Abstract
Background
Frailty can be operationalised using the deficit accumulation approach, which considers health deficits across multiple domains. We aimed to develop, validate and compare three different frailty indices (FI) constructed from self-reported health measures (FI-Self Report), blood-based biomarkers (FI-Blood) and examination-based assessments (FI-Examination).
Methods
Up to 30,027 participants aged 45–85 years from the baseline (2011–2015) comprehensive cohort of the Canadian Longitudinal Study on Aging were included in the analyses. Following standard criteria, three FIs were created: a 48-item FI-Self Report, a 23-item FI-Blood and a 47-item FI-Examination. In addition a 118-item FI-Combined was constructed. Mortality status was ascertained in July 2019.
Results
FI-Blood and FI-Examination demonstrated broader distributions than FI-Self Report. FI-Self Report and FI-Blood scores were higher in females, whereas FI-Examination scores were higher in males. All FI scores increased nonlinearly with age and were highest at lower education levels. In sex and age-adjusted models, a 0.01 increase in FI score was associated with a 1.08 [95% confidence interval (CI): 1.07,1.10], 1.05 (1.04,1.06), 1.07 (1.05,1.08) and a 1.13 (1.11,1.16) increased odds of mortality for FI-Self Report, FI-Blood, FI-Examination and FI-Combined, respectively. Inclusion of the three distinct FI types in a single model yielded the best prognostic accuracy and model fit, even compared to the FI-Combined, with all FIs remaining independently associated with mortality.
Conclusion
Characteristics of all FIs were largely consistent with previously established FIs. To adequately capture frailty levels and to improve our understanding of the heterogeneity of ageing, FIs should consider multiple types of deficits including self-reported, blood and examination-based measures.
Keywords: Frailty, ageing, Canadian Longitudinal Study on Aging (CLSA), epidemiology, older people
Key Points
The frailty indices examined demonstrated some differences in characteristics.
All frailty indices were independently associated with higher mortality risk.
Mortality prediction was strongest in the model that included all three deficit types.
Future frailty assessments should consider self-reported, blood and examination-based measures.
Introduction
Examining the frailty levels of middle-aged and older adults can improve our understanding of the heterogeneity of ageing and the subsequent impact on health and mortality. As people age, the risk of mortality increases. Even so, this risk varies considerably for those of the same age. The deficit accumulation approach to frailty suggests that heterogeneity in mortality risk with age reflects that people accumulate deficits at varying rates, and that, at any age, the risk is greatest in those with the greatest number of deficits [1].
This approach operationalises frailty with a frailty index (FI) score, which considers the number of deficits present as a proportion of all possible health deficits [2, 3]. Compared with a single health measure, continuous FI scores can accurately reflect an individual’s current state and better capture variability between individuals and within individuals over time [4]. This approach, which is strengthened by its reproduceable mathematical and clinical characteristics [1, 5], has been widely applied across clinical and research settings in community-dwelling and clinical samples of all ages [6–15].
FIs were historically constructed as a single continuous score to capture health across multiple domains [2, 4]. Studies have demonstrated meaningful variability in constructing FIs from different measurement types [16–18]. For example, FIs based on blood or urine biomarkers reflect an accumulation of damage at a physiological level [17–20]; this subclinical dysregulation is hypothesised to scale up and manifest clinically in the form of disability or comorbidity [21, 22]. Previous comparison of self-reported and examination-based FIs has suggested that self-reported deficits may underreport frailty [16]. Often, measures such as pulse or pressure have been included in a lab-based FI to achieve a minimum number of deficits required for an FI [16, 18, 22]. This has made it challenging to ascertain differences between subclinical and both objective and subjective clinical deficits.
Despite differences in the measurement, manifestation and temporality by which different deficits may arise, there is very little evidence comparing FIs that consist solely of each item type. Using data from the Canadian Longitudinal Study on Aging, our aim was to develop, validate and compare three frailty indices consisting of self-reported, blood biomarker and objective examination-based deficits. In addition, we report results for males and females separately and provide reference documentation to promote future investigation of frailty in this cohort.
Methods
Sample
The Canadian Longitudinal Study on Aging (CLSA) is a longitudinal study of 51,338 community-dwelling Canadians aged 45–85 years at baseline. CLSA has a tracking cohort, with telephone-based questionnaires, and a comprehensive cohort, with clinical, biological and physical assessments from both home and data collection site (DCS) visits. Between 2010 and 2015, 30,097 participants were randomly selected to take part in the comprehensive baseline data collection. Participants were eligible if they lived within 25–50 km of 1 of 11 DCSs across 7 Canadian provinces. Additional exclusion criteria included those living on federal First Nations reserves, full-time members of the Canadian Armed Forces, those unable to conduct an interview in English or French, those with cognitive impairment at the time of recruitment and institutionalised individuals. Further information on the CLSA objectives, protocol and sample is available elsewhere [23, 24].
