research was supported by The National Key Research and Development Program of China (No. 2016YFC1301202).
J Diabetes Investig. 2022; 13: 868–877
REFERENCES
- 1. Federation ID. IDF Diabetes Atlas, 9th edn. Brussels, Belgium, International Diabetes Federation, 2019. [Google Scholar]
- 2. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846. [DOI] [PubMed] [Google Scholar]
- 3. Ye J. Mechanisms of insulin resistance in obesity. Front Med 2013; 7: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neeland IJ, Ross R, Després J‐P, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019; 7: 715–725. [DOI] [PubMed] [Google Scholar]
- 5. Wang T, Zhang R, Ma X, et al. Causal association of overall obesity and abdominal obesity with type 2 diabetes: a Mendelian randomization analysis. Obesity 2018; 26: 934–942. [DOI] [PubMed] [Google Scholar]
- 6. Caspard H, Jabbour S, Hammar N, et al. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: an analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab 2018; 20: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shungin D, Winkler TW, Croteau‐Chonka DC, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015; 518: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu H, Jin C, Guan Q. Causal effects of overall and abdominal obesity on insulin resistance and the risk of type 2 DM: a two‐sample Mendelian Randomization Study. Front Genet 2020; 11: 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emdin CA, Khera AV, Natarajan P, et al. Genetic association of waist‐to‐hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 2017; 317: 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashwell M, Lejeune S, McPherson K. Ratio of waist circumference to height may be better indicator of need for weight management. BMJ 1996; 312: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guerrero‐Romero F, Rodríguez‐Morán M. Abdominal volume index. An anthropometry‐based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch Med Res 2003; 34: 428–432. [DOI] [PubMed] [Google Scholar]
- 12. Thomas DM, Bredlau C, Bosy‐Westphal A, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013; 21: 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity 2011; 19: 1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu F, Ho V, Fraser BJ, et al. Predictive utility of childhood anthropometric measures on adult glucose homeostasis measures: a 20‐year cohort study. Int J Obes 2018; 42: 1762–1770. [DOI] [PubMed] [Google Scholar]
- 15. Feng J, He S, Chen X. Body adiposity index and body roundness index in identifying insulin resistance among adults without diabetes. Am J Med Sci 2019; 357: 116–123. [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, He S, Chen X. Capacity of different anthropometric measures to predict diabetes in a Chinese population in southwest China: a 15‐year prospective study. Diabetes Med 2019; 36: 1261–1267. [DOI] [PubMed] [Google Scholar]
- 17. Mamtani MR, Kulkarni HR. Predictive performance of anthropometric indexes of central obesity for the risk of type 2 diabetes. Arch Med Res 2005; 36: 581–589. [DOI] [PubMed] [Google Scholar]
- 18. Hu H, Nagahama S, Nanri A, et al. Duration and degree of weight change and risk of incident diabetes: Japan Epidemiology Collaboration on Occupational Health Study. Prev Med 2017; 96: 118–123. [DOI] [PubMed] [Google Scholar]
- 19. Stokes A, Collins JM, Grant BF, et al. Obesity progression between young adulthood and midlife and incident diabetes: a retrospective cohort study of U.S. adults. Diabetes Care 2018; 41: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee AK, Woodward M, Wang D, et al. The risks of cardiovascular disease and mortality following weight change in adults with diabetes: results from ADVANCE. J Clin Endocrinol Metab 2020; 105: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Popkin BM, Du S, Zhai F, et al. Cohort Profile: The China Health and Nutrition Survey–monitoring and understanding socio‐economic and health change in China, 1989–2011. Int J Epidemiol 2010; 39: 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang B, Zhai FY, Du SF, et al. The China Health and Nutrition Survey, 1989‐2011. Obes Rev 2014; 15: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Diabetes Association . Diagnosis and classification of DM. Diabetes Care 2010; 33: S62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He J, Fang A, Yu S, et al. Dietary nonheme, heme, and total iron intake and the risk of diabetes in adults: results from the China Health and Nutrition Survey. Diabetes Care 2020; 43: 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Proust‐Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw 2015; 78: 1–56. [Google Scholar]
