Abstract
The mouse bcg host resistance gene is known to control the activation of host macrophages for killing of intracellular parasites like Leishmania donovani as well as intracellular bacteria, including Mycobacterium bovis BCG and Salmonella enterica serovar Typhimurium. The Nramp1 gene has been mapped to this locus and affects the efficiency of macrophage activation. It has been shown that imidazoquinoline compounds, including S28463, are able to improve the clearance of a number of intracellular pathogens such as herpes simplex virus 2, human papillomavirus, and Leishmania. The goal of this study was to determine whether S28463 is efficient against infection with another intracellular pathogen, M. bovis BCG, and to determine the molecular basis underlying this effect. To achieve this, B10A.Nramp1r and B10A.Nramp1−/− mice were infected with M. bovis BCG and treated with S28463. The bacterial content in the spleen from these mice was assayed by a colony-forming assay. In addition, in vitro experiments were performed using bone marrow-derived macrophage cell lines from these mice. These cells were treated with S28463 and/or gamma interferon (IFN-γ), and nitric oxide (NO) production was measured. Our study was able to show that S28463 acts in synergy with IFN-γ to increase the production of NO in vitro. We were also able to demonstrate that mice that carried the resistant allele of the Nramp1 gene and were infected with M. bovis BCG responded to treatment with S28463, resulting in a decreased bacterial load after 2 weeks of treatment. Mice that do not express the Nramp1 gene responded only to a very large dose of S28463, and the response was not as efficient as that observed in mice carrying a wild-type Nramp1 allele. Our data provide evidence for the potential of S28463 as an immunomodulator that may be helpful in designing efficient strategies to improve host defense against mycobacterial infection.
S28463 (R-848), an analog of imiquimod, is a member of the imidazoquinoline family that have been described as immune response modifiers. Numerous members of this family of compounds exhibit potent antiviral (8, 32), tumoricidal (10, 27, 37), and adjuvant activities (9, 46). An imiquimod cream, Aldara, is currently being administered in humans for the treatment of external genital warts caused by the human papillomavirus (HPV) (11, 19, 41).
The imidazoquinolines have no inherent antiviral or cytotoxic effect but seem to stimulate the innate and acquired arm of the immune response (3). They were first described as potent inducers of alpha and beta interferons (IFN-α/β) in the sera of mice (35), chickens (28), and humans (51) that had been orally treated with these compounds. The imidazoquinolines have also been shown to induce the secretion of a whole spectrum of cytokines, such as interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) in a number of animal models such as the mouse, the guinea pig, and the monkey (26, 29, 35, 42, 51). Imidazoquinolines can also induce cytokine secretion as well as maturation of dendritic cells, as shown by increased T-cell proliferation in the presence of imiquimod-treated dendritic cells (1, 17). B-cell maturation and antibody production are also enhanced by these compounds (45). Furthermore, Langerhans cell migration to the draining lymph node is increased in imiquimod-treated mice (40).
The exact mechanism by which the imidazoquinolines exert their effect is currently unknown. A number of transcription factors and protein tyrosine kinases have been shown to be important for some but not all of the effects of these compounds. Some of these crucial factors include STAT1 (13), NF-κB, and AP-1 (15), which are transcription factors controlling the expression of a number of immune response genes. Protein kinase C (31), Jun kinase, and the mitogen-activated protein kinase p38 (45) have all been shown to play a role in the response to the imidazoquinolines, although the exact biochemical pathway used to activate them remains to be elucidated.
The macrophage has been characterized as the main target for the imidazoquinolines (20), and the cytokines produced in response to these compounds, such as IL-12, are able to skew the immune response toward a TH1 phenotype (50). This type of response is crucial against intracellular pathogens such as viruses and intracellular parasites. In accordance with these findings, S28463 has been shown to effectively activate macrophage killing of Leishmania both in vivo and in vitro through inducing the synthesis of nitric oxide (NO) (15). More recently, through a gene array approach, it was revealed that S28463 induced the expression of numerous genes which are associated with macrophage activation and the inflammatory response (16). Based on these observations, we hypothesized that S28463 may also be effective against Mycobacterium bovis BCG.
The susceptibility to infection with Leishmania donovani, M. bovis BCG, and Salmonella enterica serovar Typhimurium was shown to be regulated by an autosomal dominant gene located on chromosome 1 in mice (7, 18, 47). The natural resistance-associated macrophage protein 1 (NRAMP1) encodes a macrophage-restricted transmembrane protein that shows homology with other eukaryotic transporter proteins of the non-ATP-binding cassette type (4, 21). In mice, two alleles of the Nramp1 gene have been described. The wild-type allele (Nramp1r) conferring resistance to infection has a glycine residue at position 169, while the other allele (Nramp1s) has an aspartic acid residue at the same position, leading to susceptibility to intracellular pathogens (49). This aspartic acid substitution was found to be in absolute association with the M. bovis BCG susceptibility phenotype in all tested mouse strains.
