Abstract
Background
Fibrillin-1 (FBN1) is an extracellular matrix glycoprotein essential to the structural component of microfibrils and FBN1 gene polymorphisms can be associated with adolescent idiopathic scoliosis (AIS) susceptibility. This study aimed to evaluate the potential role of the FBN1 rs12916536 polymorphism in AIS development or severity and the variation in Cobb angle in relation to patient’s characteristics.
Methods
DNA from 563 subjects (185 AIS patients and 378 controls) were genotyped using a validated TaqMan allelic discrimination assay. A multivariate logistic regression model evaluated the association between polymorphism and AIS, using the adjusted odds ratios (OR) with their respective 95% confidence intervals (95% CI). A linear regression analysis evaluated the variation in Cobb angle according to the patient’s age and body mass index (BMI).
Results
Among the AIS group there was a predominance of females (12:1), low or normal BMI (90%), 58% had a Cobb angle greater than 45° and 74% were skeletally mature. Age was a risk factor (4-fold) for curve progression higher than BMI (P < 0.001). The allelic frequency of the rs12916536 G > A polymorphism was 40% in controls and 31% in AIS cases; and this difference was statistically significant (P = 0.004). FBN1 rs12916536 GA + AA genotypes were associated with a lower risk of AIS susceptibility (OR = 0.58 and 95% CI = 0.35–0.98), after adjustment for age, sex and BMI. However, no significant differences were detected in polymorphism distribution with the severity of the disease (Cobb < 45° or ≥ 45°).
Conclusion
Age was a risk factor for progression of the scoliotic curve and FBN1 rs12916536 polymorphism a protective factor for AIS susceptibility.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-022-05370-1.
Keywords: Adolescent idiopathic scoliosis, Fibrillin-1, Polymorphism
Introduction
Adolescent idiopathic scoliosis (AIS), a three-dimensional deformity of the spine, typically becomes evident near the onset of puberty, with no apparent cause and prevalence around 3% [1]. Most patients have non-progressive deformity, and factors associated with curve progression and disease development are not fully understood. Thus, several distinct pathogenesis theories have been proposed for AIS development, including biomechanical, neurological, hormonal, growth-related and genetic [2–5].
Genome-wide association studies (GWAS) have identified variants in genes related to muscle, cartilage, bone, intervertebral discs, and connective tissue development that appear to be associated with AIS susceptibility [6–17]. However, the molecular genetic origin of AIS development remains unknown. GWAS of rare variants using exome sequence analysis identified fibrillin-1 (FBN1) as a susceptible gene of AIS [9]. In addition, common variant rs12916536 of FBN1 was significantly associated with AIS development in Chinese population, suggested a regulatory role in the expression of gene [18].
The FBN1 gene, localized at chromosome 15q21.1, comprising aroud 200 kb and containing 65 exons [19], codes for an extracellular matrix glycoprotein crucial to extracellular microfibrils organization in skeletal muscle cells and dermal fibroblasts. Spinal stability needs the biomechanical properties of the ligaments, discs and connective-tissue components [20, 21]. Several inherited connective tissue disorders (e.g., syndromes: Marfan, Ehlers-Danlos, Beals and Weill-Marchesani and osteogenesis imperfecta) that show clinical features of structural scoliosis were associated with rare mutations in FBN1 [20–25]. As far as we know, no studies have investigated the association between common genetic variants of FBN1 and AIS in Brazilians. This population is extraordinarily heterogenic and extrapolation of genetic data from well-defined ethnic groups may not be applicable to most Brazilians [26–28].
Therefore, due to the relationship between genetic variants in connective-tissue genes and scoliosis, this study was designed to investigate the association between the FBN1 rs12916536 polymorphism and the susceptibility of AIS in Brazilians, as well as its influence on the severity of the disease. In addition, was evaluate the variation in Cobb angle in dependence to the patient’s age and BMI.
Materials and methods
Study population
The current study comprises a retrospective case-control evaluation of 563 subjects (185 AIS patients and 378 controls) from a reference center in Orthopedics. The study protocol was approved by the Human Research Ethics Committees of the Instituto Nacional de Traumatologia e Ortopedia Jamil Haddad – INTO (protocol numbers 637.973 and 2.767.503), and all subjects provided written informed consent. The study was conducted in accordance with the Helsinki Declaration. All sociodemographic and clinical data were obtained during the recruitment process between 2018 and 2020. The body mass index (BMI) was calculated as the weight status (kg) divided by the square of height (m2) and the weight status is classified into five groups: underweight (BMI < 18.5), normal weight (18.5 ≤ BMI ≤ 24.9), overweight (25 ≤ BMI ≤ 29.9), obesity (30 ≤ BMI < 40) and morbid obesity (BMI ≥ 40).
