Abstract
Rifampin is the most potent drug used in the treatment of disease due to Mycobacterium kansasii. A 69-bp fragment of rpoB, the gene that encodes the β subunit of the bacterial RNA polymerase, was sequenced and found to be identical in five rifampin-susceptible clinical isolates of M. kansasii. This sequence showed 87% homology with the Mycobacterium tuberculosis gene, with an identical deduced amino acid sequence. In contrast, missense mutations were detected in the same fragment amplified from five rifampin-resistant isolates. A rifampin-resistant strain generated in vitro also harbored an rpoB gene missense mutation that was not present in the parent isolate. All mutations detected (in codons 513, 526, and 531) have previously been described in rifampin-resistant M. tuberculosis isolates. Rifampin MICs determined by E-test were <1 mg/liter for all rifampin-susceptible isolates and >256 mg/liter for all rifampin-resistant ones. In addition, four of the five rifampin-resistant isolates were also resistant to rifabutin. We have thus shown a strong association between rpoB gene missense mutations and rifampin resistance in M. kansasii. Although our results are derived from a small number of isolates and confirmation with larger numbers would be useful, they strongly suggest that mutations within rpoB form the molecular basis of rifampin resistance in this species.
Mycobacterium kansasii is second only to Mycobacterium avium-intracellulare as a cause of nontuberculous mycobacterial disease in the United States (11). Since its introduction into clinical practice in 1971, rifampin has become a key component of multidrug regimens used to treat M. kansasii. Clinical use of this drug is probably the main reason for the success of short-course regimens and consequently the abandonment of surgical resection as a treatment for pulmonary disease (12). Although initial clinical isolates of M. kansasii are almost universally susceptible to rifampin in vitro, acquired resistance in vivo has been well documented, usually occurs against a background of suboptimal therapy, and significantly compromises medical treatment options (2, 5, 15).
The mechanism of rifampin resistance in this species is unknown. Mutations in the rpoB gene, which encodes the β subunit of the RNA polymerase (the rifampin target), are found in around 95% of rifampin-resistant M. tuberculosis isolates, mainly concentrated in a 69-bp pair region (13, 14). Like Mycobacterium tuberculosis, M. kansasii is usually highly susceptible to rifampin, acquired resistance is usually high level, and strains of intermediate susceptibility are uncommon (15, 19). It was on this basis that we hypothesized that rifampin resistance in M. kansasii was likely to be due to single mutational events affecting the rpoB gene, leading to the development of high-level resistance. To test this hypothesis, we analyzed a 69-bp segment of the rpoB gene in rifampin-susceptible and -resistant M. kansasii isolates.
MATERIALS AND METHODS
M. kansasii isolates.
Five rifampin-susceptible clinical isolates of M. kansasii were obtained form our own laboratory (nos. 1, 2, 4, and 5) and from the Scottish Mycobacteria Reference Laboratory (no. 3). Five rifampin-resistant isolates were obtained from our laboratory (no. 11), the Scottish Mycobacteria Reference Laboratory (no. 12), and the Mycobacterium Reference Unit, London (nos. 8, 9, and 10). All isolates were derived from sputum samples obtained from different patients apart from one rifampin-resistant strain (no. 12) isolated from homograft washing fluid. All four patients with rifampin-resistant organisms had previously been infected with rifampin-susceptible strains.
In vitro generation of rifampin-resistant strain.
A heavy suspension of a rifampin-susceptible isolate (no. 1) was obtained by inoculating culture material into Middlebrook 7H9 broth (Difco, Hemel Hempstead, U.K.) and incubating at 37°C until heavy growth was visible. A 4-ml aliquot of this suspension was put into a plastic tube and centrifuged at 13,500 rpm for 5 min, and the supernatant was discarded. The pellet was resuspended in 0.5 ml of distilled water, and plated onto Middlebrook 7H11 (Difco) slants containing 8 mg of rifampin per liter. The latter slants were prepared by adding rifampin solution (Sigma) to molten agar, which was then poured into sterile Universal containers and allowed to set. After incubation in room air for 3 weeks at 37°C, a single colony (M1) was subcultured and maintained on Löwenstein-Jensen slants.
Phenotypic rifamycin susceptibility testing.
Susceptibility to rifampin and rifabutin was determined for all isolates using the BACTEC 460 radiometric system (Becton Dickinson, Oxford, U.K.) using 2 mg/liter as the critical concentration for both drugs. E-test strips (AB Biodisk, Solna, Sweden) were used in accordance with the manufacturer's instructions (1) to estimate MICs of rifampin for four susceptible (nos. 1 to 4) and five resistant (nos. 8, 9, 10, 12, and M1) isolates.
