Abstract
Background:
We implemented an infantile spasms management guideline recommending standard therapies and, early start of next treatment. After six years, we determined (1) our compliance with standard therapies, (2) time to next treatment, and (3) rate of initial and three-month electroclinical remission with first, second, and third treatments.
Methods:
This is a retrospective record review of newly diagnosed spasms from September 2012 to September 2018, with the onset age of two months to two years.
Results:
Standard therapies (hormone or vigabatrin) were the first treatments in 114 of 115 consecutive patients. The second and third treatments were started within 14 days of failed treatment in only 21% and 24%, respectively. Remission with the first and second treatments was similar (41% and 40%). Remission was lower for the third treatment (15%), although higher if standard therapy was used (36%). Initial and three-month remission by the first treatment was significantly higher for adrenocorticotropic hormone (ACTH, 66% and 79%, respectively) and prednisolone (53% and 83%, respectively) than for vigabatrin (19% and 40%, respectively). There were no significant differences in patient characteristics or rates of remission between ACTH and prednisolone.
Conclusions:
Although we achieved excellent compliance with standard therapies as initial treatment, a next treatment often started after two weeks. Given the superiority of hormone therapies over vigabatrin and standard therapies over nonstandard therapies, as well as the potentially negative impact of delays in effective treatment, future interventions need to focus on increasing the use of hormone over vigabatrin (for patients without tuberous sclerosis complex), use of standard therapies as second and third treatments, and reducing delays to next treatment.
Keywords: Infantile spasms, West syndrome, Hypsarrhythmia, Quality measure, Standard therapy
Introduction
Infantile spasms are seizures associated with West syndrome, a severe developmental and epileptic encephalopathy of infancy.1 Although epilepsy and developmental outcomes are largely determined by the associated etiology, early effective treatment of spasms and the epileptic encephalopathy (electroclinical remission) is associated with better electroclinical and neurodevelopmental outcomes.2–4 A Cochrane review of multiple randomized trials best supports early treatment with adrenocorticotropic hormone (ACTH), high-dose prednisolone, or vigabatrin.5 The American Academy of Neurology and Child Neurology Society recommend these medications as the first treatment for infantile spasms unless refused, contraindicated, enrolled in research, or better treated with epilepsy surgery.6 In addition, more rapid initiation of standard therapy after onset of spasms is correlated to better outcomes.2,4,7 Therefore, experts recommend early treatment and starting a new treatment within two weeks for nonresponders.8,9
Unfortunately, the use of nonstandard therapies as the initial treatment of spasms remains commonplace,7,8 and treatment is often delayed,10 highlighting choice and timing of treatment as critical knowledge-practice gaps in infantile spasm management. To address these gaps, we developed a standardized guideline for the management of infantile spasms at the Nationwide Children’s Hospital (NCH). The most important feature of the NCH guideline was the recommendation to use standard therapies (as first, second, and third treatments if feasible), which led to improvements in the institution’s rate of electroclinical remission at three months.11
We report our compliance with recommended best practices and electroclinical outcomes since guideline implementation with focus on time to next treatment and rates of treatment with standard therapies as first, second, and third treatments within a contemporary consecutive cohort.
Methods
Our methodology, including intent for publication, was reviewed by the NCH Institutional Review Board. In light of our aim to use the acquired data to optimize our practice and improve outcomes for our patients, this study was designated Institutional Review Board exempt for quality improvement.
From September 2012 to September 2018, there were 115 consecutive newly diagnosed children with spasms (age of onset two months to two years) at NCH. Study data were acquired through retrospective medical record review. We excluded patients with a prior diagnosis of Ohtahara syndrome or early myoclonic encephalopathy as well as those with late infantile epileptic encephalopathy.12 Although patients with Ohtahara syndrome, early myoclonic encephalopathy, and late infantile epileptic encephalopathy may evolve to or have coexisting spasms, these syndromes represent a population distinct from primary-onset infantile spasms in the setting of West syndrome. There were no exclusions for missing data.
Patient characteristics included sex, race, gestational age, prior seizures, normal versus abnormal development before onset of spasms (based on clinician impression or developmental assessment), spasm-associated etiology, length of diagnostic hospitalization associated with first treatment, and relevant dates (spasm onset, treatment start, next treatment, last follow-up, post-treatment electroencephalography [EEG]). Age-based patient characteristics were corrected for prematurity (less than 37 weeks). Consistent with the known delays in recognition of spasm onset,13 the age at spasm onset could not be determined for all patients.