All patients had idiopathic scoliosis diagnosed by clinical and radiographic examination with the spinal curve Cobb angle measuring ≥10°. Posteroanterior radiographs were used to measure the major curve angles employing the Cobb method [29]. AIS cases were separated into two groups: Cobb < 45° and Cobb ≥45° according to the magnitude of the Cobb angle and indication of surgical treatment for scoliosis. The Risser sign was used to estimate skeletal maturity and is a predictor of scoliosis progression [30]. The skeletal maturity was assessed by evaluating ossification of the iliac apophysis and patients with a Risser’s sign of zero to 3 were classified as skeletally immature and those with a Risser’s sign of 4 or 5 were classified as skeletally mature. All measurements were independently made by two investigators (GBLA and AEPAJ or LAMM) who are experienced spine surgeons and blinded to the clinical information to avoid bias.
The control group (N = 378) consisted of healthy volunteers recruited at INTO’s blood bank when they appeared to donate blood. An orthopedic spine surgeon evaluated them to rule out any spine deformity.
Polymorphisms genotyping
Genomic DNA was obtained from oral mucosa collected by swab or from peripheral blood samples using an extraction kit (Qiagen) following the procedures recommended by the manufacturer. The genotyping analyses of FBN1 rs12916536 polymorphism (chr15:48414374) were performed using a TaqMan allelic discrimination assay (C__31343379_20) by 7500 Real-Time System (Applied Biosystems, Foster City, CA, USA). PCR amplification was performed in 8 μL reactions with 30 ng of template DNA, 1x TaqMan Universal Master Mix, 1x each primer and probe assay. Thermal cycling was initiated with a first denaturation step of 10 min at 95 °C, followed by 40 cycles of denaturation at 92 °C for 15 s and annealing at 60 °C for 1 min. To assure genotyping quality, in each reaction, two standardized negative and positive controls of each polymorphism genotype were used, as previously described [28].
Statistical analysis
A sample size calculation was performed using Epi Info 7, version 7.2.4.0 (http://wwwn.cdc.gov/epiinfo/html/downloads.htm) capable of detecting differences between cases and controls, assuming an odds ratio of 0.5 with a power of 0.8 and 5% type I error.
A descriptive study of the population was conducted, presenting relative frequencies for each categorical variable. The categorical data were expressed as percentages and evaluated by the Chi-square (χ2) test or Fisher’s exact test, when applicable. A linear regression analysis was performed using Cobb angle and age or BMI to evaluate the variation in Cobb angle in relation to the patient’s age and BMI.
Genotypic frequency of FBN1 polymorphism was derived by direct gene counting, and the adherence to the Hardy–Weinberg principle was evaluated by the Chi-square test for goodness-of-fit. The magnitude of association from each comparison was estimated by calculating crude odds ratios (ORs) with 95% confidence intervals (95% CIs). As a final regression model used to control possible confounding factors (sociodemographic and clinical features), each variable was introduced considering the biological and statistical significance of the univariate analysis, which an input significance level less than 0.25 (P ≤ 0.25) and output significance was 0.05 (P ≤ 0.05) at the regression model, as previously described [28, 31]. The difference was statistically significant when P < 0.05. All analyses were performed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA, version 20.0).
Results
Table 1 presents the main sociodemographic and clinical characteristics of the AIS case population. The mean age and BMI (and ranges) were 18.6 ± 6.7 (10–48) years and 20.4 ± 4.0 (14.0–39.9) kg/m2, respectively. There was a predominance (89.6%) of low or normal BMI values (≤24.9) among the AIS patients group and the ratio of female to male patients affected by the disease was 12.2:1. Of the 185 AIS patients, 129 (73.7%) were classified as skeletally mature, according to the Risser grade. The mean major spinal curve magnitude was 47.7 ± 16.3 (11–110) degrees and 58% (N = 102) had curves greater than 45°.
Table 1.
Demographics and clinical characteristics of 185 patients with adolescent idiopathic scoliosis
| Variables | Idiopathic scoliosis cases |
|---|---|
| N (%) | |
| Agea,b (years old) | |
| ≤ 18 | 115 (62.8) |
| 19 a 28 | 51 (27.9) |
| ≥ 29 | 17 (9.3) |
| Sex | |
| Female | 171 (92.4) |
| Male | 14 (7.6) |
| BMIc (kg/m2) | |
| ≤ 18.50 | 64 (35.2) |
| 18.51 a 24.9 | 99 (54.4) |
| 25.0 a 29.9 | 12 (6.6) |
| ≥ 30 | 7 (3.8) |
| Risser scaled | |
| Immature (0-III) | 46 (26.3) |
| Mature (IV-V) | 129 (73.7) |
| Cobb (degrees)e | |
| < 45 | 74 (42.0) |
| ≥ 45 | 102 (58.0) |
aInformation obtained from 183 cases
bValues categorized according to the quartile distribution of the total study population (N = 563)
cInformation obtained from 182 cases
dInformation obtained from 175 cases
eInformation obtained from 176 cases
The mean age in AIS patients with Cobb < 45° (15.6 ± 6.3 years 10–29) was significantly smaller (P < 0.001) than patients with Cobb ≥45° (22.5 ± 6.7 years 11–48).