FI construction
Three distinct FIs were constructed from self-reported items (FI-Self Report), blood biomarkers (FI-Blood) or physical and cognitive examination-based deficits (FI-Examination). In addition, an FI-Combined was derived to include all deficits. Indices were constructed based on four standard criteria [3]; deficits must (i) be health-related; (ii) increase with age; (iii) not saturate too early and (iv) cover a range of health domains. Additional criteria were followed with two minor modifications [21]. First, although deficits with a low prevalence (<1%) are typically excluded, items with low prevalence remained eligible for inclusion. This follows a previous approach to a CLSA-based FI [25], which argues that the prevalence of these items is expected to meaningfully increase over 20-years of follow-up in the CLSA given the young age of participants. Second, deficits with missing data in >5% of the study population are typically excluded. However, as there were higher levels of missingness for entire testing domains (e.g. blood, spirometry), exclusion of health domains would have violated the 4th criterion above and these items were included. Details of all items are provided in Appendix 1.
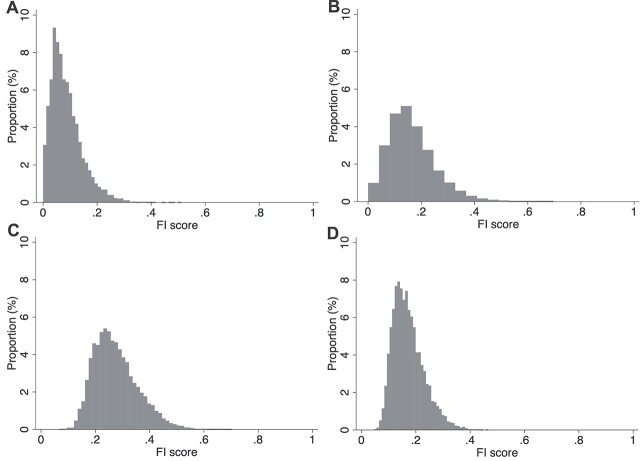
Figure 1.
Distribution of (A) FI-Self Report; (B) FI-Blood; (C) FI-Examination and (D) FI-Combined.
Deficits were coded on a 0–1 scale, where 0 indicates no deficit and 1 indicates the highest level of deficit, and could be binary, ordinal or continuous. For example, chronic conditions such as diabetes were binary, where 0 indicates no diabetes and 1 indicates the presence of diabetes. Ordinal deficits included items such as self-reported health where 1 indicated poor health, 0.75 indicated fair health, 0.5 good, 0.25 very good and 0 excellent health. For continuous variables with meaningful variation in performance (e.g. physical performance, cognitive scores), data were normalised such that 0 indicated no deficit and 1 indicated the maximal deficit.
FI scores were calculated as the number of deficits present divided by the number of deficits considered (e.g. the FI score of an individual with 20/50 deficits = 0.40). Scores ranged from 0 to a theoretical maximum of 1. Individuals must have information for at least 80% of the deficits to calculate an FI score.
Outcomes
Mortality was ascertained by CLSA using three methods: linkage to provincial vital statistics, attempted contact with participants between data collection waves or direct contact from next of kin. Censoring or mortality time was calculated from the date of the DCS visit to 1 July 2019. Exact time to death was not available.
Statistical analysis
Distributions of each FI were examined using histograms and summary measures (means ±SD, ranges, 99th percentiles). One-way analysis of variances and Bonferroni post hoc tests examined differences in FI scores across sex, age groups and education levels. Mean FI scores for males and females were plotted at each year of age and sympercents were used to assess the annual rate of increase in FI score [26]. Here, FI scores were transformed using the natural log (+0.0001 added to scores to circumvent zero scores) and regressed as the dependent variable to estimate percent change in FI per 1-year age increase. Correlations between FIs were assessed using Pearson’s r. Sex and age-adjusted logistic regression models examined associations between FI score and mortality. Each FI was first considered in an individual model; subsequently, the three individual FIs were included in the same model. Areas under the curves (AUCs) and Akaike information criterion (AIC) were calculated for each model. The difference between AUCs indicated the prognostic accuracy of the models, whereas AICs allowed comparison of model fit, with a lower value suggesting a better model fit. Sensitivity analyses reported all results separately for males and females, examined differences in characteristics between the analytical and excluded samples and replicated all analyses using the maximal sample size for each FI. Following CLSA recommendations [27], we used inflation weights for descriptive statistics and analytic weights for regression models to provide population-representative estimates. Data analysis was conducted in Stata 16. An alpha of 0.05 was used to determine statistical significance.
Results
FI derivation
We screened 204 health-related items, including 71 self-reported deficits, 31 blood-based deficits and 109 examination-based deficits. Across the three FIs, 118 items were included (Table 1). A comprehensive data dictionary of FI-Blood and FI-Examination deficits including a list of excluded variables is provided in Appendix 1; the corresponding data dictionary for the FI-Self Report has been previously published [25]. Stata syntax for all FIs is available in Appendix 2.
Table 1.
