Abstract
Oxygen is essential for the survival, function, and fate of mammalian cells. Oxygen tension controls cellular behaviour via metabolic programming, which in turn controls tissue regeneration, stem cell differentiation, drug metabolism, and numerous pathologies. Thus, oxygen-releasing biomaterials represent a novel and unique strategy to gain control over a variety of in vivo processes. Consequently, numerous oxygen-generating or carrying materials have been developed in recent years, which offer innovative solutions in the field of drug efficiency, regenerative medicine, and engineered living systems. In this review, we discuss the latest trends, highlight current challenges and solutions, and provide a future perspective on the field of oxygen-releasing materials.
The Effect of Oxygen Tension on Cellular Metabolism and Behaviour
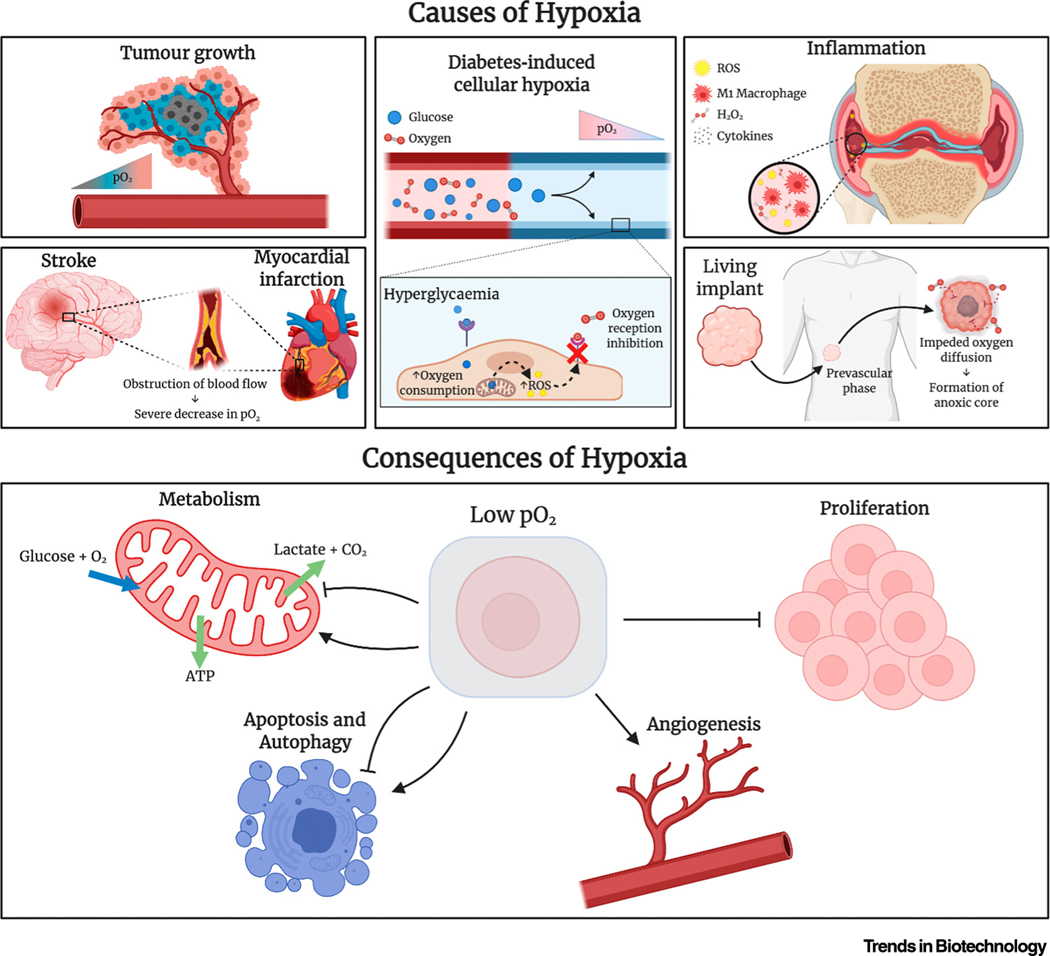
Oxygen is an essential metabolite for the survival and function of aerobic organisms. A key role of oxygen in the human body is the oxidation of nutrients, which enables efficient energy production. For example, oxygen is required for aerobic respiration, in which a single molecule of glucose is metabolised to produce approximately 30 molecules of ATP, which is the main biomolecule for energy transfer. In contrast, anaerobic (see Glossary) respiration results in the production of only two molecules of ATP [1]. Therefore, limited oxygen availability, such as in poorly vascularised microenvironments, can lead to limited ATP availability. This in turn induces metabolic stress, formation of reactive oxygen species (ROS), and autophagy, which eventually causes cell death via apoptosis and necrosis [2–4]. Moreover, when the partial pressure of oxygen (pO2) of a tissue falls to <5% oxygen, the tissue becomes hypoxic, which potently influences cellular signalling pathways that significantly alter cellular function. For example, hypoxia-induced alteration in activity of hypoxia-inducible factors 1, 2α (HIF-1α and HIF-2α; Figure 1) and nuclear factor kappa B results in changed expression of >100 genes that are involved in erythropoiesis, angiogenesis, cell migration, inflammation, apoptosis, cell motility, and proliferation (Box 1) [2,5–10].
Figure 1.
Schematic Overview of the Causes and Consequences of Hypoxia on Cellular Behaviour. Abbreviations: pO2, partial pressure of oxygen; ROS, reactive oxygen species. Figure created with BioRender.com.
Box 1. Mechanism of Hypoxic Mediators HIF-1α and HIF-2α.
In hypoxia, HIF-1α and HIF-2α is stabilized due to reduced prolyl hydroxylation, leading to the activation of the anaerobic metabolism for cell survival, the recruitment of new vasculature, the activation of Glut1 and glycolytic enzymes, the upregulation of the apoptotic pathway (depending on hypoxia severity) and the inhibition of mitochondrial respiration [2,6,8]. Mild hypoxia will lead to the promotion of pro-survival functions of the p53 response, while severe or continued hypoxia will lead to the p53-dependent apoptosis activation [9].
The pO2 within healthy tissues, also named physioxia, is relatively stable because it is required to maintain homeostasis. However, pO2 levels vary significantly between the different tissues in the human body. pO2 values can be as high as 100 mmHg in arterial blood and 70 mmHg in kidney and as low as 20 mmHg in cartilage [11–13]. Regardless, the pO2 pressure of a tissue can be severely disturbed by pathological conditions, such as disturbed blood flow, inflammation, or an increase in tissue mass, which occur in tissue trauma, cancer, diabetes, stroke, and coronary heart disease, among others (Figure 1) [11]. While in the short term, this shift toward hypoxia associates with regenerative responses, such as neovasculogenesis, stem cell differentiation, and tissue regeneration, prolonged hypoxia associates with adverse effects, such as slowed healing, ischaemic heart disease, pulmonary hypertension, cerebral ischaemia, loss of hepatic metabolic function, systemic inflammatory response, liver fibrosis, cystic fibrosis, glaucoma, arthritis, and tissue necrosis [7,14–17]. Consequently, gaining control over the local pO2 in vivo can stimulate tissue regeneration and mitigate severe adverse effects on tissue structure and organ function; thus, this represents a potent yet largely unexplored therapeutic avenue for a variety of diseases. Moreover, oxygen diffusion within implanted tissues is limited to only ~200 μm [18], resulting in severe oxygen deficiencies in the core of implanted tissues. Therefore, implant vascularisation is essential to overcome oxygen diffusion limitations, but this is a slow process, with only 5 mm of vascular penetration in 25 days into porous implanted scaffolds [19], during which implant viability needs to be maintained.
In recent years, various materials have been developed that offer a gradual and consistent release of oxygen over time [20,21]. There are two main classes of oxygen-releasing biomaterials: oxygen-carrying biomaterials (OCBs) and oxygen-generating biomaterials (OGBs). In this review, we provide a critical, comparative, and comprehensive analysis of the advantages, drawbacks, and mechanisms of oxygen release for each class, highlight suitable areas of application in the biotechnology field, and discuss anticipated future development.