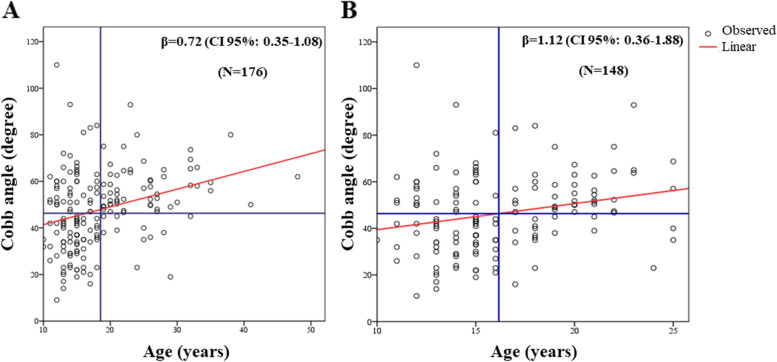
The statistical analysis estimated a mean increase in the Cobb angle of 0.72° and 1.12° for every additional year of age, considering all AIS cases and only patients up to 25 years of age, respectively. The relative regression lines, with 95% confidence intervals for the estimated mean Cobb angle values, are shown in Fig. 1A and B. The comparison of the standardized regression coefficients β, which determines the contribution linked to various independent variables expressed in different measurement scales, showed that age contributed 4-fold more than BMI to the Cobb angle increase (Table 2).
Fig. 1.
Relationship between the Cobb angle and the age in adolescent idiopathic scoliosis. Legend: The circles represent each patients. The red line is linear function coefficient (A, R2 = 0.09 with P < 0.001 and B, R2 = 0.05 with P = 0.004) and the blue lines are means of th age and Cobb angle. Information obtained from 176 cases (A) and 148 AIS patients up to 25 years of age (B)
Table 2.
Linear regression analysis of the Cobb angle with age and BMI of adolescent idiopathic scoliosis patients
| Variables | Regression Coefficient | P-value | Standardized coefficient β | (CI 95%) |
|---|---|---|---|---|
| Cobb (N = 176) | ||||
| Age | 0.72 | < 0.001 | 0.29 | 0.35–1.08 |
| Sex | −6.21 | 0.17 | −0.10 | −15.12 – 2.71 |
| BMI | 0.33 | 0.31 | 0.08 | −0.30 – 0.96 |
| r2 ajusted | 0.11 | |||
| P-modela | < 0.001 | |||
aP-model calculated by ANOVA test. Information obtained from 176 cases
The rate of successful genotyping was 100% and the genotypic distribution in the entire study population was in Hardy-Weinberg equilibrium. A statistically different frequency was observed between AIS cases and controls in genotype and allele comparison of the FBN1 rs12916536 G > A polymorphism (Fig. 2). After adjustment for confounding factors (age, sex and BMI), the FBN1 rs12916536 genotypes (GA + AA) were associated with a lower risk of developing AIS, since the variant allele was more frequent in the control group. No significant associations were found in FBN1 rs12916536 polymorphism with severity of the disease, considering either AIS cases with a Cobb angle < 45° or a Cobb angle ≥45° (Table 3).
Fig. 2.
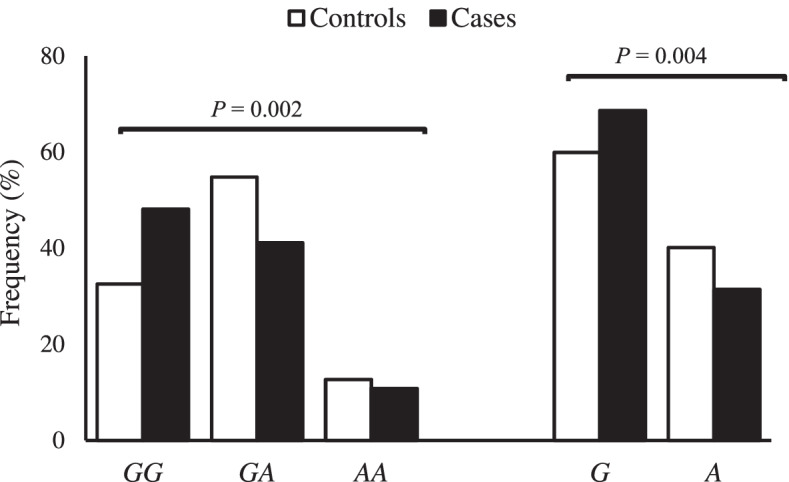
Allelic and genotypic distribution of the FBN1 rs12916536 polymorphism in adolescent idiopathic scoliosis cases and controls. Legend: Number of controls = 378 and cases = 185. P-value calculated by Chi-Square Teste or Fisher’s exact test, when necessary
Table 3.
