Abstract
Sterol 14α-demethylase (ERG11) is the target enzyme of azole antifungals that are widely used for the treatment of fungal infections. Candida glabrata is known to be less susceptible to fluconazole than most Candida albicans strains, and the incidence of C. glabrata infection has been increasing mostly in conjunction with the use of azole antifungals. Recently, it has been reported that C. glabrata can rescue the defect of ergosterol biosynthesis by incorporating cholesterol from serum. To explore the effect of inactivating Erg11p in C. glabrata, we generated mutant strains in which the ERG11 gene was placed under the control of tetracycline-regulatable promoters. In these mutants, expression of the ERG11 gene can be repressed by doxycycline (DOX). All mutants showed a growth defect in the presence of DOX. The numbers of CFU of the mutants were lowered by only 1/10 with DOX treatment. In these mutants, accumulation of 4,14-dimethylzymosterol, which differs from an accumulated abnormal sterol detected in C. albicans and Saccharomyces cerevisiae treated with fluconazole, was observed by DOX treatment. Although such phenotypes were also observed in serum-containing media by DOX treatment, they were alleviated. Furthermore, the mutant could grow in DOX-treated mice without a severe reduction in the number of cells. Thus, depleting the expression of the ERG11 gene lowered the number of CFU by only 1/10 due to the accumulation of 4,14-demethylzymosterol in vitro, and it did not result in the defective growth of fungal cells in mice. These results suggested that Erg11p is not an ideal target molecule of antifungals for C. glabrata.
The incidence of life-threatening fungal infections has been increasing, particularly among patients who are immunocompromised by human immunodeficiency virus infection and those who are receiving immunosuppressive therapy for organ transplantation or chemotherapy for cancer. Azole antifungals are widely used in current therapies against such infections. Fluconazole, a water-soluble triazole with greater than 90% bioavailability after oral administration, has been used extensively to treat a wide range of Candida infections (30). Azole antifungals, including fluconazole, selectively inhibit the sterol 14α-demethylase gene (ERG11), which is an essential participant in ergosterol biosynthesis. The nitrogens in an azole ring form a complex with a heme iron component of the cytochrome group, resulting in the inhibition of the enzyme (46). Ergosterol is an essential component of the fungal plasma membrane. In Saccharomyces cerevisiae and Candida albicans, sterols have been shown to be important in membrane fluidity, membrane permeability, cell morphology, enzyme activity, and cell cycle progression (3, 16–18).
It has been a matter of concern that an increasing number of azole-resistant isolates are recovered in many immunocompromised and immunosuppressed patients (39). The mechanisms of azole resistance have been identified and classified mainly into three categories at this time. Mutation or disruptions of ERG3, which encodes Δ5,6 sterol desaturase, are associated with increased resistance to azole in S. cerevisiae (44) since the loss of function of Erg3p exerts a suppressor effect on the phenotype strain harboring erg11 mutations (2, 38). Increased efflux of drugs, mediated by multidrug pumps, including the major facilitators and the ATP-binding cassette (ABC) transporters, also confers resistance to azole antifungals (1, 21, 33, 35–37, 42, 43, 45). In addition, alteration of the target enzyme Erg11p, including point mutations (15, 19, 20, 34, 40) and overexpression (13, 45), causes resistance to azole antifungals.
Candida glabrata, which always grows as a haploid yeast cell, is a common pathogen in immunocompromised persons or those with diabetes mellitus. Although C. albicans is the best known of the pathogenic Candida group, the frequency of other Candida species that are isolated from clinical infections has been increasing during the past few years. Among the infections of the non-albicans Candida species, the incidence of C. glabrata infection has been increasing, mostly in conjunction with the use of azole antifungals (25, 27, 28). Consistently, this organism has been reported to be an intrinsically resistant Candida species, as is Candida krusei (5, 29). Other recent studies reported that C. glabrata is often the second or third most common cause of candidiasis after C. albicans and that C. glabrata infections have been linked to the death of compromised, at-risk hospitalized patients (4). Recently, we reported that squalene synthase (ERG9), which is also essential for ergosterol biosynthesis, is not required for the growth of C. glabrata in mice since C. glabrata has the ability to incorporate sterol from serum even under aerobic conditions (24). However, since a disruption study has proven that the ERG11 gene is also required for the aerobic growth of C. glabrata (6), the following question is raised: how can the inactivation of Erg11p, which may mimic fluconazole treatment, affect fungal growth in mice? To answer the question, we studied the effect of diminishing the expression of the ERG11 gene on growth in both in vitro and in vivo settings. For this study, we used tetracycline-regulatable promoters (23) to repress expression of the ERG11 gene. Although the generated strains showed a growth defect by repressing the expression of the ERG11 gene by using doxycycline (DOX), severe reduction of the number of viable cells could not be observed in either in vitro or in vivo culture settings. We also showed that abnormal sterol, which is different from the accumulated abnormal sterol detected in fluconazole-treated C. albicans and S. cerevisiae (11, 12), accumulated in such strains. Based on these results, Erg11p is not an ideal target molecule of antifungals for C. glabrata infection.
MATERIALS AND METHODS
Strains and growth media.
The C. glabrata strains used in this study are shown in Table 1. The C. glabrata strains were grown at 37°C on yeast extract-peptone dextrose (YEPD) complex medium containing 2% (wt/vol) glucose, 2% (wt/vol) Bacto peptone (Difco Laboratories), and 1% (wt/vol) yeast extract (Difco). The YEPD agar plates contained 2% (wt/vol) agar (Difco) as a supplement. Yeast nitrogen base (0.67%; Difco) with 2% (wt/vol) glucose, 2% (wt/vol) agar (Difco), and appropriate amino acids and bases was used as the selective medium after the transformation of ACG4. Yeast transformations were carried out by the modified lithium acetate method (8, 10). Escherichia coli DH5α was used as the host strain for all plasmid constructions and was grown on standard media.
TABLE 1.
