Abstract
Background:
To evaluate the association of statins and co-morbidities with new onset type 2 diabetes mellitus (T2DM) in patients 65 years and older.
Methods:
This retrospective study used de-identified administrative healthcare claims and enrolment data from a Medicare Advantage Prescription Drug (MAPD) health plan offered by a large multistate healthcare company. The plan covered >2.4 million individuals, of whom >1.7 million individuals were ≥65 years. Of these, 265 554 individuals had continuous MAPD enrolment January 2008 to December 2015. The unadjusted model assessed demographic, pharmacy and T2DM comorbidities as covariates. Significant variables (P < .05) in the unadjusted model were then included in the adjusted model. The adjusted model used Cox proportional hazards to evaluate covariate effects. Matched propensity score analysis was used to analyse the association of statins and T2DM onset.
Results:
The cumulative rate of diagnosed T2DM onset in the study cohort was 4.82% (4314/89 390). Annualised incidence of T2DM diagnosis was 0.82%, 0.88%, 1.04% and 2.09% in 2012, 2013, 2014 and 2015, respectively. T2DM onset was associated with male sex, non-white (African American or Hispanic ethnicity), statin use, hypertension, hyperlipidaemia, heart failure, lower limb ulceration, atherosclerosis, other retinopathy, angina pectoris, poor vision and blindness and absence ischaemic heart disease (IHD). Matched propensity score analysis showed that statin use was significantly associated with T2DM onset (Odds Ratio = 1.26, 95% Confidence Interval: 1.12–1.41, P < .0001) in the adjusted model.
Conclusions:
Analyses indicated that statin usage was associated with new onset T2DM after adjusting for covariates.
Keywords: diabetes, Medicare, onset, propensity, retrospective, statin
1 |. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a chronic condition caused by failure of pancreatic beta cells to produce enough insulin, or the body does not use insulin efficiently (also called insulin resistance). In some patients, there is complete absence of insulin production. T2DM is associated with deleterious macrovascular and microvascular outcomes1 and is the seventh leading cause of death in the United States affecting 29.1 million people.2 In 2012, 25.9% or 11.8 million Americans 65 and older had diagnosed or undiagnosed diabetes.3 The total estimated national cost of diabetes in the United States in 2012 was approximately $245 billion and increased to $327 billion in 2017, a 26% increase in adjusted dollars.4,5
T2DM can have a long asymptomatic phase, that is, an individual may be unaware that they have T2DM for 4 to 7 years before an actual diagnosis.6 The onset (or first occurrence of T2DM) is generally preceded by pre-diabetes, a condition where an individual’s blood sugar is higher than normal, but not high enough to be classified as T2DM.7 Without intervention, pre-diabetes will progress to T2DM onset.8 When unmanaged, T2DM is a progressive disease and continues to advance from onset to development of comorbidities and can eventually lead to death.9
Several risk factors are associated with T2DM onset such as: family history of T2DM; age; obesity; physical inactivity; prior history of gestational diabetes; impaired glucose tolerance; dipocytokines; inflammatory factors and hepatocyte factors; race/ethnicity; alcohol consumption; tobacco smoking; diet10–14; high blood pressure15; appendectomy16 and hypothyroidism.17 In addition to the above factors, medications such statins or 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) inhibitors have also been linked with onset of T2DM.18–21
The novel aspect of this study is its focus on Medicare members. The study uses claims data for patients enrolled in Medicare Advantage Prescription Drug (MAPD) plan. Medicare population differs from the population at large in age, life expectancy, frequency of pre-existing comorbid conditions and comorbidities due to progression of T2DM. Such differences affect the types of pharmacological and non-pharmacological interventions appropriate for this population.22 Understanding these factors will help design better T2DM prevention programmes.
In the present study, the factors associated with the new onset of T2DM in Medicare are investigated, especially the association of statins with new onset type 2 diabetes.
2 |. MATERIALS AND METHODS
2.1 |. Study design
This retrospective study used de-identified administrative healthcare claims and enrolment data from a MAPD health plan offered by a large multistate healthcare company. The data were collected during standard operations. It was not originally collected for the purpose of research or any new study on human or animal subjects. Prior to the beginning of the study, this study protocol was reviewed and approved by the Institutional Review Board at University of Louisville (Reference # 573220). The study was also reviewed by the healthcare company’s privacy and ethics committee.
2.2 |. Study cohort
The large multi-state healthcare company insured >2.4 million individuals who had continuous MAPD coverage from January to December of 2015. Of these >2.4 million individuals, there were more than 1.7 million individuals who had “age ≥65” as the original reason for Medicare entitlement. The remainder qualified under Medicare disability or ESRD eligibility provisions and were excluded from this study. Of these 1.7 million individuals, 265 554 individuals had continuous MAPD coverage from January 2008 to December 2015. Starting with the sampling frame of these 265 554 individuals for which longitudinal data were available, the following individuals were excluded: Those who had bariatric surgery (including gastric bypass, laparoscopic gastroenterostomy (n = 619), women with polycystic ovary syndrome (n = 131) and those with lipodystrophy (n = 718). They were excluded because medications to treat these diseases may have competing indications for use in T2DM. Individuals with any diagnostic codes related to nephropathy were excluded because a previous study published by Kaiser Permanente indicated that worsening renal function could lead to improved glycaemic control.23 In the present study, chronic kidney disease (CKD) was excluded (n = 109 243) in order to identify a population without end organ damage. In the current study, we excluded pre-existing nephropathy (41.3%) of the study sample. Previous research suggested the rate of CKD among individuals 65 years and older was 61%. The CKD rate in the current study was likely lower because individuals with “disability” and “ESRD” as their initial reason for Medicare enrolment was excluded from the sampling frame.24 Individuals who had drug and/or steroid-induced diabetes (determined by ICD codes) were excluded because the pathway for T2DM onset in these individuals may be different from normal pathways for T2DM onset (n = 3436). Type 1 diabetes patients (determined by ICD codes) were excluded because it has a different aetiology from T2DM (n = 25 535). Those who had T2DM-related diagnostic codes only during inpatient hospital stay(s) and do not have T2DM diagnostic claims or T2DM-related pharmacy claims in a non-hospital setting were excluded to account for potential surgery induced dysglycaemia (n = 1772). Finally, certain groups with contractual restrictions with the health plan prohibiting research are also excluded (n = 2238).
2.3 |. Inclusion criteria
To be included in the study cohort, individuals were required to have no T2DM-related claims in years 2008, 2009, 2010 and 2011 and have either no T2DM-related claims in years 2012, 2013, 2014 and 2015; or T2DM onset in any year between 2012 and 2015. Upon onset, individuals were also required to have T2DM-related claims every year until the end of the study (Table S1). This resulted in a final analytical sample of 89 390 Medicare patients of whom 4314 individuals had T2DM onset (T2DM onset cohort) and 85 076 individuals had no T2DM onset (no T2DM onset cohort) during the study period (Table S1). The T2DM onset baseline year was 2011, because by design the entire study cohort had no T2DM in 2008, 2009, 2010 and 2011.
2.4 |. T2DM definition
T2DM was identified with administrative medical and pharmacy claims data using the following criteria, measured per calendar year: (a) at least two diagnoses of T2DM (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] and/or (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM]; (b) at least two prescriptions related to T2DM; and (c) at least two claims (medical or pharmacy) related to T2DM. A minimum of two claims were used in the identification of T2DM as a means of excluding patients who were potentially miscoded and patients who were suspected to have T2DM but were never formally diagnosed and consistently treated.