Key Methods of Oxygen Generation
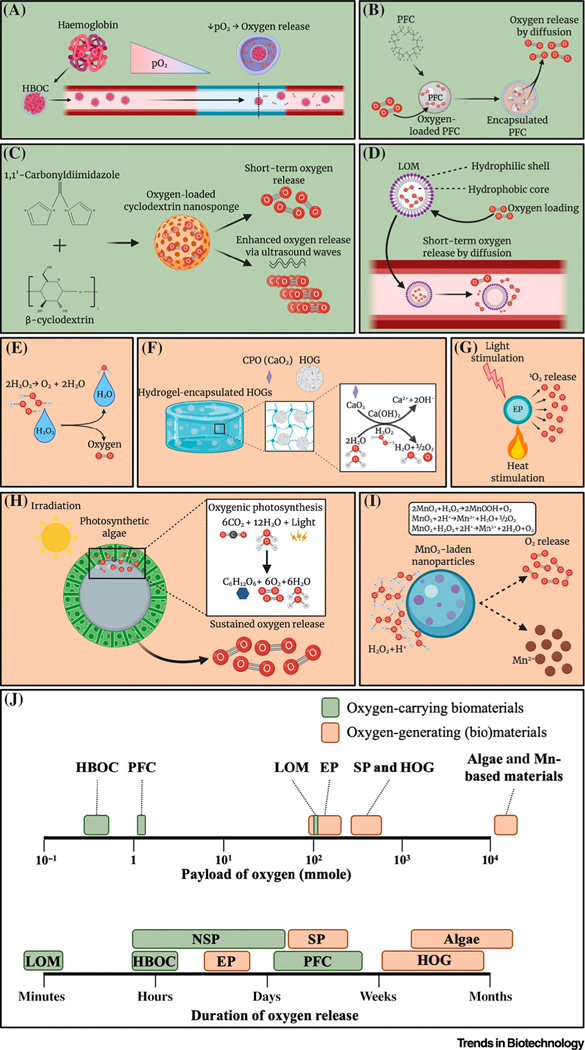
Multiple material fabrication strategies have been investigated for the production of OCBs and OGBs (Figure 2, Key Figure). Haemoglobin-based oxygen carriers (HBOCs), perfluorocarbons (PFCs), and peroxides have been topics of interest for over a decade, while novel technologies, such as lipid-based oxygen microbubbles (LOMs), oxygen-laden nanosponges (NSPs), hydrophobic oxygen generators (HOGs), metallic nanoparticles (NPs), and photosynthetic algae have only been explored in the recent past. Here, we discuss the various OCB and OGB biomaterials and provide the first comprehensive overview of their respective theoretical maximum payload and their empirically observed maximum oxygen-release duration.
Figure 2.
Key Figure Oxygen Release from Oxygen-Carrying and Oxygen-Generating Biomaterials (OCBs and OGBs)
(A–I) Oxygen-release mechanism of various OCBs and OGBs: (A) haemoglobin-based oxygen carrier (HBOC), (B) perfluorocarbon (PFC), (C) nanosponge, (D) lipid-based oxygen microbubble, (E) liquid peroxide, (F) solid peroxide (SP), (G) endoperoxide, (H) algae-based biomaterials, and (I) manganese-based materials. (J) Comparison of oxygen payload [35,38,40,53,102–104] and duration of OCBs and OGBs [23,24,31,32,35,37,39,47,53–55,57,66,102,105–110]. Abbreviations: 1O2, Singlet oxygen; CPO, calcium peroxide; EP, endoperoxide; HOG, hydrophobic oxygen generator; LOM, lipid-based oxygen microbubble; Mn, manganese; MnO2, manganese (IV) oxide; pO2, partial pressure of oxygen. Figure created with BioRender.com.
Oxygen-Carrying Biomaterials
HBOCs use natural haemoglobin (Hb) or myoglobin to reversibly bind oxygen. The haem group within these molecules can bind oxygen, and the extent of oxygen loading is dependent on the pO2 (Figure 2A). Hb and myoglobin are often encapsulated within biocompatible polymeric carriers (e.g., conjugated polymers or liposomal NPs) to enhance their stability and vascular residence time [22–24]. Despite delivering a proof-of-principle and initial success in improving the pO2 of blood, the tested formulations have often been associated with severe adverse effects, and short durations and inaccurate control over oxygen release. Most notably, owing to the production of Hb derivates, such as methaemoglobin and ferryl Hb, these formulations can significantly increase the mortality rate and myocardial infarction in multiple patient cohorts [25,26]. To address this challenge, polydopamine-coated Hb (Hb-PDA) NPs were designed by Wang and colleagues [23]. In this work, Hb-PDA NPs were developed with decreased production of Hb derivatives, which reduced cytotoxicity, increased biocompatibility, and demonstrated effective scavenging of free radicals and ROS.
PFCs have attracted much attention for their ability to serve as potential artificial blood substitutes due to their chemical and biological inertness, ease of sterilisation, and high oxygen solubility [27]. Similar to HBOCs, oxygen loading in PFCs is dependent on the pO2 (Figure 2B). In blood, oxygen extraction from PFC emulsions can reach 90% of oxygen content [28]. However, oxygen release can also be controlled by external stimuli, such as irradiation for photodynamic therapy (PDT) [29]. Niu and colleagues used PFC-containing N-isopropylacrylamide-based hydrogels to enhance cell survival by preventing occurrence of anoxia in hydrogels [30]. The hydrogels had higher oxygen levels and promoted the survival and proliferation of mesenchymal stem cells (MSCs). Yet, the duration of oxygen release remained relatively short and the control over the oxygen release was poor [29].
Cyclodextrin NSPs saturated with oxygen (Figure 2C) provide a relatively novel method for oxygen delivery [31,32]. Cavalli and colleagues developed three different cyclodextrin NSPs, which were all able to entrap and release oxygen for approximately 1 h [31]. The rate of oxygen release could be enhanced using ultrasound as an external stimulus, which increased the oxygen permeation through the NSPs in vitro. Recently, Femminò and colleagues showed sustained release of oxygen in cyclodextrin nanospheres for >2 days, resulting in a higher cell viability under anoxic conditions compared with the control group [32].
LOMs comprise an oxygen-laden core with a monolayer shell, which can be readily conjugated with a surfactant [i.e., poly(ethylene glycol)], to increase stability (Figure 2D). LOMs have been proposed as a new route of intravenously injectable materials to address hypoxaemia because they can transport and release oxygen through the bloodstream for short periods of time [33,34]. However, LOMs with the ability to deliver a clinically meaningful amount of oxygen typically caused adverse effects due to altered haemodynamics following systemic introduction [33]. To overcome this challenge, Peng and colleagues used interfacial nanoprecipitation to manufacture and investigate a microbubble-based intravenous oxygen carrier, which improved the survival rate of pathologic animal models with asphyxial cardiac arrest [35].
Oxygen-Generating Biomaterials
Liquid peroxides, such as hydrogen peroxide (H2O2), decompose into water and oxygen, thereby offering a mechanism of oxygen generation (Figure 2E). They have a fast-paced oxygen release rate due to their high water solubility and decomposition rate [36]. To increase the maximal oxygen payload and allow for more gradual release of oxygen, solid peroxides (SPs) have been explored as a way to generate oxygen by producing H2O2 as an intermediate product following their hydrolysis (Figure 2F). The three most commonly explored SPs are calcium peroxide (CPO), magnesium peroxide (MPO), and sodium percarbonate (SPC) [37–41]. The extent and duration of oxygen release can be tailored by tuning other factors, such as temperature, solubility, pH, and catalysts [42,43]. Initial studies showed oxygen release from peroxides up to several days, but this was associated with severe cytotoxicity because high levels of H2O2 and ROS are still being formed owing to the fast-paced hydrolysis of SPs. To address this challenge, both liquid peroxides and SPs are being encapsulated in a hydrophobic material [e.g., poly (caprolactone) (PCL) or poly(lactic-co-glycolic acid) (PLGA)] [38,44,45] or an enzyme-modulated material [poly(1,3-trimethylene carbonate)] [46] to control their hydrolysis rate by limiting their exposure to water molecules. These HOGs offer prolonged and gradual release rates for H2O2 and, thus, oxygen, which significantly decreases the cytotoxicity of SPs due to minimised accumulation of peroxide- and free radical-based toxic compounds [38,44–48]. Although this approach has been proven effective for a low concentration (<1%) of HOGs in living tissues, higher concentrations of HOGs still associate with substantial levels of cytotoxicity. Consequently, catalysts that accelerate the decomposition of H2O2, such as catalase and magnesium dioxide, have been found to be effective in further lowering the cytotoxicity of solid SPs [41,42,44,49,50].
