Abstract
The movement correlates of unit activity in medullary reticular formation cells were observed in unrestrained cats. Fifty-four percent of these cells had “laterally asymmetrical” movement relations and 38% had “laterally symmetrical” movement relations. All cells that discharged in relation to active lateral movement of the spinal column discharged preferentially in relation to ipsilateral movements, while all cells responding to passive lateral movement discharged preferentially in relation to contralateral movement. Cells related to movements of the vertebral column in the vertical plane and a small number of units related to facial, laryngeal, paw, and other movements were also found. The specific motor relations of reticular formation cells may explain the findings of previous lesion, stimulation, and recording studies.
Conclusions about the functional role of the medial reticular formation (RF) have been derived largely from investigations in anesthetized or restrained animals. Those studies proposed that RF cells mediate a number of behaviors including locomotion (10), orienting (7), REM sleep (8), pain perception (1, 2), control of motor tone (6), sensory integration (14), and sexual activity (3, 13). Microwire recording techniques now make it possible to record from these cells in freely moving animals. Using such techniques, we previously reported on the behavioral relations of pontine RF cells (17, 18). The present study reports on the behavioral relations of medullary RF cells.
Units were recorded in six unrestrained cats using bundles of 32-μm microwires (20). Unit activity was observed polygraphically and on an audio monitor. All units considered had initially negative spikes and were observed for at least 4 h. Electrooculograms (EOGs) were derived from screws in the orbit and electromyograms (EMGs) from stranded stainless-steel wires placed bilaterally in the splenius muscles.
Unit discharge increased during movement detected by EOG and EMG activity, as reported in pontine RF units (18). However, these measures alone were insufficient to characterize the cells’ specific movement relations. In order to differentiate between cells related to different components of motor behavior, we developed a series of behavioral testing procedures which included observations of passive and active movements of all limb joints and of the vertebral column. The procedures and samples of unit activity during movement were presented elsewhere (17, 18, 20). In selected cases, results of the behavioral tests were confirmed by filming the cats and the associated unit discharge with an 8-mm sound ciné-camera and subjecting the film to frame by frame vector analysis of movement-unit discharge relations, as previously reported (16).
Medullary RF cells could be divided into two major classes (Table 1) on the basis of the behavioral correlates of their discharge; cells with laterally asymmetrical movement relations, i.e., cells that discharged during movements to the left or on the left side of the body at rates that differed from the rates during corresponding movements to the right or on the right side of the body, and cells with laterally symmetrical movement relations, i.e., cells that discharged equally during left and right movements. Fifty-four percent of encountered cells (N = 21) had laterally asymmetrical movement relations and 38% (N = 15) had laterally symmetrical movement relations. The remaining cells (N = 3, 8% of the total) did not relate to any specific motor behavior.
TABLE 1.
Frequencies of Subtypes of Behavioral Relations in the Medullary Reticular Formation
| Cell type | Movement relation | N | Percent |
|---|---|---|---|
|
| |||
| A—Cells with laterally asymmetrical movement relations | 21 | 54 | |
| Lateral head–neck movement | 16 | 41 | |
| Other ipsilateral movement | 2 | 5 | |
| Contralateral paw and scapula | 3 | 8 | |
| B—Cells with laterally symmetrical movement relations | 15 | 38 | |
| Spinal movements | 12 | 31 | |
| Miscellaneous | 3 | 8 | |
| C—Unclassified cells | 3 | 8 | |
| Total | 39 | 100 | |
Laterally Asymmetrical Cells.
Cells with laterally asymmetrical movement relations could be further divided into three types on the basis of the part of the body involved in the movement. The largest class (N = 16) consisted of cells related to movements of the head or vertebral column in the horizontal plane. Of these, 12 responded when the head was passively moved in the horizontal plane. In all of these cells, increased discharge was related to passive head movement to the contralateral side (i.e., to the cat’s right if the cell was localized to the left medullary RF). None responded to passive movement to the ipsilateral side. This asymmetry was highly significant (P < 0.0005, binomial test, two-tailed). When these head movement relations were first seen, we assumed that they were vestibular responses and made no further observations of spontaneous movements. We were therefore surprised later in the study to find that three cells responding to passive head movement to the contralateral side did not discharge when the same movement was made spontaneously by the cat. Instead these cells discharged only when the cat turned to the ipsilateral side. An additional four units that showed no response to passive head movement also discharged during active movements to the ipsilateral side. No lateral head movement units which discharged when the cat moved spontaneously to the contralateral side were found. This asymmetry of active movement relations was significant (P < 0.016, binomial test, two-tailed).