Mice lacking the Nramp1 gene (Nramp1−/−) exhibit numerous defects at the immunological level and have been reported to be as susceptible to M. bovis BCG infection as Nramp1s mice (48). Availability of Nramp1−/− mice has provided a genetically homogenous model to study the consequence of Nramp1 gene deletion on susceptibility to infection with M. bovis BCG, S. enterica serovar Typhimurium, and L. donovani. In fact, for all these pathogens, the kinetics of infection in Nramp1−/− mice was the same as that observed previously in Nramp1s mice (48). In Nramp1s macrophages, induction of major histocompatibility complex (MHC) class II expression by IFN-γ is much less pronounced then what is observed in Nramp1r macrophages (6, 30, 52). Nramp1s macrophages treated with IFN-γ are less efficient in phosphorylating STAT1 (52) and consequently exhibit lower levels of a number of cytokines, such as inducible NO synthase (iNOS), IL-1β, and TNF-α compared to Nramp1r macrophages (5, 12, 34, 36, 38).
Studies of the yeast homologs of Nramp1, smf1 and smf2, suggest that the NRAMP1 protein might act to transport divalent cations such as Fe2+, Mn2+, and Zn2+ (33). Since NRAMP1 is localized in the late phagosomal/early endosomal vesicle (22), it could modulate the intravesicular environment and control the proliferation of some intracellular pathogens.
Since imidazoquinolines (S28463 and imiquimod) were shown to be effective against Leishmania spp. (15), and because resistance to this pathogen is controlled at the Nramp1 locus (14), we hypothesized that imidazoquinolines may affect the Nramp1-dependent macrophage activation during the course of infection with M. bovis BCG or other intracellular pathogens such as L. donovani.
In this study, we demonstrated that expression of the NRAMP1 protein is required for the optimal induction of nitric oxide production in macrophages treated with S28463. Our results also demonstrate that Nramp1 gene expression plays an important role in the regulation of the efficiency of imidazoquinoline-induced macrophage activation in mice as seen by the inability of Nramp1−/− mice to reduce M. bovis BCG proliferation.
MATERIALS AND METHODS
Bacteria and reagent.
M. bovis BCG substrain Montreal was cultivated using constant rotation at 37°C for 2 weeks in Middlebrook 7H9 broth supplemented with 10% Middlebrook OADC enrichment (Becton Dickinson, Cockeysville, Md.) and containing 0.05% Tween 80. After the culture reached an optical density at 600 nm of 0.6 to 1.0, cells were collected, briefly sonicated to disrupt bacterial clumps, and filtered through a 5-μm syringe filter to eliminate remaining clumps. After estimation of cell concentration, the culture was aliquoted and frozen in 15% glycerol solution. S28463 is a propriety of 3M Pharmaceuticals and was kindly provided by R. Miller (3M Pharmaceuticals). IFN-γ was purchased from Invitrogen (Burlington, Ontario, Canada).
Mice.
B10A mice were purchased from the National Cancer Institute (Frederick, Md.). B10A.Nramp1r mice, expressing the wild-type allele of the Nramp1 gene, and B10A.Nramp1−/− mice generated from 129/J mice which had the Nramp1 gene disrupted (48) and backcrossed for 16 generations to the B10A.Nramp1r genetic background were bred according to the animal care committee protocol in the Montreal General Hospital Research Institute Animal Facility under specific-pathogen-free (SPF) condition.
Cells.
Macrophage cell lines were derived from the bone marrow of B10A.Nramp1r mice (B10A.Nramp1r cell line) and of B10A.Nramp1−/− mice (B10A.Nramp1−/− cell line). Cell lines were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah) and 1% penicillin-streptomycin antibiotic mixture (Invitrogen). Subconfluent cell cultures were used for all experiments.
Quantification of nitrite production by macrophages.
Two to four hours prior to stimulation, B10A.Nramp1r and B10A.Nramp1−/− cell lines were plated at a concentration of 1 million cells/ml. The cells were subsequently treated with IFN-γ (10 U/ml) and/or S28463 (25 ng/ml) for 24 h. The estimation of NO2− in supernatants of stimulated and nonstimulated macrophages was performed by colorimetric spectrophotometry at 543 nm using the Griess reagent. Background values for the media were subtracted from those obtained for all experimental samples. Results are expressed as the micromolar concentration of nitrite per microgram of protein. Protein concentrations were determined using the Bio-Rad protein assay.