An alternative peroxide-based strategy to generate oxygen involves the decomposition of various aromatic molecules (e.g., naphthalene and 2-pyridones) into endoperoxides (EPs). These constructs generate singlet oxygen (1O2) upon warming following a retro-Diels-Alder reaction (Figure 2G) [51]. 1O2 molecules are reactive and can be harmful to living systems, thus they have been used in combination with antioxidants, such as ascorbic acid, to quench 1O2. EPs can also be covalently linked to a polymer or scaffold to gain control over the rate of 1O2 release [51–53]. Furthermore, EPs require a heat source (i.e., laser therapy) to generate 1O2, which severely hinders their useability for numerous in vivo applications. Besides, the maximal therapeutic activity window of EPs is limited by their short half-life [52]. This half-life of EPs can be tuned depending on the temperature, stability, and size and steric effects of the aromatic molecules [51,52,54], with reported half-lives of only 8.5 h and 13 h [53,54]. Therefore, EPs are unlikely to be suitable for applications that require long-lasting release of 1O2.
Photosynthetic algae have been generating oxygen for millions of years, and have recently been applied as OGBs. A unique advantage is their ability to generate a constant amount of oxygen owing to their mechanism of oxygenic photosynthesis (Figure 2H) [55–57]. Several research groups have used genetically engineered photosynthetic algae to generate oxygen and secrete growth factors [e.g., vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF)] to stimulate a pro-regenerative microenvironment for wound-healing purposes [56,57]. Centeno-Cerdas and colleagues showed stable oxygen release for >30 days of culture to bridge the prevascular phase in vivo [55]. However, because exposure to light is essential to the photosynthetic release of oxygen, the applications of these living materials are limited to areas of the human body that are naturally exposed to light (e.g., skin and, to a lesser extent, subcutaneous locations). Moreover, in-depth investigations of immune compatibility and toxicity are required to ensure the safety of this material because this strategy is based on the use of living xenogenic matter (e.g., production of phycotoxin) and has yet to be performed. Regardless, these living oxygen-generating systems could be engineered to avoid detection and subsequent clearance by the body’s immune system. For example, algae can be endowed with red blood cell (RBC) membranes to reduce macrophage uptake and systemic clearance [58].
Manganese (IV) oxide (MnO2) can be used as inorganic (nano)particles capable of scavenging H2O2 and releasing oxygen as a by-product (Figure 2I) [59–61]. A major advantage of these materials is their ability to significantly reduce oxidative stress while generating oxygen. These materials have primarily been used in PDT, because they can both lower H2O2 in tumour tissue and simultaneously improve MRI by releasing Mn2+ [59,61]. Manganese ferrite, another manganese-based oxygen-generating material, also generates oxygen using a similar mechanism as MnO2 [62–64]. However, excessive concentrations of manganese-based materials can lead to cell and tissue cytotoxicity and a decrease in oxygen generation [64,65].
Comparison of Oxygen-Release and Oxygen-Generation Platforms
When comparing the theoretical maximum oxygen payload (maxO2) and the duration of oxygen release of the various oxygen-releasing biomaterials, a clear trend can be observed (Figure 2J). In general, a higher theoretical maxO2 closely correlates with an extended duration of oxygen release. For example, OGBs are able to theoretically achieve a higher oxygen payload and, thus, offer longer release times, with a range from several weeks up to a month [47,55,57,66]. By contrast, OCBs have significantly lower (two to four orders of magnitude) theoretical maxO2, which associates with a shorter duration of oxygen release. OCBs are generally able to provide oxygen in the range from minutes to several days. Of note, while EPs and LOM-based OCBs offer a high potential oxygen payload, they still associate with the short duration of oxygen release that is typical of OCBs. Consequently, OCBs and OGBs are each suited for a distinct set of applications owing to their notable difference in maxO2 capacity and release duration.
Applications of OCBs and OGBs
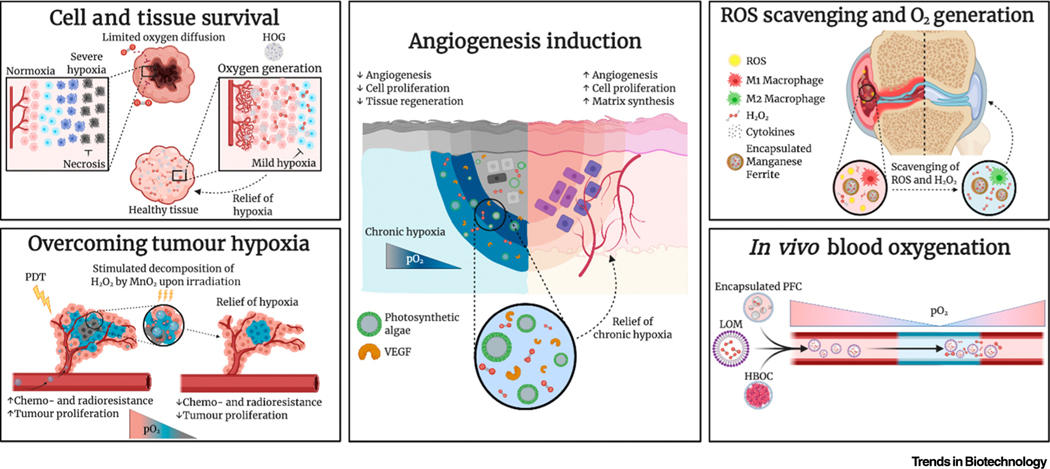
All OCBs and OGBs rely on a distinct oxygen-release mechanism that offers a unique set of advantages and disadvantages, which effectively dictates whether a material is suited for a specific application (Table 1). Thus, the development of an extensive variety of oxygen-releasing materials has opened a range of possibilities within the field of bioengineering. Specifically, OCBs and OGBs have been found to play a key role in multiple areas of applications (Figure 3): (i) in vivo implant and cell/tissue survival; (ii) OCB and OGB-induced metabolic reprogramming of cell fate and tissue function; (iii) cancer therapies; (iv) oxygen-generating and ROS-scavenging biomaterials; and (v) overcoming asphyxial cardiac arrest.
Table 1.
Advantages and Drawbacks of Various Oxygen-Releasing Biomaterials
| Method | Mechanism | Advantages | Drawbacks | Refs |
|---|---|---|---|---|
| Oxygen-carrying biomaterial | ||||
| HBOC | Binding of oxygen with haem groups | Use of natural human protein (i.e., Hb) Enhanced stability of encapsulated Hb Encapsulation of higher amounts of Hb compared with RBCs Long shelf life |
Short half-life (and oxygen release) in blood circulation Binding of Hb to nitric oxide (can result in vasoconstriction) Oxidative damage by increase of free radicals, causing adverse effects |
[23,24,111] |
| PFC | Dissolving oxygen in oil via Van der Waals forces |
Does not require blood-type matching May be used as contrast agents with MRI Low cost Long shelf life |
Rapid plasma clearance Low oxygen-carrying ability at physiological oxygen levels Induces flu-like symptoms Can bind to nitric oxide (which may result in vasoconstriction) Causes severe adverse effects |
[105–107,112] |
| NSP | Binding of oxygen to cyclodextrins | Oxygen release can be enhanced externally (offers more control) Biocompatible, biodegradable, and nontoxic | Short release times of oxygen limits applicability Needs external stimulus for enhanced oxygen generation |
[31,32] |
| LOM | Encapsulation of gaseous oxygen in microbubbles | Can be used as contrast agents for ultrasound imaging Lipid bubble increases oxygen-loading capacity Able to release high amounts of oxygen in short time |
Short release times of oxygen Often have relatively large sizes (> 10μm) Limited shelf life Can cause adverse haemodynamic effects |
[33,35,110] |
| Oxygen-generating biomaterials | ||||
| Liquid peroxides | Decomposition of H2O2 | Faster oxygen release due to higher solubility in water Can be bound to high-molecular-weight polymers to tailor oxygen generation Catalysts can be used to catalyse reaction |
Catalyst needed to lower cytotoxic effect of H2O2 and ROS Hard to control release rate and burst release |
[36] |
| SP | Generation and decomposition of H2O2 |
Ease of use High payload CPO: high purity for sustained release SPC: biocompatible products MPO: slowest oxygen formation |
Hard to control release rate and burst release Catalyst needed to lower cytotoxic effects of by-products By-products (e.g., calcium hydroxide) increase local pH CPO: lowest solubility SPC: less purity MPO: less purity |
[39,43] |
| HOG | Generation and decomposition of H2O2 in microparticles |
Ease of use Better control over oxygen release Extended release duration High payload |
Catalyst needed to lower cytotoxic effects of by-products | [38,44,45,47] |
| EP | Decomposition of aromatic molecules in endoperoxides to generate 1O2 | No cytotoxic by-products Can be linked to polymer or scaffold to control oxygen release |
Toxic main product (1O2) Requires energy (e.g., heat) to generate 1O2 Long treatment times needed for effective therapeutic treatment Short half-life |
[51–54] |
| Algae-based biomaterials | Oxygenic photosynthesis | Theoretically infinite oxygen production Can be genetically engineered to secrete growth factors | Limited applications due to required light exposure Not extensively tested in vivo for safety Likely not immunocompatible |
[55–58] |
| Mn-based materials |
Catalysed decomposition of H2O2 | Lower ROS-related cytotoxicity Enhanced MRI performance due to reduction of MnO2 into Mn2+ |
Oxygen release dependent on H2O2-rich environments More extensive (human) in vivo studies needed to determine safety Excessive Mn2+ can lead to oxidative damage in liver |
[60,62,65] |
Figure 3.