Strains used in this study
| Strain | Parent strain | Genotypea | Reference |
|---|---|---|---|
| ACG4 | ATCC 2001 | his3 trp1 PScADH1::tetR::GAL4AD::TRP1 | 23 |
| 97ERG11 | ACG4 | his3 trp1 PScADH1::tetR::GAL4AD::TRP1 97t::ERG11-HIS3 | This study |
| 98ERG11 | ACG4 | his3 trp1 PScADH1::tetR::GAL4AD::TRP1 98t::ERG11-HIS3 | This study |
| 99ERG11 | ACG4 | his3 trp1 PScADH1::tetR::GAL4AD::TRP1 99t::ERG11-HIS3 | This study |
PScADH1, S. cerevisiae ADH1 promoter; GAL4AD, GAL4 activation domain.
Construction of plasmids and strains.
All primers in this study are shown in Table 2. Plasmids p97ERG11, p98ERG11, and p99ERG11 were constructed by introducing region A (nucleotides [nt] −417 to −156) or region B (nt −6 to 330) of CgERG11 (6) into SacII and XbaI sites or EcoRI and SalI sites, respectively, of p97CGH, p98CGH, and p99CGH (23). Region A or region B of the C. glabrata ERG11 gene was amplified with PCR using the primer pairs ERG11AF and ERG11AR or P5ERG11 and P3ERG11, respectively. To replace the endogenous promoter of ERG11 with tetracycline-regulatable promoters (97t, 98t, and 99t) by homologous recombination (Fig. 1A), p97ERG11, p98ERG11, and p99ERG11 linearized with SacII and SalI were used to transform ACG4, yielding strains 97ERG11, 98ERG11, and 99ERG11 (designated controllable strains), respectively. Approximately 0.2 μg of genomic DNA and the primer pair ERG11CH5 (nt −548 to −529) and ERG11CH3 (nt 430 to 450) were used for PCR to confirm correct integration of tetracycline-regulatable promoters in these controllable strains.
TABLE 2.
Linkers and primers used in this study
| Name | Designed C. glabrata ERG11 region (orientation) | Sequence (5′-3′)a |
|---|---|---|
| ERG11AF | 5′-end flanking (sense) | TTTTTTCCGCGG ATTTATCGCTTCTTCTCTAATCCA |
| ERG11AR | 5′-end flanking (antisense) | TTTTTTTCTAGA TGAGTATACGGGTTCTTCG |
| P5ERG11 | N terminus (sense) | TTTTTTGAATTC AATAACATGTCCACTGAAAAC |
| P3ERG11 | N terminus (antisense) | TTTTTTGTCGAC GTGACCCTTTGGACCCAAG |
| ERG11CH5 | 5′-end flanking (sense) | CCAACTACAATCGAGTGAGC |
| ERG11CH3 | N terminus (antisense) | TTCCATTAGTCTGTGGTTTGG |
Italic letters indicate restriction enzyme sites.
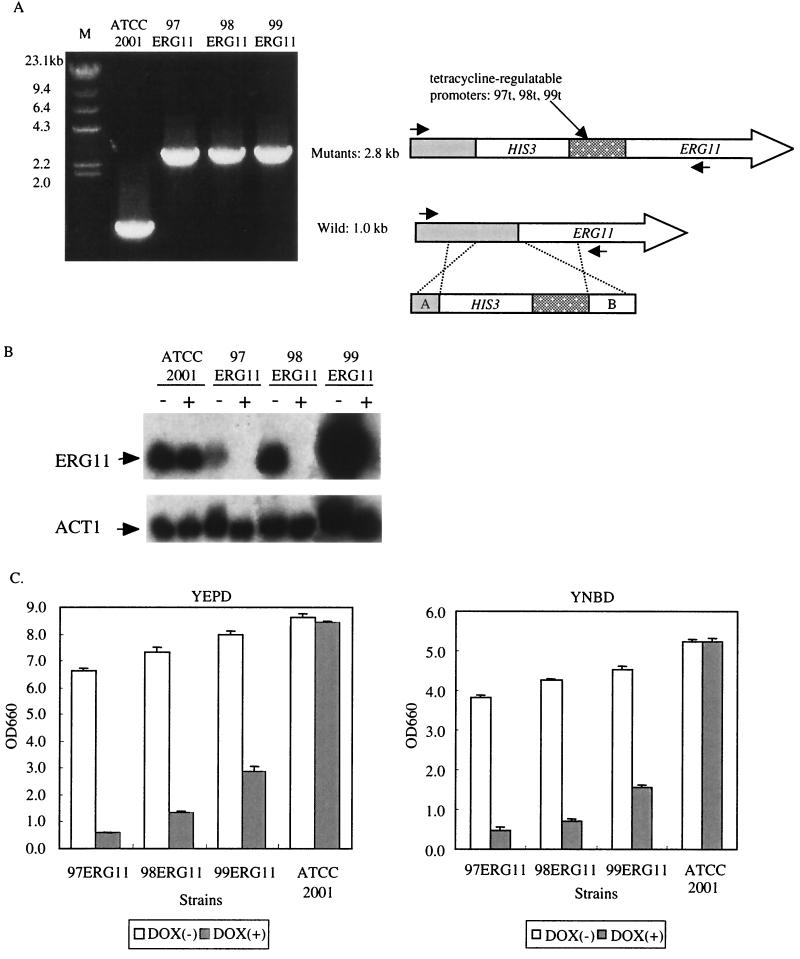
FIG. 1.
Effect of overexpression and repression of the ERG11 gene. (A) Construction of the controllable strains 97ERG11, 98ERG11, and 99ERG11. We generated 97ERG11, 98ERG11, and 99ERG11 by replacing the endogenous ERG11 promoter with a tetracycline-regulatable promoter as shown on the right. Its replacement was confirmed by PCR (on the left). (B) Northern blot analysis of 97ERG11, 98ERG11, and 99ERG11. The actin gene (ACT1) was used as an internal control to quantify the amount of RNAs. Minus signs show total RNA extracted from cells not treated with DOX; plus signs show total RNA extracted from DOX-treated cells. (C) Growth defects of 97ERG11, 98ERG11, and 99ERG11 produced by DOX. 97ERG11, 98ERG11, 99ERG11, and ATCC 2001 cells (105 cells each) were inoculated into YEPD medium and cultured at 37°C with or without DOX (10 μg/ml). The results for three independent experiments were averaged. OD660, optical density at 660 nm.
Northern blot analyses.