Lateral head movement cells would typically respond during a variety of movements to the ipsilateral side. For example, a cell might discharge as the cat made head movements to track an object moving to the ipsilateral side, during head movement in response to a noise localized to the ipsilateral side, and rhythmically during grooming movements to the ipsilateral side. The topography of the movement, not the nature of the stimulus, was the best correlate of the neural response. Only one of the seven lateral head movement cells tested discharged in relation to spontaneous quick head shakes or to the stereotyped, quick head shake responses induced by placing a pellet into the concha of the ear. This was consistent with our observations that activity in most of these cells was more closely correlated with movement of the lower neck (lower cervical and thoracic vertebrae) than with movements of the head and upper cervical vertebrae.
Another type of laterally asymmetrical movement relation was observed in two cells related to intrinsic head musculature. One responded during tongue protrusions to the ipsilateral side. The other cell was related to movements of the ipsilateral nostril and upper lip.
A third type of laterally asymmetrical cell discharged in relation to contralateral movements. Three such cells were observed. One discharged in conjunction with contralateral forepaw movements, a second during movements of the contralateral scapula, and a third during forceful thrusts of the contralateral rear paw.
Laterally Symmetrical Cells.
Cells without lateralized behavioral relations (N = 15) could be divided into those related to head or spinal movements and those related to other movements. The first group could be subdivided into two categories. The largest (N = 8) consisted of cells that did not respond to passive movements of the head. Of these, six discharged in relation to active forward movements of the head when it was in a lowered position, such as movements that would occur when the cat was approaching objects put on the cage floor. Associated movements of the paws or sniffing movements were not required to activate these cells. A seventh cell discharged only during active ventral movements of the lumbar spine, and an eighth discharged during fixation of the lumbar region.
The second category (N = 4) consisted of cells that increased discharge during passive ventroflexion of the head. Three of these cells responded most vigorously during spontaneous upward spinal movements, such as those which occurred when the cat stood up. They did not discharge during spontaneous downward movements. The fourth cell responded during either active or passive downward movement.
Cells not related to spinal movement included (i) a cell which discharged tonically during purring and in a burst during swallowing. This cell appeared to be related to laryngeal activity and (ii) two cells that discharged only during swallowing. These three cells were situated immediately dorsolateral to the inferior olive, not, as might be expected, near the nucleus ambiguus.
We looked for, but did not observe, correlations between medullary RF discharge and eye movement, although we have seen a small number of eye movement related cells in pontine RF regions (19). We saw no cell that increased discharge rate selectively in response to painful stimuli. All cells were tested with a mild noxious stimulus, a cotton swab irritating each cornea until the nictitating membrane response occurred and a more intense noxious stimulus, manual pressure applied to the forepaw until withdrawal occurred. In no case was cell discharge selective for these stimuli. Although cell discharge did not appear to be related to the noxious stimulus itself, the application of such a stimulus usually produced movements. These movements were accompanied by increased unit discharge if, and only if, they included the movement with which the cell was associated during spontaneous behavior. No cell appeared to discharge selectively during startle responses or in relation to arousal or REM sleep. The modulation of activity in these cells during the sleep cycle was described elsewhere (21).
No medullary RF cell responded to stroboscopic visual stimulation. Six cells responded to auditory stimulation. Four of these six were related to lateral head movement and the other two to dorsoflexion of the head during spontaneous behavior. The auditory response latency was 13 to 45 ms.
Twelve cells responded to somatic stimuli. Ten of these were cells with laterally asymmetrical behavioral relationships, and the other two were cells with symmetrical relations to spinal movement. The center of the receptive field was stimulated electrically with 0.2-ms square wave pulses in seven of the cells having somatic receptive fields. Despite the use of stimuli sufficiently intense to produce twitching in adjacent musculature, only three of these cells responded. The latencies were 15 to 30 ms.
Seventeen cells responded to head acceleration. These included 13 of the cells related to lateral head movement. Acceleration in directions opposite to the preferred one produced inhibition or no response.
Repeated stimulation at rates in excess of 2/s resulted in attenuation of response within 5 s in all 12 cells responding to somatic stimuli, in five of six cells responding to auditory stimuli, and in 11 of the 15 cells responding to vestibular stimulation. The response reduction was often abrupt and coincident with a change in motor activity. For example, vestibular response attenuation usually occurred when the cat either increased its resistance to head movement, or when it relaxed its head musculature.
Histology.
