Abstract
Background and objectives:
Concurrent use of cocaine and opioids is a persistent and challenging problem, particularly within methadone maintenance settings, and there are no approved pharmacotherapies for this population. Galantamine, a cholinesterase inhibitor, was found in a randomized clinical trial to reduce cocaine use among methadone-maintained individuals who were also cocaine dependent. Because of the potential of galantamine to reduce multiple drugs of abuse, it may also reduce opioid use.
Methods:
We conducted a secondary analysis of a randomized, double-blind, placebo-controlled trial of 120 methadone-maintained individuals with concurrent cocaine dependence. Participants were randomized to galantamine or placebo in a 12-week trial with a 6-month follow-up (97% of intention to treat sample reached for final follow-up).
Results:
There was a significant main effect for galantamine over placebo on percent of urine specimens that were negative for opioids, both within treatment (77% for galantamine versus 62% for placebo, F=5.0, p=.027) and through a 6-month follow-up (81% versus 59%, respectively, F=10.8, p=.001). This effect was seen regardless of whether participants used non-prescribed opioids during the baseline period. Galantamine effects were seen early in treatment, with participants in placebo submitting the first opioid positive urine specimen significantly sooner than participants in galantamine (median day 15 vs. 53, Wilcoxon= 5.7, p =.02).
Conclusions and scientific significance:
If these results are supported in future trials, galantamine may hold promise across multiple drugs of abuse, including opioids.
Introduction
Opioid use remains at epidemic levels in the US, and access to and retention in medication-assisted treatment is a key component of the public health strategy 1. In a recent randomized, placebo-controlled trial, we tested the efficacy of galantamine and computerized cognitive behavioral therapy as a treatment for cocaine use disorder in patients stabilized on methadone for co-occurring opioid use disorder 2. As galantamine is a cognitive enhancer used for the treatment of mild to moderate Alzheimer’s dementia and similar neuropsychiatric disorders with cognitive deficits 3, we hypothesized that it would improve outcomes via improvement of attention and memory, and hence, the ability to benefit from cognitive behavioral therapy. Results indicated significant reductions in frequency of cocaine use over time, with significant treatment by time effects for both galantamine over placebo and computerized cognitive behavioral therapy over standard methadone treatment, but no evidence of significant benefit of the combination over either treatment alone. Moreover, although cognitive indicators such as memory and attention were strongly associated with cocaine use outcomes, there was no evidence that galantamine improved cognitive functioning as assessed by these indicators. Given that galantamine had no discernable effect on cognitive functioning, it is possible that it reduces cocaine use via other mechanisms.
At the synapse, galantamine increases acetylcholine (ACh) concentrations by inhibiting acetylcholinesterase, the enzyme that breakdowns ACh. Galantamine also allosterically potentiates nicotinic cholinergic receptors 4. ACh impacts a broad range of CNS functions including sleep, nociception, mood, stress response, cognitive and reward functions 5–7. For example, it has been suggested that dopamine (DA)/ACh balance in the nucleus accumbens may affect reward and aversion spectrum such that, while increases in DA/ACh ratio facilitates reward, decreases in DA/ACh ratio facilitates aversive states 8. In preclinical studies, administration of cholinesterase inhibitors reduces self-administration of cocaine or opioids in primates and rodents 9–11. Cholinesterase inhibitors also attenuate conditioned place preference induced by opioids or cocaine 12. Consistent with these preclinical studies, galantamine reduced the number of drinks in recently detoxified alcoholics 13 and reduced smoking behavior in overnight abstinent smokers 14. Together, these data indicate that the acetylcholine system may be related to a broad range of substance-use behaviors and that, correspondingly, galantamine may be effective in reducing substance use in general 15.
To test whether galantamine might also reduce opioid use, we conducted a secondary analysis of the aforementioned trial. We hypothesized, first, that there would be less illicit opioid use during the trial among participants treated with galantamine versus placebo and that the effect would be seen soon after medication initiation. Because not all participants used illicit opioids during the baseline period, we also stratified the sample by the presence of indicators of non-prescribed/illicit opioid use (urine toxicology screens or self-report), to explore possible interactions of medication with baseline illicit opioid use. Finally, based on the preclinical studies suggesting that galantamine may target a common mechanism across drugs of abuse, we hypothesized that reductions in cocaine and illicit opioids would be related.