Bacterial DNA extraction and PCR amplification of rpoB gene fragments.
Cells were suspended in 10 mM Tris–1 mM EDTA–1% Triton X-100 at pH 8. Suspensions were heated to 95°C for 20 min and centrifuged at 13,500 rpm for 5 min. The supernatant was then used as a template in the PCR. A 409-bp PCR product was amplified from the rpoB gene using the following primers, designed from published M. kansasii rpoB gene sequences: MK1 (5′GCG GAT GAC CAC CCA GGA CG 3′) and MK2 (5′ GCG CGG TCC TC[C/T] TCG TCG GC 3′). This amplicon included the region homologous to the 69-bp rifampin resistance-determining region of M. tuberculosis. The 50-ml reaction mixtures contained 2 U of Taq polymerase (Dynazyme II; Flowgen, Kent, U.K.), 0.2 mM each of the four deoxynucleoside triphosphates, 0.4 mM each primer (Pharmacia, St. Albans, U.K.), 1.5 mM MgCl2, and 50 mM KCl. A Perkin-Elmer thermocycler was used for the PCR with the following parameters: 95°C for 2 min, then 30 cycles of 95°C (15 s) and 70°C (60 s), followed by a 5-min extension period at 72°C. PCR products were electrophoresed on a 1.5% agarose gel containing ethidium bromide and visualized using UV illumination.
DNA sequencing of PCR products.
Forward and reverse strands of the PCR products were sequenced directly using a Thermo-sequenase cycle sequencing kit (Amersham, Little Chalfont, U.K.) and an ALF DNA sequencer (Pharmacia, St. Albans, U.K.). The following 5′-fluorescein-labeled primers were used to sequence an approximately 290-bp part of the amplicon: MKF1 (5′ GGA GGC GAT CAC [A/G]CC GCA GAC 3′) and MKF2 (5′ CGT GCG TAC ACC GAC AGC GA 3′). A Perkin-Elmer thermocycler was used for the sequencing reaction with the following parameters: 25 cycles of 98°C for 15 s and 65°C for 30 s. A 69-bp region encompassing the rifampin resistance-determining region of M. tuberculosis (codons 511 to 533, using numbering derived from the Escherichia coli rpoB gene) was analyzed.
RESULTS
Phenotypic susceptibility to rifampin and rifabutin.
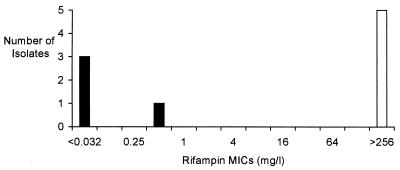
Using BACTEC technology, there was complete concordance between rifampin and rifabutin susceptibility in all isolates with the exception of isolate 12, which was rifabutin susceptible but rifampin resistant. The in vitro-generated strain was also found to be rifampin and rifabutin resistant. Using E-test, rifampin MICs were <1 mg/liter for four rifampin-susceptible isolates and >256 mg/liter for five rifampin-resistant ones (Fig. 1). Thus, mutations at all three loci in the rifampin-resistant isolates appeared to be associated with high-level resistance.
FIG. 1.
Rifampin MICs as determined by E-test for four rifampin-susceptible isolates (nos. 1 to 4, solid bars) and five rifampin-resistant isolates (nos. 8, 9, 10, 12, and M1, open bar).
Nucleotide sequence analysis of rpoB gene of rifampin-susceptible isolates.
The nucleotide sequence of the 69-bp fragment of the rpoB gene was identical in all five rifampin-susceptible isolates and showed 87% homology with the M. tuberculosis gene sequence (GenBank accession no. L27989) (Fig. 2). The deduced amino acid sequence was identical to that of M. tuberculosis, reflecting the conserved nature of this gene within mycobacteria. In addition, the nucleotide sequence was identical to previously published M. kansasii rpoB gene sequences (GenBank accession no. AF060301).
FIG. 2.
rpoB gene sequences of five rifampin-susceptible and six rifampin-resistant strains of M. kansasii, with the M. tuberculosis sequence shown for comparison. Codons commonly involved in rifampin resistance in M. tuberculosis are shown in bold. The codon numbering corresponds to the homologous gene in E. coli. Nucleotide identity is indicated by a dash. Missense mutations are shown in bold, with the amino acid substitution indicated below.
Nucleotide sequence analysis of rpoB gene of rifampin-resistant isolates.