Items included in the FI-Self Report, FI-Blood and FI-Examination
| FI-SELF REPORT (48 ITEMS) | FI-BLOOD (23 ITEMS) | FI-EXAMINATION (47 ITEMS) | ||
|---|---|---|---|---|
| Chronic conditions | Self-rated health | 1. Albumin | Physical performance | Hearing and vision |
| 1. Osteoarthritis | 31. General health | 2. Cholesterol | 1. Standing balance | 24. Visual acuity, left eye |
| 2. Arthritis | 32. Vision | 3. Creatinine | 2. Timed 4-metre walk | 25. Visual acuity, right eye |
| 3. Chronic obstructive pulmonary disease4. High blood pressure5. Diabetes mellitus6. Chronic heart failure7. Angina8. Acute myocardial infarction9. Peripheral vascular disease10. Stroke11. Transient ischemic attack12. Memory problem13. Alzheimer’s disease14. Parkinson’s disease15. Peptic ulcer disease16. Colitis17. Bowel incontinence18. Urinary incontinency19. Cataracts20. Glaucoma21. Macular degeneration22. Cancer23. Osteoporosis24. Back pain25. Hypothyroidism26. Hyperthyroidism27. Kidney failure28. Pneumonia29. Urinary tract infection30. Falls | 33. HearingActivities of daily living34. Dressing35. Grooming36. Walking37. Getting in/out of bed38. BathingInstrumental activities ofdaily living39. Using the phone40. Using transport41. Shopping42. Cooking43. Doing housework44. Taking medicine45. Managing moneyMental health46. Effort47. Felt lonely48. Could not get going | 4. Estimated Glomerular Filtration Rate5. Ferritin6. Free thyroxine7. Granulocytes8. Haemoglobin A1c9. Haematocrit10. Haemoglobin11. High Sensitivity C-Reactive Protein12. Lymphocytes13. Mean corpuscular haemoglobin14. Mean corpuscular volume15. Monocytes16. Mean platelet volume17. Platelets18. Red blood cells19. Red blood cell distributionwidth20. Triglycerides21. Thyroid-Stimulating hormone22. 25-hydroxyvitamin D23. White blood cells | 3. Chair rise4. Timed Get Up and Go5. Grip strengthCognition6. Immediate recall7. Delayed recall8. Mental Alteration Test9. Animal fluency10. Controlled Oral Word Association11. Time-based memory12. Event-based memory13. Choice reaction time14. Stroop interference timeAnthropometric measures15. Body mass index16. Waist-hip ratio17. Whole body bone mineral density, T-score18. Bone mineral density in multiple body regions19. Appendage lean mass20. Body fat percent21. High adiposity in multiple body regionsSpirometry22. Forced Vital Capacity (FVC)23. Forced Expiratory Volume 1 / FVC Ratio | 26. Intraocular pressure, right27. Intraocular pressure, left28. Corneal hysteresis, right29. Corneal hysteresis, left30. Mean ocular perfusion pressure31. Hearing pure tone average, right32. Hearing pure tone average, leftCardiac33. Systolic BP34. Diastolic BP35. Pulse36. Pulse pressure37. Average carotid intima thickness, right side38. Average carotid intima thickness, left side39. Presence of plaques (max carotid intima thickness)40. ECG diagnosis summary41. ECG, PQ interval42. ECG, QRS duration43. ECG, QT interval44. ECG, P axis45. ECG, R axis46. ECG, T axis47. ECG, P duration |
The FI-Self Report was adapted from an FI constructed for use in both the tracking and pooled CLSA cohorts [25]. It consists of 48 self-reported deficits across five domains: self-rated health, chronic conditions, activities of daily living, instrumental activities of daily living and mental health. Cognitive scores from the original 52-item FI were excluded. Of 30,097 eligible participants, 30,027 (99.8%) had a valid score (≥39 items).
The FI-Blood consists of 23 biomarkers from chemistry and haematological reports (Table 1). Although 27,341 (90.8%) had blood tests, only 25,418 (84.5%) had haematological analyses. Of these, 25,253 (99.4%; 83.9% of full sample) had a valid FI-Blood score (≥19 items).
The FI-Examination consists of 47 deficits across six domains: physical performance, cognition, cardiac, anthropometric, spirometry, and hearing and vision (Table 1). Due to the lack of clinical reference ranges and the informative variability in performance across most assessments, normative coding of many deficits was used (Appendix 1). Valid FI-Examination scores (≥38 items) could be calculated for 29,341 (97.5%) individuals.
The FI-Combined consists of all 118 self-reported, blood and examination-based deficits. To ensure items from all three FIs were included in the FI-Combined calculation, scores could only be derived for the individuals who had blood tests in addition to the minimum ≥95 total items (n = 26,921; 89.4%). The primary analytical sample consists of the 24,780 (82.3%) individuals with a valid score for all four FIs.
