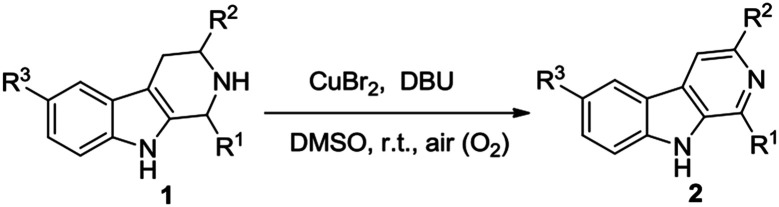
Abstract
A mild, efficient and environmentally benign method for synthesis of aromatic β-carbolines via Cu(ii)-catalyzed oxidation of 1,2,3,4-tetrahydro-β-carbolines (THβCs) was developed, in which air (O2) was used as the clean oxidant. This method has advantages such as environmentally friendliness, mildness, very good tolerance of functional groups, high yielding and easy experiment operation. In addition, this new methodology was successfully applied in the efficient and practical total syntheses of β-carboline alkaloids perlolyrine and flazin.
A mild, efficient and ecofriendly method for synthesis of β-carbolines via Cu-catalyzed aerobic oxidation of 1,2,3,4-tetrahydro-β-carbolines (THβCs) was developed. In addition, this method was successfully applied in the practical total syntheses of perlolyrine and flazin.
Introduction
The aromatic β-carboline skeleton is ubiquitous in many alkaloids,1 bioactive congeners,2 agrochemicals3 and functional materials.4 In view of the significance of β-carbolines in drug discovery, agricultural chemistry and material science, development of practical, efficient and environmentally benign methods for syntheses of β-carbolines is of considerable interest and has attracted much attention from chemists.5
Since 1,2,3,4-tetrahydro-β-carbolines (THβCs) can be readily and efficiently prepared via the Pictet–Spengler reaction,6 so the aromatization of THβCs via dehydrogenation or oxidation is an easy and good method to obtain β-carbolines. Dehydrogenation of THβCs usually employed precious metallic catalysts including Pd,7 Pt8 and complexes of Ru9 and Ir.10 Oxidation of THβCs normally needed stoichiometric strong oxidants such as KMnO4,11 MnO2,12 SeO2,13 DDQ,14 TCCA15 and IBX.16 However, uses of expensive metallic catalysts and hazardous strong oxidants are sometimes lack of cheapness, practicality and environmental friendliness, therefore novel practical, efficient and environmentally benign methods for the conversion of THβCs to β-carbolines are highly desirable.
Oxygen is an important component of air, which is one of the most abundant resources on the earth, so the air has been used as a clean and eco-friendly oxidant for various oxidation reactions in recent decades.17 On the other hand, copper is one of the most abundant metals on the earth's crust, thus copper salts are normally very cheap, and moreover copper is a low toxic transition metal, so an increasing amount of copper-catalyzed reactions have been developed recently.18 Herein, we describe a new practical and very mild Cu(ii)-catalyzed oxidative conversion of THβCs to β-carbolines using air as the clean oxidant.
Results and discussion
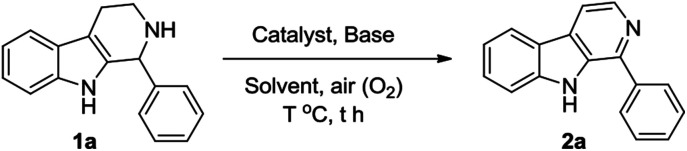
At first, we attempted to find out the optimized reaction conditions for the Cu-catalyzed oxidative conversion of THβCs 1 to β-carbolines 2. With the conversion 1-phenyl-THβC 1a to 1-phenyl-β-carboline 2a as the model reaction, we tried the reaction under various conditions, and results are summarized in Table 1. As can be seen from Table 1, various copper reagents have been tested as catalysts (Table 1, Entries 2–11), and it was found that cupric bromide is the best catalyst for the reaction. When 0.2 equivalents of CuBr2 was used as the catalyst, the aerobic oxidation of compound 1a took place smoothly at room temperature in DMSO in the presence of 2 equivalents of 1,8-diazabicyclo[5,4,0]undec-7-ene (DBU) to afford the desired compound 2a in high yield (Entry 2). Several amines such as DBU, 1,5-diazabicyclo[4,3,0]non-5-ene (DBN), 4-dimethyl-aminopyridine (DMAP), pyridine and trimethylamine have been tested as bases (Entries 2 and 12–15), DBU was found to be the most appropriate base for the reaction. Amount of DBU has significant effect on the reaction (Entries 2 and 16–19), almost no desired product 2a could be obtained in the absence of DBU (Entry 16); and the reaction was hard to be complete even for a longer time, if less than 2.0 equivalents of DBU were used (Entries 17–19). Several other solvents such as N,N-dimethylformamide (DMF), acetonitrile, ethanol, tetrahydrofuran (THF), dichloromethane, ethyl acetate and acetone have also been examined (Entries 20–26); DMSO is obviously better than them. Additionally, when the reaction temperature was elevated, the reaction velocity increased, but the yield of desired product 2a obviously decreased (Entries 27–29).