Association between the FBN1 rs12916536 polymorphism with adolescent idiopathic scoliosis and its severity (N = 563)
| FBN1 | Controls | All cases | OR ajustedb | Cobb < 45° | OR ajustedc | Cobb ≥ 45° | OR ajustedd | OR ajustede |
|---|---|---|---|---|---|---|---|---|
| rs12916536 | (N = 378) | (N = 185) | (CI 95%) | (N = 74) | (CI 95%) | (N = 102) | (CI 95%) | (CI 95%) |
| N (%) N (%) N (%) | ||||||||
| GG | 123 (32.5) | 89 (48.1) | 1a | 37 (50.0) | 1a | 48 (47.1) | 1a | 1a |
| GA | 207 (54.8) | 76 (41.1) | 0.52 (0.30–0.90) | 29 (39.2) | 0.54 (0.22–1.33) | 43 (42.1) | 0.68 (0.37–1.24) | 1.12 (0.55–2.26) |
| AA | 48 (12.7) | 20 (10.8) | 0.95 (0.40–2.26) | 8 (10.8) | 2.40 (0.45–12.75) | 11 (10.8) | 0.91 (0.34–2.45) | 1.28 (0.42–3.90) |
| GG | 123 (32.5) | 89 (48.1) | 1a | 37 (50.0) | 1a | 48 (47.1) | 1a | 1a |
| GA + AA | 255 (67.5) | 96 (51.9) | 0.58 (0.35–0.98) | 37 (50.0) | 0.65 (0.28–1.55) | 54 (52.9) | 0.71 (0.40–1.27) | 1.15 (0.60–2.23) |
| G | 453 (59.9) | 254 (68.6) | 1a | 103 (69.6) | 1a | 139 (68.1) | 1a | 1a |
| A | 303 (40.1) | 116 (31.4) | 0.79 (0.54–1.16) | 45 (30.4) | 0.96 (0.51–1.81) | 65 (31.9) | 0.85 (0.56–1.30) | 1.14 (0.69–1.88) |
OR Odds ratio, ajusted by age, sex and BMI, CI confidence interval 95%
aReference group
bAssociation analysis between controls and all AIS cases
cAssociation analysis between controls and Cobb < 45° AIS cases
dAssociation analysis between controls and Cobb ≥45° AIS cases
eAssociation analysis between Cobb < 45° and Cobb ≥45° AIS cases
Discussion
The FBN1 rs12916536 polymorphism was associated with the susceptibility of AIS in the Brazilian population, but it was not influence on the severity of the disease. Age was an independent risk factor for scoliotic curve progression. The molecular mechanisms involved in the pathogenesis and development of AIS have been the subject of many research studies; however, the etiology remains an enigma [5, 32, 33]. For example, low BMI has been associated with AIS and it is hypothesized that this association may be related to the pathogenesis of the disease [34–38]. The majority of our patients (~ 90%) had low or normal BMI and 58% had curves with Cobb angles exceeding 45°, a parameter commonly used to indicate the need for surgical correction of scoliosis [39]. Our results corroborate with Miyagi and colleagues, as patients with curves with Cobb angles exceeding 45° had significantly lower BMI than patients with curves under 45° [35]. Furthermore, of the significant correlations between anthropometric parameters and the scoliotic curve severity [34], low BMI also was associated with increased risk of all poor outcomes, including brace treatment failure and the need for surgical intervention [37].
The risk of scoliosis curve progression was also associated with female sex, age, skeletal maturity and curve Cobb angle exceeding 30° at presentation [39–41]. The female-to-male ratio (12:1) observed here is consistent with previous studies [9, 42]. In addition, most of our AIS patients were skeletally mature with curve Cobb angles exceeding 45°. This is likely due to the fact that all cases were recruited at a public orthopedic referral hospital in Brazil, where the majority of patients were referred from other facilities for a surgical indication and sometimes the waiting times were surgery were long. Thus, age contributed 4-fold more than BMI to the Cobb angle increase in our cases. Our results corroborate with previous findings that show progression of curves above 30° in skeletally mature patients at rates of < 1° per year [39, 43, 44]. However, patients with Cobb angles exceeding 45° were more likely come back for follow up and the association found might just be due to follow-up bias. So, intervening early is vital because patients with curves below 30° can have a quality of life similar to a healthy population [44, 45] and they are amenable for brace treatment, which was not the case for most of our patients.
Genetic factors have been largely considered to play an important role in its onset and progression of AIS [5]. Rare variants in FBN1 were first identified in severe AIS North American patients with European ancestry (n = 344), other ancestral backgrounds (n = 47) and replicated in an independent cohort of Han Chinese (n = 370), suggesting that this variant can be predictive of curve severity and promising as a new option for early diagnosis and more timely treatment of severe AIS [9]. FBN1 variants were also associated with Marfan syndrome’s skeletal features, autosomal dominant disorder, which frequently requires orthopedic surgical intervention [25, 46]. FBN1 is richly distributed in structural elements of elastic and non-elastic tissues, responsible for connective tissue disorders, such as scoliosis [46, 47]. Furthermore, FBN1 variants can influence in upregulated transforming growth factor-beta signaling in paravertebral muscles [48, 49].
AIS patients showed significantly decreased FBN1 mRNA expression and common variants (polymorphisms) of FBN1 were associated with increased individual risk of AIS [18]. The inheritable susceptibility to AIS justifies the growing interest in identifying genetic polymorphisms that could lead to an increased risk or severity of the disease [5]. The present results indicate a negative association between FBN1 rs12916536 G > A and the risk of developing AIS, since cases showed a significantly lower frequency of genotypes GA + AA than the controls. This polymorphism probably plays an important role in the regulation of FBN1 expression, since it was marked by enhancer histone in multiple cell lines [50]. It is noteworthy that our result is in agreement with Sheng and colleagues [18], who found that FBN1 rs12916536 was associated with the development of AIS in a large study (952 cases and 1499 controls) of Chinese.