FI characteristics
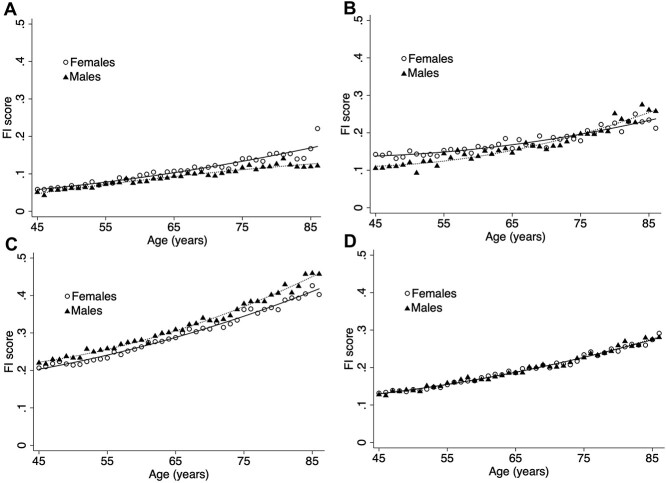
FI distributions differed (Figure 1). FI-Self Report exhibited a strong positive skew, with a long right tail (range: 0.00–0.47). FI-Blood, also right-skewed, showed a broader distribution (range 0.00–0.70). Finally, the FI-Examination (range: 0.07–0.70) and the FI-Combined (range: 0.05–0.47) were well-fitted by gamma distributions. The 99th percentiles were 0.26, 0.43, 0.49 and 0.34, respectively. Mean frailty scores (±SD) were higher in females compared with males for FI-Self Report (0.09 ± 0.06 vs. 0.08 ± 0.05) and FI-Blood (0.16 ± 0.09 vs. 0.14 ± 0.10), but lower for FI-Examination (0.27 ± 0.08 vs. 0.28 ± 0.08) (Table 2). There were minimal sex differences in FI-Combined scores, with slightly higher scores in males (Table 2). Sex differences were constant across the full age range for FI-Self Report and FI-Examination; however, the pattern appeared to reverse after age 75 for FI-Blood (Figure 2).
Table 2.
Mean frailty scores ±SD by age, sex and education
| Sample size (n, weighted %) |
FI-Self Report | FI-Blood | FI-Examination | FI-Combined | |
|---|---|---|---|---|---|
| Sex: | |||||
| Male | 12,326 (48.2) | 0.08 ± 0.05 | 0.14 ± 0.10 | 0.28 ± 0.08a | 0.17 ± 0.05 |
| Female | 12,454 (51.8) | 0.09 ± 0.06a | 0.16 ± 0.09a | 0.27 ± 0.08 | 0.17 ± 0.06ab |
| Age group: | |||||
| 45–54 | 6,293 (39.5) | 0.06 ± 0.04c | 0.13 ± 0.08c | 0.23 ± 0.06c | 0.14 ± 0.04c |
| 55–64 | 8,228 (31.0) | 0.08 ± 0.05 | 0.15 ± 0.09 | 0.27 ± 0.06 | 0.17 ± 0.05 |
| 65–74 | 6,064 (18.3) | 0.10 ± 0.06 | 0.17 ± 0.10 | 0.32 ± 0.07 | 0.20 ± 0.05 |
| 75+ | 4,195 (11.1) | 0.13 ± 0.06 | 0.21 ± 0.10 | 0.38 ± 0.07 | 0.25 ± 0.05 |
| Education: | |||||
| Less than secondary school graduation | 1,317 (16.7) | 0.11 ± 0.06d | 0.18 ± 0.10d | 0.32 ± 0.08d | 0.20 ± 0.06d |
| Secondary school graduation | 2,360 (11.4) | 0.09 ± 0.05 | 0.16 ± 0.10 | 0.28 ± 0.08 | 0.18 ± 0.05 |
| Some post-secondary | 1,823 (9.2) | 0.09 ± 0.06 | 0.16 ± 0.10 | 0.27 ± 0.08 | 0.17 ± 0.06 |
| Post-secondary degree/diploma | 19,244 (62.7) | 0.08 ± 0.05 | 0.14 ± 0.09 | 0.26 ± 0.08 | 0.16 ± 0.05 |
Higher FI score (P < 0.001)
Mean FI-Combined scores: 0.170 in males; 0.173 in females (P < 0.001)
Differences between all age groups (Bonferroni post hoc; all P < 0.001)
Differences between education groups except secondary school and some post-secondary (Bonferroni post hoc; all p < 0.001)
Figure 2.
Age association by sex of the (A) FI-Self Report; (B) FI-Blood; (C) FI-Examination and (D) FI-Combined.