Optimization of reaction conditions for the Cu-catalyzed aerobic oxidative conversion of 1-phenyl-THβC 1a to 1-phenyl-β-carboline 2aa.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Catalystb | Basec | Solvent | T (°C) | t (h) | Yieldd (%) |
| 1 | None | DBUe | DMSOf | 25 | 18 | 0 |
| 2 | CuBr2 | DBU | DMSO | 25 | 18 | 95 |
| 3 | CuCl2 | DBU | DMSO | 25 | 18 | 90 |
| 4 | Cu(OAc)2 | DBU | DMSO | 25 | 18 | 86 |
| 5 | CuBr | DBU | DMSO | 25 | 18 | 85 |
| 6 | CuCl | DBU | DMSO | 25 | 18 | 84 |
| 7 | CuI | DBU | DMSO | 25 | 18 | 82 |
| 8 | CuSO4 | DBU | DMSO | 25 | 24 | <5 |
| 9 | CuCO3 | DBU | DMSO | 25 | 24 | 11 |
| 10 | CuO | DBU | DMSO | 25 | 24 | <5 |
| 11 | Cu | DBU | DMSO | 25 | 24 | <5 |
| 12 | CuBr2 | DBNg | DMSO | 25 | 20 | 88 |
| 13 | CuBr2 | DMAPh | DMSO | 25 | 20 | 19 |
| 14 | CuBr2 | Py | DMSO | 25 | 20 | 12 |
| 15 | CuBr2 | Et3N | DMSO | 25 | 20 | 10 |
| 16 | CuBr2 | None | DMSO | 25 | 18 | 0 |
| 17 | CuBr2 | DBU (0.5)i | DMSO | 25 | 60 | 75 |
| 18 | CuBr2 | DBU (1.0)j | DMSO | 25 | 30 | 83 |
| 19 | CuBr2 | DBU (1.5)k | DMSO | 25 | 25 | 92 |
| 20 | CuBr2 | DBU | DMFl | 25 | 24 | 82 |
| 21 | CuBr2 | DBU | CH3CN | 25 | 24 | 65 |
| 22 | CuBr2 | DBU | EtOH | 25 | 24 | 23 |
| 23 | CuBr2 | DBU | THFm | 25 | 24 | 30 |
| 24 | CuBr2 | DBU | CH2Cl2 | 25 | 24 | 27 |
| 25 | CuBr2 | DBU | EtOAc | 25 | 24 | 12 |
| 26 | CuBr2 | DBU | Me2CO | 25 | 24 | 14 |
| 27 | CuBr2 | DBU | DMSO | 45 | 13 | 90 |
| 28 | CuBr2 | DBU | DMSO | 65 | 9 | 84 |
| 29 | CuBr2 | DBU | DMSO | 85 | 6 | 76 |
All reactions were performed at T °C for t hours under an atmosphere of air.
20% (mol%) of the catalyst was used.
2.0 equivalents of the base were used unless otherwise indicated.
Isolated yields.
1,8-Diazabicyclo[5,4,0]undec-7-ene.
Dimethyl sulfoxide.
1,5-Diazabicyclo[4,3,0]non-5-ene.
4-Dimethylaminopyridine.
0.5 equivalent of DBU was used.
1.0 equivalent of DBU was used.
1.5 equivalents of DBU was used.
N,N-Dimethylformamide.
Tetrahydrofuran.
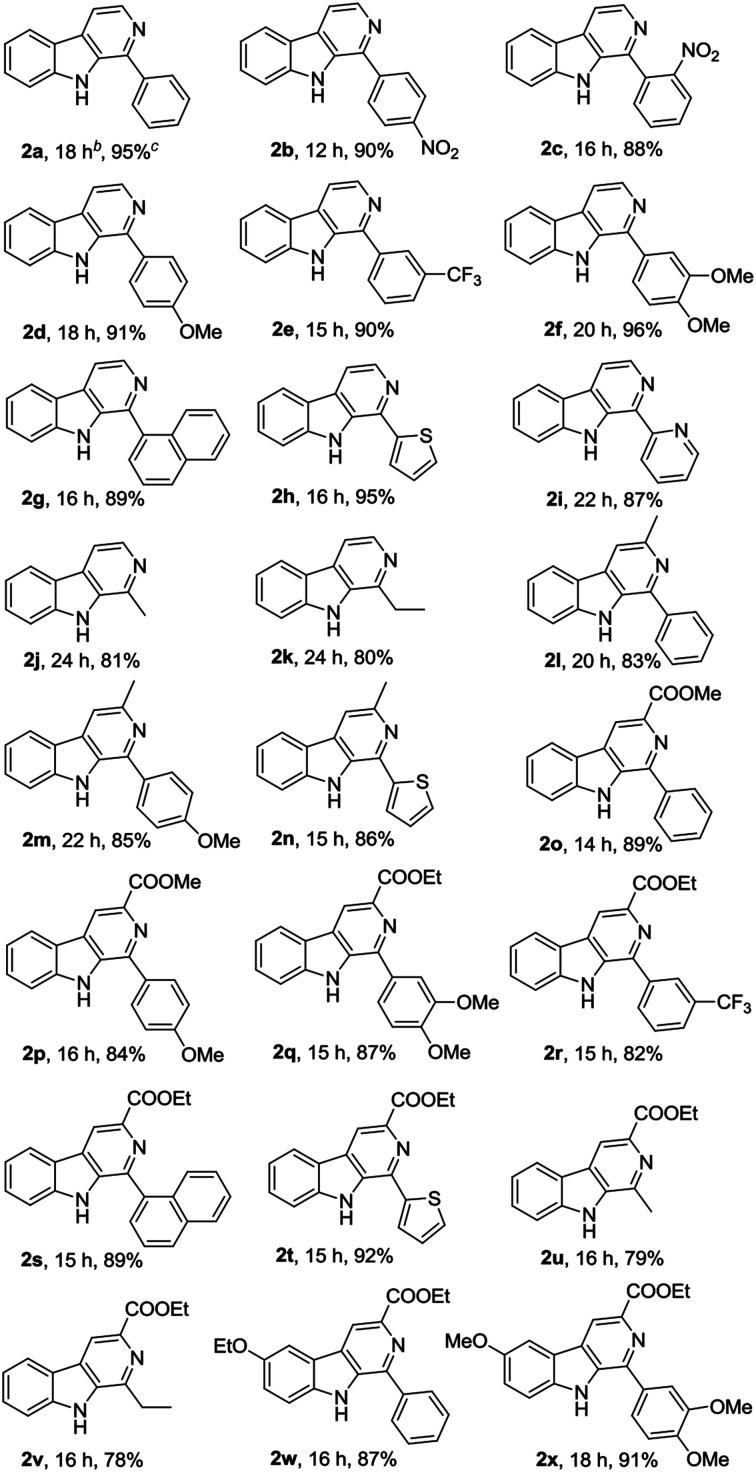
Subsequently, we attempted the CuBr2-catalyzed oxidative conversion of variously substituted THβCs 1 to β-carboline 2 in DMSO with DBU as the base, and the results are summarized in Table 2. The scope of the reaction is very wide; almost all of the tested THβCs could be smoothly converted to β-carbolines in good to excellent yields. All the conversions were performed under the standard reaction conditions (see footnote of the Table 2), and a total of twenty-four variously substituted β-carbolines 2a–2x were obtained in 78–96% yields.