2.4.1 |. Covariates
T2DM co-morbidities were defined using administrative claims data as described previously using both ICD-9 and ICD-10 diagnostic codes.25 The following co-morbidities were included in the study: (a) retinopathy: other retinopathy, retinal oedema, cystoid macular oedema/degeneration (CSME), other retinal disorders, retinal detachment and blindness; (b) neuropathy: amyotrophy, cranial nerve palsy, neurogenic bladder, gastroparesis/diarrhoea and orthostatic hypotension; (c) cerebrovascular: transient ischemic attack, stroke; (d) cardiovascular diseases: atherosclerosis, other ischemic heart disease (IHD), angina pectoris, other chronic IHD, myocardial infarction, ventricular fibrillation and arrest, atrial fibrillation, arrest, other atherosclerotic cardiovascular disease, prior myocardial infarction, heart failure, atherosclerosis severe and aortic aneurysm/dissection; (e) peripheral vascular disease (PVD): other aneurysm of lower extremities, foot wound and complications, embolism/thrombosis in lower extremities, ulcer of lower limbs; (f) hypertension and (g) hyperlipidaemia.
Demographic information (sex and race) was based on enrolment data. T2DM-related pharmacy and statin use were determined using administrative pharmacy data.
2.5 |. Statistical analysis
Data aggregation and the implementation of inclusions and exclusions were done in Hadoop (using Spark, Scala and Hive). Hadoop is an open-source software framework for distributed processing and distributed storage of very large datasets.26 The processing was done in Hadoop because of the volume of the data, which included ≥2.4 million individuals’ medical and pharmacy claims data from 2008 to 2015. Means and frequencies to generate patient profiles were calculated using Hadoop. The analytical dataset was created in a counting process input style.27,28 These data were then imported into SAS 9.2. (SAS Institute Inc., Cary, North Carolina) to calculate rate of T2DM onset. Cox proportional hazard models in PROC PHREG were specified to identify the significant variables associated with T2DM onset.
The unadjusted model was developed using demographic, pharmacy and T2DM co-morbidities. The level of significance for unadjusted models was P < .05. The model was developed using Cox regression proportional hazards. Variables that were significant in the unadjusted model were then included in the adjusted model. In the final analysis, the adjusted model included demographic variables (sex and race), pharmacy (statin use) and clinical condition variables (hypertension, hyperlipidaemia, heart failure, ulcer of lower limbs, atherosclerosis, retinal oedema, gastroparesis, orthostatic hypotension, cerebrovascular, atherosclerosis, other IHD, other chronic IHD, myocardial infarction (MI), ventricular fibrillation and arrest, other atherosclerotic cardiovascular disease (ASCVD), old MI, atherosclerosis severe, embolism, blindness and low vision, other retinopathy and angina pectoris). The level of significance for all statistical tests was P < .05.
A logistic propensity score model was constructed using the covariates from the adjusted model as independent variables and statin usage as outcome variable. The scores were then used to create non-overlapping partition of patients into two groups with identical propensity scores, with a matching tolerance at 0.001. These two groups were used to evaluate the prevalence of T2DM onset for statin patients and non-statin patients. Approximately 10.5% (n = 454) were excluded from the onset group because these members did not have a record of statin usage prior to diabetes and started on statins after T2DM onset. 36.0% (n = 1555) of T2DM patients did not match on the propensity score with a non-T2DM participant and were not included in the matched PSA analysis.
Within the propensity matched cohort, each patient was associated with only one statin based on their most frequently utilized statin. The statin dose and distribution were done as described previously.29 The patients in the study cohort were prescribed atorvastatin, rosuvastatin, lovastatin, pravastatin and simvastatin. The study did not have pharmacy claims related to pitavastatin, likely because they were not prescribed to the patients in this study cohort.
A meta-analysis model was computed to analyse statin types and T2DM onset because it is likely that the true effect size varies (ie, age, gender, co-morbidities differ across studies). The random effects model is the appropriate one to use in this instance because it is likely that the true effect of statins on T2DM onset is different between populations.30 The Comprehensive Meta-Analysis Software Version 3 (Biostat Inc., Englewood, New Jersey) was used for the analysis. SAS V9.4 (SAS Institute) was used for statistical analyses.
3 |. RESULTS
There were 89 390 people 65 years of age and older who met study inclusion criteria. Of these, 4314 were diagnosed with T2DM between 2012 and 2015, while the remaining 85 076 continued to have no T2DM diagnosis until the end of study (ie, 2015). The cumulative incidence of diagnosed T2DM onset in the study cohort was 4.82% (4314/89390) (Table 1). The incidence of diagnosed T2DM was 0.82%, 0.88%, 1.04% and 2.09% in years 2012, 2013, 2014 and 2015, respectively.
TABLE 1.
Baseline demographic and T2DM onset characteristics
| Characteristic | No Onseta (n = 85 076) | Onsetb (n = 4314) | P value |
|---|---|---|---|
| Age, mean (SD), years | 73.0 (5.6) | 72.1 (5.1) | <.0001 |
| Female sex | 63.5% | 60.3% | <.0001 |
| Race/ethnicity | <.0001 | ||
| White | 75 051 (88.3) | 3465 (80.5) | |
| Black | 6316 (7.4) | 542 (12.5) | |
| Others | 3640 (4.3) | 300 (7.0) |
Note: Data are represented as n (%).
Missing data in no onset cohort: race (n = 69).
Missing data in onset cohort: race (n = 7).
In the baseline year (2011), the average age of patients in the T2DM onset cohort (n = 4314) was 72.1 (SD, 5.1) years compared to the average age of 73.0 (SD, 5.6) years for the no-onset cohort (n = 85 076) (P < .0001). The T2DM onset cohort consisted of 60.3% females compared to 63.5% females in the no-onset cohort (Table 1) (P < .0001).
At baseline, there were significantly more non-white (African American, Hispanic) individuals (19.5%) in the T2DM onset sub-group compared to the T2DM no onset sub-group (11.7%) (P < .0001) (Table 1). The T2DM onset sub-group had significantly higher prevalence of disease comorbidities compared to the no-onset cohort. Individuals in the T2DM onset cohort had higher prevalence of clinical conditions such as hypertension (70.1% vs 59.1%), hyperlipidaemia (69.4% vs 59.5%), cardiovascular diseases (ie, atherosclerosis [5.0% vs 7.2%], other IHD [0.8% vs 1.5%], angina pectoris [2.1% vs 3.6%], other chronic IHD [12.3% vs 15.4%], other atherosclerotic cardiovascular disease [0.9% vs 1.3%], history of myocardial infarction [3.2% vs 4.6%], heart failure [3.1% vs 4.8%], [P < .05 for all]) (Table 2).
TABLE 2.