All FI scores increased nonlinearly with age (Table 2, Figure 2). Each additional year of age was associated with a 3.39% [95% confidence interval (CI): 3.27,3.50] increase in FI-Self Report, 2.77% (2.59,2.95) increase in FI-Blood, 1.78% (1.75,1.81) increase in FI-Examination and 1.95% (1.92,1.98) increase in FI-Combined. Across all FIs, those with less than secondary school graduation had the highest frailty scores, and those with post-secondary degree/diploma had the lowest frailty scores (Table 2). There were no differences between those who graduated from secondary school and those who completed some post-secondary education. FI-Self Report was most strongly correlated with FI-Examination (r = 0.48), whereas FI-Blood had weaker correlations with each (r = 0.33 for both).
FI validation
As of July 2019, 2.2% (702/24,780) of the analytical sample had died. The average time between baseline data collection and the date of mortality ascertainment was 5.5 ± 0.8 years (range: 4.1–7.2). In individual sex and age-adjusted models, a 0.01 increase in FI score was associated with a 1.08 (95% CI: 1.07,1.10), 1.05 (1.04,1.06) and 1.07 (1.05,1.08) increased odds of mortality for the FI-Self Report, FI-Blood and FI-Examination, respectively (Models 1–3, Table 3). A 0.01 increase in the 118-item FI-Combined score was associated with a 1.13 (1.11,1.16) increased odds of mortality (Model 4). AUCs for the three individual FIs did not have statistically significant differences when compared (all AUCs = 0.79; Table 3). However, the AUC for the FI-Combined model was significantly higher than any individual FI (AUC: 0.805 (0.789,0.822); all P < 0.001).
Table 3.
Logistic regression results demonstrated increased risk of mortality as of July 2019 per 0.01 increase in FI score (n = 24,780)
| FI-Self Report | FI-Blood | FI-Examination | FI-Combined | |
|---|---|---|---|---|
| MALES AND FEMALES | ||||
| Models 1-4: adjusted for age and sex | ||||
| Odds ratio (95% CI) | 1.08 (1.07, 1.10) | 1.05 (1.04, 1.06) | 1.07 (1.05, 1.08) | 1.13 (1.11, 1.16) |
| AUC | 0.793 (0.776, 0.809) | 0.787 (0.770, 0.804) | 0.785 (0.767, 0.802) | 0.805 (0.789, 0.822) |
| Model 5: adjusted for age, sex, FI-Self Report, FI-Blood and FI-Examination | ||||
| Odds ratio (95% CI) | 1.05 (1.03, 1.07) | 1.03 (1.02, 1.04) | 1.04 (1.02, 1.05) | — |
| AUC | 0.807 (0.791, 0.823) | |||
| MALES | ||||
| Models 1-4: adjusted for age | ||||
| Odds ratio (95% CI) | 1.09 (1.07, 1.12) | 1.05 (1.03, 1.06) | 1.08 (1.06, 1.10) | 1.14 (1.11, 1.17) |
| AUC | 0.797 (0.777, 0.817) | 0.794 (0.774, 0.815) | 0.789 (0.768, 0.810) | 0.814 (0.794, 0.834) |
| Model 5: adjusted for age, FI-Self Report, FI-Blood and FI-Examination | ||||
| Odds ratio (95% CI) | 1.06 (1.03, 1.08) | 1.03 (1.02, 1.04) | 1.05 (1.03, 1.07) | — |
| AUC | 0.816 (0.796, 0.835) | |||
| FEMALES | ||||
| Models 1-4: adjusted for age | ||||
| Odds ratio (95% CI) | 1.08 (1.05, 1.10) | 1.05 (1.03, 1.07) | 1.06 (1.03, 1.08) | 1.12 (1.08, 1.16) |
| AUC | 0.774 (0.747, 0.802) | 0.761 (0.721, 0.790) | 0.763 (0.735, 0.792) | 0.782 (0.755, 0.809) |
| Model 5: adjusted for age, FI-Self Report, FI-Blood and FI-Examination | ||||
| Odds ratio (95% CI) | 1.05 (1.02, 1.08) | 1.04 (1.02, 1.06) | 1.02 (1.00, 1.05) | — |
| AUC | 0.782 (0.755, 0.810) | |||
When the three individual FIs were included in the same model (Model 5, Table 3), each remained independently associated with mortality. Estimates attenuated to 1.05 (1.03, 1.07) for FI-Self Report, 1.03 (1.02,1.04) for FI-Blood and 1.04 (1.02,1.05) for FI-Examination. Comparison of the difference between AUCs suggested that the model with all 3 FI scores had stronger prognostic discriminatory ability (AUC: 0.807 (0.791,0.823); P < 0.001) compared with any individual model, including FI-Combined (P = 0.04). AICs were 5,556, 5,577, 5,593 and 5,431 for FI-Self Report, FI-Blood, FI-Examination and FI-Combined, respectively; the lowest AIC appeared when all three FIs were included in the same model (5425).