Total RNA was extracted by the glass bead lysis method (32). Ten micrograms of total RNA was separated on an agarose gel, transferred onto a nylon membrane (Hybond-N; Amersham-Pharmacia Biotech), and hybridized with a radiolabeled probe. The DNA fragments used for hybridization were ERG11 region B and the 0.5-kb MunI fragment of S. cerevisiae ACT1 exon 2. The signal obtained from ACT1 was used to normalize the mRNA signals. Radiolabeling of DNA was carried out by the random priming method using [α-32P]dCTP. The RNA bands that hybridized with the radiolabeled probe were visualized by autoradiography or with a BAS 1000 image analyzer (Fuji film). The intensity of each ERG11 signal was measured with an analyzing program installed in BAS 1000.
Effect of DOX on growth and the number of viable cells.
To investigate the effect of DOX on growth, approximately 105 of the cells were inoculated in YEPD medium with or without 10 μg of DOX per ml and cultured at 37°C. After a 14-h culture, their growth was monitored by measuring optical density at 660 nm. In order to determine the number of viable cells at several time points, 105 98ERG11 cells were inoculated into YEPD with or without DOX (10 μg/ml). The number of viable cells was determined by counting the number of colonies on an agar plate in which 20 μl of diluted cultures had been spread after a 24-h incubation at 37°C.
Analyses of sterol composition of C. glabrata cells.
We prepared samples for analyzing the sterol content as described previously (24). Approximately 106 cells/ml were inoculated into YEPD medium or YEPD medium containing 5% (vol/vol) human serum and cultured at 37°C with or without DOX (10 μg/ml). The cells were harvested at the times indicated in Table 3. We used 4,4-diphenyl-1-benzyl-piperidine as the internal control for the analyses.
TABLE 3.
Effect of DOX and human serum on sterol composition of controllable strains and ATCC 2001 cellsa
| Strain | Incubation time (h) | Medium supplementation
|
Amt of sterol (μg/g [dry weight] of cell)
|
|||||
|---|---|---|---|---|---|---|---|---|
| DOX (μg/ml) | Fluconazole (μg/ml) | Serum (%) | Cholesterol | Ergosterol | 4,14-Dimethyl-zymosterol | Lanosterol | ||
| 97ERG11 | 8 | 0 | 0 | 0 | 0 | 180.6 ± 26.2 | 58.7 ± 7.0 | 221.0 ± 19.7 |
| 8 | 10 | 0 | 0 | 0 | 26.1 ± 3.2 | 359.8 ± 33.2 | 1405.8 ± 103.2 | |
| 99ERG11 | 8 | 0 | 0 | 0 | 0 | 963.6 ± 65.5 | 42.3 ± 6.4 | 25.2 ± 6.8 |
| 8 | 10 | 0 | 0 | 0 | 108.5 ± 12.3 | 337.6 ± 7.2 | 1201.2 ± 17.9 | |
| 98ERG11 | 8 | 0 | 0 | 0 | 0 | 277.5 ± 25.2 | 12.1 ± 2.9 | 12.1 ± 3.0 |
| 8 | 10 | 0 | 0 | 0 | 33.2 ± 2.3 | 376.7 ± 43.0 | 1380.1 ± 100.3 | |
| 8 | 0 | 0 | 5 | 1101.9 ± 118.9 | 671.4 ± 63.9 | 30.5 ± 2.4 | 41.8 ± 14.4 | |
| 8 | 10 | 0 | 5 | 1200.9 ± 172.4 | 45.5 ± 1.3 | 305.2 ± 38.4 | 1185.3 ± 159.7 | |
| ATCC 2001 | 8 | 0 | 0 | 0 | 0 | 301.4 ± 27.9 | 12.0 ± 0.6 | 5.9 ± 0.8 |
| 8 | 0 | 30 | 0 | 0 | 18.3 ± 1.6 | 183.7 ± 16.1 | 630.8 ± 46.5 | |
| 8 | 0 | 0 | 5 | 808.1 ± 43.3 | 254.5 ± 16.1 | 7.9 ± 2.5 | 5.0 ± 2.0 | |
| 98ERG11 | 16 | 0 | 0 | 0 | 0 | 591.1 ± 19.5 | 20.3 ± 1.4 | 11.2 ± 2.1 |
| 16 | 10 | 0 | 0 | 0 | 28.1 ± 2.8 | 246.8 ± 9.5 | 1202.0 ± 97.9 | |
| 16 | 0 | 0 | 5 | 93.0 ± 1.5 | 562.3 ± 33.1 | 22.6 ± 4.9 | 32.8 ± 16.2 | |
| 16 | 10 | 0 | 5 | 388.7 ± 19.8 | 45.9 ± 9.8 | 187.1 ± 11.2 | 906.0 ± 56.2 | |
| ATCC 2001 | 16 | 0 | 0 | 0 | 0 | 265.8 ± 12.3 | 10.2 ± 1.0 | 9.4 ± 2.2 |
| 16 | 0 | 0 | 5 | 48.4 ± 2.4 | 245.0 ± 6.3 | 8.3 ± 1.5 | 6.6 ± 0.6 | |
Values are means ± standard deviations (three independent samples per group).
Effect of serum on the growth defect produced by DOX.
Approximately 106 98ERG11 cells were inoculated into YEPD medium with or without DOX (10 μg/ml) and cultured at 37°C with or without 5% (vol/vol) human serum (Irvine Scientific). Their growth was monitored by measuring optical density at 660 nm at the indicated times.
Determination of the number of viable C. glabrata cells in mice.
To generate immunocompromised mice, male CD-1 mice were treated as described previously (23, 24). Each mouse was intravenously inoculated with 105 ATCC 2001 or 98ERG11 cells after having been given 5% (wt/vol) sucrose solution with or without DOX (2 mg/ml) as drinking water from 3 days before the infection. In this dose regimen, each mouse drank approximately 5 ml of sucrose solution every day. Results show that the concentrations of DOX in serum, liver, and kidney were maintained at more than 2 μg of serum per ml, 8 μg of liver per g, and 10 μg of kidney per g. Such concentrations can be enough to repress the gene expression regulated by tetracycline-regulatable promoters (23, 24). The five pairs of mouse kidneys per group were sacrificed and homogenized 5 h, 7 days, and 14 days after the infections. The homogenates were spread on YEPD plates containing penicillin G (200 U/ml) and streptomycin (200 μg/ml), which were used for preventing bacterial infection. The number of colonies that had appeared after culturing the cells for 24 h at 37°C was counted.