In addition, FBN1 expression levels were correlated with AIS curve severity [18]. Here, patients were divided into two Cobb angle groups (< 45° and ≥ 45°); however, FBN1 rs12916536 polymorphism was not associated with severity of AIS, only with the development of disease. The total sample size was adequate to detect significant associations with 80% statistical power; the small number of patients in Cobb angle or Lenke classification groups and the biased in term of the age effect because most of AIS cases were skeletally mature with curve Cobb angles exceeding 45° were the main limitations of this study. We believe that the role of FBN1 polymorphisms in the AIS curve severity and development still needs further investigation. Thus, it is necessary to be accounted for by other variables, such as polymorphisms in LBX1, GPR126, BNC2, PAX1, LBX1-AS1, BCL2 and PAX3 genes [6, 8, 10–12, 14], or other yet unknown. Furthermore, no family history data was collected to evaluated the differences between familial and non-familial AIS cases. In addition to providing the first report of the distribution of FBN1 rs12916536 polymorphism in the heterogeneous Brazilian population and validated the same association observed in Chinese patients, our study has distinct strengths. First, this study reflects real-life community for diagnosing and treating AIS in a public hospital in a developing country. Second, all patients recruited (cases and controls) were evaluated by experienced spine surgeons, excluding spine deformities in the control group.
Whereas AIS is a multifactorial disease, including environmental and genetic factors, it is essential to study this possible association in patients from different populations, including the Brazilian population, which stands out for the extensive admixture and heterogeneity [26, 27]. It is becoming increasingly important to derive data from different populations to validate the role of genetic polymorphisms in AIS and to build a database that can then be used in future investigations (replication study) to better understand and identify modifiable non-modifiable risk factors associated with AIS development and progression.
Conclusion
In summary, FBN1 rs12916536 polymorphism play a protective role in the development of AIS, which represents an advance in understanding etiology of disease. Age was an independent risk factor for curve progression. Due to chronic pain, poor health quality of life and the high treatment costs experienced by AIS patient, the genetic information could assist in the diagnosis of the disease, as well as contribute to an individualized treatment for monitoring the at-risk individuals.
Supplementary Information
Additional file 1: Supplementary table. Dataset of case-control study: FBN1 rs12916536 G > A polymorphism associated with susceptibility of adolescent idiopathic scoliosis in Brazilian population. Legend: Age in years. Body mass index (BMI) was calculated as the weight status (kg) divided by the square of height (m2). Magnitude of the Cobb angle. The Risser sign was used to estimate skeletal maturity: zero to III were classified as skeletally immature and those with a Risser’s sign of IV or V were classified as skeletally mature.
Acknowledgments
The authors thank all staff members of the recruitment hospital especially the INTO Blood Bank staff for collaborating and enabling this study to happen. We also thank our dear students Lucas Rafael Lopes, Giuliana Rodrigues de Souza and Camilla Manso for their technical assistance. This work was supported by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq.
Abbreviations
- AIS
Adolescent idiopathic scoliosis
- 95% CI
95% Confidence interval
- χ2
Chi-square
- BMI
Body mass index
- FBN1
Fibrillin-1
- GWAS
Genome-wide association studies
- HWE
Hardy–Weinberg equilibrium
- mRNA
Messenger RNA
- OR
Odds ratio
- PCR
Polymerase chain reaction
- R2
Degree of correlation
- SD
Standard deviation
- SPSS
Statistical Package for Social Sciences
Authors’ contributions
GBLA, AEPAJ, HLAD and JAP participated in conception and design of study. GBLA, AEPAJ, LAMM, RMA and JAP collated the data and developed the database. JAP performed the experiments and statistical analysis. GBLA, AEPAJ, HLAD and JAP analysis and interpretation of data. GBLA and JAP wrote the manuscript. JAMG and HLAD critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This study was supported by the Brazilian agency Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, Brazil and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq. Funding body contributed to acquisition of research inputs.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the Supplementary material.
Declarations
Ethics approval and consent to participate
This study was approved by the Human Research Ethics Committee of the Instituto Nacional de Traumatologia e Ortopedia, Rio de Janeiro, Brazil (protocol number 637.973 and 2.767.503). All participating provided written informed consent. The study was conducted in accordance with the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gustavo Borges Laurindo de Azevedo and Jamila Alessandra Perini contributed equally to this work.