Material and methods
Overview of parent trial
Participants were recruited from individuals stabilized on methadone maintenance at Recovery Network of Programs (Bridgeport, Connecticut) between September 2009 and April 2015 (clinicaltrials.gov #NCT0080935). Individuals were included as participants if they were 18 years or older and met DSM-IV-R criteria for current cocaine dependence or abuse, as assessed by the Structured Clinical Interview for DSM-IV-R (SCID)16 and provided at least one cocaine-positive urine test during screening. Individuals were excluded if they (1) were currently dependent on another illicit drug (other than opioids) or had principal drug use that was not cocaine (n=1), (2) had a current medical condition contraindicating galantamine (e.g. asthma, chronic obstructive lung disease, history of or current gastrointestinal ulcer, hepatic or renal impairment, cardiac rhythm disturbance, or pregnancy) (n=3), as assessed by baseline physical examination (EKG, urinalysis and blood work), (3) had a screening liver function test greater than 3 times normal (n=5), (4) used medications (including beta-blockers and nonsteroidal anti-inflammatory drugs) contraindicated with galantamine (n=1), or (5) were not sufficiently stable for outpatient treatment (n=2). Two individuals were incarcerated prior to randomization and 16 did not complete the screening process (Figure 1).
Figure 1.
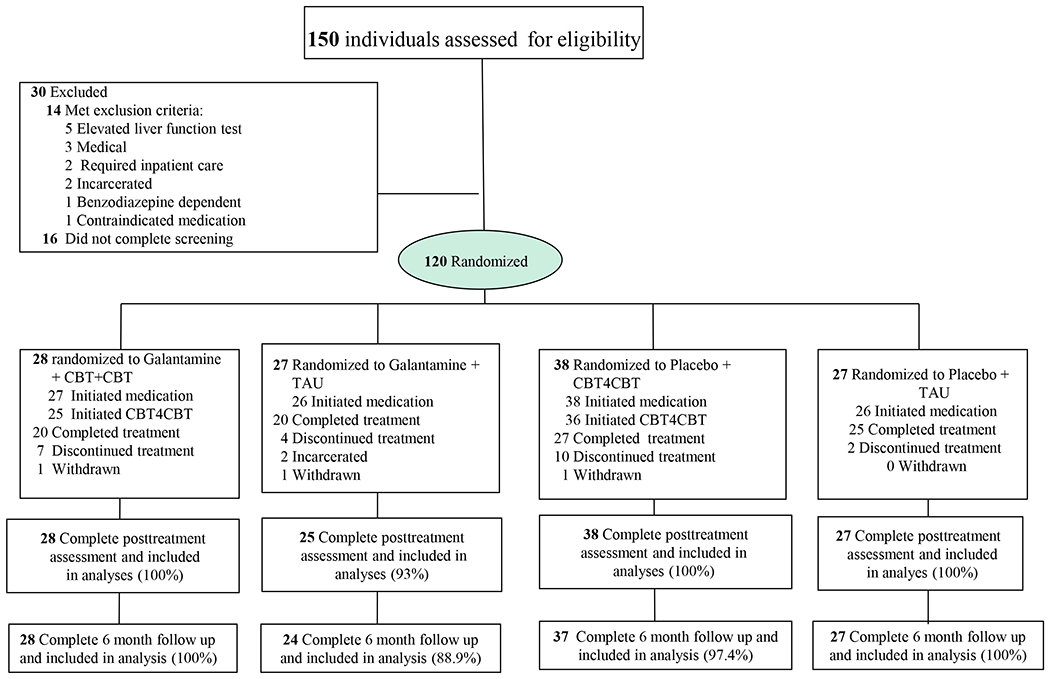
CONSORT flow diagram of participants through the trial. CBT, cognitive behavioral therapy.
Note : Completing treatment defined as taking at least 1 day of study medication in week 12
One hundred twenty of the 150 individuals screened were determined to be eligible, provided written informed consent approved by the Yale School of Medicine IRB and were randomized. A masked, computerized urn randomization program 17 used in previous trials 18 was used to produce equivalent group size and balance groups with respect to baseline level of cocaine use (more or less than 11 days per month), gender, ethnicity (ethnic minority/non-minority), age (over/under 40), and baseline Shipley estimated IQ score (over/under 100) 19.
Treatments
All participants received standard methadone treatment, consisting of daily methadone and weekly individual or group counseling, with access to other program services. Participants met twice weekly with research staff blind to medication condition who collected urine and breath samples and monitored other clinical symptoms. Adverse events and blood pressure were monitored weekly.
Galantamine:
Participants assigned to galantamine were prescribed a maximum dose of 8 mg/day galantamine extended release (ER), given limited tolerability of the 16 mg/day dose in our previous pilot study in cocaine users 20. Galantamine or matched placebo capsules were dispensed daily at the time of methadone dosing and observed by program nurses.