CuBr2-catalyzed oxidation of variously substituted THβCs 1 β-carbolines 2 using air as the clean oxidanta.
|
|---|
|
Reaction conditions: THβCs 1 (2 mmol), CuBr2 (0.4 mmol), DBU (4 mmol), DMSO (6 mL), stirring at room temperature (25 °C) under an atmosphere of air.
Reaction time.
Isolated yields.
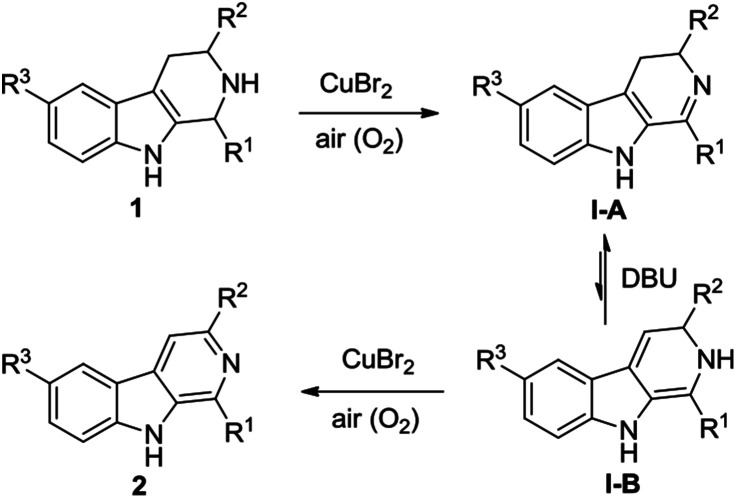
A possible mechanism for the CuBr2-catalyzed oxidative conversion of THβCs 1 to β-carbolines 2 is proposed in Scheme 1. As can be seen from Scheme 1, THβCs 1 would first undergo CuBr2-catalyzed aerobic oxidation of C–N single bond to C N double bond to produce 3,4-dihydro-β-carbolines I-A according to Adimurthy's reports.19 3,4-Dihydro-β-carbolines I-A would then undergo DBU-promoted reversible tautomerization20 to form unstable enamine intermediates I-B, which would also undergo the CuBr2-catalyzed aerobic oxidation of C–N single bond to afford β-carbolines 2. Actually, dihydro-β-carbolines I-A could be isolated if the reaction was stopped in the midway. For example, when the CuBr2-catalyzed oxidative conversion of 1-phenyl-THβC 1a to 1-phenyl-β-carboline 2a was stopped at 8 h, 1-phenyl-3,4-dihydro-β-carboline was obtained in 36% yield.
Scheme 1. Possible mechanism for the CuBr2-catalyzed oxidative conversion of THβCs 1 to β-carbolines 2.
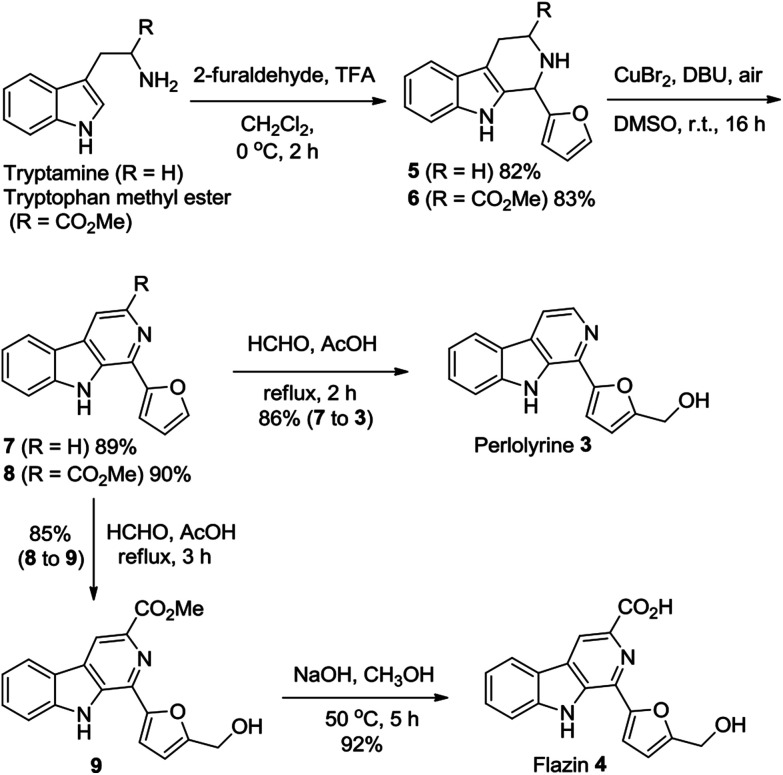
To showcase the synthetic utility of the above-described method for the CuBr2-catalyzed oxidative conversion of THβCs 1 to β-carbolines 2, we have successively applied the methodology to the efficient and practical total syntheses of two β-carboline alkaloids perlolyrine 3 and flazin 4.