Missense mutations were found within the 69-bp region amplified from all six rifampin-resistant isolates (Fig. 2). Four isolates (three clinical and one environmental) had a C-to-T mutation leading to a Ser531-Leu substitution. This is the commonest mutation found in rifampin-resistant clinical isolates of M. tuberculosis and Mycobacterium leprae (8, 14). One clinical isolate had a mutation leading to a Gln513-Leu substitution, and the in vitro-generated rifampin-resistant strain had an identical sequence to the parent isolate apart from a mutation encoding a His526-Tyr substitution. Mutations in one of these three codons have been found in over 80% of rifampin-resistant M. tuberculosis isolates (14).
DISCUSSION
To our knowledge, this is the first study to examine the molecular basis of rifampin resistance in M. kansasii. We have shown an association between missense mutations in a short segment of the rpoB gene and phenotypic rifampin resistance in five M. kansasii isolates. Moreover, an in vitro-derived rifampin-resistant strain was shown to have an rpoB mutation that was not present in the susceptible parent isolate. Although we cannot exclude other mechanisms, in the context of the recognized role of rpoB mutations in rifampin resistance in other mycobacteria, our data provide strong evidence for these being the primary basis for rifampin resistance in M. kansasii.
Rifampin resistance in M. tuberculosis has been shown to be predominantly due to mutations in an 81-bp region of the rpoB gene designated the rifampin resistance-determining region. Similar mechanisms appear to operate in M. leprae (8), although other mechanisms (such as cell membrane impermeability) may explain rifampin resistance in other mycobacteria, such as Mycobacterium avium-intracellulare (7, 18). Although we only studied six rifampin-resistant isolates, it is notable that four had the same nucleotide substitution in codon 531. This mutation is the most commonly found in rifampin-resistant M. tuberculosis and M. leprae clinical isolates and has recently been shown to be the most commonly found mutation in in vitro-selected rifampin-resistant mutants of M. tuberculosis (10). This may be explained by an intrinsically higher mutation frequency at this site, or by increased “fitness” associated with particular amino acid substitutions. In vitro evidence for the latter has been published for rifampin-resistant M. tuberculosis mutants (3), although in vivo data are lacking. Interestingly, the substituted amino acids in our rifampin-resistant strains reflected the most commonly found residues in clinical rifampin-resistant M. tuberculosis isolates.
In order to examine the degree of resistance conferred by each mutation, we determined the MICs using E-test. Although this is not standard methodology, the test appears to perform well for M. tuberculosis (16) and has been used for susceptibility testing of M. kansasii (6). In this study the E-test distinguished clearly between susceptible and resistant strains. The data also show that rpoB gene mutations in M. kansasii are associated with high-level resistance to rifampin, and in a single laboratory-generated mutant, we showed a rise in the MIC from <0.032 mg/liter in the parent isolate to >256 mg/liter associated with a mutation in the rpoB gene. There is some evidence that different rpoB gene mutations lead to different degrees of rifamycin resistance in M. tuberculosis. However, the commonest mutations detected in clinical isolates generally show high-level rifampin resistance and cross-resistance to other rifamycins such as rifabutin (4, 17). From our limited data, the three mutations detected in M. kansasii appear to be associated with high-level rifampin resistance, and all but one isolate showed cross-resistance to rifabutin.
In a large study from Texas, rifampin resistance occurred in approximately 4% of M. kansasii isolates, increased in frequency over the period from 1981 to 1992, and was strongly associated with previous suboptimal therapy and human immunodeficiency virus coinfection (15). Person-to-person transmission of this organism has never been confirmed, and consequently primary rifampin resistance has not been described. Treatment regimens for rifampin-resistant infections, although frequently successful, are complex and prolonged (2). If the molecular basis of rifampin resistance is confirmed to result from rpoB gene mutations in a larger number of M. kansasii isolates, hybridization assays could be designed to detect rifampin resistance in this organism at an earlier stage than is possible using conventional techniques. In view of recent studies using rpoB gene sequencing to determine mycobacterial species (9), analysis of this gene has the potential to provide rapid information on both species and rifampin susceptibility of organisms such as M. kansasii and M. tuberculosis.
ACKNOWLEDGMENTS
We are grateful to Andrew Middleton, who performed the BACTEC sensitivity testing, and to Francis Drobniewski and Brian Watt for providing isolates.