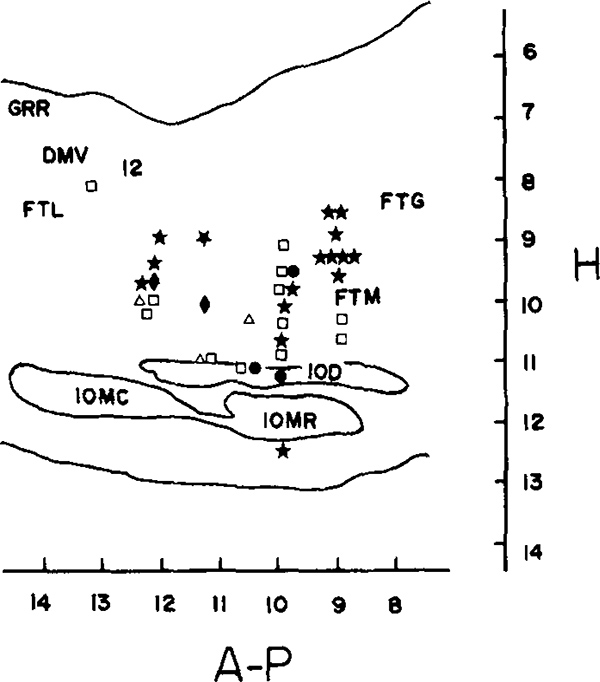
Figure 1 presents the anatomical distribution of RF unit-movement relations. There was considerable overlap in the loci of cell types. All but seven of the units were located between 0.8 and 1.6 mm lateral to the midline. These seven, between P10.5 and P11.5, were located 1.6 to 2.7 mm from the midline (21).
FIG. 1.
Anatomical distribution of medullary behavioral relations on sagittal section of the cat brain stem. Filled symbols are cells with laterally asymmetrical movement relations and unfilled symbols are cells with laterally symmetrical movement relations. Symbols—stars—lateral head movement cells, diamonds—ipsilateral facial and tongue movement, circles—contralateral paw, ear, and scapula, squares—nonlateralized spinal movements, triangles—pharyngeal and laryngeal movements. Overlapping symbols indicate cells recorded from adjacent or same microwires. Abbreviations: GRR—rostral gracile nucleus, DMV—dorsal motor nucleus of the vagus, 12-hypoglossal nucleus, FTL—lateral tegmental field, IOD—dorsal accessory nucleus of the inferior olive, IOMC—caudal division of the medial accessory inferior olive, IOMR—rostral division of the medial accessory inferior olive.
These results indicate that discharge in single units in the medullary RF, like that in pontine RF units, is related to specific movements. Most medullary RF cells discharged in relation to movements of the spinal column. Although these cells may be driven by activity in muscle spindles or tendon receptors, it appears unlikely that most of their movement relations are a reflection of activity in vestibular or joint receptors, for the following reasons. (i) In most cases passive movements which caused unit discharge were opposite in direction to those associated with unit discharge during spontaneous movement. Medullary RF-mediated muscle resistance to forced head movement may explain their discharge during passive movement. (ii) Response amplitude to passive movement was highly variable and attenuated rapidly. In contrast, repetitive spontaneous movements, such as those that occurred during grooming, produced repeated unit discharge as long as similar movements were repeated. (iii) There was no response to spontaneous rapid head-shake movement, presumably an effective vestibular and proprioceptive stimulus. (iv) Head restraint did not prevent phasic discharge bursts in association with EMG activation.
The asymmetrical relationship of medullary RF unit activity and lateral head movement is somewhat surprising. Although medullary reticulo-spinal neurons project largely in the ipsilateral white matter of the cord (9, 22), they also branch bilaterally and extensively, innervating both ventral horns (11, 12). The behavioral relations shown by these cells indicate that many may have a pattern of synaptic relationships that coordinates excitation and inhibition of several motoneuron pools involved in producing ipsilateral movement.
Lawrence and Kuypers (5) reported that bilateral lesions in the medulla, interrupting outflow from medial brain stem systems, produce a loss of control in postural muscles, while limb muscles remain unaffected. The finding that most medullary RF cells, whose axons would be cut by this procedure, discharge in relation to contractions of axial musculature may explain those findings. Similarly the present results may reveal the cellular mechanism underlying the classic “tegmental response” of ipsilateral curvature of the spinal column after electrical stimulation of the RF (4).
The motor relations of RF cells may help explain their discharge during painful stimulation, locomotion, REM sleep, sexual activity, and other functions to which they have been related. Spinal movement would be expected to be closely linked to all these behaviors and may be the common element mediating medullary RF activity. A recent review (15) discussed this possibility.
Acknowledgments
The author thanks Rebecca Wheeler for assistance. Supported by the Medical Research Service of the Veterans Administration and National Institutes of Health research grant NS14610.