Perlolyrine 3 and flazin 4 are both strongly fluorescent 1-furanyl-β-carboline alkaloids. These two particular alkaloids are widespread in nature, and have been isolated from plants, bacteria, Japanese sake and soy sauce.1h,3b,21 Several total syntheses of perlolyrine 3 and flazin 4 have been reported hitherto,22 we herein report novel practical total syntheses of them, which are depicted in Scheme 2. Pictet–Spengler reaction of tryptamine and tryptophan methyl ester with 2-furaldehyde first gave THβCs 5 and 6 in 82% and 83% yields, respectively. CuBr2-catalyzed oxidation of compounds 5 and 6 with air then furnished β-carbolines 7 and 8 in 89% and 90% yields, respectively. Next, hydroxymethylation of compounds 7 and 8 with HCHO in AcOH produced perlolyrine 3 and compound 9 in 86% and 85% yields, respectively. Hydrolysis of the ester 9 afforded flazin 4 in 92% yield. Thus, perlolyrine 3 was synthesized from tryptamine in 3 steps in 63% overall yield; and flazin 4 was synthesized from tryptophan methyl ester in 4 steps in 58% overall yield.
Scheme 2. Novel concise total syntheses of β-carboline alkaloids perlolyrine 3 and flazin 4.
Conclusions
In conclusion, we have developed a novel method for the CuBr2-catalyzed oxidative conversion of THβCs to β-carbolines. This method has some advantages as follows: (a) the process was carried out at room temperature with air as the clean oxidant, so it is very mild and environmentally benign; (b) CuBr2 as the catalyst is inexpensive and low-toxic; (c) all products were obtained in good to excellent yields; (d) the reaction has very good tolerance of functional groups, it seemed to be applicable to all the tested substrates; (e) experiment operation is quite easy. In addition, novel practical total syntheses of β-carboline alkaloids perlolyrine and flazin were performed with the above-described CuBr2-catalyzed mild oxidation of THβCs as the key step.
Experimental
General
1H NMR and 13C NMR spectra were acquired on a Bruker AM 400 instrument, chemical shifts are given on the δ scale as parts per million (ppm) with tetramethylsilane (TMS) as the internal standard. IR spectra were recorded on a Nicolet Magna IR-550 instrument. Mass spectra were performed with a HP1100 LC-MS spectrometer. Melting points were measured on a Mei-TEMP II melting point apparatus. Column chromatography was performed on silica gel (Qingdao Chemical Factory). All reagents and solvents were analytically pure, and were used as such as received from the chemical suppliers.
General procedure for the preparation of various THβCs 1
1-Substituted tryptamine (3 mmol) was dissolved in CH2Cl2 (10 mL), an aldehyde (3.3 mmol) was added. The resulting solution was cooled to 0 °C, TFA (0.686 mg, 6.016 mmol) was added. The mixture was then stirred at 0–5 °C for 2–6 h. After the reaction was complete (checked by TLC, eluent: EtOAc/CH2Cl2 = 1 : 4), solvent was removed under vacuum, the residue was then partitioned between EtOAc (30 mL) and an aqueous solution of K2CO3 (10% w/w, 20 mL). Two phases were separated, and the aqueous phase was extracted again with EtOAc (20 mL). Organic extracts were combined, and dried over anhydrous MgSO4. Evaporation of solvent under vacuum gave crude product, which was purified by flash chromatography (eluent: MeOH/CH2Cl2 = 1 : 20–1 : 10) to afford THβCs 1a–1x in 70–95% yields.
General procedure for the CuBr2-catalyzed oxidative conversion of THβCs 1 to β-carbolines 2
THβCs 1 (2 mmol) was dissolved in DMSO (5 mL). DBU (609 mg, 4.000 mmol) and anhydrous CuBr2 (89.5 mg, 0.401 mmol) were added. The resulting solution was then stirred under air at room temperature (around 25 °C) for 12–24 h (see Table 2). After the reaction was complete (checked by TLC, EtOAc/CH2Cl2 = 1 : 1 to 1 : 4), the reaction solution was poured into the mixed solution of EtOAc (30 mL) and aqueous ammonia (5% w/w, 25 mL). After the mixture was stirred for 5 minutes, two phases were separated. The aqueous layer was extracted again with EtOAc (20 mL). The organic extracts were combined and washed with brine (10 mL). The organic solution was dried over anhydrous MgSO4, and then was concentrated under vacuum to give the crude product, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1 : 2 to 1 : 15) to afford pure β-carbolines 2 in 78–96% yield as indicated in the Table 2. Characterization data of the β-carbolines 2a–2x are given in the ESI† of this paper.
Total syntheses of perlolyrine 3 and flazin 4
1-(Furan-2-yl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole 5
A mixture of tryptamine (1.604 g, 10.01 mmol), 2-furaldehyde (1.076 g, 11.20 mmol) and CH2Cl2 (15 mL) was stirred and cooled to 0 °C by an ice-bath. TFA (2.285 g, 20.04 mmol) was slowly added, and then the reaction mixture was further stirred at 0 °C for 2 h. After the reaction was complete (checked by TLC, eluent: EtOAc/CH2Cl2 = 1 : 3), ethyl acetate (60 mL) and an aqueous solution of K2CO3 (10% w/w, 30 mL) were added. The mixture was vigorously stirred for 5 min, and then two phases were separated. Organic phase was extracted again with ethyl acetate (20 mL). Organic extracts were combined and dried over anhydrous MgSO4, and then was concentrated under vacuum to give the crude product, which was purified by flash chromatography (eluent: MeOH/CH2Cl2 = 1 : 10) to afford pure compound 5 (1.957 g, 8.213 mmol) in 82% yield as white solid, mp 138–139 °C. 1H NMR (CDCl3, 400 MHz) δ 8.17 (s, 1H, NH in indole), 7.94 (s, 1H), 7.64 (d, J = 7.8 Hz, 1H), 7.48 (d, J = 3.2 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.18 (dd, J1 = 8.0 Hz, J2 = 7.9 Hz, 1H), 7.11 (dd, J1 = 7.8 Hz, J2 = 7.9 Hz, 1H), 6.96 (brs, 1H, NH), 6.66 (d, J = 3.7 Hz, 1H), 6.44 (dd, J1 = 3.7 Hz, J2 = 3.2 Hz, 1H), 3.90 (t, J = 7.3 Hz, 2H), 3.17 (t, J = 7.3 Hz, 2H). 13C NMR (CDCl3, 100 MHz) δ 154.51, 142.52, 135.87, 131.84, 127.23, 121.91, 119.37, 118.34, 111.04, 110.35, 109.94, 107.76, 50.73, 41.46, 22.31. HRMS (ESI) m/z calcd for C15H14N2ONa [M + Na]+: 261.1004, found: 261.1008. IR (KBr, film) 3393, 3145, 2922, 2846, 1450, 1298, 1141, 1010, 739 cm−1.