Infection of mice and determination of spleen CFU.
B10A.Nramp1r, B10A.Nramp1−/−, and F1 B10A.Nramp1r× B10A.Nramp1−/− mice were infected intravenously with M. bovis BCG at the dose indicated in the Results section. They were subsequently injected intraperitoneally every 2 days with various doses of S28463 for 2 weeks (see Results). The mice were then sacrificed by CO2 inhalation. The spleens were removed and homogenized in 4 ml of 0.25% saponin solution using a Polytron. Subsequently, various dilutions of the homogenized samples were plated on Dubos solid agar. Plates were incubated at 37°C for 2 weeks, and the number of CFU was counted to assess the bacterial burden.
Statistical analysis.
A Mann and Whitney nonparametric test was performed using the SigmaStat software (SPSS, Chicago, Ill.) to calculate statistical significance. Potential differences among treatments are considered significantly different when the P values are lower than 0.05.
RESULTS
S28463 induces NO production alone in B10A.Nramp1r and B10A.Nramp1−/− macrophage cell lines and acts synergistically with IFN- γ.
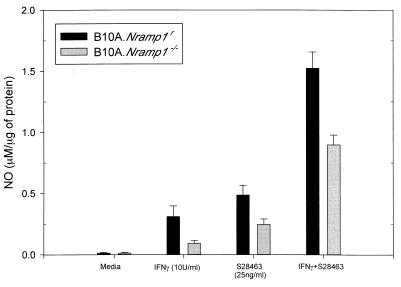
It has previously been shown that imidazoquinolines could induce the secretion of numerous cytokines by various cell types, including macrophages (26, 35, 39, 51), and it has been observed that Nramp1s macrophages do not respond efficiently to IFN-γ treatment, resulting in low levels of NO upon stimulation (5). To determine whether the presence of the Nramp1 gene was essential for activation of macrophages by S28463 and also to study the possible interplay between imidazoquinolines and IFN-γ, B10A.Nramp1r and B10A.Nramp1−/− cells were treated for 24 h with S28463 (25 ng/ml) with or without IFN-γ (10 U/ml) (Fig. 1). S28463 alone or in combination with IFN-γ induced a significant increase in NO production (P < 0.001 in all cases) in B10A.Nramp1r (0.5 μM/μg of protein and 1.5 μM/μg of protein, respectively) and B10A.Nramp1−/− (0.29 and 0.84 μM/μg of protein, respectively). Nramp1−/− macrophages produced significantly less NO in response to S28463 and IFN-γ (P < 0.001 in both cases). Synergy between S28463 and IFN-γ could still be observed in B10A.Nramp1−/− albeit to a lower extent than seen in B10A.Nramp1r macrophages (P < 0.001).
FIG. 1.
Effect of S28463 on NO production. B10A.Nramp1r and B10A.Nramp1−/− macrophages were plated on 24-well plates (106 cells/well) and allowed to adhere for 2 to 4 h. The cells were then treated for 24 h with S28463 (25 ng/ml) with or without IFN-γ (10 U/ml). Each stimulus was done in quadruplicate. The amount of NO2− produced per amount of total protein was determined by using the Griess reagent. Data are presented as the mean + standard deviation (SD) of two independent experiments. There was a significant difference in NO production between B10A.Nramp1r and B10A.Nramp1−/− macrophages following S28463 treatment alone (P < 0.001) or following treatment with IFN-γ and S28463 (P < 0.001).
S28463 reduces M. bovis BCG load in B10A.Nramp1r and in F1 B10A.Nramp1r × B10A.Nramp1−/− mice.
S28463 was shown to be effective against various intracellular pathogens (15, 19, 23–25, 39, 44). To determine whether it could be effective against M. bovis BCG infection, B10A.Nramp1r and F1 B10A.Nramp1r × B10A.Nramp1−/− mice were infected intravenously with 5 × 105 CFU of M. bovis BCG and injected intraperitoneally every 2 days with S28463 (2 μg/mouse) for a period of 2 weeks. The spleens were homogenized, plated, and incubated at 37°C, and the CFU were determined 2 weeks later. Treatment with S28463 significantly reduced the amount of CFU present in the spleen of B10A.Nramp1r from 13,432 to 4,959 CFU per spleen (P < 0.001, n = 13 for PBS-injected and n = 12 for S28463 treatment) (Fig. 2A). Since the B10A.Nramp1r mice are naturally resistant to M. bovis BCG infection, an infectious dose five times higher than that used with the B10A.Nramp1−/− mice was administered, although similar effects were observed in these mice at an infectious dose of 105 CFU of M. bovis BCG (data not shown). F1 B10A.Nramp1r × B10A.Nramp1−/− mice also showed a significant reduction in the bacterial load from 10,400 to 5,600 CFU (P < 0.001, n = 7 for both groups) (Fig. 2B). These findings show that S28463 is effective against M. bovis BCG infection and that the presence of one functional allele of the Nramp1 gene is sufficient for complete responsiveness to this compound.