Fluconazole sensitivity.
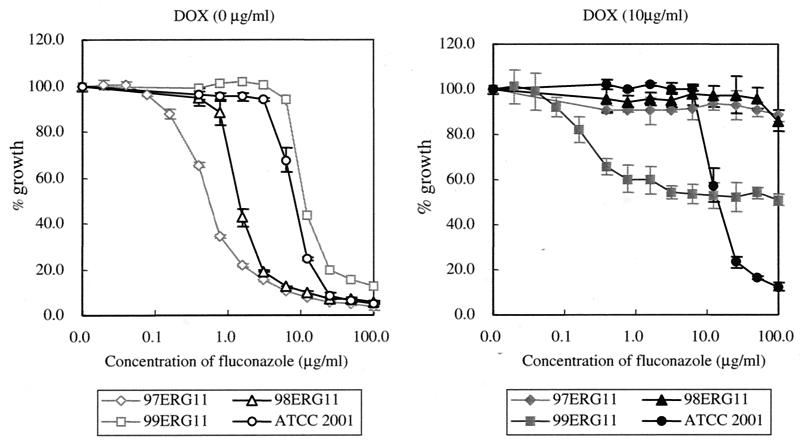
Approximately 105 cells (each) of strains 97ERG11, 98ERG11, and 99ERG11 were inoculated into YEPD medium containing the concentrations of fluconazole indicated in Fig. 5 in the absence or presence of DOX (10 μg/ml). After 14 h at 37°C, optical density at 660 nm was determined.
FIG. 5.
Fluconazole sensitivity. The controllable strains and ATCC 2001 cells were inoculated into YEPD and were cultured for 14 h with the indicated concentrations of fluconazole at 37°C in the absence (left panel) or presence (right panel) of DOX (10 μg/ml). The y axis shows the growth rate relative to that of each strain without fluconazole treatment. Therefore, each value was calculated by dividing the optical density value of the fluconazole-treated strain with that of the untreated strain. Error bars show standard deviations for the averages of results of three independent experiments.
RESULTS
Effect of altering expression of the ERG11 gene on cell growth.
To investigate the effect of deactivating Erg11p on C. glabrata growth, we generated three ERG11-controllable strains in which the endogenous ERG11 promoter was replaced with three tetracycline-regulatable promoters, 97t, 98t, and 99t (23), and which were designated 97ERG11, 98ERG11, and 99ERG11, respectively. The correct replacement from the endogenous promoter to the tetracycline-regulatable promoter in each strain was confirmed by PCR (Fig. 1A). Since it had been shown that the promoters 97t, 98t, and 99t have different activities (22, 23), we first compared the expression level of the ERG11 gene in each controllable strain with that of their isogenic wild-type strain ATCC 2001. Northern analysis showed that the expression level of the ERG11 gene in the absence of DOX varied among the three controllable strains. The 98ERG11 cells could express the ERG11 gene at almost the same level as that in ATCC 2001, and the level of 98ERG11 cells was approximately fivefold higher than that of 97ERG11 and was one-fifth lower than that of 99ERG11 (Fig. 1B and see Table 4). We next investigated the effect of DOX on the expression of the ERG11 gene. The expression of the ERG11 gene was almost completely repressed in all controllable strains (Fig. 1B). The growth defect was consistently observed in all three controllable strains when they were cultured with DOX both in YEPD and a synthetic medium, yeast nitrogen base and dextrose (YNBD). In both media, 97ERG11 showed a slight growth defect even in the absence of DOX (Fig. 1C and data not shown). Thus, the decrease in ERG11 gene expression was able to affect growth.
TABLE 4.
Comparison of fluconazole sensitivities and expression levels of the ERG11 gene in controllable strains
| Presence of DOX | Strain | Relative % of mRNAa | IC50 of fluconazole (μg/ml)b |
|---|---|---|---|
| Yes | 97ERG11 | 18.9 | 0.4 (27.0) |
| 98ERG11 | 100.0 | 1.4 (100.0) | |
| 99ERG11 | 536.6 | 9.7 (681.2) | |
| No | 97ERG11 | NDd | >100 |
| 98ERG11 | ND | >100 | |
| 99ERG11 | ND | >100 |
Each value shows the percentage of mRNA relative to the amount in 98ERG11 in the absence of DOX after normalization by the ACT1 signal.
IC50, 50% inhibitory concentration for the cells not treated with fluconazole. Values in parentheses show percentages of the 50% inhibitory concentration for 98ERG11 in the absence of DOX.
ND, not detected.
Effect of DOX on sterol composition.
We anticipated that altering the sterol composition by deactivating Erg11p resulted in a growth defect, since it was shown that abnormal sterol, 14α-methylergosta-8,24(28)-dien-3β,6α-diol, was produced in azole-treated S. cerevisiae and C. albicans and that this accumulation leads to cell death (11, 12). We therefore investigated the sterol compositions of the controllable strains and ATCC 2001 cells in the absence or presence of DOX. As shown in Table 3, in the absence of DOX, the amount of ergosterol correlated with the activity of tetracycline-regulatable promoters and the composition of 98ERG11 resembled that of ATCC 2001. Although the amount of ergosterol in all controllable strains was dramatically reduced in the presence of DOX, the amount of ergosterol in 99ERG11 treated with DOX was obviously much larger than that of 97ERG11 and 98ERG11 treated with DOX, suggesting that the DOX-dependent repression activity of the tetracycline-regulatable promoter 99t was lower than that of 97t or 98t. In addition to decreasing ergosterol, large amounts of lanosterol and 4,14-dimethylzymosterol were accumulated in the strains treated with DOX. Furthermore, even in the absence of DOX, we could also detect 4,14-dimethylzymosterol in 97ERG11, which showed a slight growth defect. Thus, sterol analysis implied that accumulation of the abnormal sterol 4,14-dimethylzymosterol might cause a growth defect, since other sterol fractions excluding these three sterols could not be detected in this analysis. In the absence of DOX, we could also detect this sterol in 99ERG11 without the accumulation of lanosterol, although 99ERG11 could grow as well as ATCC 2001 (data not shown). This result implied that the toxicity of 4,14-dimethylzymosterol can be alleviated by the overproduction of ergosterol and that the accumulation of lanosterol can also participate in a growth defect by DOX.