- 26. Muthen LK, Muthen BO. Mplus User's Guide, 7th edn. Los Angeles, CA: Muthen & Muthen, 1998; 2016. [Google Scholar]
- 27. Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018; 61: 1249–1260. [DOI] [PubMed] [Google Scholar]
- 28. Chin Y‐T, Lin W‐T, Wu P‐W, et al. Characteristic‐grouped adiposity indicators for identifying metabolic syndrome in adolescents: develop and valid risk screening tools using dual population. Nutrients 2020; 12: 3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu L, Zhu W, Qiao Q, et al. Novel and traditional anthropometric indices for identifying metabolic syndrome in non‐overweight/obese adults. Nutr Metab 2021; 18: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perona JS, Schmidt‐RioValle J, Fernández‐Aparicio Á, et al. Waist circumference and abdominal volume index can predict metabolic syndrome in adolescents, but only when the criteria of the international diabetes federation are employed for the diagnosis. Nutrients 2019; 11: 1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu S, An S, Li W, et al. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw Open 2019; 2: e194758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Dong B, Huang S, et al. Body mass index trajectory and incident hypertension: results from a longitudinal cohort of Chinese children and adolescents, 2006–2016. Am J Public Health 2020; 110: 1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu AW, Song SO, Hayashi T, et al. Change in CT‐measured abdominal subcutaneous and visceral but not thigh fat areas predict future insulin sensitivity. Diabetes Res Clin Pract 2019; 154: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wander PL, Boyko EJ, Leonetti DL, et al. Change in visceral adiposity independently predicts a greater risk of developing type 2 diabetes over 10 years in Japanese Americans. Diabetes Care 2013; 36: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang M, Zheng LI, Li P, et al. 4‐year trajectory of visceral adiposity index in the development of type 2 diabetes: a prospective cohort study. Ann Nutr Metab 2016; 69: 142–149. [DOI] [PubMed] [Google Scholar]
- 36. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008; 29: 2959–2971. [DOI] [PubMed] [Google Scholar]
- 37. The NS, Richardson AS, Gordon‐Larsen P. Timing and duration of obesity in relation to diabetes: findings from an ethnically diverse, nationally representative sample. Diabetes Care 2013; 36: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abdullah A, Wolfe R, Mannan H, et al. Epidemiologic merit of obese‐years, the combination of degree and duration of obesity. Am J Epidemiol 2012; 176: 99–107. [DOI] [PubMed] [Google Scholar]
- 39. Davis SR, Castelo‐Branco C, Chedraui P, et al. Understanding weight gain at menopause. Climacteric 2012; 15: 419–429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Flowchart of screening process of the study population.
Figure S2 | The geographical distribution of the population included in the study.
Figure S3 | Estimated and observed mean value of abdominal volume index.
Figure S4 | The predicted trajectory of abdominal volume index with age in different sex presented by the solid line, and 95% of the confidence interval were represented by shading.
Figure S5 | Area under the curve of receiver operating characteristic for the comparison among abdominal volume index slope, body mass index and waist circumference in the prediction of diabetes mellitus.
Table S1 | Incidence of diabetes mellitus according to different sex and age at enrollment.
Table S2 | Fitting parameters and results of different classifications in latent class growth mixed model.
Table S3 | Estimated mean of slope, intercept and quadratic parameters for each class, and the odds ratio of the incidence of diabetes mellitus.
Table S4 | Linear slope of abdominal volume index by incidence of diabetes mellitus with age.
Table S5 | Odds ratio and 95% confidence interval of incident diabetes mellitus according to tertiles of baseline abdominal volume index.
Table S6 | Odds ratio and 95% confidence interval of incident diabetes mellitus adjusting for covariate and baseline abdominal volume index.
Table S7 | Comparison between enrolled and excluded participants.