Sensitivity analyses
When stratified by sex, differences in FI scores were reflected in a slight shift in distribution (Appendix 3). Differences in scores by age and education level were consistent between males and females, although there was some evidence to suggest that associations between frailty and mortality, particularly for FI-Examination, were stronger in males (Appendix 4). Compared with the analytical sample, those who were excluded from analyses due to missing one or more FI scores (n = 5,317) were older, had lower levels of education, were more commonly female and had higher mortality rates (Appendix 5). A subset of those with valid scores for any FI was compared with the main analytical sample; all FI scores were higher in the excluded sample (P < 0.001; Appendix 5). Finally, analyses were replicated in the maximal available sample for each FI. Results did not change, although there were minor increases in effect size and improvement in prognostic accuracy indicators (Appendix 6).
Discussion
Using nationally representative data from the CLSA, FIs were constructed from self-reported measures (FI-Self-Report), blood biomarkers (FI-Blood) and examination-based scores (FI-Examination). Each FI demonstrated characteristics consistent with previously established FI properties, with some differences between FI type. Notably, blood and examination-based deficits were detectable at younger ages than self-reported deficits, as demonstrated by the wider distribution and higher intercept. Females had higher FI-Blood and FI-Self Report scores, with slight evidence of higher FI-Examination scores in males. Finally, higher scores in all FIs were independently associated with greater mortality risk. Prognostic accuracy and model fit were highest when all three FIs were included in a single model suggesting that there is benefit in considering individual FI types. We have shared the coding syntax to promote reproducibility and encourage further investigation of frailty in the CLSA cohorts; researchers should consider one or more of the FIs to best support their research question.
Previous studies investigating FI type have frequently combined the items explored in this study; for example, clinical FIs have included self-reported and objective deficits [28, 29] and assessment and laboratory-based FIs have each included examination and blood-based deficits [16–18, 28, 29]. Given the overlap of deficit type within these FIs, direct comparison to studies is limited due to differences in the accuracy (e.g. recall bias for self-reported items, missing data in clinical records) and nature of individual items. For example, other FIs may have collected similar comorbidities using other methods such as electronic health records (eFI) [9] or clinician assessments (e.g. FI-CGA) [30]. However, it is notable that we report similar differences in FI-Self Report and FI-Blood as seen in comparisons of clinical and laboratory-based FIs [17, 18, 28, 29]. These studies reported that laboratory-based FIs have higher mean scores, wider distributions and smaller associations with mortality. This is consistent with the hypothesis that blood or laboratory-based FIs may capture deficit accumulation at the cellular level before clinical deficits arise. The reversal of sex differences in FI-Blood scores was also observed in a US-based cohort study, which reported an earlier reversal between ages 60 and 70 [31].
Our findings are also consistent with self-reported and assessment-based FIs constructed in the Irish Longitudinal study on Ageing including a wider distribution and higher scores in males for the assessment-based FI [16]. The assessment-based FI had stronger associations with mortality; here we did not observe this, despite a similar age range and mortality rate. Further investigation of sex differences in self-reported and examination-based assessments may improve understanding of the male–female health survival paradox that is widely observed in frailty [32–34]. Differences in associations with mortality may reflect underreporting of self-reported items in males or greater likelihood of health-seeking behaviours in females that could lead to better recognition and diagnosis of health problems [35, 36]. Finally, cultural or socioeconomic differences, differences in health seeking behaviours and access to health care, could partially explain discordance between subjective and objective health measures [35, 37–39].
Although dissimilarities between FIs may be partially attributed to both the level of deficit accumulation (e.g. subclinical, clinical) or the measurement type (e.g. objective, subjective), examination-based frailty deficits may provide an additional intermediary process between subclinical FI-Blood and clinical FI-Self Report deficits. Early variability in certain examination-based measures may reflect deficits at the organ or system level that have scaled up from damage at the cellular level (e.g. slower walking speed, higher intraocular pressure) [40, 41]. Normalised coding facilitates the measurement of this variability at early stage and could allow it to be captured before it arises clinically, for example, in the form of mobility or visual impairment.
Although adding more deficits to an FI can strengthen its predictive validity, it is not well understood whether this arises from increased information value or from the type of items added [42, 43]. The independent contribution of each FI in predicting mortality risk was a significant finding as the three FI model provided statistically improved prognostic accuracy and model fit compared with the combined 118-item FI. This suggests that, in addition to improved risk prediction with a greater number of items, the nature and diversity of items may improve the predictive validity of FI scores. Self-reported, blood and examination-based health deficits capture different components of frailty and should each be considered when measuring frailty. Further understanding of the information value of FI type may expand our understanding of how deficit types contribute to overall frailty assessment and related outcomes.
There are some methodological limitations of the study. Although the CLSA is designed as a nationally representative cohort study, individuals with cognitive impairment, full-time members of the Canadian Armed Forces, those in long-term care institutions or those living on reserves or other Aboriginal settlements were not eligible to participate. In addition, individuals who lived in the three territories, Prince Edward Island, New Brunswick, Saskatchewan or in more rural settings (e.g. >25–50 km from a DCS) could not be included in the comprehensive cohort. Investigation of representativeness at a municipal-level suggests that the CLSA may not be fully representative of ethnic diversity nor of lower socioeconomic position [44]. CLSA is a rich resource for studying associations; however, precision around estimates requires caution in interpretation. The exact time to death is not available and as such, we were limited to logistic regressions which may have overestimated associations with mortality. In addition, the number of deaths was small, although sufficient to demonstrate associations between frailty and mortality with effect sizes comparable to other studies [16–18, 28]. Although nearly 10% of the sample were missing blood samples, the sample size remained large and sensitivity analyses indicated that associations may be larger in the maximal sample.