FIG. 2.
Bacterial load of B10A.Nramp1r and F1 B10A.Nramp1r × B10A.Nramp1−/− mice treated with S28463. B10A.Nramp1r (A) or F1 B10A.Nramp1r × B10A.Nramp1−/− mice (B) were infected intravenously with 5 × 105 M. bovis BCG and injected intraperitoneally every 2 days with S28463 (2 μg/mouse). Once the mice were sacrificed, the spleen was removed, homogenized, plated at various dilutions, and incubated for 2 weeks. The colonies were then counted, which enabled the determination of the total amount of bacteria. Data are presented as the median from three and two independent experiments for panels A and B, respectively. Differences in CFU between PBS-treated and S28463-treated mice were significant (P < 0.001) in both panel A and panel B.
S28463 fails to reduce bacterial load in B10A.Nramp1−/− mice.
Nramp1s and Nramp1−/− mice have previously been shown to display numerous differences in their immunological response compared to wild-type mice. Some of these differences include the production of some crucial cytokines, such as IFN-γ (43). To determine the role of Nramp1 in the responsiveness to imidazoquinolines in vivo, B10A.Nramp1−/− mice were infected intravenously with 105 M. bovis BCG and injected intraperitoneally every 2 days with different doses of S28463 (from 2 to 50 μg). After 2 weeks of treatment the animals were sacrificed, and the spleens were collected, homogenized, and plated. The CFU were subsequently counted after 2 weeks. No significant reduction in bacterial load could be observed with the dose of S28463 shown to be effective in B10A.Nramp1r mice (2 μg) or doses 4, 8, or 25 times higher (P > 0.05 for all the doses tested) (Fig. 3). These results and those presented in Fig. 2 demonstrate that the Nramp1 gene plays an important role in the regulation of imidazoquinoline-induced protection against mycobacterial infection.
FIG. 3.
Bacterial load of B10A.Nramp1−/− mice treated with various doses of S28463. B10A.Nramp1−/− mice were infected intravenously with 105 M. bovis BCG and injected intraperitoneally every 2 days with 2 μg (panel A), 8 μg (panel B), 16 μg (panel C), or 50 μg (panel D) of S28463. The mice were sacrificed and the spleens were removed. The spleens were homogenized, and various dilutions were plated and left to grow for 2 weeks. The colonies were then counted, and the total amount of bacteria was determined. Results from four different experiments are shown. Filled symbols, PBS; open symbols, S28463. No significant decrease in CFU could be detected for any of the doses tested (P > 0.05 in all cases).
High dose of imidazoquinolines leads to a small reduction in bacterial load in B10A.Nramp1−/− mice infected with M. bovis BCG.
Even though B10A.Nramp1−/− mice failed to respond to a dose of S28463 effective in B10A.Nramp1r mice (2 μg), it is still possible that dramatically higher doses of imidazoquinolines could rescue these mice from their lack of responsiveness. In order to test this hypothesis, B10A.Nramp1−/− mice were infected with 105 M. bovis BCG and treated every 2 days with 500 μg of S28463, which is 250 times higher than the effective dose used in mice carrying a wild-type allele of Nramp1. After 2 weeks, spleens were collected to measure bacterial load, and the splenic ratio was calculated. Treatment with the high dose of S28463 led to a small but significant decrease in the amount of bacteria found in the spleen from 232,500 to 175,250 CFU (P = 0.034, n = 11 for PBS treatment and n = 12 for S28463 treatment) (Fig. 4A), albeit to a much lower level than what is observed in B10A.Nramp1r (data not shown). At this dose (500 μg), S28463 induced a twofold increase in the splenic ratio of both B10A.Nramp1−/− (P < 0.001) (Fig. 4B) and B10A.Nramp1r mice (data not shown). Taken together, these results demonstrate that at much higher doses, B10A.Nramp1−/− mice are able to respond to S28463, although less efficiently than in B10A.Nramp1r mice.
FIG. 4.