Standard therapies included ACTH, prednisolone, vigabatrin, and epilepsy surgery in accordance with current recommendations.6 As some patients who fail to respond to prednisolone will respond to ACTH (and vice versa),14,15 patients treated with ACTH or prednisolone were eligible to receive the alternative hormone therapy. All other treatments were designated as nonstandard and were grouped together for analysis.
We modeled our etiology categories and standard dosing regimens after Knupp and colleagues’ previously published large North American cohort.10 In brief, the etiology was separated into the following five categories: (1) genetic/metabolic, (2) prior brain injury, (3) malformation of cortical development/other structural, (4) unknown etiology abnormal development, and (5) unknown etiology normal development. Only three patients in our cohort had tuberous sclerosis complex and were included in the other structural group as recommended by the International League Against Epilepsy.16 To assess patient characteristics by three-month electroclinical remission, we grouped patients into the following three categories: (1) genetic/metabolic/unknown etiology abnormal development, (2) prior brain injury/malformation of cortical development/other structural, and (3) unknown etiology normal development.
Our spasms management guideline included standardized dosing regimens for high-dose natural ACTH (150 IU/m2/day), high-dose prednisolone (40 to 60 mg/day), and vigabatrin (150 mg/kg/ day). All patients who received standard therapies were initially prescribed the recommended regimen. In the rare circumstance that patients received both standard and nonstandard treatments simultaneously (e.g., ACTH for spasms and levetiracetam for focal seizures), electroclinical remission was attributed to standard therapy. For patients with sustained clinical remission without electrographic remission and later confirmed electrographic remission with a subsequent treatment, remission was attributed to the latter treatment. Although no patients received initial dual treatment (hormone plus vigabatrin) with standard therapies, some patients receiving vigabatrin continued to receive it during hormone therapy.
We defined initial electroclinical remission (“remission”) as resolution of spasms beginning within two weeks of treatment and remaining without spasms for a minimum of 28 days with remission of hypsarrhythmia or similar pattern on EEG.17 To determine electrographic remission, we utilized the Burden of Amplitudes and Epileptiform Discharges score18 with a modification-for the amplitude assessment, we excluded bipolar channels that include occipital electrodes; this was done in light of a subsequent report that 83% and 34% of normal children commonly have posterior background slow waves of greater than 200 or greater than 300 μV respectively.19 The Burden of Amplitudes and Epileptiform Discharges score is a simplified grading scale ranging from 0 (normal) to 5 (most epileptic) with excellent inter-rater agreement. We defined electrographic remission when a pretreatment score of 4 or 5 improved to 3 or less. If the pretreatment score was 3, we required an improvement to 2 or less. We defined three-month remission as remission for a minimum of 28 days with this duration overlapping with day 90 after treatment start. If patients died or transitioned care before confirmation of initial or three-month remission, these children were categorized as non-responders and included in our analysis. In patients with clinical spasm remission, the lead author reviewed all post-treatment EEG tracings to confirm electrographic remission.
The NCH guideline recommended that clinicians strongly consider a next treatment within two weeks in the presence of continuing spasms. We recommended clinical and video-EEG (60 minutes with sleep captured) follow-up no later than 14 to 21 days after the start of treatment. If this EEG suggested remission, our guideline recommended a subsequent overnight video-EEG (designated long-term monitoring [LTM]) for confirmation. Compliance with this recommendation was not necessary for inclusion. We collected post-treatment EEG characteristics such as the timing from treatment start to EEG, study duration, presence of sleep, and number of patients who underwent post-treatment LTM.
For the analysis of time to next treatment, we excluded treatments started because of a relapse of spasms given that this was not a modifiable cause of delay to next treatment. In contrast, we included children with initial clinical remission only (without electrographic remission) given that this may be a modifiable factor. Treatments initiated for the treatment of nonspasm seizure types were excluded.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC, USA). Patients’ characteristics were summarized using mean (S.D.), median (interquartile range [IQR]), and frequency (percentage) based on the distributions of the variables. The differences of these variables between treatments were evaluated using appropriate parametric tests such as Student’s t test and chi-square test or nonparametric tests such as Kruskal-Wallis test, Wilcoxon rank sum test, and Fisher’s exact test. We analyzed patients’ remission rates after receiving first, second, and third treatments using chi-square tests or Fisher’s exact tests. The transition times between treatments were assessed using continuous days and categorized days (transition by 14, 21, and 30 days). To further understand the potential factors associated with remission, patients who had remission at three months were compared with those who did not have remission for differences in baseline factors and types of first, second, and third treatments. Finally, to reduce the bias from confounding variables that could affect the assignment of first treatment, patients who received vigabatrin, ACTH, and prednisolone as initial treatment were pairwise matched using the propensity score method.20 For each comparison, patients were matched 1:1 using the nearest neighbor matching (caliper = 0.1), without replacement, on the propensity score generated from a model that included age at spasm onset, spasm onset to first treatment, etiology, prior seizure, and abnormal development at diagnosis. Remission rates after first treatment and remission at three months were then analyzed among the matched cohorts.