Computerized Cognitive Behavioral Therapy (CBT4CBT):
As described in detail in previous publications 21,22, the CBT4CBT program uses video vignettes, quizzes and interactive exercises to model effective use of skills and strategies. Although there was a significant effect of CBT4CBT on reduction of cocaine use in the trial, we found no evidence of galantamine’s hypothesized effect of cognitive functioning enhancing learning or response to CBT4CBT.
Assessments
Participants were assessed before treatment, weekly during treatment, at the 12-week treatment termination point and at in-person follow-up interviews conducted 1, 3, and 6 months after the end of treatment. In cases where a randomized participant did not initiate (n=3), was withdrawn from treatment (n=3; one for elevated blood pressure, one for suicidal ideation, one for deliberately breaking the medication blind), or dropped out of treatment (n=25), he or she was interviewed at the 12-week point to collect data from the intent-to-treat sample, regardless of level of treatment involvement. Thus, complete 12-week self-report data were available for 118 of 120 (98.3%) of the randomized sample; complete follow-up data were available on (116/120) 96.7% of the randomized sample.
The Timeline Follow Back 23 method was used to collect detailed day-by-day self-reports of substance use throughout the 84-day treatment period. Analyses of cocaine and opioid use self-reports were verified through onsite urine toxicology screens (ToxCup™ Drug Screen Cup 5 with adulterant checks), obtained twice weekly. Self-reports of opioid use were highly consistent results of urine toxicology tests: Of 1976 urine specimens collected, 8.3% of urine samples tested indicated recent opioid use when the participant denied it (87.2% were consistent with self-report; 4.6% were negative although the participant reported recent use in the past 3 days). Multiple cognitive tasks, drawn from the CANTAB 24 were administered at baseline and end of treatment to evaluate effects of study treatments on indicators of cognitive function. These included potential effects of galantamine on sustained attention (Rapid Visual Information Processing, RVP A`), response inhibition (Stop Signal Reaction Time) and visual memory (Pattern Recognition Memory, PRM percent correct). Sleep was measured using the Pittsburgh Sleep Quality Index 25 and general functioning was measured using the SF-36 26.
Data analyses
For this secondary analysis focused on opioid use outcomes, we used general linear models evaluating self-report as well as urine toxicology screens negative for opioids (other than methadone), both within treatment and during follow-up. Survival analyses were conducted to evaluate differences in time from medication induction to first non-prescribed use. These analyses were conducted for the full intention to treat sample, as well as with relevant subgroups (e.g., participants with evidence of non-prescribed opioid use during the baseline period). As there was no evidence of differential effects of the behavioral therapy condition on opioid use outcomes (either main or interactive effects), we collapsed behavioral therapy conditions (CBT4CBT versus standard care) for the analyses presented here.
Results
Sample description and protocol adherence.
Overall, the sample was predominantly male (67%); about half were white, 21% were African-American, and 27% were Latino; most (73%) were unemployed. Participants reported they used cocaine an average of 14 days of the 28 prior to baseline; their mean methadone dose at baseline was 70.5 mg. Because study medication was dispensed at the same time as the daily methadone dose, adherence to study medication was excellent (participants took their study medication on 92% of study days).
Seventy-one individuals reported non-prescribed opioid use during the baseline period prior to randomization or submitted one or more urine screens positive for any opioid other than methadone, whereas the remaining 49 individuals had no evidence of non-prescribed opioid use in the month prior to treatment. As shown in Table 1, there were no significant baseline differences between these two groups in all but one of the many baseline variables evaluated, including demographic characteristics; rates of DSM-IV psychiatric disorders; frequency or chronicity of non-opioid substance use, including alcohol, nicotine, alcohol, or benzodiazepine use; baseline scores on the cognitive tests and multiple other indicators of sleep, stress, and psychological symptoms. The only statistically significant difference found was for number of previous inpatient treatment episodes, which were higher among those who used opioids during the baseline period (4.0 for the opioid users versus 1.8 for those who did not use opioids at baseline). Thus, although participants status regarding use of non-prescribed opioids during baseline was not included in the randomization program as an urn, or balancing, variable, those who used nonprescribed opioids at baseline did not appear to differ systematically from those who did not on multiple variables that might be associated with response to treatment in general or galantamine in particular; including dose of methadone at baseline or end of treatment. Finally, there was no significant difference between these groups in terms of likelihood of assignment to galantamine versus placebo.