Baseline comorbidities
| Characteristic | No Onset (n = 85 076) | Onset (n = 4314) | P value |
|---|---|---|---|
| Hypertension | 50 336 (59.2) | 3024 (70.1) | <.0001 |
| Hyperlipidaemia | 50 636 (59.5) | 2993 (69.4) | <.0001 |
| Retinopathy | |||
| Other retinopathy | 795 (0.9) | 42 (1.0) | .7950 |
| Retinal oedema | 351 (0.4) | 15 (0.3) | .5150 |
| CSME | 430 (0.5) | 26 (0.6) | .3820 |
| Other retinal disorders | 294 (0.3) | 10 (0.2) | .2100 |
| Retinal detachment | 647 (0.8) | 37 (0.9) | .4750 |
| Blindness | 535 (0.6) | 32 (0.7) | .3620 |
| Neuropathy | |||
| Neurogenic bladder | 154 (0.2) | <10 | .7770 |
| Gastroparesis/diarrhoea | 174 (0.2) | 12 (0.3) | .3000 |
| Orthostatic hypotension | 1226 (1.4) | 57 (1.3) | .5190 |
| Cerebrovascular | |||
| TIA | 1486 (1.7) | 78 (1.8) | .7640 |
| Stroke | 5104 (6.0) | 288 (6.7) | .0690 |
| Cardiovascular | |||
| Atherosclerosis | 4216 (5.0) | 311 (7.2) | <.0001 |
| Other IHD | 675 (0.8) | 66 (1.5) | <.0001 |
| Angina pectoris | 1773 (2.1) | 155 (3.6) | <.0001 |
| Other chronic IHD | 10 445 (12.3) | 664 (15.4) | <.0001 |
| Myocardial infarction | 588 (0.7) | 37 (0.9) | .2000 |
| Ventricular fibrillation, arrest | 5508 (6.5) | 308 (7.1) | .0840 |
| Atrial fibrillation, arrest | 78 (0.1) | <10 | .3220 |
| Other ASCVD | 761 (0.9) | 55 (1.3) | .0100 |
| Old myocardial infarction | 2761 (3.2) | 197 (4.6) | <.0001 |
| Heart failure | 2673 (3.1) | 208 (4.8) | <.0001 |
| Atherosclerosis, severe | 38 (0.0) | <10 | .9590 |
| Aortic aneurysm/dissection | 1307 (1.5) | 52 (1.2) | .0830 |
| Peripheral vascular disease | |||
| Other aneurysm, LE | 35 (0.0) | <10 | .3770 |
| Foot wound + complication | 26 (0.0) | <10 | .7860 |
| Embolism/thrombosis (LE) | 75 (0.1) | <10 | .5520 |
| Ulcer of lower limbs | 493 (0.6) | 22 (0.5) | .5560 |
Note: Data are expressed as n (%). Baseline year = 2011.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; IHD, ischemic heart disease; LE, lower extremity; TIA, transient ischemic attack.
In bivariate and multivariate analyses, male sex, non-white (African American, Hispanic), statin use, hypertension, hyperlipidaemia, heart failure, ulcer of lower limbs, atherosclerosis, other retinopathy, angina pectoris, blindness and low vision, absence of other IHD were associated with T2DM onset (P < .05) (Figures 1, 2 and Table S2).
FIGURE 1.
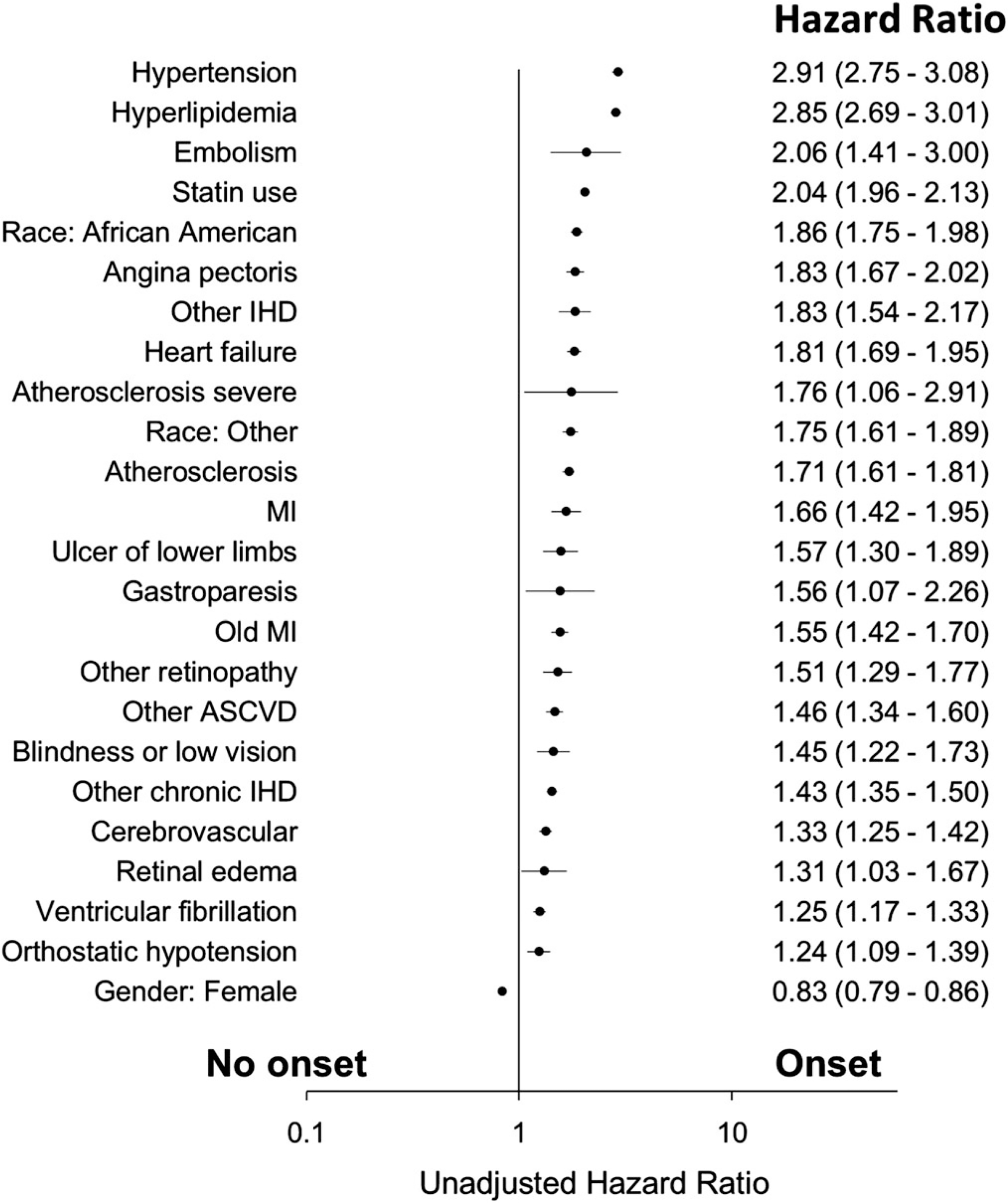
Unadjusted Cox proportional hazard for T2DM onset (MI, Myocardial infarction; ASCVD, atherosclerotic cardiovascular disease; IHD, ischemic heart disease; Race other, non-white and non-African American)
FIGURE 2.