Results
Patient characteristics
Patient characteristics by first treatment are presented in Table 1. Children receiving vigabatrin as the first treatment were significantly more likely to have prior seizures and have abnormal development before spasm onset.
TABLE 1.
Characteristics by First Treatment (Standard Only)
| Characteristic | Variable | N (%)/Median (Q1-Q3) | P | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Vigabatrin (N = 52) | ACTH (N = 32) | Prednisolone (N = 30) | All (N = 114) | |||
|
| ||||||
| Sex | Female | 17 (32.7) | 13 (40.6) | 10 (33.3) | 40(35.1) | 0.74 |
| Male | 35 (67.3) | 19 (59.4) | 20 (66.7) | 74 (64.9) | ||
| Race | White | 40 (76.9) | 23 (71.9) | 17 (56.7) | 80 (70.2) | 0.20 |
| Black | 11 (21.2) | 7 (21.9) | 9 (30) | 27 (23.7) | ||
| Other | 1 (1.9) | 2 (6.3) | 4 (13.3) | 7(6.1) | ||
| Abnormal development | No | 6(11.5) | 12 (37.5) | 9 (30) | 27 (23.7) | 0.02 |
| Yes | 46 (88.5) | 20 (62.5) | 21 (70) | 87 (76.3) | ||
| Gestational age (weeks) | 38 (35.5–40) | 39 (37–40) | 38.5 (34–40) | 38 (36–40) | 0.69 | |
| Corrected age of spasm onset (months) | 6.4 (5.2–9.9) | 5.8 (5–7.5) | 6.1 (5–8.2) | 6.3 (5–8.7) | 0.57 | |
| Spasm onset to first treatment (days) | 14 (5–23) | 10 (4–32) | 17 (9–29) | 14 (5–28) | 0.46 | |
| Spasm onset to diagnosis (days) | 12 (2–22) | 7(3–31) | 15.5 (8–29) | 12 (3.5–25) | 0.23 | |
| Diagnosis to first treatment (days) | 1 (1–2.5) | 1 (1–1.5) | 1 (0–1) | 1 (0–2) | 0.06 | |
| Prior seizures | No | 28 (53.9) | 25 (78.1) | 22 (73.3) | 75 (65.8) | 0.045 |
| Yes | 24 (46.2) | 7 (21.9) | 8 (26.7) | 39 (34.2) | ||
| Etiology | Genetic/metabolic | 9(17.3) | 4(12.5) | 4 (13.3) | 17 (14.9) | 0.04* |
| Prior brain injury | 24 (46.2) | 13 (40.6) | 10 (33.3) | 47 (41.2) | ||
| MCD/other structural† | 15 (28.9) | 3 (9.4) | 6 (20) | 24(21.1) | ||
| Unknown abnormal | 1 (1.9) | 5 (15.6) | 3(10) | 9 (7.9) | ||
| Unknown normal | 3 (5.8) | 7 (21.9) | 7 (23.3) | 17 (14.9) | ||
| Follow-up after first treatment (months) | 19(7–41) | 29.5 (16–59) | 18.5 (7–40) | 21 (9–49) | 0.06 | |
Abbreviations:
ACTH = Adrenocorticotropic hormone
MCD = Malformation of cortical development
Values are N (column %) or median (interquartile range).
Missing values: Age of spasm onset (N = 18), spasm onset to first treatment (N = 18) and spasm onset to diagnosis (N = 18).
Kruskal-Wallis tests used for continuous variables and chi-square tests used for categorical variables.
Fisher’s exact tests used instead of chi-square tests.
Three of 114 patients had tuberous sclerosis complex (categorized as other structural etiology); all three received vigabatrin as the first treatment.
Twelve children died within the follow-up period. Of the 12 deaths,11 were attributed to the spasm-associated etiology and one death was related to treatment (sepsis in a child treated with prednisolone). Four of 12 children experienced both an initial and three-month remission before death. An additional patient achieved initial remission but died before the three-month evaluation.