Research Applications of Oxygen-Carrying and Oxygen-Generating Biomaterials (OCBs and OGBs). Abbreviations: HBOC, haemoglobin-based oxygen carrier; HOG, hydrophobic oxygen generator; LOM, lipid-based oxygen microbubble; MnO2, manganese (IV) oxide; PDT, photodynamic therapy; PFC, perfluorocarbon; pO2, partial pressure of oxygen; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor. Figure created with BioRender.com.
Transplant and Tissue Survival
The survival and functional performance of living implants depend on the mitigation or avoidance of anoxia because this detrimental condition induces cell death, which has historically plagued in vivo experimentation and cell-based therapies. In situ oxygenation offers a first-of-its kind solution to alleviate anoxia by sustaining aerobic metabolism. Studies focussing on alleviating anoxic stress have been carried out on different cell-containing transplants in different animals, including nonhuman primates.
Baharvand and colleagues developed core-shell oxygen-carrying MPs with a poly(vinylpyrrolidone)/ H2O2 core and a PLGA shell [44]. Co-implantation of these microparticles with pancreatic islets reduced hypoxia-induced cell dysfunction, inactivation of the HIF-1α pathway, and improved graft function (i.e., higher rate of glucose clearance restored physiological glucose levels by higher rate of glucose clearance). In two similar studies, CPO was used in small poly(dimethylsiloxane) discs that could enhance cellular viability and function of β-cells and implanted islet transplants [67,68]. Furthermore, CPO was incorporated into collagen-based cryogels that were able to improve the glycaemic control offered by implanted islets in diabetic mice [39].
In addition to islet transplantation, tissue-oxygenating strategies have been used to enhance the survival of living implants that were implanted to repair or regenerate the muscle, heart, bone, and skin. For example, Li and colleagues incorporated a H2O2-laden microshell in a poly(N-isopropylacrylamide) hydrogel to augment cardiac cell survival and cardiac differentiation under hypoxia [69]. In another study, Laurencin and Daneshmandi demonstrated that CPO/PLGA matrices could enhance the regeneration of vascularised bone by stimulating the migration of host cells to the interior of the OGB matrix, which allowed these cells to survive for >8 weeks [45]. In conclusion, OGBs offer a favourable approach to maintain cellular metabolism, and thereby enable the survival of in vivo tissues and living implants when prolonged periods of oxygen release are necessitated.
Oxygen-releasing materials have also been explored to improve cell survival in ex vivo scenarios. Barralet and colleagues used CPO- and MnO2-based OGBs to sustain oxygen metabolism during organ preservation time, which is currently limited to several hours only. The study showed an increase in cell survival in aorta explants after 3 weeks with 96 ± 3% of cells surviving when OGB were used compared with 9 ± 6% when conventional vascular preservation media was used [70].
Metabolic Reprogramming of Cell Fate and Tissue Function
The oxygen tension manipulates the angiogenic processes, which further govern wound healing, bone remodelling, and female reproduction, among others, in a multifaceted manner [71,72]. Although hypoxia is considered as the main inducer of angiogenesis, anoxia and prolonged hypoxia can hinder these regenerative processes [68,73]. Interestingly, the presence of oxygen can stimulate or support angiogenesis in hypoxic and injured tissues. Although the underlying mechanism is still not entirely understood, the presence of oxygen contributes to the generation of ROS, which are hypothesised to act as signalling molecules for angiogenesis (Box 2) [37,71,74–76].
Box 2. Physiology of Angiogenesis.
All vascular cells can produce ROS through oxygen metabolism [71], which correlates with promoted signalling of, for example, VEGF, angiopoietin [75], and fibroblast growth factor [74]. Moreover, ROS facilitate the hypoxic response by decreasing prolyl hydroxylase domain protein activity, thus contributing to the stabilisation of HIF-1α [74,75]. Therefore, it has been hypothesised that oxygen-releasing materials could contribute to controlling the angiogenic process.
Several studies have reported on the positive effect of oxygen-generating materials on angiogenesis. Chavez and colleagues incorporated Chlamydomonas reinhardtii microalgae into an integra dermal regeneration template for oxygenic photosynthesis [56]. This led to significantly increased VEGF expression of genetically engineered algae 2 weeks after implantation into mice. This in turn led to an increased recruitment of vascular endothelial cells and alpha smooth actin-positive vessels. Recently, Shiekh and colleagues formulated an exosome-laden oxygen-releasing cryogel that promoted the healing of infected diabetic wounds (faster wound closure, fibroblast proliferation, and increased collagen deposition) in a diabetic rat model [77]. Moreover, implantation of thiolated gelatin containing CPO as well as the application of CPO in combination with SPO in a PCL-poly (vinyl alcohol) wound patch resulted in increased oxygen-induced vascular endothelial cell infiltration, which formed capillary-like structures [37]. Chandra and colleagues showed that this wound patch increased the formation of large vessels in a full-thickness wound model in pigs after 8 weeks [38]. These experimental findings provide a proof-of-concept that oxygen generation materials have the ability to control and guide physiological processes, such as angiogenesis.
Controlling the oxygen tension locally in vivo also offers the possibility to steer the behaviour of the immune system by, for example, manipulating the functional performance of macrophages (Box 3). Kim and colleagues formulated a dual-responsive biomaterial that increased the oxygen content while simultaneously scavenging ROS. Specifically, they showed that manganese ferrite and ceria NP-anchored mesoporous silica NPs relieved hypoxia, reduced inflammation, and induced M2 macrophage polarisation following intra-articular administration in mouse joints [62]. While a shift towards the M2 phenotype is often regarded as beneficial owing to its anti-inflammatory consequences, other applications desire the presence of M1 macrophages. Multiple studies that focused on relieving tumour hypoxia and tumour drug resistance showed that the polarisation from tumour-associated macrophages (M2 subtype) to the M1 phenotype could increase the efficacy of chemotherapies and immunotherapies [60,78]. Thus, the ability of OCBs or OGBs to induce M2 polarisation could also yield adverse effects for a specific set of applications.
Box 3. Macrophage Polarisation by Oxygen Tension.
Upregulation of HIF-1α by hypoxia as well as an increase in ROS in macrophages have shown to induce an M1 phenotype, while downregulation of HIF-1α and relief of hypoxia and ROS can induce the proinflammatory M2 phenotype [113]. Therefore, OCBs and OGBs have been hypothesised to have the potential to control macrophage polarisation.
Multiple studies have also investigated the role of oxygen tension on human MSC and embryonic stem cell behaviour, which demonstrated the influence of oxygen on motility, differentiation potential, and the secretion of angiogenic and immunomodulatory factors [79,80]. Moreover, the efficiency of iPSC reprogramming improved fourfold when adult human fibroblasts were reprogrammed under a hypoxic oxygen tension [81]. Since the physioxic oxygen tension of in vivo stem cell niches is <9% [80], higher in vitro oxygen tensions (~20%) are considered unnatural and have been shown to disturb gene expression and metabolism of various stem cells [82,83]. Despite the undisputable role of oxygen tension in stem cell development, research on the utilisation of oxygen-generating biomaterials to aid in the differentiation of stem cells has remained scarce [45,69].
Overcoming Tumour Hypoxia for Enhanced Photodynamic Therapy
Solid malignant tumours often alter their microenvironment to become hypoxic due to their high oxygen consumption, which fuels their aggressive growth. This also results in hypoxia-driven immunosuppression that can endow tumours with an increased level of drug resistance [78,84]. Thus, restrictions in the available amount of oxygen can limit the effectiveness of tumour treatments, and increasing the oxygen tension has been hypothesised as a potential strategy to resolve this clinically relevant challenge (Box 4) [85].
Box 4. Role of Oxygen within Photodynamic Therapy.