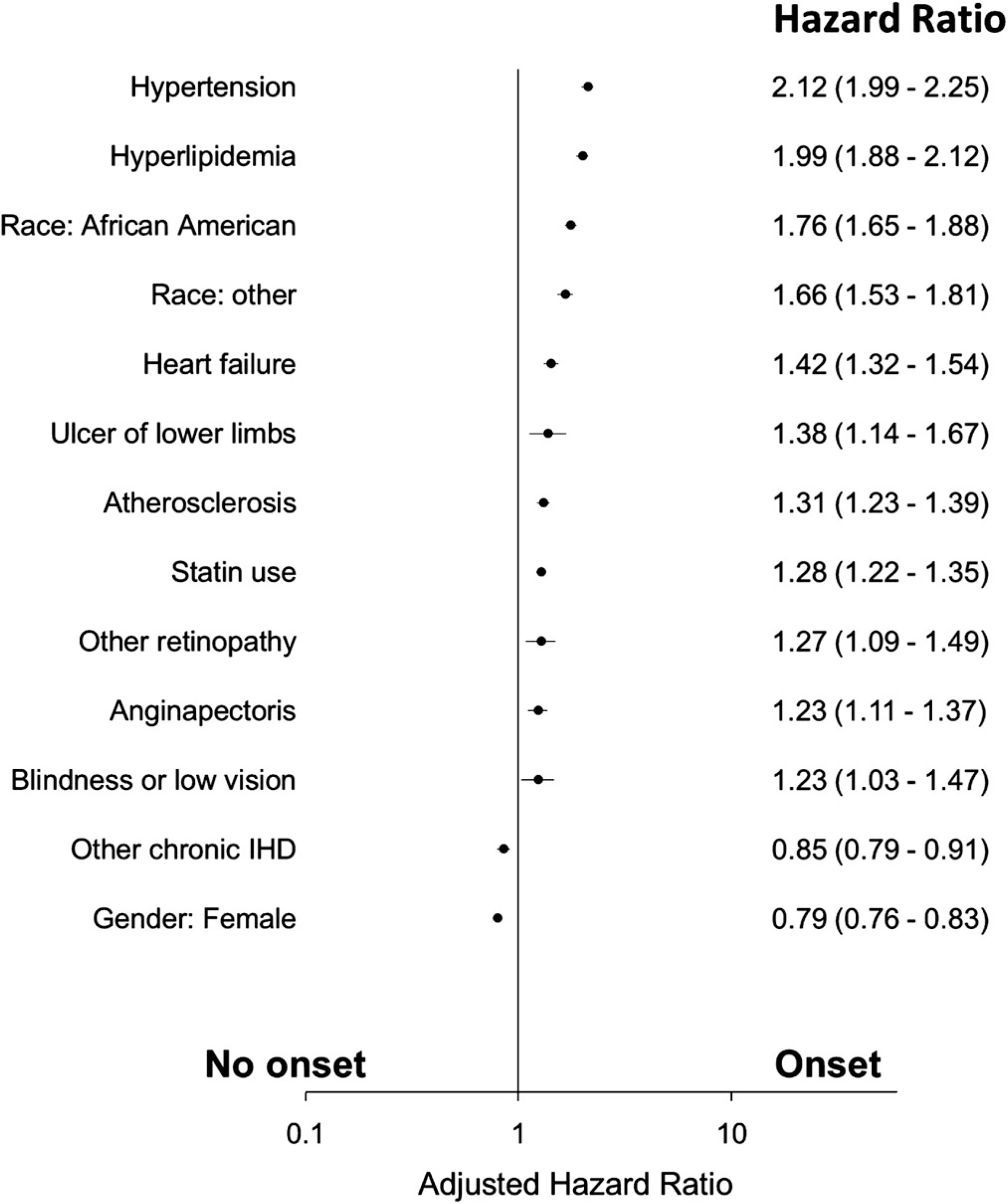
Adjusted Cox proportional hazard for T2DM onset (IHD, ischemic heart disease; Race other, non-white and non-African American)
Propensity scores were used to match cases and controls to control for confounders. The result indicated that, following adjustment for covariates, statins remained significantly associated with new onset T2DM (Table 3). The specific statins included in the present investigation indicate an over-representation of atorvastatin, rosuvastatin, lovastatin, pravastatin and simvastatin in the T2DM incident group (Table 4).
TABLE 3.
Matched propensity score analysis of statins and new onset T2DM
| No T2DM Onset | T2DMOnset | Total | |
|---|---|---|---|
| No statins | 1110 | 979 | 2089 |
| Statin use | 1195 | 1326 | 2521 |
| 2305 | 2305 | 4610 |
Note: OR = 1.26 (95% CI: 1.12–1.41). P < 0.001 with McNemar test.
TABLE 4.
Comparison of statins used by patients in this study between T2DM and non-T2DM: Propensity score matched
| OR | Lo | Hi | P (McNemar’s Test) | P (χ2) | ||
|---|---|---|---|---|---|---|
| Overall | Overall | 1.26 | 1.12 | 1.41 | <.0001 | .0001 |
| Atorvastatin | Overall | 1.33 | 1.09 | 1.63 | <.0001 | .0051 |
| Moderate | 1.26 | 0.99 | 1.61 | <.0001 | .0562 | |
| High | 1.46 | 1.07 | 1.99 | <.0001 | .0155 | |
| Rosuvastatin | Overall | 1.52 | 1.14 | 2.04 | <.0001 | .0045 |
| Moderate | 1.40 | 0.99 | 1.98 | <.0001 | .0571 | |
| High | 1.83 | 1.11 | 3.01 | <.0001 | .0168 | |
| Lovastatin | Overall | 1.54 | 1.21 | 1.96 | <.0001 | .0005 |
| Low | 1.45 | 1.05 | 2.01 | <.0001 | .0244 | |
| Moderate | 1.63 | 1.17 | 2.27 | <.0001 | .0033 | |
| Pravastatin | Overall | 1.35 | 1.09 | 1.67 | <.0001 | .0057 |
| Low | 1.26 | 0.91 | 1.75 | <.0001 | .1585 | |
| Moderate | 1.40 | 1.08 | 1.81 | <.0001 | .0099 | |
| Simvastatin | Overall | 1.46 | 1.27 | 1.68 | .8323 | <.0001 |
| Low | 1.76 | 1.22 | 2.53 | <.0001 | .0021 | |
| Moderate | 1.44 | 1.24 | 1.66 | .0942 | <.0001 |
The odds ratio (OR) point estimate associated with specific statins varied somewhat, with atorvastatin having the lowest OR at 1.33 (95% CI: 1.09–1.63), lovastatin having OR at 1.54 (95% CI: 1.21–1.96) and rosuvastatin having OR = 1.52 (95% CI: 1.14–2.04). Although the higher intensity dose and higher strength statins had higher ORs, they are not significantly different within or between statin drugs. The point estimates are within the 95% CI of all other comparator drugs in all instances (Table 4). Thus, in our cohort, variation in statin type or dose was not an important contributor to the onset of T2DM. Statin use per se was the major predictor of new onset T2DM (Table 4).
Statin use was significantly associated with 26% increased odds of T2DM onset in the present analysis of the data (OR = 1.26, 95% CI: 1.12–1.41, P < .0001). Meta-analysis of previously published matched propensity score analysis studies of incident T2DM onset and statin use showed the random effects model OR = 1.43 (95% CI: 1.31–1.56, Z = 7.94, P < .0001) (Table 5). Meta-analysis of prior studies that used propensity scores in some way other than matching to control for covariates indicated random effects model results OR = 2.23 (95% CI: 1.76–2.84, Z = 6.62, P < .0001) (Table 6).