Use of standard therapies
First, second, and third treatments are presented in Table 2. The number of patients receiving a first, second, and third treatment was 115, 62 and 33, respectively. ACTH, prednisolone, or vigabatrin were the first treatments in 114 of 115 patients. One patient received levetiracetam as the first treatment for parent refusal of standard therapy. Of those receiving standard therapy as the first treatment, 113 of 114 started within seven days of diagnosis; one patient was treated eight days after diagnosis because vigabatrin was not immediately available. The use of standard therapies decreased to 71% for the second treatment and 42% for the third treatment. Of the 18 patients who did not receive a standard therapy as the second treatment, seven (all initially treated with vigabatrin) were not eligible for a subsequent standard therapy (due to medical fragility and risk of immunosuppression with hormone therapy), five families refused standard therapies due to concern about side effects, and six were eligible for standard therapy but did not receive it. Of the 19 patients who did not receive a standard therapy as the third treatment, five were not eligible for a subsequent standard therapy (due to medical fragility and risk of immunosuppression with hormone therapy), one family refused standard therapy due to concern about side effects, three were enrolled in a clinical trial, and 10 were eligible for standard therapy but did not receive it.
TABLE 2.
Initial Remission by Treatment
| Variable | Remission* (%) | P † | Relapse (%) |
|---|---|---|---|
|
| |||
| Response to first treatment | 47/115 (41) | - | 5/47 (11) |
| Standard | 47/114 (41) | ||
| Vigabatrin | 10/52 (19) | ||
| ACTH | 21/32 (66) | 3‡/21 (14) | |
| Prednisolone | 16/30 (53) | 2§/16 (13) | |
| Nonstandard | 0/1 (0) | ||
| Levetiracetam | 0/1 (0) | ||
| Response to second treatment | 25/62 (40) | 0.004 | 4/25 (16) |
| Standard | 23/44 (52) | ||
| Vigabatrin | 10/22 (45) | 2║/10 (20) | |
| ACTH | 3/5 (60) | ||
| Prednisolone | 10/17 (59) | 2¶/10 (20) | |
| Nonstandard | 2/18 (11) | 0 | |
| Levetiracetam | 0/1 | ||
| Clobazam | 1/9 (11) | 0 | |
| Zonisamide | 1/5 (20) | 0 | |
| Topiramate | 0/2 | ||
| Oxcarbazepine | 0/1 | ||
| Response to third treatment | 5/33 (15) | 0.008 | 1/5 (20) |
| Standard | 5/14 (36) | ||
| Vigabatrin | 0/2 (0) | ||
| ACTH | 2/5 (40) | ||
| Prednisolone | 2/6 (33) | 1#/2 (50) | |
| Surgery | 1/1 (100) | ||
| Nonstandard | 0/19 (0) | ||
| Zonisamide | 0/6 | ||
| Clobazam | 0/4 | ||
| Ketogenic diet | 0/4 | ||
| Cannabidiol | 0/3 | ||
| Topiramate | 0/2 | ||
Abbreviation:
ACTH = Adrenocorticotropic hormone
Values are N (row %)
Clinical remission beginning within two weeks of starting treatment and persisting for a minimum of 28 days plus electrographic remission.
Difference in remission between standard and nonstandard therapies tested using chi-square tests (Fisher’s exact test used if any group had fewer than five remissions).
After relapse, remission by three months with epilepsy surgery (1), prednisolone (1), or vigabatrin (1).
Both with remission at three months but later relapsed without subsequent remission.
One with three-month remission and one without, both without subsequent remission.
Both with remission at three months but later relapsed without subsequent remission.
Remission at three months but later relapse without subsequent remission.
Of the 30 patients who were initially treated with a hormone therapy and received any subsequent treatment, 29 (97%) received a subsequent standard therapy. In contrast, of the 31 who were initially treated with vigabatrin and received any subsequent treatment, only 17 (55%) received a subsequent standard therapy.
Initial remission for first, second, and third treatments
The rate of initial remission by treatment is presented in Table 2. The rates of remission with first and second treatments were similar (41% and 40%, respectively), whereas the rate of remission with a third treatment was less (15%). However, the rate of remission with a third treatment was higher if standard therapy was used (36%). Remission with the first treatment was significantly higher for hormone therapy compared with vigabatrin, and no difference between ACTH and prednisolone occurred (Table 3). It is noteworthy that the rate of remission with vigabatrin was significantly higher when used as a second treatment (45%) compared with when used as a first treatment (19%, P = 0.02). For second and third treatments, remission with standard treatments was significantly higher than remission with nonstandard treatments (Table 2). Remission with nonstandard treatment was rare whether used as a first, second, or third treatment (5%).
TABLE 3.