Effect of high doses of S28463 on bacterial load in B10A.Nramp1−/− mice. (A) B10A.Nramp1−/− mice were infected intravenously with 105 M. bovis BCG and injected intraperitoneally every 2 days with S28463 (500 μg/mouse). Once the mice were sacrificed, the spleen was removed, homogenized, plated at various dilutions, and incubated for 2 weeks. The colonies were then counted, which enabled the determination of the total amount of bacteria. Data are presented as the median from three independent experiments. S28463 treatment led to a significant reduction in CFU (P = 0.034). (B) Splenic ratios were calculated from the PBS- and S28463-treated mice by dividing the weight of the spleen by the total body weight of the mouse. Data are presented as the median from three independent experiments. Treatment with 500 μg of S28463 led to a significant increase in the splenic ratio of B10A.Nramp1−/− (P < 0.001).
DISCUSSION
The study presented here demonstrates that S28463 alone can induce NO production in B10A.Nramp1r macrophages and that a synergistic effect is observed when S28463 is combined with IFN-γ to increase NO secretion. Although it had previously been demonstrated that S28463 alone could induce NO production in bone marrow-derived macrophages (15), the effect of S28463 in conjunction with IFN-γ has never been reported.
The response to S28463 in an Nramp1−/− macrophage cell line was also analyzed. It was observed that lower levels of NO were produced in response to IFN-γ, which is in agreement with what has previously been observed in our laboratory (5). Treatment with S28463 induced production of NO, but to a lesser extent than that observed in B10A.Nramp1r macrophages. Treatment with IFN-γ and S28463 induced a significant production of NO from these cells, indicating that S28463 may be bypassing the IFN-γ signaling pathway and activating downstream effector molecules. These components may include the NF-κB transcription factor as well as AP-1 and c-Fos, all of which were shown to be activated by S28463 (15, 45).
The effect of S28463 on M. bovis BCG infection was also tested in this study. We demonstrated that S28463 is able to reduce the amount of M. bovis BCG present in the spleen of B10A.Nramp1r and F1 B10A.Nramp1r × B10A.Nramp1−/− mice 14 days postinfection. This effect is probably due to the immunomodulatory activity of S28463. By inducing proinflammatory cytokines and favoring a TH1 response, S28463 increases the amount of IFN-γ present, and from our results, this would lead to an increase in NO production by macrophages. Since NO was found to be directly toxic to M. bovis BCG, an increase in NO production would result in more efficient destruction of the bacteria.
We showed that S28463 was able to reduce the bacterial load in B10A.Nramp1r mice, but not in B10A.Nramp1−/− mice at a dose of 2 μg per injection. A number of factors may explain this discrepancy. First, it was previously shown that Nramp1s mice produce less IFN-γ early in infection compared to Nramp1r mice (43), which could have an effect on NO production by the macrophages. Second, S28463 was shown to induce IL-12 production (50), which triggers CD4 T cells to produce IFN-γ. Susceptible mice may have a defect in the production of IL-12 that would decrease the amount of IFN-γ present. Another explanation for the difference observed is that the kinetic of M. bovis BCG infection for susceptible mice may be different than for resistant mice. Since Nramp1−/− mice exhibit 100-fold more CFU in their spleens than B10A.Nramp1r mice, the state of infection, which is controlled by the Nramp1 gene, differs between these two mouse strains, which could interfere with the immunomodulatory function of S28463.
Of note is the observation that treatment of B10A.Nramp1−/− mice with a dose 250 times higher than that used for the B10A.Nramp1r mice leads only to a small but significant decrease in bacterial load at the end of infection with M. bovis BCG. This clearly demonstrates that the lack of the Nramp1 gene in these mice leads to a defect that cannot be overcome by using a higher dose of imidazoquinolines. Since Nramp1 is expressed almost exclusively in macrophages (21) and since these cells are also the main effectors in the response to imidazoquinolines (20), any defect in Nramp1 gene expression or function will lead to a severe impairment in the in vivo response to these compounds. It was also observed after exposure to a high dose of S28463 that the splenic ratio increased twofold in both B10A.Nramp1r and B10A.Nramp1−/− mice. This increase could be due to proliferation of lymphocytes, especially B cells, which were shown to proliferate in response to imidazoquinolines (45). Although this increase in B cells could be beneficial for certain types of infection, it did not have a major effect on the course of M. bovis BCG infection in B10A.Nramp1−/− mice.
Studies have already shown that responsiveness to imidazoquinolines varies from patient to patient and that the interferon response might be involved (2). Our model allows us to study these differences in an animal model as well to search for the critical pathway used by the imidazoquinolines. The data presented in this study suggest an important role for the Nramp1 gene in controlling the response to imidazoquinolines. This may be of crucial importance in determining the outcome of treatment for patients receiving imidazoquinolines as treatment for warts induced by HPV or for cancer treatment.
ACKNOWLEDGMENTS
This work was supported by Canadian Institutes of Health Research Grant 36337. J.M. is a recipient of an FRSQ scholarship.