TABLE 5.
Meta-analysis of propensity score matched analyses
|
95% CI
|
||||||
| Assessment | N | OR/HR | lo | hi | P < | Investigators |
| Any statin | 9043 | 1.27 | 1.14 | 1.41 | .001 | Lin et al68 |
| Any statin | 3351 | 1.87 | 1.67 | 2.01 | .001 | Mansi et al69 |
| Any statin | 6728 | 1.34 | 1.24 | 1.44 | .001 | Mansi et al51 |
| Atorvastatin | 818 | 1.99 | 1.00 | 3.98 | .05 | Park et al70 |
| Any statin | 1699 | 1.99 | 1.36 | 2.92 | .001 | Rha et al71 |
| Any statin | 1609 | 1.66 | 1.14 | 2.42 | .001 | Vande Woestijne72 |
| Any statin | 500 | 1.61 | 1.10 | 2.37 | .001 | Yamakazi73 |
| Any statin | 4610 | 1.26 | 1.12 | 1.41 | .0001 | This study |
| 95% CI | ||||||
| Meta-analysis | OR | Lo | Hi | Z-value | P-value | |
| Fixed | 1.33 | 1.29 | 1.37 | 17.842 | .0001 | |
| Random | 1.43 | 1.31 | 1.56 | 7.94 | .0001 | |
| Heterogeneity | ||||||
| Q-value | Q df | P-value | I-squared | |||
| 26.79 | 6 | <.0001 | 77.606 | |||
| Tau squared | SE | Variance | Tau | |||
| 0.008 | 0.008 | 0.000 | 0.090 | |||
TABLE 6.
Meta-analysis of propensity score adjusted, not matched analyses
| 95% CI | ||||||
| Not matched | N | OR | Lo | Hi | P < | Investigators |
| Specific statin: | ||||||
| Atorvastatin× | 1065 | 2.80 | 1.74 | 4.49 | .001 | Chen et al74 |
| Pravastatin | 3.41 | 1.66 | 7.04 | 0.001 | .001 | Chen et al74 |
| Rosuvastatin | 4.69 | 2.78 | 7.92 | 0.001 | .001 | Chen et al74 |
| Simvastatin | 4.09 | 2.52 | 6.64 | 0.001 | .001 | Chen et al74 |
| Any statin | 4460 | 1.19 | 1.05 | 1.35 | .007 | Castro et al75 |
| Any statin | 7076 | 1.43 | 1.28 | 1.58 | .05 | Culver et al49 |
| Any statin | 2 016 094 | 1.57 | 1.54 | 1.59 | .01 | Macedo76 |
| Any statin | 53 212 | 2.07 | 1.77 | 2.42 | .0001 | Olotu et al77 |
| Atorvastatin | 27 155 | 1.95 | 1.62 | 2.35 | .0001 | Olotu et al77 |
| Fluvastatin | 1638 | 1.95 | 1.28 | 2.96 | .003 | Olotu et al77 |
| Lovastatin | 3570 | 2.45 | 1.82 | 3.25 | .0001 | Olotu et al77 |
| Pravastatin | 5870 | 1.40 | 1.04 | 1.87 | .0001 | Olotu et al77 |
| Rosuvastatin | 2766 | 1.75 | 1.19 | 2.56 | .0001 | Olotu et al77 |
| Simvastatin | 12 213 | 1.79 | 1.43 | 2.24 | .0001 | Olotu et al77 |
|
95% CI
|
||||||
| Meta-analysis | OR | lo | hi | Z | P < | |
| Fixed | 1.66 | 1.59 | 1.74 | 21.34 | .0001 | |
| Random | 2.23 | 1.76 | 2.84 | 6.617 | .0001 | |
| Heterogeneity | ||||||
| Q-value | Q df | P-value | I-squared | |||
| 214.349 | 10 | <.0001 | 95.335 | |||
| Tau squared | SE | Variance | Tau | |||
| 0.148 | 0.096 | 0.009 | 0.385 | |||
The results of the present analysis indicate an association between statin use and new onset T2DM. For the meta-analysis, individual data acquired for each study were not available. Therefore, in the meta-analysis, only summary data were used. Meta-analysis of seven prior investigations and the present study that used matched propensity score analysis indicated an average 43% increased risk for incident T2DM (random effects), similar to the 26% point estimate from the present study (Table 5). The Q statistic (index of heterogeneity) was 26.8 (I-squared 77.60, P < .0001, Qdf = 6) indicate the effects between studies vary. The Q statistic may be biased and have limited ability to evaluate homogeneity because of the small number of studies and the matching criteria to maximise methodological heterogeneity. Significant heterogeneity exists in the meta-analysis of only propensity matched studies (Table 5). In our meta-analysis, Q was 8-fold higher than among studies that used propensity scores to adjust for confounding in some other way than for matching (Table 6).
4 |. DISCUSSION
The incidence of T2DM onset in the present population was 12.05 per 1000 population/year, and the 4-year rate of T2DM onset was 48.2 per 1000 population in the present investigation. This rate was consistent with the unadjusted incidence of T2DM of 11.5 per 1000 in the United States 65 years or older population.2 Previously identified risk factors for T2DM include family history of T2DM, age, obesity and physical inactivity, prior history of gestational diabetes, race, alcohol consumption, tobacco smoking and diet.10–14 Known risk factors that characterise metabolic syndrome include impaired glucose tolerance, dipocytokines, inflammatory factors and hepatocyte factors, and hypertension.15,31
Risks observed in the present study for T2DM include being male, African American, Hispanic (without regard to sex), statin use, hyperlipidaemia and hypertension, consistent with previously reported onset of T2DM risk factors.
In the present investigation, non-white (African American, Hispanic) ethnicity and male gender were significantly associated with T2DM onset in patients 65 years and older. Previous studies found that males were more likely to develop T2DM than females.32,33 This trend has changed over the last 50 years. Females were more likely to be diagnosed with T2DM in recent years, associated with increasingly sedentary lifestyles and high energy, carbohydrate fatty diets.34,35 Consistent with prior studies, the current investigation found that African Americans and Hispanics were more likely to have an onset of T2DM than Caucasians in the Medicare population.36
High blood pressure is a common yet modifiable risk factor for T2DM onset. Those with hypertension are 2.5 times more likely to develop T2DM.37,38 In the present study, hypertension is associated with T2DM onset (P < .05) (Figure 2). Hyperlipidaemia is a proximate cause of hypertension, and a key component of the metabolic syndrome. Metabolic syndrome includes elevated triglycerides, low high-density lipoprotein [HDL] levels, high blood pressure, elevated glucose level and abdominal obesity with pro-thrombotic and proinflammatory states.39 Individuals with metabolic syndrome are at a significantly higher risk of developing T2DM.40 In the present investigation, hyperlipidaemia was associated with T2DM onset in Medicare patients (P < .05) (Figure 2). Hyperlipidaemia and hypertension are significant predictors of T2DM onset, and are proxies for metabolic syndrome as a predictor for T2DM onset.41 Hyperlipidaemia had an adjusted HR of 1.99 (95% CI: 1.88–2.12) in the present study (Figure 2).