Oxygen has been shown to facilitate DNA double-strand breaks and expose cells to oxidative stresses via ROS generation [58,84,88]. Moreover, oxygen is an essential component of several anticancer therapies, such as PDT, where photosensitisers use oxygen to generate cytotoxic ROS and singlet oxygen [84] to destroy proteins and nucleic acids in tumour cells, which induces apoptosis and necrosis [93]. PDT is an important treatment modality for solid tumours owing to its minimally invasive properties, superior spatiotemporal selectivity [93], and potential to initiate an antitumour immune response [88].
To relieve hypoxia-induced multidrug resistance in solid tumours, OGBs have been explored to locally increase the oxygen tension by decomposing endogenous intracellular H2O2 [86], which is overproduced by hypoxic tumours [87]. To this end, a few strategies based on biocompatible composite materials with low toxicity, targeted delivery, and high catalyst loading (i.e., photosensitiser) have been developed for PDT [86]. For example, MnO2 was delivered in a hyaluronic acid NP in combination with various photosensitisers, such as indocyanine green [59], gold nanocages [87], acriflavine [61], and black phosphorus [88]. The reaction of MnO2 with H2O2, which is induced upon irradiation (i.e., laser or X-ray) of the photosensitisers in tumour-bearing mice, resulted in significantly reduced tumours and even tumour eradication, whereas tumour growth in non-irradiated mice was not significantly reduced [89]. Other oxygen-generating approaches that have been explored for PDT include microalgae covered in a RBC membrane [58], haem dimer coupled to BP [90], and porous platinum NPs [84]. In a recent study, Colombani and colleagues constructed CPO-based cryogels to increase CD4+ T cell and CD8+ T cells to induce a shift from immunosuppression to antitumour immunity. Using an in vivo mouse model, this approach promoted natural killer cell infiltration and enhanced immune cell survival in the tumour microenvironment, which are both involved in the antitumour response [91].
All these various oxygen-generating approaches consistently contributed to tumour-size reduction or even eradication. Consequently, elevating the oxygen tension of a tumour towards normoxic levels offers a promising avenue to improve tumour treatments.
Multifunctional Oxygen-Generating and ROS-Scavenging Biomaterials
Although the primary and most intuitive goal of OCBs and OGBs is to elevate pO2, other applications for these materials have been identified in which oxygen generation is considered a harmless by-product of its primary function (i.e., ROS scavenging) [23,39,42,49,92,93]. While the extremely oxidizing nature of ROS can be utilised for increasing cytotoxicity in tumour tissue, it can be detrimental to the viability of healthy tissues, and has been found to have a major role in several pathological diseases [3,4]. Thus, multiple studies have focussed on developing (bio)materials, including OGBs, to reduce the ROS levels in living tissues.
Oxidative stress has a key role in osteoarthritis, among others, where increased ROS (i.e., peroxides, hydroxylated radicals, and nitric oxide) levels drive extracellular matrix degradation, joint inflammation, and chondrocyte death [94]. To protect cartilage from inflammation-induced oxidative stress, intra-articular injectable MnO2 NPs were applied [49]. These were demonstrated to have a substantial retention time in the intra-articular space (at least 7 days) and, thus, allowed chondroprotection of articular cartilage in an osteoarthritic rat model. Tapeinos and colleagues developed biodegradable PLGA microspheres that were coated with type I collagen and decorated with MnO2 NPs to protect rat fibroblast cells from oxidative stress [92]. Thermally responsive 2,2,6,6-tetramethylpiperidinyloxy (TEMPO) hydrogels were explored to evaluate the protective effect of these hydrogels in a rat myocardial infarction/reperfusion model, resulting in significant reduction of local ROS accumulation in cardiomyocytes [95]. Moreover, the ROS-scavenging and oxygen-generating TEMPO hydrogel reduced ROS-mediated damage and maintenance of physiological cell function, shown by reduced left ventricle dilation and increased left ventricle wall thickness.
ROS have also been strongly linked to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with higher levels of ROS measured in the respiratory epithelium of hospitalised patients compared with the respiratory epithelium of healthy patients [96]. In a recent study, Qin and colleagues encapsulated catalase in a nanocapsule to reduce ROS-mediated immune responses, which were indicated by an increase in the number of leukocytes and further release of ROS and proinflammatory cytokines. The catalase-containing nanocapsules were able to repress replication of SARS-CoV-2 in rhesus macaques by downregulating leukocyte-mediated cytokine production [97].
Consequently, OGBs that operate via ROS decomposition have great therapeutic potential to mitigate the damaging oxidative stress associated with a variety of soft tissue diseases.
Synthetic RBC for In vivo Blood Oxygenation
OCBs, such as HBOCs and PFCs, have been widely explored as artificial RBC substitutes. The development of suitable RBC substitutes is indispensable, because they can have a vital role as an RBC alternative in case of depleted storage of blood supplies, which is an increasing problem in developing countries. Moreover, they represent a viable alternative to conventional blood transfusions, which are becoming progressively more costly due to the steadily growing list of disease (e.g., HIV, Ebola, and swine flu) detection tests that are mandated, among others [98]. Consequently, the demand for artificial blood products is rapidly increasing.
RBC substitutes have also found their way into other medical areas. For example, patients with severe and prolonged oxygen deprivation can experience asphyxial cardiac arrest. When the oxygen in blood is being depleted, the heart stops beating, which further reduces the oxygen delivery to all vital organs. Failure to rapidly restore blood oxygen levels can lead to severe organ injury and even death within minutes. HBOCs and PFCs have been intensively investigated to restore the oxygen tension of blood, but both have been associated with severe adverse effects [25–27]. Recently, an injectable foam suspension containing self-assembling lipid-based microparticles that encapsulated gaseous oxygen were intravenously administered to increase the oxygen tension of the blood [99]. The oxygen-releasing microparticles significantly decreased the degree of hypoxaemia in rabbits, which reduced the incidence of cardiac arrest and other organ injuries compared with the control group. LOMs have also been devised to release oxygen within the bloodstream. Yet, a key limitation of using LOMs within the circulatory system is their associated risk of causing acute pulmonary vascular obstruction due to lipid shedding, resulting in the subsequent formation of large lipid aggregates [100]. Recently, dextran-acetyl-succinate LOMs were adopted to address this challenge. This material was investigated for its ability to improve the survival rate of a rodent model of asphyxial cardiac arrest. Strikingly, compared with results from control groups, all animal models survived the observation period and remained haemodynamically stable. Blood mixed with dextran-acetyl-succinate LOMs did not exhibit evidence of haemolysis or complement activation, and its coagulation profile was within the acceptable range [35].
Noticeably, restoring the oxygen tension of blood has relied almost exclusively on the use of OCBs. The exploration of OGBs could also be of interest, because they allow long-term release of oxygen without the need to recharge the RBC substitute. Moreover, OGBs offer heart–lung loop-independent blood oxygenation, which could prove advantageous for clinical scenarios in which the capacity of the lung to oxygenate blood is temporarily impaired.
Concluding Remarks and Future Perspectives
The research focus of past years has been primarily on the development of new OCBs and OGBs, which has provided the field with an extensive and versatile toolbox for local oxygenation. However, a shift in research focus is foreseen from the development of novel materials toward improving their safety, improving their control, and realizing their useability (see Outstanding Questions). The current toolbox of oxygen-releasing biomaterials offers a range of oxygen-release mechanisms, durations, payloads, and (toxic) by-products. Yet, almost all materials offer poor control over their oxygen release. Specifically, current materials intensively release oxygen (e.g., burst release), which dwindles over time. Endowing solid and liquid peroxides with a coating of hydrophobic materials (i.e., PCL or PLGA) or encapsulation of Hb in carriers (i.e., liposomal or micelles) has already enhanced the duration of oxygen release, but true controllable release of stable oxygen concentrations has remained largely unattainable. Gaining true control over oxygen generation is anticipated to offer more accurate and reliable control over local oxygen tensions and tissue behaviours. The more extensive use of smart (bio)materials that are sensitive to, for example, heat stimulation [66] or enzymatic cleavage [46], is expected. These materials could be applied as sacrificial coatings around OCBs or OGBs, which would prevent oxygen generation until they are triggered to degrade. On-demand oxygen release, as used in PDT, where oxygen is released upon light stimulation, may also have a larger role in the coming years. Furthermore, an increase in combinations of existing tissue fabrication techniques with OGBs for middle to long-term oxygen generation is foreseen, which could be applied for printing vessel structures in scaffolds with oxygen-generating properties. Moreover, the safety of both OCBs (adverse effects) and OGBs (toxicity and oxidative by-products) can still be significantly improved, which is vital for the routine clinical use of these materials. Antioxidants (e.g., ascorbic acid), enzymes (e.g., catalase), or ROS-scavenging materials (MnO2) are expected to have a key role in reducing ROS-mediated cytotoxicity, and to bridge the gap between in vivo studies and clinical use.