Table 1:
Baseline characteristics of sample, by baseline use of nonprescribed opioids
| Pretreatment nonprescribed opioid use N=71 |
No nonprescribed opioid use pretreatment N=49 |
Total N=120 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Categorical variables, number (%) | n | % | % | % | df | F or X2 | p-value | ||
|
| |||||||||
| Assigned to galantamine | 31 | 44 | 24 | 49 | 55 | 46 | 1 | .33 | .57 |
| Female | 19 | 27 | 21 | 43 | 40 | 33 | 1 | 3.38 | .07 |
| Race and ethnicity | |||||||||
| Caucasian | 35 | 49 | 27 | 55 | 62 | 52 | 3 | 1.54 | .67 |
| African-American | 14 | 20 | 11 | 22 | 25 | 21 | |||
| Hispanic | 21 | 30 | 11 | 22 | 32 | 27 | |||
| Multiracial | 1 | 1 | 0 | 1 | 1 | ||||
| Completed high school | 53 | 75 | 33 | 67 | 86 | 72 | 1 | .76 | .38 |
| Unemployed | 54 | 76 | 33 | 67 | 87 | 73 | 1 | 1.10 | .29 |
| On public assistance | 51 | 72 | 34 | 69 | 85 | 71 | 1 | .08 | .77 |
| Major depression -Lifetimea | 5 | 7 | 5 | 10 | 10 | 8 | 1 | .38 | .54 |
| Anxiety disorder -Lifetime | 10 | 14 | 5 | 10 | 15 | 13 | 1 | .40 | .53 |
| Antisocial personality disorder | 9 | 13 | 7 | 14 | 16 | 13 | 1 | .07 | .80 |
| Alcohol use disorder-lifetime | 38 | 54 | 28 | 57 | 66 | 55 | 1 | .15 | .70 |
| Post traumatic stress disorder | 2 | 3 | 1 | 2 | 3 | 3 | 1 | .07 | .79 |
| Continuous variables, mean and SD | M | SD | M | SD | M | SD | |||
| Days paid for working, past 28 | 8.41 | 9.89 | 6.18 | 9.49 | 7.50 | 9.75 | 1, 118 | 1.52 | .22 |
| Age | 38.41 | 9.27 | 38.29 | 9.74 | 38.36 | 9.43 | 1, 118 | .01 | .94 |
| Days marijuana use, past 28 | 3.56 | 7.11 | 2.84 | 7.70 | 3.27 | 7.34 | 1, 118 | .28 | .60 |
| Days cocaine use, past 28 | 14.00 | 8.30 | 14.33 | 8.44 | 14.13 | 8.32 | 1, 118 | .04 | .83 |
| Days cigarette use, past 28 | 26.27 | 6.53 | 26.53 | 5.98 | 26.38 | 6.29 | 1, 118 | .05 | .82 |
| Days alcohol use, past 28 | 3.30 | 6.46 | 1.59 | 3.59 | 2.60 | 5.52 | 1, 118 | 2.81 | .10 |
| Days benzodiazepine use, past 28 | .45 | 1.39 | .20 | .71 | .35 | 1.16 | 1, 118 | 1.30 | .26 |
| Years of regular cocaine use | 9.87 | 8.75 | 9.69 | 8.36 | 9.80 | 8.56 | 1, 118 | .01 | .91 |
| Years of regular opiate use | 10.77 | 7.57 | 8.57 | 8.09 | 9.88 | 7.83 | 1, 118 | 2.32 | .13 |
| Lifetime number of arrests | 7.86 | 10.45 | 6.51 | 11.30 | 7.31 | 10.78 | 1, 118 | .45 | .50 |
| Prior Treatment Episodes: | |||||||||
| Number of outpatient episodes | 2.87 | 3.52 | 2.35 | 2.50 | 2.66 | 3.14 | 1, 118 | .81 | .37 |
| Number of inpatient episodes | 3.96 | 6.10 | 1.78 | 2.49 | 3.07 | 5.06 | 1, 118 | 5.60 | .02 |
| Estimated IQ from Shipley | 100.71 | 11.62 | 100.31 | 11.84 | 100.55 | 11.67 | 1, 118 | .03 | .85 |
| Baseline methadone dose, mg/day | 68.10 | 29.00 | 73.65 | 28.01 | 70.45 | 28.60 | 1, 118 | 1.07 | .30 |
| Posttreatment methadone dose, mg. day | 67.77 | 31.17 | 71.04 | 34.15 | 69.11 | 32.32 | 1, 115 | .29 | .59 |
|
| |||||||||
All psychiatric diagnoses from SCID interviews
Galantamine effects on opioid use
Within treatment there was a significant main effect of galantamine over placebo on percentage of urine samples that were negative for opioids (galantamine 76.7% versus placebo 62.4%, p=.03) and a significant difference for self-reported days of abstinence from illicit opioids (galantamine 92.5% versus placebo 86.3%, p=.03). Survival analyses showed that participants in the placebo condition provided an opioid-positive urine specimen significantly earlier in treatment than those in the galantamine condition (median day 14.5 for placebo, day 52.5 for galantamine, Wilcoxon statistic= 5.72, df 1, p = .02).