We thank S. Daly for review of the manuscript.
REFERENCES
- 1.Ahonen C L, Gibson S J, Smith R M, Pederson L K, Lindh J M, Tomai M A, Vasilakos J P. Dendritic cell maturation and subsequent enhanced T-cell stimulation induced with the novel synthetic immune response modifier R-848. Cell Immunol. 1999;197:62–72. doi: 10.1006/cimm.1999.1555. [DOI] [PubMed] [Google Scholar]
- 2.Arany I, Tyring S K, Brysk M M, Stanley M A, Tomai M A, Miller R L, Smith M H, McDermott D J, Slade H B. Correlation between pretreatment levels of interferon response genes and clinical responses to an immune response modifier (imiquimod) in genital warts. Antimicrob Agents Chemother. 2000;44:1869–1873. doi: 10.1128/aac.44.7.1869-1873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arany I, Tyring S K, Stanley M A, Tomai M A, Miller R L, Smith M H, McDermott D J, Slade H B. Enhancement of the innate and cellular immune response in patients with genital warts treated with topical imiquimod cream 5% Antiviral Res. 1999;43:55–63. doi: 10.1016/s0166-3542(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson P G, Blackwell J M, Barton C H. Nramp1 locus encodes a 65 kDa interferon-gamma-inducible protein in murine macrophages. Biochem J. 1997;325:779–786. doi: 10.1042/bj3250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrera L F, Kramnik I, Skamene E, Radzioch D. Nitrite production by macrophages derived from BCG-resistant and -susceptible congenic mouse strains in response to IFN–gamma and infection with BCG. Immunology. 1994;82:457–464. [PMC free article] [PubMed] [Google Scholar]
- 6.Barrera L F, Kramnik I, Skamene E, Radzioch D. I-A beta gene expression regulation in macrophages derived from mice susceptible or resistant to infection with M. bovis BCG. Mol Immunol. 1997;34:343–355. doi: 10.1016/s0161-5890(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy R. The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1999;1:23–27. doi: 10.1016/s1286-4579(99)80010-0. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein D I, Harrison C J. Effects of the immunomodulating agent R837 on acute and latent herpes simplex virus type 2 infections. Antimicrob Agents Chemother. 1989;33:1511–1515. doi: 10.1128/aac.33.9.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein D I, Miller R L, Harrison C J. Adjuvant effects of imiquimod on a herpes simplex virus type 2 glycoprotein vaccine in guinea pigs. J Infect Dis. 1993;167:731–735. doi: 10.1093/infdis/167.3.731. [DOI] [PubMed] [Google Scholar]
- 10.Beutner K R, Geisse J K, Helman D, Fox T L, Ginkel A, Owens M L. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J Am Acad Dermatol. 1999;41:1002–1007. doi: 10.1016/s0190-9622(99)70261-6. [DOI] [PubMed] [Google Scholar]
- 11.Beutner K R, Spruance S L, Hougham A J, Fox T L, Owens M L, Douglas J M., Jr Treatment of genital warts with an immune-response modifier (imiquimod) J Am Acad Dermatol. 1998;38:230–239. doi: 10.1016/s0190-9622(98)70243-9. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell J M, Searle S. Genetic regulation of macrophage activation: understanding the function of Nramp1 (=Ity/Lsh/Bcg) Immunol Lett. 1999;65:73–80. doi: 10.1016/s0165-2478(98)00127-8. [DOI] [PubMed] [Google Scholar]
- 13.Bottrel R L, Yang Y L, Levy D E, Tomai M, Reis L F. The immune response modifier imiquimod requires STAT-1 for induction of interferon, interferon-stimulated genes, and interleukin-6. Antimicrob Agents Chemother. 1999;43:856–861. doi: 10.1128/aac.43.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley D J. Genetic control of natural resistance to Leishmania donovani. Nature. 1974;250:353–354. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- 15.Buates S, Matlashewski G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J Infect Dis. 1999;179:1485–1494. doi: 10.1086/314782. [DOI] [PubMed] [Google Scholar]
- 16.Buates S, Matlashewski G. Identification of genes induced by a macrophage activator, s-28463, using gene expression array analysis. Antimicrob Agents Chemother. 2001;45:1137–1142. doi: 10.1128/AAC.45.4.1137-1142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns R P, Jr, Ferbel B, Tomai M, Miller R, Gaspari A A. The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans' cells. Clin Immunol. 2000;94:13–23. doi: 10.1006/clim.1999.4804. [DOI] [PubMed] [Google Scholar]