Outstanding Questions.
How can control over the release of oxygen be realised to enable the release of a user-defined amount of oxygen in a manner that is stable over time?
How can the safety of various oxygen-releasing materials be improved to allow them to reach clinical approval and routine clinical use?
What are the most promising research areas for multiple OCBs and OGBs?
What are the long-term consequences of introducing oxygen-releasing materials within living implants or hosts?
Given that oxygen has a central role in many pathological conditions and cellular behaviours, it is anticipated that novel areas of applications will be identified. As an example, oxygen was generated a side product of glucose-sensing enhancement, and could be used as an enhancer or quencher of sensing approaches [101]. Due to the versatility of the already developed toolbox of oxygen-releasing materials, it is likely that current materials can already offer viable solutions as a logical first step to address these new challenges. For example, OCBs have a rather short duration of oxygen release and, therefore, should primarily be used for processes demanding immediate oxygen release for a short period of time, such as temporarily relieving tumour hypoxia to enhance chemotherapeutic response, where reactive by-products can also be harnessed to increase cytotoxicity against tumour and steer macrophage polarisation from M2 to M1. By contrast, OGBs are ideal for applications that require a longer duration of oxygen release and a higher total payload. For example, bioengineering of tissues requires continued survival to ensure function and anastomosis of implanted tissues, which requires oxygen release for prolonged periods of time. Moreover, OGBs could have a relevant role in the steering of (stem) cell fate, which may take several weeks to complete.
In conclusion, research on oxygen-releasing biomaterials has notably increased in its intensity and abundancy over the past few years. To maintain the increasing impact of oxygen-releasing biomaterials, research should shift toward in vivo and clinical translation. In recent years, many materials have been developed and numerous novel research fields have been discovered for these materials, yielding promising results. However, gaining temporal control over oxygen release, improving the safety of the (bio)material, and selecting the right oxygen-releasing (bio)material for the right application are anticipated to be of high relevance to increase the development pace as well as to boost impactful and clinically applied solutions to oxygen deficits.
Highlights.
Oxygen tension is a crucial factor in many diseases, and gaining control over local partial pressure of oxygen (pO2) using oxygen-releasing materials can have a significant role in overcoming these pathological conditions.
Novel oxygen-releasing strategies have extended the oxygen-generating window from several hours to several weeks and offer improved control over oxygen-release profiles by shifting away from pO2-based diffusion towards biomaterial-controlled oxygen release.
Oxygen-releasing biomaterials hold potential to improve many (novel) biomedical areas, including improving in vivo survival of living implants, offering metabolic control over cell fate and tissue function, enhancing the effectivity of photodynamic anticancer therapies, reducing reactive oxygen species-mediated cellular damage, and acting as synthetic red blood cells (RBCs) for in vivo blood oxygenation.
Acknowledgements
Je.L. acknowledges financial support from an Innovative Research Incentives Scheme Vidi award (#17522) from The Netherlands Organization for Scientific Research (NWO), European Research Council (ERC, Starting Grant, #759425), and Health-Holland (#LSHM19074). This paper was funded by the National Institutes of Health (R01AR074234) and AHA Innovative Project Award (19IPLOI34660079).
Glossary
- Anaerobic
living in the absence of free oxygen.
- Anastomosis
infiltration of blood vessels into an implanted scaffold or integration with the vasculature of the host.
- Angiogenesis
process in which new blood vessels grow from existing vasculature.
- Asphyxial cardiac arrest
cause of death induced by insufficient oxygen in the blood.
- Autophagy
mechanism of a cell in which it removes its unnecessary components.
- Catalase
enzyme that decomposes H2O2 into oxygen.
- Cytokine
small protein essential in cell signalling.
- Cytotoxicity
toxic to cells.
- Emulsion
mixture of two liquids that are immiscible.
- Erythropoiesis
process in the body for producing red blood cells.
- Exosome
extracellular vesicles produced in the endosomes of cells.
- Graft
tissue moved from one place to another by transplantation.
- Glaucoma
eye disease that results in nerve damage and vision loss.
- Haemodynamics
dynamics of blood flow.
- Homeostasis
stable internal state of cells or tissues.
- Hydrogel
a water-swollen crosslinked polymer network.
- Hydrolysis
chemical breakdown of a substance by water.
- Hypoxaemia
low level of oxygen in the blood.
- Infarction
death of tissue due to insufficient blood supply.
- Intra-articular
into an artery.
- Intravenous
into a vein.
- Ischaemia
local reduction in oxygen and nutrient availability leading to severe tissue damage.
- Macrophage polarisation
process in which macrophages adopt different functions depending on extracellular signals. They can adopt a M1 phenotype, with a role in defence against pathogens, and a M2 phenotype, which regulates the repair of damaged tissue.
- Metabolism
chemical reactions within a cell that are involved in keeping cells alive by converting nutrients into energy.
- Necrosis
cell death caused by irreversible damage.
- Neovasculogenesis
formation of new functional vascular networks.
- Platelet-derived growth factor (PDGF)
growth factor with a role in, among others, cell growth and division.
- Photodynamictherapy (PDT)
cancer treatment that combines drugs with light to destroy tumour cells.
- Singlet oxygen (1O2)
electronically excited form of oxygen.
- Solubility
ability of a substance to dissolve in a liquid.
- Subcutaneous
under the skin.
- Surfactant
compounds that reduce the surface tension between two phases.
- Vascular endothelial growth factor (VEGF)
growth factor essential in angiogenesis.
- Xenogenic
a foreign substance introduced into an organism.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Alberts B. et al. (2013) Essential Cell Biology (4th edn), Garland Science [Google Scholar]
- 2.Altman BJ and Rathmell JC (2012) Metabolic stress in autophagy and cell death pathways. Cold Spring Harb. Perspect. Biol 4, a008763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergamini C. et al. (2004) Oxygen, reactive oxygen species and tissue damage. Curr. Pharm. Des 10, 1611–1626 [DOI] [PubMed] [Google Scholar]
- 4.Barbieri E. and Sestili P. (2012) Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct 2012, 982794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins ND (2006) Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 25, 6717–6730 [DOI] [PubMed] [Google Scholar]
- 6.Mazure NM and Pouysségur J. (2010) Hypoxia-induced autophagy: Cell death or cell survival? Curr. Opin. Cell Biol 22, 177–180 [DOI] [PubMed] [Google Scholar]
- 7.D’Ignazio L. and Rocha S. (2016) Hypoxia induced NF-κB. Cells 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JW et al. (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 [DOI] [PubMed] [Google Scholar]
- 9.Feng X. et al. (2011) P53 directly suppresses BNIP3 expression to protect against hypoxia-induced cell death. EMBO J. 30, 3397–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culver C. et al. (2010) Mechanism of hypoxia-induced NF-κB. Mol. Cell. Biol 30, 4901–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreau A. et al. (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med 15, 1239–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz-Prado E. et al. (2019) Partial pressure of oxygen in the human body: a general review. Am. J. Blood Res 9, 1–14 [PMC free article] [PubMed] [Google Scholar]
- 13.Reuther MS et al. (2012) In vivo oxygen tension in human septal cartilage increases with age. Laryngoscope 122, 2407–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegfried CJ and Shui YB (2019) Intraocular oxygen and antioxidant status: new insights on the effect of vitrectomy and glaucoma pathogenesis. Am J. Ophthalmol 203, 12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C. et al. (2016) A new approach for on-demand generation of various oxygen tensions for in vitro hypoxia models. PLoS ONE 11, e0155921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery ST et al. (2017) Hypoxia and sterile inflammation in cystic fibrosis airways: mechanisms and potential therapies. Eur. Respir. J 49, 1600903 [DOI] [PubMed] [Google Scholar]