4.1 |. Statins use and T2DM onset
This study also found a significant increase in the odds of developing T2DM after statin exposure, OR = 1.26 (95% CI: 1.12–1.41) matched propensity score analysis (Table 3). The statin-specific analyses by the same techniques revealed that each drug had an OR within the 95% CI of the overall OR of 1.26 (Table 4). The difference in OR between statins by dose or within statin type was not significant. The differences between lower intensity (pravastatin, lovastatin) and higher (atorvastatin, rosuvastatin, simvastatin) intensity statins was not significant.
The HMG-CoA inhibitors (statins) are one of the most commonly prescribed drugs worldwide to reduce cholesterol and prevent or manage coronary heart disease.21,42 In a meta-analysis of 13 randomised trials, the use of statins was associated with a 12% increase in the risk of T2DM onset, with the risk being the highest in older populations ≥65 years.43 In an analysis of the World Health Organisation’s pharmacovigilance database (n = 177 323 statin-exposed subjects), the OR = 1.75 (95% CI: 1.72–1.78) was reported.44
The association between statin therapy and T2DM onset was first observed in a 2008 study.45 Computed from Ridker’s reported data, the statin unadjusted OR for T2DM (1.34, 95% CI: 1.10–1.63, P < .004), indicates a 34% increased risk for diabetes with statin therapy (n = 8901) compared to those not on statin therapy (n = 8901) in a prospective trial to test statin effects on C-reactive protein. A slightly increased risk (9%-11%) for T2DM was found in two summaries of 94 938 individuals from several studies.46,47 Analysis of 76 RCTs involving 170 255 participants revealed a modestly increased incident diabetes among statin users (OR = 1.09, 95% CI: 1.02–1.17).48 Women’s Health Initiative investigators reported a 48% increased risk of new onset T2DM in 7076 women prescribed a statin, after adjusting for potential confounding factors.49 In a large population based study (n = 471 250), a dose-response association of the increased risk (10%–22%) for new onset T2DM for atorvastatin, simvastatin and rosuvastatin.50 Another study reported that statin users (n = 10 910) had a 87% higher frequency of new onset T2DM than non-statin users (n = 49 545), including diabetic complications after adjustment for potential confounders.51
Comparison of our results to previously published studies includes only those studies that matched case and control on propensity scores. Seven such studies met the criteria and were included in the meta-analysis. The meta-analysis shows that statins had a significant effect on T2DM onset (Random Effects OR = 1.43, 95% CI: 1.31–1.56, Z = 7.94 P < .0001) (Table 5). In analyses that used propensity scores but not in matched analyses (ie, used the probability as an independent variable/covariate), the random effect result was higher in prior meta-analyses. Our meta-analysis of propensity score unmatched studies found a significantly higher OR (Random Effects OR = 2.23, 95% CI:1.76–2.84, Z = 6.62 P < .0001) for new onset T2DM (Table 6), perhaps reflecting inadequate adjustment for confounding by using an adjustment method other than matching to use propensity scores as covariates. These data strongly support an association between statin use and subsequent new T2DM onset. This is a difficult determination, however, because approximately 70% of T2DM patients are dyslipidaemic. Some may be diagnosed with hypercholesteraemia before being diagnosed with T2DM. By design the first 4 years of the study population was T2DM-free. The effects of all types of statins were within the 95% CI of other statins, indicating no significant differences among the types of statins and risk of T2DM onset.
Possible mechanisms involve more than 15 pathways through which statins may plausibly affect risk of new onset T2DM.21,52–54 Statins up regulate low density lipoprotein receptor gene (LDLP) via sterol regulatory element-binding protein 2 (SREBP2). Increased LDLP causes cellular cholesterol accumulation and dysfunction in pancreatic islets.54 Fat accumulation in liver and pancreas has been associated with onset of T2DM. The excess liver fat worsens hepatic insulin response, which in-turn causes increased glucose production. Excess pancreatic fat causes fat induced metabolic stress, which results in pancreatic β cells entering survival mode and apoptosis. Therefore, the pathway for statins effects on T2DM may involve inflammatory response, LDL toxicity, increased cholesterol in cells and β cell dormancy/apoptosis.55,56 The risk: benefit evaluation of new onset T2DM risk and use of statins considering the cardiovascular benefits do not suggest discontinuation of medication with a known therapeutic advantage.57 The risk of incident diabetes showed a continuous increasing trend with higher levels of adherence, compared to dyslipidaemia patients with low adherence, supporting the role of the drug but not dose in the 2014 meta-analysis.58 In another study, increasing statin dose was associated with an increased risk of incident TDM.59 However, considering its effect on reducing coronary events, previous studies have recommended no change in clinical decision making for medium to high risk of cardiovascular diseases.46
4.2 |. T2DM co-morbidities
The study also identified several new associations with T2DM onset such as other retinopathy, blindness and low vision, ulcer of lower limbs, angina pectoris, absence of other chronic IHD, heart failure and atherosclerosis. Prior investigations suggest that nearly 21% of individuals who are diagnosed with T2DM also have some level of retinopathy at the time of diagnosis.60 In individuals who do not have T2DM-related retinopathy, symptoms are generally a manifestation of either retinal micro aneurysms or blot haemorrhages. Advanced age and hypertension are associated with the development of non-diabetic retinopathy but hypertension and T2DM are risk factors for retinopathy in individuals who are <65 years of age years.61
In the present study, it was found that retinopathy (other retinopathy, blindness or low vision) was associated with T2DM onset in Medicare patients (P < .05) (Figure 2). One possible explanation is that when an optometrist or other provider evaluates a Medicare beneficiary for non-diabetic retinopathy, they may recommend T2DM screening. This interaction with their provider and subsequent evaluation of blood glucose and/or HbA1c can lead to diagnosis of T2DM.
Vascular ulcers such as ulcers of lower limbs are a common cause for older patients to visit a provider.62 In the study presented here, non-diabetic ulcer of lower limbs was associated with T2DM onset in Medicare patients (P < .05).
As mentioned above, metabolic syndrome is characterised by clustering of risk factors such as prothrombotic state, hypertension and dyslipidaemia. Metabolic syndrome independently contributes to CVD and T2DM.63 This study evaluated several cardiovascular conditions associated with T2DM: angina pectoris, atherosclerosis, heart failure and other chronic IHD. Clinical and laboratory data related to metabolic syndrome were not available to further evaluate if the observed associations are due to the underlying metabolic syndrome. Despite this limitation, these findings provide valuable information to providers about the prognosis of T2DM. Cardiovascular conditions significantly associated with T2DM included: angina pectoris (angina); characterised by chest pain or discomfort, often associated with myocardial ischemia, arterial insufficiency or hypoxia. Angina is generally a symptom for an underlying cause such as coronary artery disease.64 Angina pectoris is associated with T2DM onset in the present study (P < .05). Associations of angina pectoris and T2DM onset must be re-evaluated by analysing the underlying causes. The diagnostic codes for other chronic IHD are associated with loss of elasticity and thickening of the coronary arteries, leading to progressive arterial insufficiency or “stiffness.” This study found in bivariate analysis that other chronic IHD was associated with T2DM onset. However, when adjusted for other factors, IHD had a protective effect on T2DM onset. This finding suggests that individuals who have hypertension and hyperlipidaemia and also have IHD might have different health-related behaviour (eg, medication adherence, compliance etc.) perhaps preventing or delaying onset of T2DM.65 Heart failure and atherosclerosis were associated with T2DM onset Medicare patients 65 years and older (Figure 2).