Effect of DOX on cell viability and sterol composition at several time points.
We used 98ERG11 for further experiments since it showed almost the same sterol composition and expression level of the ERG11 gene in the absence of DOX as ATCC 2001. Sterol analysis implied that DOX-dependent depletion of Erg11p could be complete, as the amount of ergosterol of 98ERG11 treated with DOX in a 16-h culture showed no increase from the amount in an 8-h culture. When we investigated the number of viable cells cultured with DOX at several time points, the number of CFU of DOX-treated cells was smaller than that of DOX-untreated cells at each time point. The maximum difference was about 1/10. However, the numbers of CFU of 98ERG11 treated with DOX (Fig. 2) in a 9-h culture and a 24-h culture were not significantly different. These results suggest that growth inhibition by diminishing the expression of the ERG11 gene had reached a plateau.
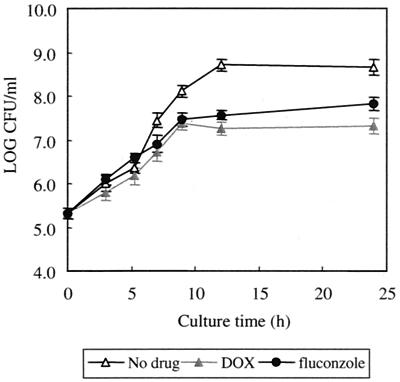
FIG. 2.
Effect of DOX or fluconazole on the number of viable cells of 98ERG11. The cells (105 each) were cultured with YEPD containing DOX (10 μg/ml) or fluconazole (30 μg/ml) at 37°C. The cells were harvested and diluted on an agar plate at the indicated times. After a 24-h incubation at 37°C, the number of colonies was counted. Error bars show standard deviations for the averages of the results of three independent experiments.
Investigation of the importance of the ERG11 gene on cell growth in serum-containing media.
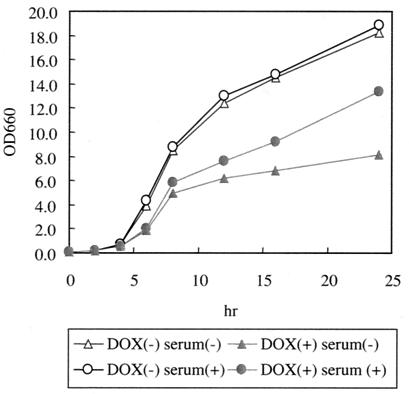
It was recently demonstrated that the C. glabrata squalene synthase (ERG9) gene, which also participates in ergosterol biosynthesis, is not essential for cell growth in either serum-containing media or mice due to the incorporation of exogenous sterol (24). We therefore investigated the importance of the ERG11 gene for the growth of C. glabrata cells in human serum-containing media. As shown in Fig. 3, the addition of serum resulted in the alleviation of the growth defect by DOX in 98ERG11 cells. A growth defect, however, was still observed. We then analyzed the sterol composition of serum-treated 98ERG11 and ATCC 2001 cells after cultivation for 8 and 16 h. Incorporation of cholesterol was observed independent of DOX treatment, although the amount of cholesterol in cells cultured for 16 h decreased compared to that in the cells cultured for 8 h. In 98ERG11 treated with DOX and human serum, the amount of ergosterol was slightly increased compared to that of 98ERG11 treated with DOX at both culturing times. However, the addition of serum could not affect depletion of the ERG11 gene by DOX, since the amount of ergosterol did not change in 8- or 16-h cultures. In 98ERG11 cultured with DOX and serum, ergosterol slightly increased and 4,14-dimethylzymosterol decreased compared to levels in 98ERG11 treated with DOX only (Table 3), presumably due to the incorporation of cholesterol from serum. Such a change in sterol composition may result in the alleviation of the growth defect by DOX.
FIG. 3.
Alleviation of the growth defect with the addition of serum. 98ERG11 cells (106 cells each) were inoculated into YEPD or 5% human serum containing YEPD and cultured with or without DOX (10 μg/ml). Optical densities at 660 nm (OD660) were measured at the indicated times. The values are averages of results for two independent experiments.
Investigation of the effect of diminishing the expression of the ERG11 gene on cell growth in mice.
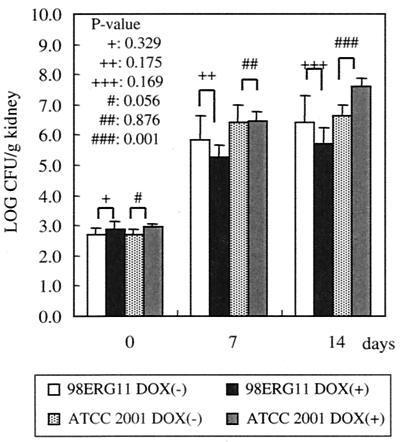
As the next step, we investigated the effect of diminishing the expression of the ERG11 gene on growth in mice. Five hours, 7 days, and 14 days after the infections, we determined the numbers of CFU in mouse kidneys treated with or without DOX (see Materials and Methods). As shown in Fig. 4, the number of CFU of 98ERG11 recovered from DOX-treated mice was not significantly different from that of DOX-untreated mice at each time point (P values were 0.329 [5 h], 0.175 [7 days], and 0.169 [14 days]). This result suggested that depleting the ERG11 gene would not affect cell growth in mice. On the other hand, the number of CFU of ATCC 2001 recovered from DOX-treated mice was higher than that of DOX-untreated mice 14 days after the infections (the P value was 0.009). At 7 and 14 days after the infection, the number of CFU of ATCC 2001 recovered from DOX-treated mice was higher than that of CFU of 98ERG11 recovered from both the DOX-treated and untreated mice. Furthermore, the number of CFU of 97ERG9 recovered from DOX-treated mice was higher than that of 97ERG9 recovered from mice not treated with DOX (24; data not shown). Taken together, these results suggested that it might be an indirect effect of DOX that ATCC 2001 in the DOX-treated mice yielded CFU numbers higher than those for the mice not treated with DOX, although we did not rule out the possibility that the difference in numbers of CFU was caused by strain differences. If DOX treatment indirectly decreased the number of CFU of C. glabrata in mice, the effect was marginal. Therefore, diminishing the expression of the ERG11 gene could not result in defective growth in mice.