Methyl1-(furan-2-yl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylate 6
Compound 6 was prepared from tryptophan methyl ester and 2-furaldehyde according to the same procedure as above for compound 5. Compound 6 was obtained as an epimeric mixture of cis and trans isomers (cis/trans = 3 : 5) in a combined yield of 83% as offwhite solid. Two epimers can be separated by chromatography, but herein the epimeric mixture was used as such for the next step. Data for cis epimer: white solid, mp 141–142 °C. 1H NMR (CDCl3, 400 MHz) δ 7.98 (s, 1H, NH in indole), 7.50 (d, J = 7.6 Hz, 1H), 7.41 (d, J = 3.5 Hz, 1H), 7.25 (d, J = 7.8 Hz, 1H), 7.15 (dd, J1 = 7.6 Hz, J2 = 7.7 Hz, 1H), 7.11 (dd, J1 = 7.7 Hz, J2 = 7.8 Hz, 1H), 6.32–6.36 (m, 2H), 5.38 (s, 1H), 3.91 (dd, J1 = 9.8 Hz, J2 = 4.5 Hz, 1H), 3.79 (s, 3H), 3.17 (dd, J1 = 15.0 Hz, J2 = 4.5 Hz, 1H), 2.95 (dd, J1 = 15.0 Hz, J2 = 9.8 Hz, 1H), 2.44 (s, 1H, NH). 13C NMR (CDCl3, 100 MHz) δ 173.06, 153.02, 142.85, 136.03, 131.79, 127.01, 122.18, 119.68, 118.28, 111.07, 110.48, 108.61, 107.78, 56.51, 52.33, 51.64, 25.46. HRMS (ESI) m/z calcd for C17H16N2O3Na [M + Na]+: 319.1059, found: 319.1057. IR (KBr, film) 3393, 2950, 1735, 1436, 1351, 1271, 1218, 1174, 1141, 1009, 740 cm−1. Data for trans epimer: white solid, mp 145–146 °C. 1H NMR (CDCl3, 400 MHz) δ 8.30 (s, 1H, NH in indole), 7.49 (d, J = 7.5 Hz, 1H), 7.34 (d, J = 3.4 Hz, 1H), 7.16 (d, J = 7.8 Hz, 1H), 7.11 (dd, J1 = 7.5 Hz, J2 = 7.6 Hz, 1H), 7.06 (dd, J1 = 7.6 Hz, J2 = 7.8 Hz, 1H), 6.23 (dd, J1 = 4.2 Hz, J2 = 3.4 Hz, 1H), 5.96 (d, J = 4.2 Hz, 1H), 5.25 (s, 1H), 3.88 (dd, J1 = 9.5 Hz, J2 = 4.7 Hz, 1H), 3.69 (s, 3H), 3.13 (dd, J1 = 15.2 Hz, J2 = 4.7 Hz, 1H), 2.88 (J1 = 15.2 Hz, J2 = 9.5 Hz, 1H), 2.59 (brs, 1H, NH). 13C NMR (CDCl3, 100 MHz) δ 173.82, 154.40, 142.67, 136.26, 130.97, 126.78, 122.14, 119.49, 118.27, 111.20, 110.28, 108.65, 108.14, 52.24, 52.23, 49.10, 25.17. HRMS (ESI) m/z calcd for C17H16N2O3Na [M + Na]+: 319.1059, found: 319.1055. IR (KBr, film) 3395, 2945, 1736, 1435, 1352, 1271, 1218, 1175, 738 cm−1.
1-(Furan-2-yl)-9H-pyrido[3,4-b]indole 7
Compound 5 (0.962 g, 4.037 mmol) was dissolved in DMSO (8 mL), DBU (1.225 g, 8.047 mmol) and anhydrous CuBr2 (0.179 g, 0.801 mmol) were added. The resulting solution was then stirred under air at 25 °C for 16 h. After the reaction was complete (checked by TLC, eluent: EtOAc/CH2Cl2 = 1 : 1), the reaction solution was poured into the mixed solution of EtOAc (50 mL) and aqueous ammonia (5% w/w, 20 mL). After the mixture was stirred for 5 minutes, two phases were separated. The aqueous layer was extracted again with EtOAc (20 mL). The organic extracts were combined and washed with brine (10 mL). The organic solution was dried over anhydrous MgSO4, and then was concentrated under vacuum to give the crude product, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1 : 3) to afford pure compound 7 (0.842 g, 3.594 mmol) in 89% yield as white solid, mp 166–167 °C. 1H NMR (DMSO-d6, 400 MHz) δ 11.50 (s, 1H, NH in indole), 8.38 (d, J = 5.2 Hz, 1H), 8.25 (d, J = 7.8 Hz, 1H), 8.08 (d, J = 5.2 Hz, 1H), 7.99 (d, J = 1.6 Hz, 1H), 7.77 (d, J = 8.2 Hz, 1H), 7.58 (dd, J1 = 7.6 Hz, J2 = 7.8 Hz, 1H), 7.23–7.32 (m, 2H), 6.79 (dd, J1 = 3.5 Hz, J2 = 3.2 Hz, 1H). 13C NMR (CDCl3, 100 MHz) δ 155.09, 143.34, 141.09, 139.47, 134.09, 131.97, 130.83, 129.17, 122.25, 121.87, 120.72, 114.26, 112.92, 112.25, 109.32. HRMS (ESI) m/z calcd for C15H11N2O [M + H]+: 235.0871, found: 235.0872. IR (KBr, film) 3458, 2998, 2920, 1624, 1557, 1493, 1424, 1377, 1235, 997, 754, 459 cm−1.