The findings from this study can be used to inform interventions for sub-groups of people at the highest risk of developing T2DM. For example, the sociodemographic and clinical profile of patients 65 years and older with T2DM onset could be used to: (a) educate patients, providers, payers and other stakeholders; (b) develop Diabetes Prevention Programs; (c) design public health policies and/or; (d) to select individuals into disease management programs in-turn; this may help to improve quality of life and reduce T2DM disease burden. The study provides considerable support for the tendency of statin use and T2DM incidence, but future definitive studies should be of a randomised placebo-controlled prospective design.
4.3 |. Study limitations
Administrative Data, as used in this study, do not include clinical and laboratory findings such as physician notes and HbA1c levels, which would have provided a more accurate measure of T2DM onset. Further, the lack of clinical and laboratory data limits the possibility of evaluating any confounding independent risk factors. Data such as nutrition, physical activity, BMI, original diagnosis date for T2DM are also not available. The study does not further split the study cohort based on pre-diabetes and/or undiagnosed individuals. Individuals with metabolic syndrome could not be identified due to lack of data such as BMI and cholesterol data. These types of data are routinely not available in claims and administration data sets. As with other similar retrospective studies, there is a potential for error in the information coded on the administrative data. Even though the claims are audited on a regular basis, it is a rare possibility that potential for error in the information coded on medical claims due to provider error, fraud, etc. may occur. Many controls are in place to manage this risk. As with similar retrospective studies, this type of analysis can only establish associations and is not intended to establish causation of T2DM onset, although matched propensity score analysis was used. Matched propensity score analysis was developed as a way to infer causality from retrospective data,66 but that property is not discussed here because a prospective study is needed to conduct a true causality analysis of the statin-T2DM association. This was a quasi-experimental study (ie, it is not a true randomised study). The factors (covariates, confounding variables, etc.) will not be random and it is not possible to eliminate bias or confounding, especially when some confounders are not recorded in the database (eg, BMI, HbA1c and other clinical chemistry are not available in the administrative data).
Exclusion criteria (disabled individuals, individuals with ESRD or chronic kidney disease) in the study cohort also limit the generalizability of the study.
In summary, longitudinal analysis of the use of statins in this large Medicare population was associated with a propensity score adjusted 26% increased risk of new T2DM onset. To maintain a realistic clinical perspective, it was estimated that the clinical benefit from statin therapy is 50 times greater than the risk of incident T2DM.67 Longitudinal analysis of the use of statins in this large Medicare population was associated with several modifiable (social or behavioural) risk factors for new onset T2DM. Several T2DM onset risk factors (atherosclerosis, hyperlipidaemia, hypertension, PVD) are modifiable and are treated by statins. Statins have a propensity score adjusted 43% increased risk of new T2DM onset across seven studies plus the current study, which used a matched propensity score study design. Statin therapy should not be discontinued to avoid the risk of T2DM. However, the association between T2DM and statins should certainly be studied further. Future studies should focus on how to minimise risk of T2DM with continued statin therapy, which has such great therapeutic benefit that one is driven to continue its use. The most informative of these future studies will be large, prospective studies in populations for which complete clinical and pharmacy data are available. This will allow effective preventive interventions while continuing highly beneficial medical therapy.
Supplementary Material
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. National Diabetes Statistics Report: estimates of diabetes and its burden in the United States. In: Services USDoHaH, ed. Atlanta, GA; 2014. [Google Scholar]
- 3.Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes A. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colagiuri S, Davies D. The value of early detection of type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2009;16:95–99. [DOI] [PubMed] [Google Scholar]
- 7.Tuso P Prediabetes and lifestyle modification: time to prevent a preventable disease. Perm J. 2014;18:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twito O, Frankel M, Nabriski D. Impact of glucose level on morbidity and mortality in elderly with diabetes and pre-diabetes. World J Diabetes. 2015;6:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16:17–23. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Basics about diabetes. 2016. http://www.cdc.gov/diabetes/basics/diabetes.html.
- 12.Choi BC, Shi F. Risk factors for diabetes mellitus by age and sex: results of the National Population Health Survey. Diabetologia. 2001; 44:1221–1231. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zivanovic D, Sipetic S, Stamenkovic-Radak M, Milasin J. Potential risk factors for developing diabetes mellitus type 2. Med Pregl. 2010;63: 231–236. [DOI] [PubMed] [Google Scholar]
- 15.Emdin CA, Anderson SG, Woodward M, Rahimi K. Usual blood pressure and risk of new-onset diabetes: evidence from 4.1 million adults and a meta-analysis of prospective studies. J Am Coll Cardiol. 2015; 66:1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei PL, Tsai MC, Hung SH, Lee HC, Lin HC, Lee CZ. Risk of new-onset type II diabetes after appendicectomy. Br J Surg. 2015;102: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 17.Gronich N, Deftereos SN, Lavi I, Persidis AS, Abernethy DR, Rennert G. Hypothyroidism is a risk factor for new-onset diabetes: a cohort study. Diabetes Care. 2015;38:1657–1664. [DOI] [PubMed] [Google Scholar]
- 18.Zigmont VA, Shoben AB, Lu B, et al. Statin users have an elevated risk of dysglycemia and new-onset-diabetes. Diabetes Metab Res Rev. 2019;35(8):e3189. [DOI] [PubMed] [Google Scholar]
- 19.Betteridge DJ, Carmena R. The diabetogenic action of statins—mechanisms and clinical implications. Nat Rev Endocrinol. 2016;12: 99–110. [DOI] [PubMed] [Google Scholar]
- 20.Carmena R, Betteridge DJ. Diabetogenic action of statins: mechanisms. Curr Atheroscler Rep. 2019;21:23. [DOI] [PubMed] [Google Scholar]
- 21.Paseban M, Butler AE, Sahebkar A. Mechanisms of statin-induced new-onset diabetes. J Cell Physiol. 2019;234:12551–12561. [DOI] [PubMed] [Google Scholar]
- 22.Koller EA, Chin JS, Conway PH. Diabetes prevention and the role of risk factor reduction in the Medicare population. Am J Prev Med. 2013;44:S307–S316. [DOI] [PubMed] [Google Scholar]
- 23.Karter AJ, Nundy S, Parker MM, Moffet HH, Huang ES. Incidence of remission in adults with type 2 diabetes: the diabetes & aging study. Diabetes Care. 2014;37:3188–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on kidney disease: improving global outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor RC. An overview of the Hadoop/MapReduce/HBase framework and its current applications in bioinformatics. BMC Bioinformatics. 2010; (11 Suppl 12):S1. https://www.ncbi.nlm.nih.gov/pubmed/21210976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Counting Process Style of Input [database on the Internet]. http://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_phreg_sect027.htm.