- 17.Kang YB et al. (2020) Progressive hypoxia-on-a-chip: an in vitro oxygen gradient model for capturing the effects of hypoxia on primary hepatocytes in health and disease. Biotechnol. Bioeng 117, 763–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain RK et al. (2005) Engineering vascularized tissue. Nat. Biotechnol 23, 821–823 [DOI] [PubMed] [Google Scholar]
- 19.Mikos AG et al. (1993) Prevascularization of porous biodegradable polymers. Biotechnol. Bioeng 42, 716–723 [DOI] [PubMed] [Google Scholar]
- 20.Oh SH et al. (2009) Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30, 757–762 [DOI] [PubMed] [Google Scholar]
- 21.Gholipourmalekabadi M. et al. (2016) Oxygen-generating biomaterials: a new, viable paradigm for tissue engineering? Trends Biotechnol. 34, 1010–1021 [DOI] [PubMed] [Google Scholar]
- 22.Bobofchak KM et al. (2008) Effect of cross-linker length on the stability of hemoglobin. Biochim. Biophys. Acta - Proteins Proteomics 1784, 1410–1414 [DOI] [PubMed] [Google Scholar]
- 23.Wang Q. et al. (Apr. 2017) Bioinspired polydopamine-coated hemoglobin as potential oxygen carrier with antioxidant properties. Biomacromolecules 18, 1333–1341 [DOI] [PubMed] [Google Scholar]
- 24.Centis V. et al. (2011) PEGylated liposomes encapsulating human hemoglobin enhance oxygen transfer and cell proliferation while decreasing cell hypoxia in fibrin. Biochem. Eng. J 55, 162–168 [Google Scholar]
- 25.Mozzarelli A. et al. (2010) Haemoglobin-based oxygen carriers: Research and reality towards an alternative to blood transfusions. Blood Transfus. 8, s59–s68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natanson C. et al. (2008) Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA - J. Am. Med. Assoc 299, 2304–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer D. and Ferenz KB (2019) Perfluorocarbons for the treatment of decompression illness: how to bridge the gap between theory and practice. Eur. J. Appl. Physiol 199, 2421–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riess JG (2001) Oxygen carriers (‘blood substitutes’) Raison d’etre, chemistry, and some physiology. Chem. Rev 101, 2797–2920 [DOI] [PubMed] [Google Scholar]
- 29.Hu H. et al. (2019) Perfluorocarbon-based O 2 nanocarrier for efficient photodynamic therapy. J. Mater. Chem B 7, 1116–1123 [DOI] [PubMed] [Google Scholar]
- 30.Niu H. et al. (2020) High oxygen preservation hydrogels to augment cell survival under hypoxic condition. Acta Biomater. 105, 56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalli R. et al. (2010) Nanosponge formulations as oxygen delivery systems. Int. J. Pharm 402, 254–257 [DOI] [PubMed] [Google Scholar]
- 32.Femminò S. et al. (2018) α-Cyclodextrin and α-cyclodextrin polymers as oxygen nanocarriers to limit hypoxia/reoxygenation injury: implications from an in vitro model. Polymers 10, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black KJ et al. (2017) Hemodynamic effects of lipid-based oxygen microbubbles via rapid intravenous injection in rodents. Pharm. Res 34, 2156–2162 [DOI] [PubMed] [Google Scholar]
- 34.Khan MS et al. (2018) Engineering oxygen nanobubbles for the effective reversal of hypoxia. Artif. Cells, Nanomedicine Biotechnol. 46, s318–s327 [DOI] [PubMed] [Google Scholar]
- 35.Peng Y. et al. (2018) Interfacial nanoprecipitation toward stable and responsive microbubbles and their use as a resuscitative fluid. Angew. Chem. Int. Ed 57, 1271–1276 [DOI] [PubMed] [Google Scholar]
- 36.Sen Lin L. et al. (2019) Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc 141, 9937–9945 [DOI] [PubMed] [Google Scholar]
- 37.Park S. and Park KM (2018) Hyperbaric oxygen-generating hydrogels. Biomaterials 182, 234–244 [DOI] [PubMed] [Google Scholar]
- 38.Chandra PK et al. (2015) Peroxide-based oxygen generating topical wound dressing for enhancing healing of dermal wounds. Wound Repair Regen. 23, 830–841 [DOI] [PubMed] [Google Scholar]
- 39.Razavi M. et al. (2020) A collagen based cryogel bioscaffold that generates oxygen for islet transplantation. Adv. Funct. Mater 30, 1902463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vrolijk MF et al. (2016) Iron supplements and magnesium peroxide: an example of a hazardous combination in self-medication. Basic Clin. Pharmacol. Toxicol 119, 412–417 [DOI] [PubMed] [Google Scholar]
- 41.Newland H. et al. (2018) Oxygen producing microscale spheres affect cell survival in conditions of oxygen-glucose deprivation in a cell specific manner: implications for cell transplantation. Biomater. Sci 6, 2571–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdi SIH et al. (2011) An enzyme-modulated oxygen-producing micro-system for regenerative therapeutics. Int. J. Pharm 409, 203–205 [DOI] [PubMed] [Google Scholar]
- 43.Wang H. et al. (2016) Properties of calcium peroxide for release of hydrogen peroxide and oxygen: a kinetics study. Chem. Eng. J 303, 450–457 [Google Scholar]
- 44.Montazeri L. et al. (2016) Improvement of islet engrafts by enhanced angiogenesis and microparticle-mediated oxygenation. Biomaterials 89, 157–165 [DOI] [PubMed] [Google Scholar]
- 45.Daneshmandi L. and Laurencin CT (2020) Regenerative engineered vascularized bone mediated by calcium peroxide. J. Biomed. Mater. Res. Part A 108, 1045–1057 [DOI] [PubMed] [Google Scholar]
- 46.Steg H. et al. (2017) Oxygen-releasing poly(trimethylene carbonate) microspheres for tissue engineering applications. Polym. Adv. Technol 28, 1252–1257 [Google Scholar]
- 47.Zhang T. et al. (2020) Cyanoacrylate-encapsulated calcium peroxide achieved oxygen-sustained release and promoted wound healing. Int. J. Polym. Mater. Polym. Biomater 69, 703–708 [Google Scholar]
- 48.Abdi SIH et al. (2013) Controlled release of oxygen from PLGA-alginate layered matrix and its in vitro characterization on the viability of muscle cells under hypoxic environment. Tissue Eng. Regen. Med 10, 131–138 [Google Scholar]
- 49.Kumar S. et al. (2019) Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 224, 119467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newland B. et al. (2017) Oxygen-producing gellan gum hydrogels for dual delivery of either oxygen or peroxide with doxorubicin. ACS Biomater. Sci. Eng 3, 787–792 [DOI] [PubMed] [Google Scholar]
- 51.Changtong C. et al. (2013) A porphyrin molecule that generates, traps, stores, and releases singlet oxygen. J. Photochem. Photobiol. A Chem 260, 9–13 [Google Scholar]
- 52.Bregnhøj M. et al. (2016) Solvent-dependent singlet oxygen lifetimes: temperature effects implicate tunneling and charge-transfer interactions. Phys. Chem. Chem. Phys 18, 22946–22961 [DOI] [PubMed] [Google Scholar]
- 53.Benz S. et al. (Dec. 2013) Controlled oxygen release from pyridone endoperoxides promotes cell survival under anoxic conditions. J. Med. Chem 56, 10171–10182 [DOI] [PubMed] [Google Scholar]
- 54.Chen J. et al. (2019) Epidermal growth factor receptor-targeted delivery of a singlet-oxygen sensitizer with thermal controlled release for efficient anticancer Therapy. Mol. Pharm 16, 3703–3710 [DOI] [PubMed] [Google Scholar]
- 55.Centeno-Cerdas C. et al. (2018) Development of photosynthetic sutures for the local delivery of oxygen and recombinant growth factors in wounds. Acta Biomater. 81, 184–194 [DOI] [PubMed] [Google Scholar]
- 56.Chávez MN et al. (2016) Towards autotrophic tissue engineering: photosynthetic gene therapy for regeneration. Biomaterials 75, 25–36 [DOI] [PubMed] [Google Scholar]
- 57.Evron Y. et al. (2015) Oxygen supply by photosynthesis to an implantable islet cell device. Horm. Metab. Res 47, 24–30 [DOI] [PubMed] [Google Scholar]
- 58.Qiao Y. et al. (2020) Engineered algae: a novel oxygen-generating system for effective treatment of hypoxic cancer. Sci. Adv 6, eaba5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao S. et al. (2017) Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials 112, 324–335 [DOI] [PubMed] [Google Scholar]
- 60.Song M. et al. (2016) Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward m1-like phenotype and attenuating tumor hypoxia. ACS Nano 10, 633–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meng L. et al. (2018) Tumor oxygenation and hypoxia inducible factor-1 functional inhibition via a reactive oxygen species responsive nanoplatform for enhancing radiation therapy and abscopal effects. ACS Nano 12, 8308–8322 [DOI] [PubMed] [Google Scholar]
- 62.Kim J. et al. (Mar. 2019) Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 13, 3206–3217 [DOI] [PubMed] [Google Scholar]
- 63.Kim J. et al. (2017) Continuous O2-evolving MnFe2O4 nanoparticle-anchored mesoporous silica nanoparticles for efficient photodynamic therapy in hypoxic cancer. J. Am. Chem. Soc 139, 10992–10995 [DOI] [PubMed] [Google Scholar]