Remission Between Standard Therapies by Treatment Order
| Treatment | Model | Odds Ratios* (95% CI), P value | ||
|---|---|---|---|---|
|
| ||||
| ACTH versus VGB | PRED versus VGB | ACTH versus PRED | ||
|
| ||||
| First | Unadjusted | 8.02 (2.94,21.88), P < 0.001 | 4.80(1.77, 12.98), P = 0.002 | 1.67 (0.60, 4.65), P = 0.33 |
| Adjusted† | 6.49 (2.27,18.55), P < 0.001 | 4.1 (1.45, 11.55), P = 0.008 | 1.58 (0.55, 4.59), P = 0.4 | |
| Second | Unadjusted | 1.80 (0.25,12.99), P = 0.56 | 1.71 (0.48, 6.16), P = 0.41 | 1.05 (0.14, 8.02), P = 0.96 |
Abbreviations:
ACTH = Adrenocorticotropic hormone
PRED = Prednisolone
VGB = Vigabatrin
For third treatment, there were not enough remissions for comparison.
Logistic regression used for the odds ratio estimates.
Adjusted for abnormal development, prior seizures, and etiology.
Three-month remission
Patient characteristics and rates of three-month remission are presented in Table 4. Spasm onset after 12 months, prior seizure, abnormal development before spasm onset, etiology other than unknown normal development, and those receiving vigabatrin as the first treatment were significantly less likely to achieve remission at three months. All patients in the group with unknown etiology and normal development before spasm onset achieved three-month remission. Patients initially treated with vigabatrin were significantly less likely (40%) to achieve three-month remission compared with those initially treated with ACTH (79%) or prednisolone (83%).
TABLE 4.
Patient Characteristics by Three-Month Electroclinical Remission
| Characteristic | Variable | 3-Month Remission* (71/115) | P |
|---|---|---|---|
|
| |||
| Sex | Female | 25/40 (62.5) | 0.90 |
| Male | 46/75 (61.3) | ||
| Race | White | 48/81 (59.3) | 0.75 |
| Black | 18/27 (66.7) | ||
| Other | 5/7 (71.4) | ||
| Gestational age | ≥37 weeks | 52/83 (62.7) | 0.75 |
| <37 weeks | 19/32 (59.4) | ||
| Age at spasm onset† | ≥12 months | 4/13 (30.8) | 0.03 |
| <12 months | 67/102 (65.7) | ||
| Spasm onset to first treatment | >30 days | 14/23 (60.9) | 0.76 |
| ≤30 days | 47/73 (64.4) | ||
| Prior seizure | No | 52/76 (68.4) | 0.04 |
| Yes | 19/39 (48.7) | ||
| Abnormal development | No | 25/27 (92.6) | <0.001 |
| Yes | 46/88 (52.3) | ||
| Etiology | Genetic/metabolic/unknown abnormal | 14/26 (53.8) | <0.001 |
| Prior brain injury/MCD/other structural | 40/72 (55.6) | ||
| Unknown normal | 17/17 (100) | ||
| First treatment | Vigabtrin | 21/52 (40.4) | <0.001 |
| ACTH | 26/32 (81.3) | ||
| Prednisolone | 24/30 (80.0) | ||
| Levetiracetam | 0/1 (0) | ||
Abbreviations:
ACTH = Adrenocorticotropic hormone
MCD = Malformation of cortical development
For etiology, normal or abnormal refers to development before spasm onset. Missing values: time from spasm onset to first treatment (N = 19).
Values are N (row %).
Chi-square tests used for sex, gestational age, spasm onset to first treatment, prior seizure, and abnormal development; due to small numbers, Fisher’s exact tests used for race, age at spasm onset, etiology, and first treatment (levetiracetam excluded for this comparison).
Defined as confirmed electroclinical remission beginning within three months of the first treatment and extending for a minimum of 28 days and including day 90 after treatment start.
If date of spasm onset unknown, age of diagnosis used only for this variable.
Propensity score matching: remission by first treatment
Outcome controlled for propensity score is presented in Table 5. This analysis confirms the significantly higher rates of initial and three-month remission with hormone therapy compared with vigabatrin as well as the similar rates of remission between prednisolone and ACTH.
TABLE 5.