Conclusion
This study has shown the utility of considering deficits from self-reported, blood and examination-based items in frailty measurement. Further research is needed to be better understand the mechanisms through which each may independently or additively contribute to risk of mortality and other health outcomes including differences in accumulation or reporting of deficits in males and females. Current work from our group is examining how baseline frailty is associated with interim health outcomes including frailty levels at follow-up, clinical diagnoses and health care use. In conclusion, the results of the present and comparable studies [16–18, 28, 29, 31] suggest that considering blood biomarkers and examination tests in addition to routinely collected self-reported data may improve frailty assessment and prediction of adverse outcomes. The feasibility of measuring such data in clinical and smaller scale research settings requires further investigation.
Supplementary Material
Acknowledgments
This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the Canadian Longitudinal Study on Aging (CLSA) is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation. This research has been conducted using the CLSA dataset, Baseline Comprehensive version 4.0 with Sample Weights version 1.2, under Application Number 1906015. The CLSA is led by Drs Parminder Raina, Christina Wolfson and Susan Kirkland.
Declaration of Sources of Funding
This work was supported by the Nova Scotia Health Research Foundation (grant number: MED-EST-2017-1,241) and the Canadian Institutes of Health Research (grant number: ACD-170302). The authors have no financial interest to declare in relation to the content of this article.
Declaration of Conflicts of Interest
The opinions expressed in this manuscript are the author’s own and do not reflect the views of the Canadian Longitudinal Study on Aging MKA reports grant funding from GSK, Sanofi, Pfizer and the Canadian Frailty Network, and honoraria from Sanofi, Pfizer and Seqirus, for work unrelated to the present study. KR asserts copyright of the Clinical Frailty Scale through Dalhousie University’s Industry, Liaison and Innovation Office; is a co-founder of Ardea Outcomes (formerly DGI Clinical), which has had contracts with pharmaceutical and device manufacturers (Biogen, Shire, Hollister, Novartis, Nutricia, Roche and Takeda) on individualised outcome measurement in the last 5 years; attended an advisory board meeting on dementia with Lundbeck in 2017; chaired a Scientific Workshop and Technical Review Panel on frailty for the Singapore National Research Foundation in 2020; received personal fees directly from event organisers for invited guest lectures, rounds, academic symposia and presentations on frailty; and is an Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes for Health Research, the Alzheimer Society of Canada and several other charities.
References
- [1]. Rockwood K, Howlett SE. Age-related deficit accumulation and the diseases of ageing. Mech Ageing Dev 2019; 180: 107–16. [DOI] [PubMed] [Google Scholar]
- [2]. Rockwood K, Song X, MacKnight Cet al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J 2001; 1: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Rockwood K, Mitnitski A. Frailty, fitness, and the mathematics of deficit accumulation. Rev Clin Gerontol 2007; 17: 1–12. [Google Scholar]
- [6]. Kehler DS, Ferguson T, Stammers ANet al. Prevalence of frailty in Canadians 18–79 years old in the Canadian health measures survey. BMC Geriatr 2017; 17: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Rockwood MR, MacDonald E, Sutton E, Rockwood K, Baron M. Canadian Scleroderma Research Group. Frailty index to measure health status in people with systemic sclerosis. J Rheumatol 2014; 41: 698–705. [DOI] [PubMed] [Google Scholar]
- [8]. Kennedy CC, Ioannidis G, Rockwood Ket al. A frailty index predicts 10-year fracture risk in adults age 25 years and older: results from the Canadian multicentre osteoporosis study (CaMos). Osteoporos Int 2014; 25: 2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Clegg A, Bates C, Young Jet al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45: 353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Guaraldi G, Brothers TD, Zona Set al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015; 29: 1633–41. [DOI] [PubMed] [Google Scholar]
- [11]. Geriatric Medicine Research Collaborative . Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing 2021; 50: 617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Abeliansky AL, Erel D, Strulik H. Aging in the USA: similarities and disparities across time and space. Sci Rep 2020; 10: 14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Li X, Ploner A, Wang Yet al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife 2020; 9: e51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Livshits G, Malkin I, Bowyer RCEet al. Multi-OMICS analyses of frailty and chronic widespread musculoskeletal pain suggest involvement of shared neurological pathways. Pain 2018; 159: 2565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Le Couteur DG, Stanaway F, Waite LMet al. Apolipoprotein E and health in older men: the concord health and ageing in men project. J Gerontol A Biol Sci Med Sci 2020; 75: 1858–62. [DOI] [PubMed] [Google Scholar]
- [16]. Theou O, O'Connell MD, King-Kallimanis BL, O'Halloran AM, Rockwood K, Kenny RA. Measuring frailty using self-report and test-based health measures. Age Ageing 2015; 44: 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med 2014; 12: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Blodgett J, Theou O, Howlett S, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geroscience 2017; 39: 447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Takeda C, Angioni D, Setphan Eet al. Age-related frailty: a clinical model for geroscience? J Nutr Health Aging 2020; 24: 1140–3. [DOI] [PubMed] [Google Scholar]
- [20]. Ritt M, Jäger J, Ritt JI, Sieber CC, Gaßmann KG. Operationalizing a frailty index using routine blood and urine tests. Clin Interv Aging 2017; 12: 1029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Rockwood K, Blodgett JM, Theou Oet al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep 2017; 7: 43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Bello GA, Lucchini RG, Teitelbaum SL, Shapiro M, Crane MA, Todd AC. Development of a physiological frailty index for the world trade center general responder cohort. Curr Gerontol Geriatr Res 2018; 2018: 3725926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Raina P, Wolfson C, Kirkland Set al. Cohort profile: the Canadian longitudinal study on Aging (CLSA). Int J Epidemiol 2019; 48: 1752–53j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Canadian Longitudinal Study on Aging (CLSA) . https://www.clsa-elcv.ca (14 March 2022, date last accessed).