- 28.Knox KL, Bajorska A, Feng C, Tang W, Wu P, Tu XM. Survival analysis for observational and clustered data: an application for assessing individual and environmental risk factors for suicide. Shanghai Arch Psychiatry. 2013;25:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 30.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 31.Prasad H, Ryan DA, Celzo MF, Stapleton D. Metabolic syndrome: definition and therapeutic implications. Postgrad Med. 2012;124:21–30. [DOI] [PubMed] [Google Scholar]
- 32.Sattar N Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract Res Clin Endocrinol Metab. 2013;27: 501–507. [DOI] [PubMed] [Google Scholar]
- 33.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37:278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upadhyay R, Robay A, Fakhro K, et al. Role of SLMAP genetic variants in susceptibility of diabetes and diabetic retinopathy in Qatari population. J Transl Med. 2015;13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. [DOI] [PubMed] [Google Scholar]
- 36.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung BM, Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep. 2012;14:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037–2114. [DOI] [PubMed] [Google Scholar]
- 39.Yamaoka-Tojo M, Tojo T, Takahira N, et al. Elevated circulating levels of an incretin hormone, glucagon-like peptide-1, are associated with metabolic components in high-risk patients with cardiovascular disease. Cardiovasc Diabetol. 2010;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayans L Metabolic syndrome: insulin resistance and prediabetes. FP Essent. 2015;435:11–16. [PubMed] [Google Scholar]
- 42.Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf. 2010;9:603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thakker D, Nair S, Pagada A, Jamdade V, Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf. 2016;25:1131–1149. [DOI] [PubMed] [Google Scholar]
- 44.Montastruc F, Benevent J, Rousseau V, Durrieu G, Sommet A, Montastruc JL. Risk of diabetes with fibrates and statins: a pharmacoepidemiological study in VigiBase([R]). Fundam Clin Pharmacol. 2019;33:108–112. [DOI] [PubMed] [Google Scholar]
- 45.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 46.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 47.Waters DD, Ho JE, DeMicco DA, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol. 2011;57:1535–1545. [DOI] [PubMed] [Google Scholar]
- 48.Mills EJ, Wu P, Chong G, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104:109–124. [DOI] [PubMed] [Google Scholar]
- 49.Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med. 2012;172:144–152. [DOI] [PubMed] [Google Scholar]
- 50.Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansi IA, Frei CR, Halm EA, Mortensen EM. Association of statins with diabetes mellitus and diabetic complications: role of confounders during follow-up. J Invest Med. 2017;65:32–42. [DOI] [PubMed] [Google Scholar]
- 52.Barylski M, Nikolic D, Banach M, Toth PP, Montalto G, Rizzo M. Statins and new-onset diabetes. Curr Pharm Des. 2014;20:3657–3664. [DOI] [PubMed] [Google Scholar]
- 53.Yoon D, Sheen SS, Lee S, Choi YJ, Park RW, Lim HS. Statins and risk for new-onset diabetes mellitus: a real-world cohort study using a clinical research database. Medicine (Baltimore). 2016;95: e5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu Q, Chen Y, Xu CB. Statins and new-onset diabetes mellitus: LDL receptor may provide a key link. Front Pharmacol. 2017;8:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor R Banting memorial lecture 2012: reversing the twin cycles of type 2 diabetes. Diabet Med. 2013;30:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor R, Al-Mrabeh A, Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019;7: 726–736. [DOI] [PubMed] [Google Scholar]
- 57.Shah RV, Goldfine AB. Statins and risk of new-onset diabetes mellitus. Circulation. 2012;126:e282–e284. [DOI] [PubMed] [Google Scholar]
- 58.Corrao G, Ibrahim B, Nicotra F, et al. Statins and the risk of diabetes: evidence from a large population-based cohort study. Diabetes Care. 2014;37:2225–2232. [DOI] [PubMed] [Google Scholar]
- 59.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. Jama. 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 60.Fong DS, Aiello L, Gardner TW, et al. Diabetic retinopathy. Diabetes Care. 2003;26:s99–s102. [DOI] [PubMed] [Google Scholar]
- 61.Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98–107. [PMC free article] [PubMed] [Google Scholar]
- 62.Chin JA, Sumpio BE. Diabetes mellitus and peripheral vascular disease: diagnosis and management. Clin Podiatr Med Surg. 2014;31: 11–26. [DOI] [PubMed] [Google Scholar]
- 63.Scott M, Grundy IJB, Gregory L, et al. Diabetes and cardiovascular disease. Circulation. 1999;100:1134–1146. [DOI] [PubMed] [Google Scholar]
- 64.Davies SW. Clinical presentation and diagnosis of coronary artery disease: stable angina. Br Med Bull. 2001;59:17–27. [DOI] [PubMed] [Google Scholar]
- 65.Morisaki N, Kawano M, Watanabe S, Saito Y, Yoshida S. Role of obesity in development of ischemic heart disease in elderly diabetic patients. Gerontology. 1992;38:167–173. [DOI] [PubMed] [Google Scholar]
- 66.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70: 41–55. [Google Scholar]
- 67.Hennekens CH, Teng B, Pfeffer MA. Statins and diabetes: current perspectives and implications for clinicians. Am J Med. 2017;130: 504–506. [DOI] [PubMed] [Google Scholar]
- 68.Li H, Lin H, Zhao H, et al. Statins use and risk of new-onset diabetes in hypertensive patients: a population-based retrospective cohort study in Yinzhou district, Ningbo city, People’s Republic of China. Ther Clin Risk Manag. 2018;14:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mansi I, Frei CR, Wang CP, Mortensen EM. Statins and new-onset diabetes mellitus and diabetic complications: a retrospective cohort study of US healthy adults. J Gen Intern Med. 2015;30:1599–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park JY, Rha SW, Choi B, et al. Impact of low dose atorvastatin on development of new-onset diabetes mellitus in Asian population: three-year clinical outcomes. Int J Cardiol. 2015;184:502–506. [DOI] [PubMed] [Google Scholar]
- 71.Rha SW, Choi BG, Seo HS, et al. Impact of statin use on development of new-onset diabetes mellitus in Asian population. Am J Cardiol. 2016;117:382–387. [DOI] [PubMed] [Google Scholar]
- 72.van de Woestijne AP, van der Graaf Y, Westerink J, Nathoe HM, Visseren FL. Effect of statin therapy on incident type 2 diabetes mellitus in patients with clinically manifest vascular disease. Am J Cardiol. 2015;115:441–446. [DOI] [PubMed] [Google Scholar]
- 73.Yamazaki K, Takahashi Y, Teduka K, Nakayama T, Nishida Y, Asai S. Assessment of effect modification of statins on new-onset diabetes based on various medical backgrounds: a retrospective cohort study. BMC Pharmacol Toxicol. 2019;20:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen CW, Chen TC, Huang KY, Chou P, Chen PF, Lee CC. Differential impact of statin on new-onset diabetes in different age groups: a population-based case-control study in women from an asian country. PLoS One. 2013;8:e71817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castro MR, Simon G, Cha SS, Yawn BP, Melton LJ 3rd, Caraballo PJ. Statin use, diabetes incidence and overall mortality in normoglycemic and impaired fasting glucose patients. J Gen Intern Med. 2016;31:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macedo AF, Douglas I, Smeeth L, Forbes H, Ebrahim S. Statins and the risk of type 2 diabetes mellitus: cohort study using the UKclinical practice research datalink. BMC Cardiovasc Disord. 2014;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olotu BS, Shepherd MD, Novak S, et al. Use of statins and the risk of incident diabetes: a retrospective cohort study. Am J Cardiovasc Drugs. 2016;16:377–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