References
- 1.Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527–1537. doi: 10.1016/S0140-6736(08)60658-3. [DOI] [PubMed] [Google Scholar]
- 2.Kouwenhoven JW, Castelein RM. The pathogenesis of adolescente idiopathic scoliosis: review of the literature. Spine (Phila Pa 1976) 2008;33(26):2898–2908. doi: 10.1097/BRS.0b013e3181891751. [DOI] [PubMed] [Google Scholar]
- 3.Burwell RG, Clark EM, Dangerfield PH, Moulton A. Adolescent idiopathic scoliosis (AIS): a multifactorial cascade concept for pathogenesis and embryonic origin. Scoliosis Spinal Disord. 2016;11:8. doi: 10.1186/s13013-016-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grauers A, Einarsdottir E, Gerdhem P. Genetics and pathogenesis of idiopathic scoliosis. Scoliosis Spinal Disord. 2016;11:45. doi: 10.1186/s13013-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Y, Wang SR, Qiu GX, Zhang JG, Zhuang QY. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin Med J. 2020;133(4):483–493. doi: 10.1097/CM9.0000000000000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi Y, Kou I, Takahashi A, Johnson TA, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, et al. A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat Genet. 2011;43(12):1237–1240. doi: 10.1038/ng.974. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Gao X, Londono D, Devroy SE, Mauldin KN, Frankel JT, Brandon JM, Zhang D, Li QZ, Dobbs MB, et al. Genome-wide association studies of adolescent idiopathic scoliosis suggest candidate susceptibility genes. Hum Mol Genet. 2011;20(7):1456–1466. doi: 10.1093/hmg/ddq571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, Qiu X, Sharma S, Takimoto A, Ogura Y, et al. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45(6):676–679. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- 9.Buchan JG, Alvarado DM, Haller GE, et al. Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis. Hum Mol Genet. 2014;23(19):5271–5282. doi: 10.1093/hmg/ddu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogura Y, Kou I, Miura S, Takahashi A, Xu L, Takeda K, et al. A functional SNP in BNC2 is associated with adolescent idiopathic scoliosis. Am J Hum Genet. 2015;97(2):337–342. doi: 10.1016/j.ajhg.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, Londono D, Eckalbar WL, Gao X, Zhang D, Mauldin K, et al. A PAX1 enhancer locus is associated with susceptibility to idiopathic scoliosis in females. Nat Commun. 2015;6:6452. doi: 10.1038/ncomms7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Roffey DM, Chen S. Genetics of adolescent idiopathic scoliosis in the post-genome-wide association study era. Ann Transl Med. 2015;3(Suppl 1):S35. doi: 10.3978/j.issn.2305-5839.2015.03.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Z, Tang NL, Xu L, Qin X, Mao S, Song Y, Liu L, Li F, Liu P, Yi L, Chang J, Jiang L, Ng BK, Shi B, Zhang W, Qiao J, Sun X, Qiu X, Wang Z, Wang F, Xie D, Chen L, Chen Z, Jin M, Han X, Hu Z, Zhang Z, Liu Z, Zhu F, Qian BP, Yu Y, Wang B, Lee KM, Lee WYW, Lam TP, Qiu Y, Cheng JC. Genome-wide association study identifies new susceptibility loci for adolescent idiopathic scoliosis in Chinese girls. Nat Commun. 2015;6:8355. doi: 10.1038/ncomms9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogura Y, Kou I, Japan Scoliosis Clinical Research Group. Scoliosis J, Matsumoto M, Watanabe K, Ikegawa S. Genome-wide association study for adolescent idiopathic scoliosis. Clin Calcium. 2016;26(4):553–560. [PubMed] [Google Scholar]
- 15.Zhu Z, Xu L, Leung-Sang Tang N, Qin X, Feng Z, Sun W, Zhu W, Shi B, Liu P, Mao S, Qiao J, Liu Z, Sun X, Li F, Chun-Yiu Cheng J, Qiu Y. Genome-wide association study identifies novel susceptible loci and highlights Wnt/beta-catenin pathway in the development of adolescent idiopathic scoliosis. Hum Mol Genet. 2017;26(8):1577–1583. doi: 10.1093/hmg/ddx045. [DOI] [PubMed] [Google Scholar]
- 16.Khanshour AM, Kou I, Fan Y, Einarsdottir E, Makki N, Kidane YH, Kere J, Grauers A, Johnson TA, Paria N, Patel C, Singhania R, Kamiya N, Takeda K, Otomo N, Watanabe K, Luk KDK, Cheung KMC, Herring JA, Rios JJ, Ahituv N, Gerdhem P, Gurnett CA, Song YQ, Ikegawa S, Wise CA. Genome-wide meta-analysis and replication studies in multiple ethnicities identify novel adolescent idiopathic scoliosis susceptibility loci. Hum Mol Genet. 2018;27(22):3986–3998. doi: 10.1093/hmg/ddx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kou I, Otomo N, Takeda K, Momozawa Y, Lu HF, Kubo M, Kamatani Y, Ogura Y, Takahashi Y, Nakajima M, Minami S, Uno K, Kawakami N, Ito M, Yonezawa I, Watanabe K, Kaito T, Yanagida H, Taneichi H, Harimaya K, Taniguchi Y, Shigematsu H, Iida T, Demura S, Sugawara R, Fujita N, Yagi M, Okada E, Hosogane N, Kono K, Nakamura M, Chiba K, Kotani T, Sakuma T, Akazawa T, Suzuki T, Nishida K, Kakutani K, Tsuji T, Sudo H, Iwata A, Sato T, Inami S, Matsumoto M, Terao C, Watanabe K, Ikegawa S. Genome-wide association study identifies 14 previously unreported susceptibility loci for adolescent idiopathic scoliosis in Japanese. Nat Commun. 2019;10(1):3685. doi: 10.1038/s41467-019-11596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng F, Xia C, Leilei X, Qin X, Nelson LST, Qiu Y, Cheng JCY, Zhu Z. New Evidence Supporting the Role of FBN1 in the Development of Adolescent Idiopathic Scoliosis. Spine (Phila Pa 1976) 2019;44(4):E225–E232. doi: 10.1097/BRS.0000000000002809. [DOI] [PubMed] [Google Scholar]