To understand these findings in terms of participants’ use of non-prescribed opioids at baseline, Table 2 shows treatment retention, rates of opioid-negative urine specimens, and self-reported opioid use by medication condition (galantamine versus placebo), baseline non-prescribed opioid use (yes/no), and their interaction. There were no significant differences for treatment retention by medication condition or baseline opioid use status. As noted above, there was a main effect of medication condition indicating more opioid-negative urine specimens among those assigned to galantamine versus placebo, as well as a main effect of opioid use at baseline. Thus, as expected, those who did not use opioids during the baseline period submitted a significantly higher percentage of opioid-negative urine specimens than those who used opioids during baseline. The interaction of medication and pretreatment opioid use was not significant, however, suggesting that galantamine’s association with more opioid-negative urine specimens occurred in both those who did and did not use opioids during baseline. This effect was also apparent for self-reported opioid use.
Table 2.
Retention and opioid use outcomes by medication condition and presence of non-prescribed opioids at baseline, N=120
| Variable | Galantamine | Placebo | TOTAL | Medication | Pretreatment opioid use | Medication by pretreatment opioid use status | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | F | p | Eta2p | F | p | Eta2p | F | p | Eta2p | |
| Within-treatment outcomes | ||||||||||||||||||
| Retention-Days in treatment (of 84 possible) | ||||||||||||||||||
| No pretreatment opioid use | 61.75 | 34.13 | 24 | 73.00 | 24.06 | 25 | 67.49 | 29.66 | 49 | 2.58 | .11 | .02 | .98 | .32 | .01 | .43 | .51 | .00 |
| Pretreatment opioid use | 69.94 | 27.04 | 31 | 74.68 | 22.68 | 40 | 72.61 | 24.61 | 71 | |||||||||
| % opioid-negative urine specimens | ||||||||||||||||||
| No pretreatment opioid use | 94.64 | 12.57 | 23 | 87.34 | 17.57 | 24 | 90.91 | 15.61 | 47 | 4.32 | .04 | .04 | 41.91 | .00 | .27 | .59 | .45 | .01 |
| Pretreatment opioid use | 62.90 | 32.99 | 30 | 47.07 | 37.70 | 39 | 53.95 | 36.34 | 69 | |||||||||
| % days of opioid abstinence-self report | ||||||||||||||||||
| No pretreatment opioid use | 98.91 | 3.68 | 24 | 95.38 | 12.45 | 25 | 97.11 | 9.34 | 49 | 3.29 | .07 | .03 | 22.36 | .00 | .16 | .30 | .58 | .00 |
| Pretreatment opioid use | 87.23 | 14.59 | 29 | 80.63 | 19.77 | 40 | 83.40 | 17.96 | 69 | |||||||||
| % cocaine-negative urine samples | ||||||||||||||||||
| No pretreatment opioid use | 18.94 | 31.26 | 23 | 22.67 | 27.69 | 24 | 20.84 | 29.23 | 47 | 1.48 | .23 | .01 | .33 | .57 | .00 | 3.55 | .06 | .03 |
| Pretreatment opioid use | 32.66 | 36.76 | 30 | 15.34 | 22.14 | 39 | 22.88 | 30.41 | 69 | |||||||||
| Six-month follow-up outcomes | ||||||||||||||||||
| Percent opioid-negative urine specimens | ||||||||||||||||||
| No pretreatment opioid use | 95.14 | 13.44 | 24 | 74.00 | 32.66 | 25 | 84.35 | 27.09 | 49 | 10.2 | .00 | .08 | 16.94 | .00 | .13 | .07 | .80 | .00 |
| Pretreatment opioid use | 68.39 | 35.73 | 29 | 50.42 | 37.65 | 40 | 57.97 | 37.66 | 69 | |||||||||
The level of illicit opioid use in the sample was not negligible: Among those who used non-prescribed opioids during baseline, 74% (23/31) of those assigned to galantamine versus 85% (34/40) of those who were assigned to placebo submitted at least one urine specimen positive for non-prescribed opioids (X2= 1.29, df 1, p =.26). Among the sample who did not report opioid use during baseline, 25% (6/24) of those assigned to galantamine versus 52% (13/25) of those assigned to placebo submitted at least one opioid-positive urine specimen (X2 = 3.76, df = 1, p = .02).