Abbreviations:
- RF
reticular formation
- EOG
electrooculogram
- EMG
electromyogram
REFERENCES
- 1.BURTON H 1968. Somatic sensory properties of caudal bulbar reticular neurons in the cat (Felis domestica). Brain Res. 11: 357–372. [DOI] [PubMed] [Google Scholar]
- 2.CASEY KL 1971. Somatosensory responses of bulboreticular units in awake cat: Relation to escape-producing stimuli. Science 173: 77–80. [DOI] [PubMed] [Google Scholar]
- 3.HORNBY JB, AND ROSE JD. 1976. Responses of caudal brain stem neurons to vaginal and somatosensory stimulation in the rat and evidence of genital nociceptive interactions. Exp. Neurol. 51: 363–376. [DOI] [PubMed] [Google Scholar]
- 4.INGRAM WR, RANSON SW, HANNETT FI, ZEISS FR, AND TERWILLIGER EH. 1932. Results of stimulation of the tegmentum with the Horsley–Clarke stereotaxic apparatus. Arch Neurol. Psychiat. 28: 513–541. [Google Scholar]
- 5.LAWRENCE DG, AND KUYPERS HGJM. 1968. The functional organization of the motor system in the monkey. Brain 91: 1–43. [DOI] [PubMed] [Google Scholar]
- 6.MAGOUN HW, AND RHINES R. 1946. An inhibitory mechanism in the bulbar reticular formation. J. Neurophysiol. 9: 165–171. [DOI] [PubMed] [Google Scholar]
- 7.MAUNZ RA, PITTS NG, AND PETERSON BW. 1978. Cat spinoreticular neurons: Locations, responses and changes in responses during repetitive stimulation. Brain Res. 148: 365–379. [DOI] [PubMed] [Google Scholar]
- 8.NETICK A, OREM J, AND DEMENT W. 1977. Neuronal activity specific to REM sleep and its relationship to breathing. Brain Res. 120: 197–207. [DOI] [PubMed] [Google Scholar]
- 9.NYBERG-HANSEN R 1965. Sites and mode of termination of reticulo-spinal fibers in the cat: an experimental study with silver impregnation methods. J. Comp. Neurol. 124: 71–100. [DOI] [PubMed] [Google Scholar]
- 10.ORLOVSKII GN 1970. Work of the reticulo-spinal neurones during locomotion. Biophysics 15: 761–771. [PubMed] [Google Scholar]
- 11.PETERSON BW, MAUNZ RA, PITTS HG, AND MACKEL RG. 1975. Patterns of projection and branching of reticulospinal neurons. Exp. Brain Res. 23: 333–351. [DOI] [PubMed] [Google Scholar]
- 12.PETERSON BW, PITTS NG, FUKUSHIMA K, AND MACKEL R. 1978. Reticulospinal excitation and inhibition of neck motoneurons. Exp. Brain Res. 32: 471–489. [DOI] [PubMed] [Google Scholar]
- 13.ROSE JD 1978. Mibrain and pontine unit responses to lordosis-controlling forms of somatosensory stimuli in the female golden hamster. Exp. Neurol. 60: 499–508. [DOI] [PubMed] [Google Scholar]
- 14.SCHEIBEL AB 1979. The Brain Stem Reticular Core and Sensory Function. In KANDEL ER, Ed., Handbook of Physiology, 2nd ed. Williams & Wilkins, Baltimore: (in press). [Google Scholar]
- 15.SIEGEL JM 1979. Behavioral functions of the reticular formation. Brain Res. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SIEGEL JM, BREEDLOVE SM, AND MCGINTY DJ. 1979. Photographic analysis of relation between unit activity and movement. J. Neurosci. Methods (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SIEGEL JM, AND MCGINTY DJ. 1976. Brainstem neurons without spontaneous unit discharge. Science 193: 240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SIEGEL JM, AND MCGINTY DJ. 1977. Pontine reticular formation neurons: Relationship of discharge to motor activity. Science 196: 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SIEGEL JM, AND MCGINTY DJ. 1978. Pontine reticular formation neurons and motor activity. Science 199: 207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SIEGEL JM, MCGINTY DJ, AND BREEDLOVE SM. 1977. Sleep and waking activity of pontine gigantocellular field neurons. Exp. Neurol. 56: 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SIEGEL JM, WHEELER RL, AND MCGINTY DJ. 1979. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.TORVIK A, AND BRODAL A. 1957. The origin of reticulospinal fibers in the cat. Anat. Rec. 128: 113–137. [DOI] [PubMed] [Google Scholar]