Propensity Score Matching
| First Treatment | N | Remission After Treatment (%) | P |
|---|---|---|---|
|
| |||
| Initial remission | |||
| Vigabatrin | 20 | 4 (20.0) | 0.006* |
| ACTH | 20 | 14 (70.0) | |
| Vigabatrin | 23 | 4 (17.4) | 0.004† |
| Prednisolone | 23 | 14 (60.9) | |
| Prednisolone | 19 | 9 (47.4) | 0.56† |
| ACTH | 19 | 11 (57.9) | |
| Three-month remission | |||
| Vigabatrin | 20 | 9 (45.0) | 0.02* |
| ACTH | 20 | 17 (85.0) | |
| Vigabatrin | 23 | 7 (30.4) | 0.002† |
| Prednisolone | 23 | 18 (78.3) | |
| Prednisolone | 19 | 15 (79.0) | 0.99* |
| ACTH | 19 | 14 (73.7) | |
Matching variables: Age of spasm onset (≤6.25, >6.25, or unknown), spasm onset to first treatment (1 to 30 days, >30 days, or unknown), etiology, prior seizure, abnormal development.
Exact McNemar’s test
McNemar’s test
Quality of electrographic outcome
Of the patients with initial electroclinical remission with first, second, or third treatments, 76 of 77 (99%) had a post-treatment routine EEG (median duration 60 minutes, IQR 60, 60) or LTM (median duration 20 hours, IQR 19, 21) that included sleep. One patient did not sleep on the post-treatment routine EEG and did not have an LTM. The median time from effective treatment to the post-treatment EEG (whether routine EEG or LTM) was 17 days (IQR 14, 20). Fifty-seven of 77 patients (74%) had a post-treatment LTM.
Length of initial diagnostic hospitalization by first treatment
Six patients were excluded from the length of hospitalization analysis either because they received a nonstandard treatment (1) or because they started the first treatment as an outpatient (1), after discharge (1), or during a hospitalization initiated for reasons unrelated to spasms (3). The median length of hospitalization was significantly shorter for patients initially treated with prednisolone (2.1 days, P < 0.001) and vigabatrin (2.3 days, P < 0.001) compared with ACTH (4.1 days).
Time to next treatment
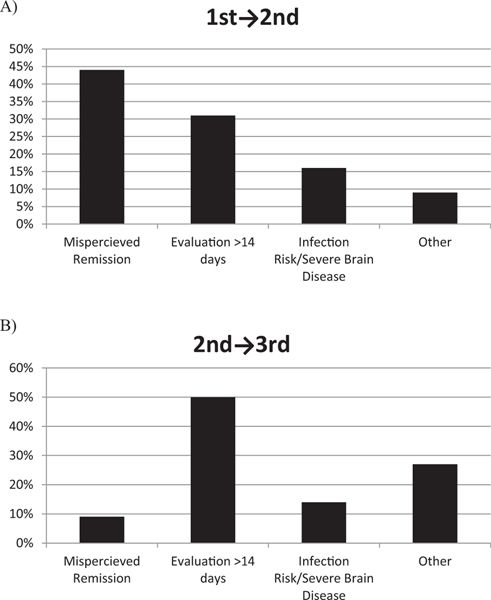
Days between treatments are presented in Supplementary Table 1. The number of patients receiving a second treatment by 14, 21, and 30 days was 21%, 47%, and 68%, respectively. The number receiving a third treatment by 14, 21, and 30 days was 24%, 45% and 59%, respectively. Primary reasons for delayed treatment are presented in Figure 1. The most common primary reasons for delay to a second treatment was misperceived initial remission (clinical without electrographic remission or spasms captured on follow-up EEG, 44%) and clinic or EEG evaluation after 14 days (31%).
FIGURE.
Reasons for treatment delay beyond 14 days for (A) first to second treatment and (B) second to third treatment.
Discussion
Implementation of a standardized management guideline resulted in excellent compliance with evidence-based recommendations regarding the timely use of standard therapies as initial treatment for infantile spasms across a large number of providers. However, whereas therapy was used as a first and second treatment for most patients, a minority of patients received standard therapy as a third treatment. Although not all patients were eligible for subsequent standard therapy, 16 patients within our cohort had no contraindication to standard therapy as the second or third treatment but did not receive it, representing an opportunity for further improvement within our institution.
Although our rates of remission with first (41%) and second (40%) treatments were similar to those reported by Knupp and colleagues (41% and 37%, respectively),10,21 this report adds data on the response rate to a third treatment (15%). However, the number of patients receiving a third treatment was small, and many received a nonstandard treatment. Thus, these results should be interpreted with caution. Yet, there were no remissions in the third treatment group unless standard therapy was used. When this finding is considered within the context of the overall low rate of remission with nonstandard treatment, our data support the use of standard over nonstandard treatment even as a third treatment.