- 18.Canonne-Hergaux F, Gruenheid S, Govoni G, Gros P. The Nramp1 protein and its role in resistance to infection and macrophage function. Proc Assoc Am Physicians. 1999;111:283–289. doi: 10.1046/j.1525-1381.1999.99236.x. [DOI] [PubMed] [Google Scholar]
- 19.Edwards L, Ferenczy A, Eron L, Baker D, Owens M L, Fox T L, Hougham A J, Schmitt K A. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group Human Papillomavirus. Arch Dermatol. 1998;134:25–30. doi: 10.1001/archderm.134.1.25. [DOI] [PubMed] [Google Scholar]
- 20.Gibson S J, Imbertson L M, Wagner T L, Testerman T L, Reiter M J, Miller R L, Tomai M A. Cellular requirements for cytokine production in response to the immunomodulators imiquimod and S-27609. J Interferon Cytokine Res. 1995;15:537–545. doi: 10.1089/jir.1995.15.537. [DOI] [PubMed] [Google Scholar]
- 21.Govoni G, Gauthier S, Billia F, Iscove N N, Gros P. Cell-specific and inducible Nramp1 gene expression in mouse macrophages in vitro and in vivo. J Leukoc Biol. 1997;62:277–286. doi: 10.1002/jlb.62.2.277. [DOI] [PubMed] [Google Scholar]
- 22.Gruenheid S, Pinner E, Desjardins M, Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison C J, Jenski L, Voychehovski T, Bernstein D I. Modification of immunological responses and clinical disease during topical R-837 treatment of genital HSV-2 infection. Antiviral Res. 1988;10:209–223. doi: 10.1016/0166-3542(88)90032-0. . (Erratum, 11:215.) [DOI] [PubMed] [Google Scholar]
- 24.Harrison C J, Miller R L, Bernstein D I. Posttherapy suppression of genital herpes simplex virus (HSV) recurrences and enhancement of HSV-specific T-cell memory by imiquimod in guinea pigs. Antimicrob Agents Chemother. 1994;38:2059–2064. doi: 10.1128/aac.38.9.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison C J, Stanberry L R, Bernstein D I. Effects of cytokines and R-837, a cytokine inducer, on UV-irradiation augmented recurrent genital herpes in guinea pigs. Antiviral Res. 1991;15:315–322. doi: 10.1016/0166-3542(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 26.Imbertson L M, Beaurline J M, Couture A M, Gibson S J, Smith R M, Miller R L, Reiter M J, Wagner T L, Tomai M A. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J Investig Dermatol. 1998;110:734–739. doi: 10.1046/j.1523-1747.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- 27.Kagy M K, Amonette R. The use of imiquimod 5% cream for the treatment of superficial basal cell carcinomas in a basal cell nevus syndrome patient. Dermatol Surg. 2000;26:577–578. doi: 10.1046/j.1524-4725.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 28.Karaca K, Sharma J M, Tomai M A, Miller R L. In vivo and In vitro interferon induction in chickens by S-28828, an imidazoquinolinamine immunoenhancer. J Interferon Cytokine Res. 1996;16:327–332. doi: 10.1089/jir.1996.16.327. [DOI] [PubMed] [Google Scholar]
- 29.Kono T, Kondo S, Pastore S, Shivji G M, Tomai M A, McKenzie R C, Sauder D N. Effects of a novel topical immunomodulator, imiquimod, on keratinocyte cytokine gene expression. Lymphokine Cytokine Res. 1994;13:71–76. [PubMed] [Google Scholar]
- 30.Lang T, Prina E, Sibthorpe D, Blackwell J M. Nramp1 transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on antigen processing and presentation. Infect Immun. 1997;65:380–386. doi: 10.1128/iai.65.2.380-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megyeri K, Au W C, Rosztoczy I, Raj N B, Miller R L, Tomai M A, Pitha P M. Stimulation of interferon and cytokine gene expression by imiquimod and stimulation by Sendai virus utilize similar signal transduction pathways. Mol Cell Biol. 1995;15:2207–2218. doi: 10.1128/mcb.15.4.2207. . (Erratum, 15:2905.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller R L, Imbertson L M, Reiter M J, Gerster J F. Treatment of primary herpes simplex virus infection in guinea pigs by imiquimod. Antiviral Res. 1999;44:31–42. doi: 10.1016/s0166-3542(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 33.Pinner E, Gruenheid S, Raymond M, Gros P. Functional complementation of the yeast divalent cation transporter family SMF by NRAMP2, a member of the mammalian natural resistance-associated macrophage protein family. J Biol Chem. 1997;272:28933–28938. doi: 10.1074/jbc.272.46.28933. [DOI] [PubMed] [Google Scholar]