- [25]. Pérez-Zepeda MU, Godin J, Armstrong JJet al. Frailty among middle-aged and older Canadians: population norms for the frailty index using the Canadian longitudinal study on Aging. Age Ageing 2021; 50: 447–56. [DOI] [PubMed] [Google Scholar]
- [26]. Cole TJ, Kryakin YV. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med 2000; 21: 2287–90. [DOI] [PubMed] [Google Scholar]
- [27]. Lara J, Cooper R, Nissan Jet al. A proposed panel of biomarkers of healthy ageing. BMC Med 2015; 13: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Blodgett JM, Theou O, Howlett SE, Wu FC, Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing 2016; 45: 463–8. [DOI] [PubMed] [Google Scholar]
- [29]. Rockwood K, McMillan M, Mitnitski A, Howlett SE. A frailty index based on common laboratory tests in comparison with a clinical frailty index for older adults in long-term care facilities. J Am Med Dir Assoc 2015; 16: 842–7. [DOI] [PubMed] [Google Scholar]
- [30]. Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 2004; 52: 1929–33. [DOI] [PubMed] [Google Scholar]
- [31]. Blodgett JM, Theou O, Mitnitski A, Howlett SE, Rockwood K. Associations between a laboratory frailty index and adverse health outcomes across age and sex. Aging Med 2019; 2: 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol 2017; 89: 30–40. [DOI] [PubMed] [Google Scholar]
- [33]. Gordon EH, Hubbard RE. Differences in frailty in older men and women. Med J Aust 2020; 212: 183–8. [DOI] [PubMed] [Google Scholar]
- [34]. Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res 2008; 20: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Boerma T, Hosseinpoor AR, Verdes E, Chatterji S. A global assessment of the gender gap in self-reported health with survey data from 59 countries. BMC Public Health 2016; 16: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract 2016; 17: 38. https://bmcprimcare.biomedcentral.com/articles/10.1186/s12875-016-0440-0#citeas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Araújo L, Teixeira L, Ribeiro O, Paúl C. Objective vs. subjective health in very advanced ages: looking for discordance in centenarians. Front Med 2018; 5: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Johnston DW, Propper C, Shields MA. Comparing subjective and objective measures of health: evidence from hypertension for the income/health gradient. J Health Econ 2009; 28: 540–52. [DOI] [PubMed] [Google Scholar]
- [39]. Zajacova A, Huzurbazar S, Todd M. Gender and the structure of self-rated health across the adult life span. Soc Sci Med 2017; 187: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Guralnik JM, Ferrucci L, Pieper CFet al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000; 55: 221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Oskarsdottir SE, Heijl A, Bengtsson B. Predicting undetected glaucoma according to age and IOP: a prediction model developed from a primarily European-derived population. Acta Ophthalmol 2019; 97: 422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Rutenberg AD, Mitnitski AB, Farrell SG, Rockwood K. Unifying aging and frailty through complex dynamical networks. Exp Gerontol 2018; 107: 26–129. [DOI] [PubMed] [Google Scholar]
- [43]. Song X, Mitnitski A, Rockwood K. Age-related deficit accumulation and the risk of late-life dementia. Alzheimers Res Ther 2014; 6: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Norberg SJ, Toohey AM, Jones S, McDonough R, Hogan DB. Examining the municipal-level representativeness of the Canadian longitudinal study on Aging (CLSA) cohort: an analysis using Calgary participant baseline data. Health Promot Chronic Dis Prev Can 2021; 41: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.