Table 2 also provides rates of opioid-negative urine specimens collected during the 6-month follow-up. During follow-up, the main effect of galantamine versus placebo on rates of opioid-negative urine specimens was statistically significant, as was the main effect of pretreatment opioid use, with no significant interaction, suggesting a durable effect of galantamine on reduced opioid use, regardless of whether the participant used non-prescribed opioids during the baseline period.
Relationship with cocaine use
When treatment effects on cocaine use (percent cocaine-negative urine specimens) were evaluated with the same model, neither the effect of medication nor pretreatment opioid use were significant, but the interaction p-value (.06) highlighted that those assigned to galantamine who had pretreatment opioid use had better a higher percentage of cocaine negative urine specimens than those on placebo who had pretreatment opioid use (32.7 vs. 15.3), which was the opposite of those on placebo who had pretreatment opioid use compared to those on placebo who had no pretreatment opioid use (18. 9 vs. 22.7). Thus, although not statistically significant, galantamine appeared to reduce both opioid and cocaine use in this sample.
Effects of galantamine versus placebo on measures of cognitive function and psychological symptoms
As the original study hypothesis was that galantamine would enhance response to treatment by improving cognitive function, we also evaluated galantamine’s effects on change in cognitive function from baseline to posttreatment overall, as well as within the subgroup who used opioids in the baseline period. None of the cognitive indicators from the CANTAB indicated a significant effect for time, medication condition, or medication condition by time, suggesting no benefit of galantamine over placebo on these measures of cognitive function in the sample. Thus, it is unlikely that the apparent benefit of galantamine on reducing opioid use in this sample was driven by its effect on cognitive function in this subgroup.
Finally, in an effort to rule out other alternative explanations for the galantamine effect on opioid use as demonstrated in these secondary analyses, we also explored whether galantamine may have exerted its apparent effect on reducing opioid use in this sample by reducing negative affect (BSI subscale and total scores), improving sleep (as indicated by the Pittsburgh Sleep Quality Index 25), or reducing pain (as indicated by the pain scale of the SF-36). This did not appear to be the case: although there was a consistent significant effect of time for these measures, indicating general improvement over time, there were no significant interactions of time with medication condition, suggesting that galantamine was not more effective than placebo in reducing these symptoms and problems (data not shown).
Discussion
In this secondary analysis of opioid use outcomes from a randomized clinical trial evaluating the effect of galantamine versus placebo within a sample of methadone-maintained individuals with concurrent cocaine use disorder, we found the following: First, there were significant main effect of galantamine relative to placebo in the time to first non-prescribed opioid use; there was a significant difference in the overall number of urine specimens negative for non-prescribed opioids; and these effects persisted into the 6-month follow-up. Second, while there was a main effect of whether or not the participant used non-prescribed opioids use during the month before screening baseline on opioid use within treatment and through follow-up; there were no significant interactions of medication with baseline opioid use status, suggesting that the effect of galantamine on non-prescribed opioid use was apparent regardless of whether or not the participant used opioids during the baseline period. Finally, there was no evidence that galantamine effects on reduced opioid use was associated with a range of other factors, including effects on cognitive or emotional functioning, overall or within the subsample who used opioids at baseline.
To our knowledge, no previous studies have suggested the impact of cholinesterase inhibitors on opioid use in humans. Reduction of opioid use is consistent with preclinical studies demonstrating that acetylcholine esterase inhibitors reduce opioid reward, assessed with the conditioned place preference (CPP) test, as well as and opioid reinforcement, assessed with self-administration paradigms 12,27. In contrast, ablation of the cholinergic system facilitates development of CPP for opioids 12. In an open label study, donepezil, a cholinesterase inhibitor, reduced opioid-induced sedation without negatively affecting the analgesic effects of opioids 28. Thus, the literature suggests a possible functional coupling between the Ach and brain opioid systems.
In this study, the effects of galantamine on opioid and cocaine use were highly correlated. Our findings are consistent with human trials indicating galantamine-associated reductions in heavy alcohol use 13 and cigarette use 14,29. Further, in preclinical studies, cholinesterase inhibitors reduced self-administration of cocaine, opioids and nicotine9–11,30. These findings led us to hypothesize that galantamine, or more generally cholinesterase inhibitors, have an “anti-addictive” effect through a common mechanism shared by different drugs of abuse31. This common mechanism may involve inhibition of DA’s effect by increased ACh levels in the brain reward circuit 32. Although the functional interaction between DA and ACh is complex 33,34 and may differ in various brain regions, increased ACh levels in the nucleus accumbens has been suggested to inhibit reward function and facilitate aversion 8. This proposed model remains to be further examined in both preclinical and clinical studies.