FIG. 4.
Effect of DOX on the growth of 98ERG11 and ATCC 2001 in mouse kidneys. The mice, infected with these cells, were sacrificed, and the C. glabrata cells in their kidneys were recovered. Each bar represents the average number of cells recovered from five mice. P values were calculated by the paired t test. The same results were obtained in two independent experiments.
Effect of altering the expression of the ERG11 gene on fluconazole sensitivity.
Since it was reported that overexpression of the ERG11 gene conferred fluconazole resistance in C. albicans and S. cerevisiae (13, 45), we investigated the fluconazole sensitivities of the three controllable strains in the absence of DOX. As shown in Fig. 5A, 99ERG11, in which the ERG11 gene is overexpressed, was most resistant to fluconazole among the tested strains. On the other hand, 97ERG11 was the most sensitive. Thus, the fluconazole sensitivity of each controllable strain was well correlated to the level of ERG11 mRNA and the amount of ergosterol (Table 4). We then investigated whether or not a further growth defect by fluconazole was observed in DOX-treated controllable strains. A fluconazole amount below 100 μg per ml could not cause a further growth defect in 97ERG11 and 98ERG11. On the other hand, a further growth defect was observed in 99ERG11 treated with DOX by adding 0.1 μg of fluconazole per ml or more (Fig. 5). However, the percentages of 99ERG11 cells showing growth, which were calculated by dividing the value for the optical density at 660 nm of fluconazole-treated 99ERG11 by that of 99ERG11 not treated with fluconazole, could not be lowered below 50%. The level of growth inhibition of 99ERG11 treated with DOX and fluconazole was the same as that of 97ERG11 or 98ERG11 treated with DOX. These results were reproducibly observed with YNBD and RPMI medium (data not shown). Although we did not measure these DOX-dependent levels of depletion at the protein level, these results strongly suggested that the depletion of Erg11p by DOX could be almost complete in 97ERG11 and 98ERG11 but that some amounts of Erg11p remained in 99ERG11. In addition, these results also suggested that the mutants, in which expression of the ERG11 gene is maintained at a very low level, show fluconazole resistance.
Comparison of fluconazole treatment and DOX-dependent depletion of ERG11.
Well-correlated fluconazole sensitivity to the expression level of the ERG11 gene in the controllable strains implied that the results of the depletion of Erg11p could mimic some of the physiological effects of fluconazole treatment. Therefore, we examined whether or not the effects of fluconazole treatment are similar to those of DOX-dependent ERG11 depletion. When the effect of fluconazole on the growth of 98ERG11 was investigated at several time points, the number of CFU of fluconazole (30 μg/ml)-treated 98ERG11 at each time point was similar to that of 98ERG11 treated with DOX. This result suggested that depletion of the ERG11 gene by DOX can mimic the growth defect produced by fluconazole with a similar time dependency (Fig. 3). Furthermore, the sterol composition of 98ERG11 treated with DOX resembled that of ATCC 2001 treated with fluconazole (Table 3). The abnormal sterol 4,14-dimethylzymosterol, detected as a unique abnormal sterol, was also detected in the other C. glabrata strains treated with fluconazole or voriconazole (14). Thus, the effects of DOX-dependent diminishment of the expression of ERG11 were quite similar to those of fluconazole treatment.
DISCUSSION
In this report, by using tetracycline-regulatable promoters, we investigated the effect of diminishing the level of expression of the ERG11 gene on the growth of C. glabrata cultured in both in vitro and in vivo settings. Sterol analysis showed that lanosterol and an abnormal sterol, 4,14-dimethylzymosterol, were accumulated by diminishing the expression of the ERG11 gene by DOX. Consistently, under all tested culture conditions, the growth defect was observed after diminishing the expression of the ERG11 gene. However, the extent of this growth defect was small: the number of CFU was lowered by only 1/10. More than a few authors have suggested that the accumulation of the abnormal sterol 14α-methylergosta-8,24(28)-dien-3β,6α-diol leads to cell death in C. albicans and S. cerevisiae (2, 11, 12, 26, 44). However, as shown here, 4,14-dimenthylzmosterol, which accumulated after the level of expression of the ERG11 gene was diminished, was found to be a unique abnormal sterol in C. glabrata. The accumulation of this methylated sterol was observed together with that of other abnormal sterols in Cryptococcus neoformans treated with azole antifungals. The treatment with azole antifungals of C. neoformans showed that the extent of the growth defect was different from that in C. glabrata, presumably due to further alterations of sterol composition compared to that of C. glabrata (7, 41). These results suggest that the growth defect by accumulation of 4,14-dimetylzymosterol may be marginal. Since production of 14α-methylergosta-8,24(28)-dien-3β,6α-diol is necessary for further modifications of 4,14-dimethylzymosterol, it is anticipated that C. glabrata late genes in the ergosterol biosynthesis pathway cannot recognize 4,14-dimethylzymosterol. This speculation suggests that the late genes in the ergosterol biosynthesis pathway of C. glabrata do not resemble those of C. albicans or S. cerevisiae.
As shown in Fig. 5, the expression level of the ERG11 gene correlates well with the fluconazole sensitivities of controllable strains. However, fluconazole sensitivities obviously differed between 98ERG11 and ATCC 2001 in spite of showing almost the same expression level of the ERG11 gene in the absence of DOX. This difference in sensitivity may depend on the kinds of promoters that regulate expression of the ERG11 gene in each strain. As Henry et al. recently reported (9), the endogenous ERG11 promoter can be up-regulated by fluconazole treatment, resulting in a two- to fourfold increase in ERG11 RNA levels. On the other hand, it can be expected that its expression in 98ERG11 cannot be affected by fluconazole treatment since the expression from a tetracycline-regulatable promoter is constitutive.