Remission rates were similar among those initially receiving ACTH or prednisolone, but ACTH resulted in longer hospitalizations; this was related to the need for insurance authorization and medication shipment via specialty pharmacy for ACTH. Among standard therapies, prednisolone was a favorable initial treatment at our center given its superior effectiveness and lower cost compared with vigabatrin as well as its similar effectiveness, lower cost, and shorter length of hospitalization compared with ACTH. It is noteworthy that three patients who failed to respond to ACTH later achieved remission with prednisolone and three patients who failed to respond to prednisolone later achieved remission with ACTH. This is consistent with prior reports of remission with the alternative hormone therapy after initial failed response.14,15
A higher rate of remission occurred when vigabatrin was used as a second treatment compared with when it was used first; this suggests that the group initially receiving vigabatrin may have been less likely to respond to treatment in the setting of more severe brain disease. For example, this group was more likely to have prior seizures and abnormal development before spasm onset.
Even with the high rate at which standard therapy was used, our data highlight areas for improvement. Vigabatrin was used more commonly than expected. The lack of a stated preference in our guideline for one standard therapy over another may have contributed to the frequent use of vigabatrin. In addition, vigabatrin has other desirable attributes such as easy acquisition (15-day starter pack available at our institution) and route of administration (compared with injectable ACTH), as well as a shorter hospitalization (compared with ACTH) and lack of immunosuppression. Yet, given the higher rate of remission with hormone therapy compared with vigabatrin in our cohort, combined with prior evidence suggesting the superiority of hormone therapy over vigabatrin,22 a future intervention will include a recommendation to use hormone therapy as initial treatment in eligible patients.
Treatment lag (spasm onset to first treatment) is an important prognostic factor.2,3,4 Compared with those patients treated within seven days of spasm onset, those treated later are at higher risk for poor developmental outcome.2 In addition, early effective treatment is associated with better developmental and epilepsy outcomes at 18 months.4 Given the importance of early remission, our guideline recommended a next treatment within 14 days of ongoing spasms. However, second and third treatments were started within 14 days of failed treatment in only 21% and 24%, respectively. We identified inaccurately perceived remission and follow-up evaluation after two weeks as common reasons for a late transition to next treatment. Initial dual therapy (hormone plus vigabatrin) would address these concerns by removing the delay to a second treatment. However, the potential benefit of dual therapy must be balanced with the risk of medication-related adverse events and drug cost. In addition, we currently lack evidence to support improved developmental outcomes with initial dual therapy over monotherapy.4 Future interventions at our center will include earlier follow-up 10 to 14 days after treatment initiation in an effort to improve our rate of transition to next treatment by two weeks.
This study includes several limitations, including the retrospective acquisition of data. Bias in treatment cannot be excluded without randomization. However, some infantile spasm randomized trials may overestimate rates of remission when compared with clinical practice. For example, the rate of clinical remission at two weeks with vigabatrin reported by Lux and colleagues was 54%. By contrast, we found that only 19% patient’s initially treated with vigabatrin experienced electroclinical remission. Some of this difference may be accounted for by our more strict definition of remission (electrographic remission and clinical remission for 28 days). Yet, many of our patients who initially received vigabatrin would not have been eligible for this clinical trial. That only 55% of these patients receiving any subsequent treatment got hormone therapy suggests that many were not eligible to receive it (e.g., risk of immunosuppression in the setting of medical fragility). Our consecutive series with all patients receiving at least one standard treatment is important as a more accurate assessment of treatment choice and short-term outcomes in clinical practice.
An additional limitation is that some patients receiving vigabatrin, such as those with incomplete clinical or electrographic improvement, continued to receive vigabatrin during hormone therapy. If remission was achieved in these circumstances, we attributed the remission to hormone therapy. However, we acknowledge the possible benefit of dual therapy in these patients. We also acknowledge that whereas the response to ACTH is typically determined within two weeks,23 remission with vigabatrin may be delayed.24 However, our recommendation for a next treatment to start within two weeks is supported by the importance of early remission. Finally, we did not have formal baseline or follow-up developmental testing for all patients. For this reason, we felt that it was more accurate to designate normal versus abnormal development as opposed to attempt to determine degrees of developmental delay based on informal assessments.
Although we achieved excellent compliance with standard therapies as initial treatment, a next treatment was often started after two weeks. Given the superiority of hormone therapies over vigabatrin and standard over nonstandard therapies, as well as the potential negative impact with delays in effective treatment, future interventions will focus on increasing the use of hormone over vigabatrin (for patients without tuberous sclerosis complex), use of standard therapies as second and third treatments, and reducing delays to next treatment. Similar guideline implementation and assessment at other centers may improve the management of infantile spasms.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Gerlach Foundation. G.N.B. was partially supported by National Institutes of Health/National Center for Advancing Translational Sciences grant UL1TR002733. We thank William Parker for his contribution to the length of hospitalization analysis.