- 19.Biery NJ, Eldadha ZA, Moore CS, Stetten G, Spencer F, Dierz HC. Revised genomic organization of FBN1 and significance for regulated gene expression. Genomics. 1999;56:70–77. doi: 10.1006/geno.1998.5697. [DOI] [PubMed] [Google Scholar]
- 20.Tsipouras P, Del Mastro R, Sarfarazi M, Lee B, Vitale E, Child AH, Godfrey M, Devereux RB, Hewett D, Steinmann B, Viljoen D, Sykes BC, Kilpatrick M, Ramirez F, The International Marfan Syndrome Collaborative Study Genetic linkage of the Marfan syndrome, ectopia lentis, and congenital contracturd arachnodactyly to the fibrillin genes on chromosomes 15 and 5. N Engl J Med. 1992;326(14):905–909. doi: 10.1056/NEJM199204023261401. [DOI] [PubMed] [Google Scholar]
- 21.Miller NH, Mims A, Child DM, Milewicz SH, Sponseller P, Blanton SH. Genetic analysis of structural elastic Fiber and collagen genes in familial adolescent idiopathic scoliosis. J Orthop Res. 1996;14(6):994–999. doi: 10.1002/jor.1100140621. [DOI] [PubMed] [Google Scholar]
- 22.Loeys B, Nuytinck L, Delvaux I, et al. Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med. 2001;161(20):2447–2454. doi: 10.1001/archinte.161.20.2447. [DOI] [PubMed] [Google Scholar]
- 23.Faivre L, Gorlin RJ, Wirtz MK, et al. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet. 2003;40(1):34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbustini E, Grasso M, Ansaldi S, et al. Identification of sixty-two novel and twelve known FBN1 mutations in eighty-one unrelated probands with Marfan syndrome and other fibrillinopathies. Hum Mutat. 2005;26(5):494. doi: 10.1002/humu.9377. [DOI] [PubMed] [Google Scholar]
- 25.Phokaew, Sittiwangkul R, Suphapeetiporn K, Shotelersuk V. Double heterozygous variants in FBN1 and FBN2 in a Thai woman with Marfan and Beals syndromes. Eur J Med Genet. 2020;63(9). 10.1016/j.ejmg.2020.103982. [DOI] [PubMed]
- 26.Pena SD, Di Pietro G, Fuchshuber-Moraes M, Genro JP, Hutz MH, Kehdy Fde S, Kohlrausch F, Magno LA, Montenegro RC, Moraes MO, de Moraes ME, de Moraes MR, Ojopi EB, Perini JA, Racciopi C, Ribeiro-Dos-Santos AK, Rios-Santos F, Romano-Silva MA, Sortica VA, Suarez-Kurtz G. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011;6(2):e17063. doi: 10.1371/journal.pone.0017063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suarez-Kurtz G, Genro JP, de Moraes MO, Ojopi EB, Pena SD, Perini JA, Ribeiro-dos-Santos A, Romano-Silva MA, Santana I, Struchiner CJ. Global pharmacogenomics: impact of population diversity on the distribution of polymorphisms in the CYP2C cluster among Brazilians. Pharmacogenomics J. 2012;12(3):267–276. doi: 10.1038/tpj.2010.89. [DOI] [PubMed] [Google Scholar]
- 28.Lopes LR, de Miranda VAR, Guimarães JAM, et al. Association of TNF-α -308G > a polymorphism with susceptibility to tendinopathy in athletes: a case–control study. BMC Sports Sci Med Rehabil. 2021;13(1):51. doi: 10.1186/s13102-021-00276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cobb JR. Outline for the study of scoliosis. The American Academy of orthopedic surgeons instructional course lectures. Ann Arbor: Edwards; 1948. [Google Scholar]
- 30.Risser JC. The classic: the iliac apophysis: an invaluable sign in the management of scoliosis. 1958. Clin Orthop Relat Res. 2010;468(3):643–653. doi: 10.1007/s11999-009-1096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goes RA, Lopes LR, Cossich VRA, de Miranda VAR, Coelho ON, do Carmo Bastos R, Domenis LAM, Guimarães JAM, Grangeiro-Neto JA, Perini JA. Musculoskeletal injuries in athletes from five modalities: a cross-sectional study. BMC Musculoskelet Disord. 2020;21(1):122. doi: 10.1186/s12891-020-3141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikanloo SR, Tarpada SP, Cho W. Etiology of adolescent idiopathic scoliosis: a literature review. Asian Spine J. 2019;13(3):519–526. doi: 10.31616/asj.2018.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaydman AM, Strokova EL, Pahomova NY, Gusev AF, Mikhaylovskiy MV, Shevchenko AI, Zaidman MN, Shilo AR, Subbotin VM. Etiopathogenesis of adolescent idiopathic scoliosis: review of the literature and new epigenetic hypothesis on altered neural crest cells migration in early embryogenesis as the key event. Med Hypotheses. 2021;151:110585. doi: 10.1016/j.mehy.2021.110585. [DOI] [PubMed] [Google Scholar]