Strengths of this study include its randomized, double-blind nature with multiple methodologic features including high rates of data availability, both within treatment and follow-up and use of urine toxicology screens to verify abstinence. The primary limitation was that this was a secondary analysis, hence the study was not designed to evaluate galantamine effects on opioid use outcomes. Thus, while all participants were receiving treatment for opioid use disorder (i.e., stabilized on methadone prior to trial entry), many, but not all, participants used non-prescribed opioids during the baseline period prior to screening. However, galantamine was associated with significantly more urine specimens that were negative for opioids for the full sample, for the full randomized sample as well as those using non-prescribed at baseline. Future studies are needed to evaluate its potential anti-addictive effects in a range of substance-using populations. In addition, the trial was not fully powered for this secondary analysis and the number of opioid outcomes evaluated raised the risk of Type I error. Finally, we used only a 5-panel urine drug screening test and thus may have missed some opioid use in the sample.
Figure 2:
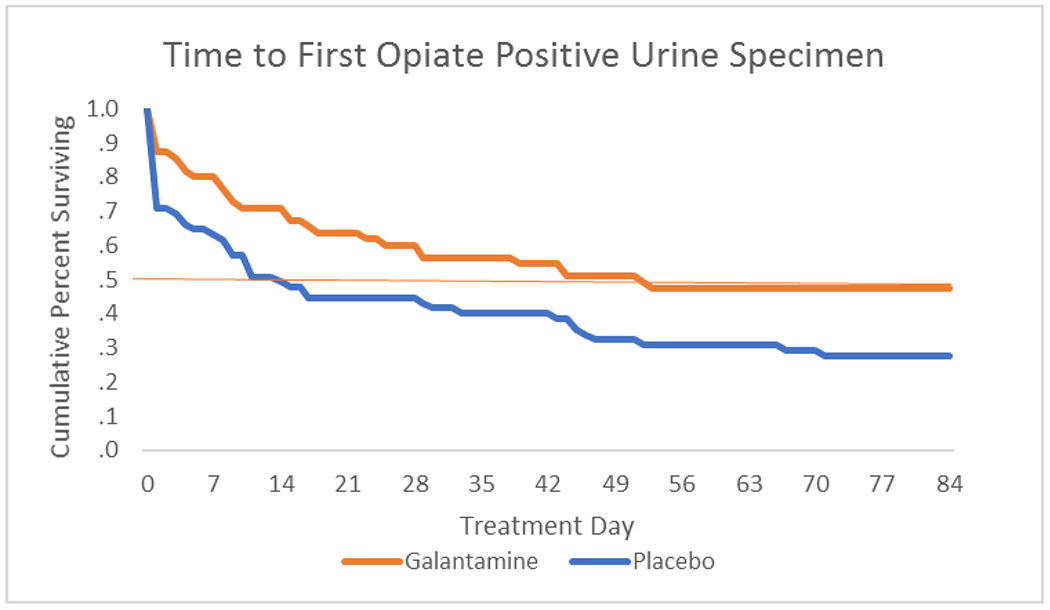
Survival analysis: Time to first submission of a non-prescribed opioid-positive urine specimen by medication condition. Red x indicates median time to relapse in each group.
Funding and Disclosures
This work was supported by grant P50-DA09241 from the National Institute on Drug Abuse.
Footnotes
Disclosure: Dr. Carroll is a member of CBT4CBT LLC, which makes validated forms of CBT4CBT available to qualified clinical providers. An approved management plan is in place with Yale University. The other authors have no disclosures.