Methyl1-(furan-2-yl)-9H-pyrido[3,4-b]indole-3-carboxylate 8
Compound 8 was prepared from compound 6 according to the same procedure as above for compound 7. Compound 8 was obtained in 90% yield as white solid, mp 168–169 °C. 1H NMR (DMSO-d6, 400 MHz) δ 11.93 (s, 1H, NH in indole), 8.90 (s, 1H), 8.44 (d, J = 8.0 Hz, 1H), 8.06 (d, J = 3.3 Hz, 1H), 7.84 (d, J = 8.2 Hz, 1H), 7.65 (dd, J1 = 8.0 Hz, J2 = 8.1 Hz, 1H), 7.32–7.40 (m, 2H), 6.85 (dd, J1 = 3.3 Hz, J2 = 3.7 Hz, 1H), 3.96 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ 165.76, 152.11, 144.23, 141.48, 136.37, 132.76, 132.11, 129.51, 128.79, 121.90, 120.82, 120.48, 116.41, 112.97, 112.34, 109.92, 52.07. HRMS (ESI) m/z calcd for C17H13N2O3 [M + H]+: 293.0926, found: 293.0922. IR (KBr, film) 3440, 3060, 2951, 1702, 1624, 1493, 1430, 1368, 1346, 1260, 738, 494 cm−1.
1-[5-(Hydroxymethyl)furan-2-yl]-9H-pyrido[3,4-b]indole (perlolyrine 3)
Compound 7 (0.470 g, 2.006 mmol) was dissolved in acetic acid (3 mL). An aqueous solution of formaldehyde (37% w/w, 1 mL) was added. The resulting solution was then heated and stirred at reflux for 2 h. After the reaction was complete (checked by TLC, eluent: EtOAc/CH2Cl2 = 1 : 3), the solvent was removed by vacuum distillation to give a viscous residue, which was partitioned between ethyl acetate (30 mL) and an aqueous solution of potassium carbonate (10% w/v, 20 mL). Two phases were separated, and aqueous phase was extracted again with ethyl acetate (20 mL). The organic extracts were combined and washed twice with brine (2 × 10 mL). After having been dried with anhydrous MgSO4, the organic solution was concentrated under vacuum to give crude solid, which was purified by flash chromatography (eluent: MeOH/CH2Cl2 = 1 : 10) to give pure perlolyrine 3 (0.457 g, 1.729 mmol) in 86% yield as pale yellow solid, mp 165–166 °C. 1H NMR (DMSO-d6, 400 MHz) δ 11.24 (s, 1H, NH in indole), 8.38 (d, J = 5.1 Hz, 1H), 8.27 (d, J = 7.8 Hz, 1H), 8.08 (d, J = 5.1 Hz, 1H), 7.78 (d, J = 8.2 Hz, 1H), 7.61 (dd, J1 = 7.8 Hz, J2 = 8.0 Hz, 1H), 7.29 (dd, J1 = 8.0 Hz, J2 = 8.2 Hz, 1H), 7.23 (d, J = 3.3 Hz, 1H), 6.60 (d, J = 3.3 Hz, 1H), 5.50 (t, J = 6.0 Hz, 1H, OH), 4.68 (d, J = 6.0 Hz, 2H). 13C NMR (DMSO-d6, 100 MHz) δ 156.73, 152.14, 140.92, 138.16, 133.14, 130.47, 129.42, 128.39, 121.59, 120.61, 119.68, 113.60, 112.40, 109.62, 109.04, 55.95. HRMS (ESI) calcd for C16H13N2O2 [M + H]+: 265.0977, found: 265.0974. IR (KBr, film) 3370, 2920, 1629, 1568, 1426, 1317, 1234, 1014, 799, 743, 634 cm−1.
Methyl1-(5-(hydroxymethyl)furan-2-yl)-9H-pyrido[3,4-b]indole-3-carboxylate 9
Compound 9 was prepared from compound 8 according to the same procedure as above for perlolyrine 3. Compound 9 was obtained in 85% yield as white solid, mp 205–206 °C. 1H NMR (DMSO-d6, 400 MHz) δ 11.63 (s, 1H, NH in indole), 8.87 (s, 1H), 8.44 (d, J = 7.9 Hz, 1H), 7.82 (d, J = 8.2 Hz, 1H), 7.66 (dd, J1 = 7.9 Hz, J2 = 8.0 Hz, 1H), 7.35 (dd, J1 = 8.0 Hz, J2 = 8.2 Hz, 1H), 7.29 (d, J = 3.4 Hz, 1H), 6.63 (d, J = 3.4 Hz, 1H), 5.50 (t, J = 6.1 Hz, 1H, OH), 4.68 (d, J = 6.1 Hz, 2H), 3.94 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ 165.92, 157.42, 151.21, 141.45, 136.65, 132.99, 132.14, 129.65, 129.01, 122.11, 121.08, 120.71, 116.43, 112.94, 110.99, 109.36, 56.07, 52.23. HRMS (ESI) m/z calcd for C18H15N2O4 [M + H]+: 323.1032, found: 323.1033. IR (KBr, film) 3406, 3297, 2922, 1727, 1565, 1435, 1351, 1251, 1118, 1005, 746 cm−1.