Footnotes
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.pediatrneurol.2019.11.016.
Conflicts of interest: The authors have no conflicts of interest to declare. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Fisher RS, Cross JH, D’Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58:531–542. [DOI] [PubMed] [Google Scholar]
- 2.O’Callaghan FJ, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52: 1359–1364. [DOI] [PubMed] [Google Scholar]
- 3.Widjaja E, Go C, McCoy B, Snead OC. Neurodevelopmental outcome of infantile spasms: a systematic review and meta-analysis. Epilepsy Res. 2015;109: 155–162. [DOI] [PubMed] [Google Scholar]
- 4.O’Callaghan FJK, Edwards SW, Alber FD, et al. Vigabatrin with hormonal treatment versus hormonal treatment alone (ICISS) for infantile spasms: 18-month outcomes of an open-label, randomised controlled trial. Lancet Child Adolesc Health. 2018;2:715–725. [DOI] [PubMed] [Google Scholar]
- 5.Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev. 2013;5:CD001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel AD, Berg AT, Billinghurst L, et al. Quality improvement in neurology: child neurology quality measure set: executive summary. Neurology. 2018;90:67–73. [DOI] [PubMed] [Google Scholar]
- 7.Yuskaitis CJ, Ruzhnikov MRZ, Howell KB, et al. Infantile spasms of unknown cause: predictors of outcome and genotype-phenotype correlation. Pediatr Neurol. 2018;87:48e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mytinger JR, Joshi S. Pediatric epilepsy research consortium, section on infantile spasms. The current evaluation and treatment of infantile spasms among members of the Child Neurology Society. J Child Neurol. 2012;27:1289–1294. [DOI] [PubMed] [Google Scholar]
- 9.Hussain SA. Treatment of infantile spasms. Epilepsia Open. 2018;3:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knupp KG, Coryell J, Nickels KC, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016;79:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedak EM, Patel AD, Heyer GL, et al. Optimizing care with a standardized management protocol for patients with infantile spasms. J Child Neurol. 2015;30:1340–1342. [DOI] [PubMed] [Google Scholar]
- 12.Nordli DR Jr. Epileptic encephalopathies in infants and children. J Clin Neurophysiol. 2012;29:420–424. [DOI] [PubMed] [Google Scholar]
- 13.Hussain SA, Lay J, Cheng E, Weng J, Sankar R, Baca CB. Recognition of infantile spasms is often delayed: the ASSIST study. J Pediatr. 2017;190: 215–221. [DOI] [PubMed] [Google Scholar]
- 14.Hussain SA, Shinnar S, Kwong G, et al. Treatment of infantile spasms with very high dose prednisolone before high dose adrenocorticotropic hormone. Epilepsia. 2014;55:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hrachovy RA, Frost JD Jr, Kellaway P, Zion TE. Double-blind study of ACTH vs prednisone therapy in infantile spasms. J Pediatr. 1983;103:641–645. [DOI] [PubMed] [Google Scholar]
- 16.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. [DOI] [PubMed] [Google Scholar]
- 17.Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia. 2004;45:1416–1428. [DOI] [PubMed] [Google Scholar]
- 18.Mytinger JR, Hussain SA, Islam MP, et al. Improving the inter-rater agreement of hypsarrhythmia using a simplified EEG grading scale for children with infantile spasms. Epilepsy Res. 2015;116:93–98. [DOI] [PubMed] [Google Scholar]
- 19.Mytinger JR, Weber A, Vidaurre J. High amplitude background slow waves in normal children aged 3 to 18 Months: implications for the consideration of hypsarhythmia. J Clin Neurophysiol. 2018;35:151–154. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knupp KG, Leister E, Coryell J, et al. Response to second treatment after initial failed treatment in a multicenter prospective infantile spasms cohort. Epilepsia. 2016;57:1834–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364:1773–1778. [DOI] [PubMed] [Google Scholar]
- 23.Mytinger JR, Weber A, Heyer GL. The response to ACTH is determined early in the treatment of infantile spasms. Epileptic Disord. 2015;17:52–57. [DOI] [PubMed] [Google Scholar]
- 24.Elterman RD, Shields WD, Bittman RM, et al. Vigabatrin for the treatment of infantile spasms: final report of a randomized trial. J Child Neurol. 2010;25:1340–1347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.