- 34.Cheung CSK, Lee WTK, Tse YK, Tang SP, Lee MK, Guo X, et al. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine (Phila Pa 1976). 2003;(28):2152–7. 10.1097/01.BRS.0000084265.15201.D5. [DOI] [PubMed]
- 35.Miyagi M, Saito W, Imura T, Nakazawa T, Shirasawa E, Kawakubo A, Uchida K, Akazawa T, Inage K, Ohtori S, Inoue G, Takaso M. Body composition in Japanese girls with adolescent idiopathic scoliosis. Spine Surg Relat Res. 2020;5(2):68–74. doi: 10.22603/ssrr.2020-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramírez M, Martínez-Llorens J, Sanchez JF, Bagó J, Molina A, Gea J, Cáceres E. Body composition in adolescent idiopathic scoliosis. Eur Spine J. 2013;22(2):324–329. doi: 10.1007/s00586-012-2465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodbody CM, Asztalos IB, Sankar WN, Flynn JM. It's not just the big kids: both high and low BMI impact bracing success for adolescent idiopathic scoliosis. J Child Orthop. 2016;10(5):395–404. doi: 10.1007/s11832-016-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeon K, Kim DI. The association between low body weight and scoliosis among Korean elementary school students. Int J Environ Res Public Health. 2018;15(12):2613. doi: 10.3390/ijerph15122613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agabegi SS, Kazemi N, Sturm PF, Mehlman CT. Natural history of adolescent idiopathic scoliosis in skeletally mature patients: a critical review. J Am Acad Orthop Surg. 2015;23(12):714–723. doi: 10.5435/JAAOS-D-14-00037. [DOI] [PubMed] [Google Scholar]
- 40.Soucacos PN, Zacharis K, Soultanis K, Gelalis J, Xenakis T, Beris AE. Risk factors for idiopathic scoliosis: review of a 6-year prospective study. Orthopedics. 2000;23(8):833–838. doi: 10.3928/0147-7447-20000801-17. [DOI] [PubMed] [Google Scholar]
- 41.Horne JP, Flannery R, Usman S. Adolescent idiopathic scoliosis: diagnosis and management. Am Fam Physician. 2014;89(3):193–198. [PubMed] [Google Scholar]
- 42.Lenke L. Idiopathic scoliosis. Philadelphia: Lippincott, Williams & Wilkins; 2004. [Google Scholar]
- 43.Weinstein SL, Dolan LA, Spratt KF, Peterson KK, Spoonamore MJ, Ponseti IV. Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. JAMA. 2003;289:559–567. doi: 10.1001/jama.289.5.559. [DOI] [PubMed] [Google Scholar]
- 44.Danielsson AJ. Natural history of adolescent idiopathic scoliosis: a tool for guidance in decision of surgery of curves above 50°. J Child Orthop. 2013;7(1):37–41. doi: 10.1007/s11832-012-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mak T, Cheung PWH, Zhang T, Cheung JPY. Patterns of coronal and sagittal deformities in adolescent idiopathic scoliosis. BMC Musculoskelet Disord. 2021;22(1):44. doi: 10.1186/s12891-020-03937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reyes-Hernández OD, Palacios-Reyes C, Chávez-Ocaña S, Cortés-Malagón EM, Alonso-Themann PG, Ramos-Cano V, Ramírez-Bello J, Sierra-Martínez M. Skeletal manifestations of Marfan syndrome associated to heterozygous R2726W FBN1 variant: sibling case report and literature review. BMC Musculoskelet Disord. 2016;15(17):79. doi: 10.1186/s12891-016-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao LG, Yao XP, Zhang L, Hui RT, Zhou XL. Molecular analysis for diagnosis of Marfan syndrome and Marfan-associated disorders. Chin Med J. 2011;124:930–934. [PubMed] [Google Scholar]
- 48.Matt P, Schoenhoff F, Habashi J, et al. Circulating transforming growth factor-beta in Marfan syndrome. Circulation. 2009;120:526–532. doi: 10.1161/CIRCULATIONAHA.108.841981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nowak R, Kwiecien M, Tkacz M, Mazurek U. Transforming growth factor-beta (TGF-beta) signaling in paravertebral muscles in juvenile and adolescent idiopathic scoliosis. Biomed Res Int. 2014;2014:594287. doi: 10.1155/2014/594287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary table. Dataset of case-control study: FBN1 rs12916536 G > A polymorphism associated with susceptibility of adolescent idiopathic scoliosis in Brazilian population. Legend: Age in years. Body mass index (BMI) was calculated as the weight status (kg) divided by the square of height (m2). Magnitude of the Cobb angle. The Risser sign was used to estimate skeletal maturity: zero to III were classified as skeletally immature and those with a Risser’s sign of IV or V were classified as skeletally mature.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the Supplementary material.