1-(5-(Hydroxymethyl)furan-2-yl)-9H-pyrido[3,4-b]indole-3-carboxylic acid (flazin 4)
Compound 9 (0.323 g, 1.002 mmol) was dissolved in methanol (5 mL), and then an aqueous solution of NaOH (3 mol L−1, 1 mL) was added. The mixture was then heated and stirred at 50 °C for 5 h. After the reaction was complete (checked by TLC, eluent: EtOAc/CH2Cl2 = 1 : 1), ethyl acetate (25 mL) and water (20 mL) were added. The mixture was vigorously stirred, and pH value of the aqueous solution was adjusted to 4–5 by adding citric acid. Two phases were separated, and the aqueous solution was extracted twice with ethyl acetate (2 × 25 mL). Organic extracts were combined and washed twice with brine (2 × 10 mL). After having been dried over anhydrous MgSO4, the organic solution was concentrated under vacuum to give a crude solid product, which was purified by flash chromatography (eluent: MeOH/CH2Cl2 = 1 : 5) to afford pure flazin 4 (0.284 g, 0.921 mmol) in 92% yield as pale yellow solid, mp 239–240 °C. 1H NMR (DMSO-d6, 400 MHz) δ 11.69 (s, 1H, NH in indole), 8.84 (s, 1H), 8.42 (d, J = 7.8 Hz, 1H), 7.87 (d, J = 8.2 Hz, 1H), 7.65 (dd, J1 = 7.8 Hz, J2 = 8.0 Hz, 1H), 7.42 (d, J = 3.2 Hz, 1H), 7.34 (dd, J1 = 8.0 Hz, J2 = 8.2 Hz, 1H), 6.62 (d, J = 3.2 Hz, 1H), 4.69 (s, 2H). 13C NMR (DMSO-d6, 100 MHz) δ 166.64, 157.45, 151.40, 141.53, 137.13, 132.63, 132.06, 129.98, 129.05, 122.17, 121.11, 120.69, 115.85, 112.94, 111.22, 109.37, 56.11. HRMS (ESI) m/z calcd for C17H12N2O4Na [M + Na]+: 331.0695, found: 331.0697. IR (KBr, film) 3242, 2922, 1735, 1624, 1612, 1576, 1360, 1321, 1018, 742 cm−1.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We are grateful to the National Natural Science Foundation of China (No. 20972048) for the financial support of this work.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13434g
Notes and references
- (a) Jiao W.-H. Chen G.-D. Gao H. Li J. Gu B.-B. Xu T.-T. Yu H.-B. Shi G.-H. Yang F. Yao X.-S. Lin H.-W. J. Nat. Prod. 2015;78:125. doi: 10.1021/np500801s. [DOI] [PubMed] [Google Scholar]; (b) Ohishi K. Toume K. Arai M. A. Koyano T. Kowithayakorn T. Mizoguchi T. Itoh M. Ishibashi M. J. Nat. Prod. 2015;78:1139. doi: 10.1021/acs.jnatprod.5b00153. [DOI] [PubMed] [Google Scholar]; (c) Furusato A. Kato H. Nehira T. Eguchi K. Kawabata T. Fujiwara Y. Losung F. Mangindaan R. E. P. Voogd N. J. d. Takeya M. Yokosawa H. Tsukamoto S. Org. Lett. 2014;16:3888. doi: 10.1021/ol5015569. [DOI] [PubMed] [Google Scholar]; (d) Kitajima M. Ohara S. Kogure N. Santiarworn D. Takayama H. Tetrahedron. 2013;69:9451. doi: 10.1016/j.tet.2013.08.070. [DOI] [Google Scholar]; (e) Huang H. Yao Y. He Z. Yang T. Ma J. Tian X. Li Y. Huang C. Chen X. Li W. Zhang S. Zhang C. Ju J. J. Nat. Prod. 2011;74:2122. doi: 10.1021/np200399t. [DOI] [PubMed] [Google Scholar]; (f) Inman W. D. Bray W. M. Gassner N. C. Lokey R. S. Tenny K. Shen Y. Y. TenDyke K. Suh T. Grews P. J. Nat. Prod. 2010;73:255. doi: 10.1021/np9005426. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Jiao W.-H. Gao H. Li C.-Y. Zhao F. Jiang R.-W. Wang Y. Zhou G.-X. Yao X.-S. J. Nat. Prod. 2010;73:167. doi: 10.1021/np900538r. [DOI] [PubMed] [Google Scholar]; (h) Fotso S. Maskey R. P. Schroder D. Ferrer A. S. Grun-Wollny I. Laatsch H. J. Nat. Prod. 2008;71:1630. doi: 10.1021/np800248s. [DOI] [PubMed] [Google Scholar]; (i) Cao R. Peng W. Wang Z. Xu A. Curr. Med. Chem. 2007;14:479. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- (a) Kamal A. Sathish M. Nayak V. L. Srinivasulu V. Kavitha B. Tangella Y. Thummuri D. Bagul C. Shankaraiah N. Nagesh N. Bioorg. Med. Chem. 2015;23:5511. doi: 10.1016/j.bmc.2015.07.037. [DOI] [PubMed] [Google Scholar]; (b) Savariz F. C. Foglio M. A. Ruiz A. L. T. G. Costa W. F. d. Silva M. d. M. Santos J. C. C. Figueiredo I. M. Meyer E. Carvalho J. E. d. Sarragiotto M. H. Bioorg. Med. Chem. 2014;22:6867. doi: 10.1016/j.bmc.2014.10.031. [DOI] [PubMed] [Google Scholar]; (c) Shi B. Cao R. Fan W. Guo L. Ma Q. Chen X. Zhang G. Qiu L. Song H. Eur. J. Med. Chem. 2013;60:10. doi: 10.1016/j.ejmech.2012.11.033. [DOI] [PubMed] [Google Scholar]