Sterol analyses suggested new insights into ergosterol biosynthesis regulation. In the absence of DOX, the amount of ergosterol of 98ERG11 cells increased by further culture whereas that of ATCC 2001 was maintained at the same level, suggesting that the Erg11p activity can be down-regulated at a late log phase, at least at a transcriptional level. In addition, the amount of 4,14-dimethylzymosterol of DOX-treated 98ERG11 in a 16-h culture was decreased compared to that in an 8-h culture, suggesting that Erg24p, which can convert from lanosterol to 4,14-dimethylzymosterol, was also down-regulated. With serum-containing medium, the following observations could not correlate with artificial expression or repression of the ERG11 gene (1). The amount of ergosterol increased when the 98ERG11 cells were cultured with serum, whereas the amount of ergosterol in ATCC 2001 was not altered (2). In spite of continuous depletion of the ERG11 gene, the amount of ergosterol in 98ERG11 treated with DOX and human serum was slightly increased compared to that in 98ERG11 treated with DOX. Although further studies are needed, these results suggested that C. glabrata cells could also incorporate precursors of ergosterol, such as episterol, fecosterol, lanosterol, and squalene, from serum.
Depletion of the level of expression of the ERG11 gene could not be a direct corollary of the result of fluconazole treatment since many other factors, such as export via efflux pumps, come into play with regard to the effect of fluconazole treatment on cell growth. However, the physiological effects of DOX-dependent diminishment of the expression of the ERG11 gene were quite similar to those of fluconazole treatment, as far as was investigated in this study. Furthermore, it has been reported that the pattern of expression observed after treatment with fluconazole most closely resembles that seen after ERG11 inhibition in S. cerevisiae (31). Therefore, the efficacy of fluconazole against C. glabrata infection might be estimated by our model, namely, depletion of the level of ERG11 expression by the tetracycline-regulatable promoter. As shown in this study, the ERG11 gene is not an ideal target molecule of antifungals for C. glabrata infection, presumably because diminishment of its level of expression results in the accumulation of a unique abnormal sterol, 4,14-dimethylzymosterol, which might not be highly toxic. This conclusion supports evidence of an intrinsic resistance of C. glabrata to fluconazole, although multiple mechanisms of azole resistance are suggested.
ACKNOWLEDGMENTS
We thank F. Ford for proofreading the manuscript and M. Sudoh, M. Kokado, and M. Izuta for experimental help.
REFERENCES
- 1.Albertson G D, Niimi M, Cannon R D, Jenkinson H F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841. doi: 10.1128/aac.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bard M, Lees N D, Turi T, Craft D, Cofrin L, Barbuch R, Koegel C, Loper J C. Sterol synthesis and viability of erg11 (cytochrome P450 lanosterol demethylase) mutation in Saccharomyces cerevisiae and Candida albicans. Lipids. 1993;28:963–967. doi: 10.1007/BF02537115. [DOI] [PubMed] [Google Scholar]
- 3.Cannon R D, Kerridge D. Correlation between the sterol composition of membranes and morphology in Candida albicans. J Med Vet Mycol. 1988;26:57–65. doi: 10.1080/02681218880000071. [DOI] [PubMed] [Google Scholar]
- 4.Fidel P L, Jr, Vazquez J A, Sobel J D. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12:80–96. doi: 10.1128/cmr.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortun J, Lopez-San Roman A, Velasco J J, Sanchez-Sousa A, de Vicente E, Nuno J, Quereda C, Barcena R, Monge G, Candela A, Honrubia A, Guerrero A. Selection of Candida glabrata strains with reduced susceptibility to azoles in four liver transplants patients with invasive candidiasis. Eur J Clin Microbiol Infect Dis. 1997;16:314–318. doi: 10.1007/BF01695638. [DOI] [PubMed] [Google Scholar]
- 6.Geber A, Hitchcock C A, Swartz J E, Pullen F S, Marsden K E, Kwon-Chung K J, Bennett J E. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob Agents Chemother. 1995;39:2708–2717. doi: 10.1128/aac.39.12.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghannoum M A, Spellberg B J, Ibrahim A S, Ritchie J A, Currie B, Spitzer E D, Edwards J E, Jr, Casadevall A. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob Agents Chemother. 1994;38:2029–2033. doi: 10.1128/aac.38.9.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gietz R D, Schiestl R H, Willems A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 9.Henry K W, Nickels J T, Edlind T D. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob Agents Chemother. 2000;44:2693–2700. doi: 10.1128/aac.44.10.2693-2700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly S L, Arnoldi A, Kelly D E. Molecular genetic analysis of azole antifungal mode of action. Biochem Soc Trans. 1993;21:1034–1038. doi: 10.1042/bst0211034. [DOI] [PubMed] [Google Scholar]
- 12.Kelly S L, Lamb D C, Corran A J, Baldwin B C, Kelly D E. Mode of action and resistance to azole antifungals associated with the formation of 14α-methylergosta-8, 24(28)-dien-3β,6α-diol. Biochem Biophys Res Commun. 1995;207:910–915. doi: 10.1006/bbrc.1995.1272. [DOI] [PubMed] [Google Scholar]
- 13.Kontoyiannis D P, Sagar N, Hirschi K D. Overexpression of Erg11p by the regulatable GAL1 promoter confers fluconazole resistance in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1999;43:2798–2800. doi: 10.1128/aac.43.11.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koul A, Vitullo J, Reyes G, Ghannoum M. Effect of voriconazole on Candida glabrata in vitro. J Antimicrob Chemother. 1999;44:109–112. doi: 10.1093/jac/44.1.109. [DOI] [PubMed] [Google Scholar]
- 15.Lamb D C, Kelly D E, Schunck W-H, Shyadehi A Z, Akhtar M, Lowe D J, Baldwin B D, Kelly S L. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole-resistance through reduced affinity. J Biol Chem. 1997;272:5682–5688. doi: 10.1074/jbc.272.9.5682. [DOI] [PubMed] [Google Scholar]
- 16.Lees N D, Broughton M C, Sanglard D, Bard M. Azole susceptibility and hyphal formation in a cytochrome P-450-deficient mutant of Candida albicans. Antimicrob Agents Chemother. 1990;34:831–836. doi: 10.1128/aac.34.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees N D, Skaggs B, Kirsch D R, Bard M. Cloning of the late genes in the ergosterol biosynthesis pathway of Saccharomyces cerevisiae—a review. Lipids. 1995;30:221–226. doi: 10.1007/BF02537824. [DOI] [PubMed] [Google Scholar]