- 64.Sen Lin L. et al. (2018) Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. Int. Ed 57, 4902–4906 [DOI] [PubMed] [Google Scholar]
- 65.Huang P. et al. (2012) Differential toxicity of Mn2+ and Mn3+ to rat liver tissues: oxidative damage, membrane fluidity and histopathological changes. Exp. Toxicol. Pathol 64, 197–203 [DOI] [PubMed] [Google Scholar]
- 66.Fan Z. et al. (2018) An injectable oxygen release system to augment cell survival and promote cardiac repair following myocardial infarction. Sci. Rep 8, 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedraza E. et al. (2012) Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. U. S. A 109, 4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coronel MM et al. (2017) Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials 129, 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z. et al. (2012) An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials 33, 5914–5923 [DOI] [PubMed] [Google Scholar]
- 70.Zhang H. et al. (2018) Preservation of blood vessels with an oxygen generating composite. Adv. Healthc. Mater 7, e1701338 [DOI] [PubMed] [Google Scholar]
- 71.Huang YJ and Nan GX (2019) Oxidative stress-induced angiogenesis. J. Clin. Neurosci 623, 13–16 [DOI] [PubMed] [Google Scholar]
- 72.Fries RB et al. (2005) Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat. Res 579, 172–181 [DOI] [PubMed] [Google Scholar]
- 73.Patel V. et al. (2005) Oxygen: From the benefits of inducing VEGF expression to managing the risk of hyperbaric stress. Antioxid. Redox Signal 7, 1377–1387 [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto N. et al. (2020) VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration. Sci. Rep 10, 2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y. et al. (2013) Reactive oxygen species in vascular formation and development. Oxidative Med. Cell. Longev 2013, 374963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hopf HW and Rollins MD (2007) Wounds: an overview of the role of oxygen. Antioxid. Redox Signal 9, 1183–1192 [DOI] [PubMed] [Google Scholar]
- 77.Shiekh PA et al. (2020) Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOB and alleviate diabetic and infectious wound healing. Biomaterials 249, 120020 [DOI] [PubMed] [Google Scholar]
- 78.He Y. et al. (2019) Tumor hypoxia relief overcomes multidrug resistance and immune inhibition for self-enhanced photodynamic therapy. Chem. Eng. J 375, 122079 [Google Scholar]
- 79.Paquet J. et al. (2015) Oxygen tension regulates human mesenchymal stem cell paracrine functions. Stem Cells Transl. Med 4, 809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simon MC and Keith B. (2008) The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol 9, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshida Y. et al. (2009) Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5, 237–241 [DOI] [PubMed] [Google Scholar]
- 82.Spyrou J. et al. (2019) Metabolomic and transcriptional analyses reveal atmospheric oxygen during human induced pluripotent stem cell generation impairs metabolic reprogramming. Stem Cells 37, 1042–1056 [DOI] [PubMed] [Google Scholar]
- 83.Kirkegaard K. et al. (2013) Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil. Steril 99, 738–744 [DOI] [PubMed] [Google Scholar]
- 84.Li Y. et al. (2019) Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials 197, 12–19 [DOI] [PubMed] [Google Scholar]
- 85.Muz B. et al. (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 3, 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang D. et al. (2020) Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun 11, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tapeinos C. et al. (2019) Cell membrane-coated magnetic nanocubes with a homotypic targeting ability increase intracellular temperature due to ROS scavenging and act as a versatile theranostic system for glioblastoma multiforme. Adv. Healthc. Mater 8, 1900612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu J. et al. (2019) A black phosphorus/manganese dioxide nanoplatform: oxygen self-supply monitoring, photodynamic therapy enhancement and feedback. Biomaterials 192, 179–188 [DOI] [PubMed] [Google Scholar]
- 89.Nurunnabi M. et al. (2014) Photoluminescent graphene nanoparticles for cancer phototherapy and imaging. ACS Appl. Mater. Interfaces 6, 12413–12421 [DOI] [PubMed] [Google Scholar]
- 90.Liu J. et al. (2018) Dual-triggered oxygen self-supply black phosphorus nanosystem for enhanced photodynamic therapy. Biomaterials 172, 83–91 [DOI] [PubMed] [Google Scholar]
- 91.Colombani T. et al. (2020) Oxygen-generating cryogels restore T cell-mediated cytotoxicity in hypoxic tumors. bioRxiv Published online October 9, 2020. 10.1101/2020.10.08.329805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tapeinos C. et al. (2018) Functionalised collagen spheres reduce H2O2 mediated apoptosis by scavenging overexpressed ROS. Nanomedicine 14, 2397–2405 [DOI] [PubMed] [Google Scholar]
- 93.Zeng W. et al. (2020) Dual-response oxygen-generating MnO2 nanoparticles with polydopamine modification for combined photothermal-photodynamic therapy. Chem. Eng. J 389, 124494 [Google Scholar]
- 94.Lepetsos P. and Papavassiliou AG (2016) ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis 1862, 576–591 [DOI] [PubMed] [Google Scholar]
- 95.Zhu Y. et al. (2018) Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 177, 98–112 [DOI] [PubMed] [Google Scholar]
- 96.Miripour ZS et al. (2020) Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens. Bioelectron 165, 112435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin M. et al. (2020) An antioxidant enzyme therapeutic for COVID-19. Adv. Mater 32, 2004901 [DOI] [PubMed] [Google Scholar]
- 98.Moradi S. et al. (2016) Artificial blood substitutes: first steps on the long route to clinical utility. Clin. Med. Insights Blood Disord 9, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kheir JN et al. (2012) Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci. Transl. Med 4, 140ra88 [DOI] [PubMed] [Google Scholar]
- 100.Peng Y. et al. (2018) Injectable oxygen: interfacing materials chemistry with resuscitative science. Chemistry 24, 18820–18829 [DOI] [PubMed] [Google Scholar]
- 101.Sun K. et al. (2020) Enhancing the long-term stability of a polymer dot glucose transducer by using an enzymatic cascade reaction system. Adv. Healthc. Mater Published online October 23, 2020. 10.1002/adhm.202001019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seekell RP et al. (2016) Oxygen delivery using engineered microparticles. Proc. Natl. Acad. Sci. U. S. A 113, 12380–12385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riess JG (2005) Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif. Cells Blood Substit. Immobil. Biotechnol 33, 47–63 [DOI] [PubMed] [Google Scholar]
- 104.Greenburg AG and Kim HW (2004) Hemoglobin-based oxygen carriers. Crit. Care 8, s61–s64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee H-Y et al. (2015) Controlling oxygen release from hollow microparticles for prolonged cell survival under hypoxic environment. Biomaterials 53, 583–591 [DOI] [PubMed] [Google Scholar]
- 106.Ma T. et al. (2013) The effect of synthetic oxygen carrier-enriched fibrin hydrogel on Schwann cells under hypoxia condition in vitro. Biomaterials 34, 10016–10027 [DOI] [PubMed] [Google Scholar]
- 107.Wijekoon A. et al. (2013) Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater. 9, 5653–5664 [DOI] [PubMed] [Google Scholar]
- 108.Li H. et al. (2014) Encapsulated neural stem cell neuronal differentiation in fluorinated methacrylamide chitosan hydrogels. Ann. Biomed. Eng 42, 1456–1469 [DOI] [PubMed] [Google Scholar]
- 109.Seekell RP et al. (2018) Tunable polymer microcapsules for controlled release of therapeutic gases. Langmuir 34, 9175–9183 [DOI] [PubMed] [Google Scholar]
- 110.Polizzotti BD et al. (2014) Optimization and characterization of stable lipid-based, oxygen-filled microbubbles by mixture design. J. Biomed. Mater. Res. - Part B Appl. Biomater 102, 1148–1156 [DOI] [PubMed] [Google Scholar]
- 111.Lai YT et al. (2015) Size-dependent interaction of cells and hemoglobin-albumin based oxygen carriers prepared using the SPG membrane emulsification technique. Biotechnol. Prog 31, 1676–1684 [DOI] [PubMed] [Google Scholar]
- 112.Xu X. et al. (2017) Microfluidic production of nanoscale perfluorocarbon droplets as liquid contrast agents for ultrasound imaging. Lab Chip 17, 3504–3513 [DOI] [PubMed] [Google Scholar]
- 113.Jeon CH et al. (2008) Hypoxia appears at pre-arthritic stage and shows co-localization with early synovial inflammation in collagen induced arthritis. Clin. Exp. Rheumatol 26, 646–648 [PubMed] [Google Scholar]