REFERENCES
- 1.AB Biodisk. Susceptibility testing of mycobacteria. Etest Technical Guide no. 5. Piscataway, N.J: AB Biodisk, N.A., Inc.; 1996. [Google Scholar]
- 2.Ahn C H, Wallace R J, Jr, Steele L C, Murphy D T. Sulfonamide-containing regimens for disease caused by rifampin-resistant Mycobacterium kansasii. Am Rev Respir Dis. 1987;135:10–16. doi: 10.1164/arrd.1987.135.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Billington O J, McHugh T D, Gillespie S H. Physiological cost or rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1999;43:1866–1869. doi: 10.1128/aac.43.8.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodmer T, Zürcher G, Imboden P, Telenti A. Mutation position and type of substitution in the β subunit of the RNA polymerase influence in-vitro activity of rifamycins in rifampicin-resistant Mycobacterium tuberculosis. J Antimicrob Chemother. 1995;35:345–348. doi: 10.1093/jac/35.2.345. [DOI] [PubMed] [Google Scholar]
- 5.Davidson P T, Waggoner R. Acquired resistance to rifampicin by Mycobacterium kansasii. Tubercle. 1976;57:271–273. doi: 10.1016/s0041-3879(76)80005-0. [DOI] [PubMed] [Google Scholar]
- 6.Fabry W, Schmid E N, Ansorg R. Comparison of the E test and a proportion dilution method for susceptibility testing of Mycobacterium kansasii. Chemotherapy. 1995;41:247–252. doi: 10.1159/000239352. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero C, Stockman L, Marchesi F, Bodmer T, Roberts G D, Telenti A. Evaluation of the rpoB gene in rifampicin-susceptible and -resistant Mycobacterium avium and Mycobacterium intracellulare. J Antimicrob Chemother. 1994;33:661–663. doi: 10.1093/jac/33.3.661-a. [DOI] [PubMed] [Google Scholar]
- 8.Honore N, Cole S T. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob Agents Chemother. 1993;37:414–418. doi: 10.1128/aac.37.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim B-J, Lee S-H, Lyu M-A, Kim S-J, Bai G-H, Kim S-J, Chae G-T, Kim E-C, Cha C-Y, Kook Y-H. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB) J Clin Microbiol. 1999;37:1714–1720. doi: 10.1128/jcm.37.6.1714-1720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morlock G P, Plikaytis B B, Crawford J T. Characterization of spontaneous, in vitro-selected, rifampin-resistant mutants of Mycobacterium tuberculosis strain H37Rv. Antimicrob Agents Chemother. 2000;44:3298–3301. doi: 10.1128/aac.44.12.3298-3301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien R J, Geiter L J, Snider D E., Jr The epidemiology of nontuberculous mycobacterial diseases in the United States. Am Rev Respir Dis. 1987;135:1007–1014. doi: 10.1164/arrd.1987.135.5.1007. [DOI] [PubMed] [Google Scholar]
- 12.Pezzia W, Raleigh J W, Bailey M C, Toth E A, Silverblatt J. Treatment of pulmonary disease due to Mycobacterium kansasii: recent experience with rifampin. Rev Infect Dis. 1981;3:1035–1039. doi: 10.1093/clinids/3.5.1035. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Musser J M. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tubercle Lung Dis. 1998;79:3–29. doi: 10.1054/tuld.1998.0002. [DOI] [PubMed] [Google Scholar]
- 14.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston M J, Matter L, Schopfer K, Bodmer T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 15.Wallace R J, Jr, Dunbar D, Brown B A, Onyi G, Dunlap R, Ahn C H, Murphy D T. Rifampin-resistant Mycobacterium kansasii. Clin Infect Dis. 1994;18:736–743. doi: 10.1093/clinids/18.5.736. [DOI] [PubMed] [Google Scholar]
- 16.Wanger A, Mills K. Testing of Mycobacterium tuberculosis susceptibility to ethambutol, isoniazid, rifampicin, and streptomycin by using Etest. J Clin Microbiol. 1996;34:1672–1676. doi: 10.1128/jcm.34.7.1672-1676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams D L, Spring L, Collins L, Miller L P, Heiferts L B, Gangadharam P R J, Gillis T P. Contribution of mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:1853–1857. doi: 10.1128/aac.42.7.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams D L, Waguespack C, Eisenach K, Crawford J T, Portaels F, Salfinger M, Nolan C M, Abe C, Sticht-Groh V, Gillis T P. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380–2386. doi: 10.1128/aac.38.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yates M D, Collins C H. Sensitivity of opportunist mycobacteria to rifampicin and ethambutol. Tubercle. 1981;62:117–121. doi: 10.1016/0041-3879(81)90019-2. [DOI] [PubMed] [Google Scholar]