- 34.Radzioch D, Kramnik I, Skamene E. Molecular mechanisms of natural resistance to mycobacterial infections. Circ Shock. 1994;44:115–120. [PubMed] [Google Scholar]
- 35.Reiter M J, Testerman T L, Miller R L, Weeks C E, Tomai M A. Cytokine induction in mice by the immunomodulator imiquimod. J Leukoc Biol. 1994;55:234–240. doi: 10.1002/jlb.55.2.234. [DOI] [PubMed] [Google Scholar]
- 36.Schurr E, Radzioch D, Malo D, Gros P, Skamene E. Molecular genetics of inherited susceptibility to intracellular parasites. Behring Inst Mitt. 1991;13:1–12. [PubMed] [Google Scholar]
- 37.Sidky Y A, Borden E C, Weeks C E, Reiter M J, Hatcher J F, Bryan G T. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 1992;52:3528–3533. [PubMed] [Google Scholar]
- 38.Skamene E. The Bcg gene story. Immunobiology. 1994;191:451–460. doi: 10.1016/S0171-2985(11)80451-1. [DOI] [PubMed] [Google Scholar]
- 39.Slade H B. Cytokine induction and modifying the immune response to human papilloma virus with imiquimod. Eur J Dermatol. 1998;8:13–16. [PubMed] [Google Scholar]
- 40.Suzuki H, Wang B, Shivji G M, Toto P, Amerio P, Tomai M A, Miller R L, Sauder D N. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Investig Dermatol. 2000;114:135–141. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 41.Syed T A, Ahmadpour O A, Ahmad S A, Ahmad S H. Management of female genital warts with an analog of imiquimod 2% in cream: a randomized, double-blind, placebo-controlled study. J Dermatol. 1998;25:429–433. doi: 10.1111/j.1346-8138.1998.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 42.Testerman T L, Gerster J F, Imbertson L M, Reiter M J, Miller R L, Gibson S J, Wagner T L, Tomai M A. Cytokine induction by the immunomodulators imiquimod and S-27609. J Leukoc Biol. 1995;58:365–372. doi: 10.1002/jlb.58.3.365. [DOI] [PubMed] [Google Scholar]
- 43.Thompson-Snipes L, Skamene E, Radzioch D. Acquired resistance but not innate resistance to Mycobacterium bovis bacillus Calmette-Guerin is compromised by interleukin-12 ablation. Infect Immun. 1998;66:5268–5274. doi: 10.1128/iai.66.11.5268-5274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomai M A, Gibson S J, Imbertson L M, Miller R L, Myhre P E, Reiter M J, Wagner T L, Tamulinas C B, Beaurline J M, Gerster J F. Immunomodulating and antiviral activities of the imidazoquinoline S-28463. Antiviral Res. 1995;28:253–264. doi: 10.1016/0166-3542(95)00054-p. [DOI] [PubMed] [Google Scholar]
- 45.Tomai M A, Imbertson L M, Stanczak T L, Tygrett L T, Waldschmidt T J. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell Immunol. 2000;203:55–65. doi: 10.1006/cimm.2000.1673. [DOI] [PubMed] [Google Scholar]
- 46.Vasilakos J P, Smith R M, Gibson S J, Lindh J M, Pederson L K, Reiter M J, Smith M H, Tomai M A. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell Immunol. 2000;204:64–74. doi: 10.1006/cimm.2000.1689. [DOI] [PubMed] [Google Scholar]
- 47.Vidal S, Gros P, Skamene E. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J Leukoc Biol. 1995;58:382–390. doi: 10.1002/jlb.58.4.382. [DOI] [PubMed] [Google Scholar]
- 48.Vidal S, Tremblay M L, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidal S M, Pinner E, Lepage P, Gauthier S, Gros P. Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J Immunol. 1996;157:3559–3568. [PubMed] [Google Scholar]
- 50.Wagner T L, Ahonen C L, Couture A M, Gibson S J, Miller R L, Smith R M, Reiter M J, Vasilakos J P, Tomai M A. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol. 1999;191:10–19. doi: 10.1006/cimm.1998.1406. [DOI] [PubMed] [Google Scholar]
- 51.Weeks C E, Gibson S J. Induction of interferon and other cytokines by imiquimod and its hydroxylated metabolite R-842 in human blood cells in vitro. J Interferon Res. 1994;14:81–85. doi: 10.1089/jir.1994.14.81. [DOI] [PubMed] [Google Scholar]
- 52.Wojciechowski W, DeSanctis J, Skamene E, Radzioch D. Attenuation of MHC class II expression in macrophages infected with Mycobacterium bovis bacillus Calmette-Guerin involves class II transactivator and depends on the Nramp1 gene. J Immunol. 1999;163:2688–2696. [PubMed] [Google Scholar]