References
- 1.Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. The New England journal of medicine. 2017;377(4):391–394. [DOI] [PubMed] [Google Scholar]
- 2.Carroll KM, Nich C, DeVito EE, Shi JM, Sofuoglu M. Galantamine and Computerized Cognitive Behavioral Therapy for Cocaine Dependence: A Randomized Clinical Trial. The Journal of clinical psychiatry. 2017;79(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prvulovic D, Hampel H, Pantel J. Galantamine for Alzheimer’s disease. Expert opinion on drug metabolism & toxicology. 2010;6(3):345–354. [DOI] [PubMed] [Google Scholar]
- 4.Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32(1):43–53. [DOI] [PubMed] [Google Scholar]
- 5.Lucas-Meunier E, Fossier P, Baux G, Amar M. Cholinergic modulation of the cortical neuronal network. Pflugers Arch. 2003;446(1):17–29. [DOI] [PubMed] [Google Scholar]
- 6.Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273–280. [DOI] [PubMed] [Google Scholar]
- 7.Smythies J Section I. The cholinergic system. Int. Rev. Neurobiol 2005;64:1–122. [DOI] [PubMed] [Google Scholar]
- 8.Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7(6):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Lai M, Zhou X, et al. Galantamine attenuates the heroin seeking behaviors induced by cues after prolonged withdrawal in rats. Neuropharmacology. 2012;62(8):2515–2521. [DOI] [PubMed] [Google Scholar]
- 10.Wilson MC, Schuster CR. Cholinergic influence on intravenous cocaine self-administration by rhesus monkeys. Pharmacology Biochemistry and Behavior. 1973;1(6):643–649. [DOI] [PubMed] [Google Scholar]
- 11.Grasing K, Yang Y, He S. Reversible and persistent decreases in cocaine self-administration after cholinesterase inhibition: different effects of donepezil and rivastigmine. Behavioural pharmacology. 2011;22(1):58–70. [DOI] [PubMed] [Google Scholar]
- 12.Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 2003;100(10):6169–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann K, Ackermann K, Diehl A, et al. Galantamine: a cholinergic patch in the treatment of alcoholism: a randomized, placebo-controlled trial. Psychopharmacology. 2006;184(1):115–121. [DOI] [PubMed] [Google Scholar]
- 14.MacLean RR, Waters AJ, Sofuoglu M. Effects of galantamine on smoking behavior and cognitive performance in treatment-seeking smokers prior to a quit attempt. Human psychopharmacology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen KP, DeVito EE, Yip SW, Carroll KM, Sofuoglu M. Acetylcholine as a treatment target for opioid use disorder. CNS drugs. 2018;32:981–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, D.C.: American Psychiatric Press; 1995. [Google Scholar]
- 17.Stout RL, Wirtz PW, Carbonari JP, DelBoca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of studies on alcohol. 1994;Supplement 12:70–75. [DOI] [PubMed] [Google Scholar]
- 18.Ball SA, Martino S, Nich C, et al. Site matters: multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. Journal of consulting and clinical psychology. 2007;75(4):556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zachary RA. The Manual of the Shipley Institute of Living Scale. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- 20.Sofuoglu M, Waters AJ, Poling J, Carroll KM. Galantamine improves sustained attention in chronic cocaine users. Exp Clin Psychopharmacol. 2011;19(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll KM, Ball SA, Martino S, et al. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. The American journal of psychiatry. 2008;165(7):881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll KM, Kiluk BD, Nich C, et al. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. The American journal of psychiatry. 2014;171(4):436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2014;28(1):154–162. [DOI] [PubMed] [Google Scholar]
- 24.Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. Journal of the International Neuropsychological Society : JINS. 1998;4(5):474–490. [DOI] [PubMed] [Google Scholar]
- 25.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of psychosomatic research. 2002;53(3):737–740. [DOI] [PubMed] [Google Scholar]
- 26.McHorney CA, Ware JE Jr., Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical care. 1994;32(1):40–66. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Lai M, Zhou X, et al. Galantamine attenuates the heroin seeking behaviors induced by cues after prolonged withdrawal in rats. Neuropharmacology. 2012;62(8):2515–2521. [DOI] [PubMed] [Google Scholar]
- 28.Slatkin NE, Rhiner M, Bolton TM. Donepezil in the treatment of opioid-induced sedation: report of six cases. J Pain Symptom Manage. 2001;21(5):425–438. [DOI] [PubMed] [Google Scholar]
- 29.Ashare R, Kimmey B, Rupprecht L, Bowers M, Hayes M, Schmidt H. Repeated administration of an acetylcholinesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Translational psychiatry. 2016;6(1):e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD. Galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic acetylcholine receptors, attenuates nicotine taking and seeking in rats. Neuropsychopharmacology. 2012;37(10):2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen KP, DeVito EE, Yip SW, Carroll KM, Sofuoglu M. The cholinergic system as a treatment target for opioid use disorder. CNS Drugs (in press). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sofuoglu M, Mooney M. Cholinergic functioning in stimulant addiction. CNS drugs. 2009;23(11):939–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aosaki T, Miura M, Suzuki T, Nishimura K, Masuda M. Acetylcholine–dopamine balance hypothesis in the striatum: An update. Geriatrics & gerontology international. 2010;10:S148–S157. [DOI] [PubMed] [Google Scholar]
- 34.Grasing K A threshold model for opposing actions of acetylcholine on reward behavior: molecular mechanisms and implications for treatment of substance abuse disorders. Behavioural brain research. 2016;312:148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]