- 18.Lees N D, Bard M, Kirsch D R. Biochemistry and molecular biology of sterol synthesis in Saccharomyces cerevisiae. Crit Rev Biochem Mol Biol. 1999;34:33–47. [PubMed] [Google Scholar]
- 19.Löffler J, Kelly S L, Hebart H, Schumacher U, Loss-Flörl C, Einsele H. Molecular analysis of cyp51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol Lett. 1997;151:263–268. doi: 10.1111/j.1574-6968.1997.tb12580.x. [DOI] [PubMed] [Google Scholar]
- 20.Marichal P, Koymans L, Willemsens S, Bellens D, Yerhasselt P, Luyten W, Borgers M, Ramaekers F C, Odds F C, Vanden Bossche H. Contribution of mutations in the cytochrome P450 14α-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology. 1999;145:2701–2713. doi: 10.1099/00221287-145-10-2701. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki H, Miyazaki Y, Geber A, Parkinson T, Hitchcock C, Falconer D J, Ward D J, Marsden K, Bennett J E. Fluconazole resistance associated with drug efflux and increased transcription of drug transporter gene, PHD1, in Candida glabrata. Antimicrob Agents Chemother. 1998;42:1695–1701. doi: 10.1128/aac.42.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagahashi S, Nakayama H, Hamada K, Yang H, Arisawa M, Kitada K. Regulation by tetracycline of gene expression in Saccharomyces cerevisiae. Mol Gen Genet. 1997;255:372–375. doi: 10.1007/s004380050508. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama H, Izuta M, Nagahashi S, Sihta E Y, Sato Y, Yamazaki T, Arisawa M, Kitada K. A controllable gene-expression system for the pathogenic fungus Candida glabrata. Microbiology. 1998;144:2407–2415. doi: 10.1099/00221287-144-9-2407. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama H, Izuta M, Nakayama N, Arisawa M, Aoki Y. Depletion of the squalene synthase (ERG9) gene does not impair growth of Candida glabrata in mice. Antimicrob Agents Chemother. 2000;44:2411–2418. doi: 10.1128/aac.44.9.2411-2418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen M H, Peacock J E, Jr, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: emergence of non-C. albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 26.Nolte F S, Parkinson T, Falconer D J, Dix S, Williams J, Gilmore C, Geller R, Wingard J R. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob Agents Chemother. 1997;41:196–199. doi: 10.1128/aac.41.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaller M A, Jones R N, Messer S A, Edmond M B, Wenzel R P. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis. 1998;30:121–129. doi: 10.1016/s0732-8893(97)00240-x. [DOI] [PubMed] [Google Scholar]
- 28.Pfaller M A, Messer S A, Houston A, Rangel-Frausto M S, Wiblin T, Blumberg H M, Edwards J E, Jarvis W, Martin M A, Neu H C, Saiman L, Patterson J E, Dibb J C, Roldan C M, Rinaldi M G, Wenzel R P. National epidemiology of mycoses survey: a multicenter study of strain variation and antifungal susceptibility among isolates of Candida species. Diagn Microbiol Infect Dis. 1998;31:289–296. doi: 10.1016/s0732-8893(97)00245-9. [DOI] [PubMed] [Google Scholar]
- 29.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C D for the NIAID Mycoses Study Group and the Candidemia Study Group. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosamond J, Allsop A. Harnessing the power of genome in the search for new antibiotics. Science. 2000;287:1973–1976. doi: 10.1126/science.287.5460.1973. [DOI] [PubMed] [Google Scholar]
- 32.Rose M D, Winston F, Hieter P. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. pp. 128–129. [Google Scholar]
- 33.Sanglard D, Ischer F, Calabrese D, Majcherczyk P A, Bille J. The ABC binding cassette transporter gene CgCDR1 from Candida glabrata is involved in resistance of clinical isolate to azole antifungal agents. Antimicrob Agents Chemother. 1999;43:2753–2765. doi: 10.1128/aac.43.11.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 37.Sanglard D, Kuchler K, Ischer F, Pagani J-L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor F R, Rodriguez R J, Parks L W. Requirement for a second sterol biosynthetic mutation for viability of a sterol C-14 demethylation defect in Saccharomyces cerevisiae. J Bacteriol. 1983;155:64–68. doi: 10.1128/jb.155.1.64-68.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanden Bossche H, Koymans L, Moereels H. P450 inhibitors of use in medical treatment: focus on mechanisms of action. Pharmacol Ther. 1995;67:79–100. doi: 10.1016/0163-7258(95)00011-5. [DOI] [PubMed] [Google Scholar]
- 40.Vanden Bossche H, Marichal P, Gorrens J, Bellens D, Moereels H, Janssen P A J. Mutation in cytochrome P450-dependent 14α demethylase results in decreased affinity for azole antifungals. Biochem Soc Trans. 1990;18:56–59. doi: 10.1042/bst0180056. [DOI] [PubMed] [Google Scholar]
- 41.Vanden Bossche H, Marichal P, Le Jeune L, Coene M C, Gorrens J, Cools W. Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethyation and reduction of 3-ketosteroids in Cryptococcus neoformans. Antimicrob Agents Chemother. 1993;37:2101–2105. doi: 10.1128/aac.37.10.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanden Bossche H, Dromer F, Improvisi I, Lozano-Chiu M, Rex J H, Sanglard D. Antifungal drug resistance in pathogenic fungi. Med Mycol. 1998;36(Suppl. I):119–128. [PubMed] [Google Scholar]
- 43.Venkateswarlu K, Denning D W, Manning N J, Kelly S L. Reduced accumulation of drug in Candida krusei accounts for itraconazole resistance. Antimicrob Agents Chemother. 1996;40:2443–2446. doi: 10.1128/aac.40.11.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson P F, Rose M E, Kelly S L. Isolation and analysis of ketoconazole mutants of Saccharomyces cerevisiae. J Med Vet Mycol. 1988;26:153–162. doi: 10.1080/02681218880000231. [DOI] [PubMed] [Google Scholar]
- 45.White T C. Increased mRNA levels of ERG16: CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolate from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida Y, Aoyama Y. Interaction of azole antifungal agents with cytochrome P45014DM purified from Saccharomyces cerevisiae microsome. Biochem Pharmacol. 1987;36:229–235. doi: 10.1016/0006-2952(87)90694-0. [DOI] [PubMed] [Google Scholar]