- (a) Song H. Liu Y. Liu Y. Wang L. Wang Q. J. Agric. Food Chem. 2014;62:1010. doi: 10.1021/jf404840x. [DOI] [PubMed] [Google Scholar]; (b) Li Y. Zhao M. Parkin K. L. J. Agric. Food Chem. 2011;59:2332. doi: 10.1021/jf104653n. [DOI] [PubMed] [Google Scholar]; (c) Herraiz T. J. Agric. Food Chem. 2007;55:8534. doi: 10.1021/jf0719151. [DOI] [PubMed] [Google Scholar]
- (a) Im Y. Lee L. Y. Chem. Commun. 2013;49:5948. doi: 10.1039/C3CC42131G. [DOI] [PubMed] [Google Scholar]; (b) Paul B. K. Ghosh N. Mukherjee S. RSC Adv. 2016;6:9984. doi: 10.1039/C5RA27050B. [DOI] [Google Scholar]
- (a) Nissen F. Richard V. Alayrac C. Witulski B. Chem. Commun. 2011;47:6656. doi: 10.1039/C1CC11298H. [DOI] [PubMed] [Google Scholar]; (b) Ding S. Shi Z. Jiao N. Org. Lett. 2010;12:1540. doi: 10.1021/ol100272s. [DOI] [PubMed] [Google Scholar]; (c) Verniest G. England D. Kimpe N. D. Padawa A. Tetrahedron. 2010;66:1496. doi: 10.1016/j.tet.2009.10.033. [DOI] [Google Scholar]; (d) Zhang H. Larock R. C. J. Org. Chem. 2002;67:7048. doi: 10.1021/jo0258857. [DOI] [PubMed] [Google Scholar]; (e) Kanekiyo N. Kuwada T. Choshi T. Nobuhiro J. Hibino S. J. Org. Chem. 2001;66:8793. doi: 10.1021/jo0105585. [DOI] [PubMed] [Google Scholar]
- (a) Cox E. D. Cook J. M. Chem. Rev. 1995;95:1797. doi: 10.1021/cr00038a004. [DOI] [Google Scholar]; (b) Stockigt J. Antonchick A. P. Wu F. Waldmann H. Angew. Chem., Int. Ed. 2011;50:8538. doi: 10.1002/anie.201008071. [DOI] [PubMed] [Google Scholar]
- (a) Kusurkar R. S. Alkobati N. A. H. Gokule A. S. Puranik V. G. Tetrahedron. 2008;64:1654. doi: 10.1016/j.tet.2007.12.008. [DOI] [Google Scholar]; (b) Kulkarni A. Abid M. Torok B. Huang X. Tetrahedron Lett. 2009;50:1791. doi: 10.1016/j.tetlet.2009.01.143. [DOI] [Google Scholar]; (c) Eagon S. Anderson M. O. Eur. J. Org. Chem. 2014:1653. doi: 10.1002/ejoc.201301580. [DOI] [Google Scholar]
- Ge D. Hu L. Wang J. Li X. Qi F. Lu J. Cao X. Gu H. ChemCatChem. 2013;5:2183. doi: 10.1002/cctc.201300136. [DOI] [Google Scholar]
- Muthaiah S. Hong S. H. Adv. Synth. Catal. 2012;354:3045. doi: 10.1002/adsc.201200532. [DOI] [Google Scholar]
- Wu J. Talwar D. Johnston S. Yan M. Xiao J. Angew. Chem., Int. Ed. 2013;52:6983. doi: 10.1002/anie.201300292. [DOI] [PubMed] [Google Scholar]
- (a) Zheng M. Yang Y. Zhao M. Zhang X. Wu J. Chen G. Peng L. Wang Y. Peng S. Eur. J. Med. Chem. 2011;46:1672. doi: 10.1016/j.ejmech.2011.02.017. [DOI] [PubMed] [Google Scholar]; (b) Ashok P. Chander S. Balzarini J. Pannecouque C. Murugesan S. Bioorg. Med. Chem. Lett. 2015;25:1232. doi: 10.1016/j.bmcl.2015.01.058. [DOI] [PubMed] [Google Scholar]
- Yin W. Sarma P. V. V. S. Ma J. Han D. Chen J. L. Cook J. M. Tetrahedron Lett. 2005;46:6363. doi: 10.1016/j.tetlet.2005.07.022. [DOI] [Google Scholar]
- Cain M. Campos O. Guzman F. Cook J. M. J. Am. Chem. Soc. 1983;105:907. doi: 10.1021/ja00342a045. [DOI] [Google Scholar]
- Kondo K. Shigemori H. Kikuchi Y. Ishibashi M. Sasaki T. Kobayashi J. J. Org. Chem. 1992;57:2480. doi: 10.1021/jo00034a052. [DOI] [Google Scholar]
- Bai B. Shen L. Ren J. Zhu H. J. Adv. Synth. Catal. 2012;354:354. doi: 10.1002/adsc.201100592. [DOI] [Google Scholar]
- Panarese J. D. Waters S. P. Org. Lett. 2010;12:4086. doi: 10.1021/ol101688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Shi Z. Zhang C. Tang C. Jiao N. Chem. Soc. Rev. 2012;41:3381. doi: 10.1039/C2CS15224J. [DOI] [PubMed] [Google Scholar]; (b) Allen S. E. Walvoord R. R. Padilla-Salinas R. Kozlowski M. C. Chem. Rev. 2013;113:6234. doi: 10.1021/cr300527g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) McCann S. D. Stahl S. S. Acc. Chem. Res. 2015;48:1756. doi: 10.1021/acs.accounts.5b00060. [DOI] [PubMed] [Google Scholar]; (d) Wendlandt A. E. Suess A. M. Stahl S. S. Angew. Chem., Int. Ed. 2011;50:11062. doi: 10.1002/anie.201103945. [DOI] [PubMed] [Google Scholar]; (e) Shaabani A. Shaabani S. Afaridoun H. RSC Adv. 2016;6:48396. doi: 10.1039/C6RA04519G. [DOI] [Google Scholar]; (f) Li C. An S. Zhu Y. Zhang J. Kang Y. Liu P. Wang Y. Li J. RSC Adv. 2014;4:49888. doi: 10.1039/C4RA09240F. [DOI] [Google Scholar]; (g) Tian H. Qiao H. Zhu C. Fu H. RSC Adv. 2014;4:2694. doi: 10.1039/C3RA44975K. [DOI] [Google Scholar]; (h) Fernandes A. E. Riant O. Jonas A. M. Jensen K. F. RSC Adv. 2016;6:36602. doi: 10.1039/C6RA05026C. [DOI] [Google Scholar]; (i) Zhang C. Zhao P. Zhang Z. Zhang J. Yang P. Gao P. Gao J. Liu D. RSC Adv. 2017;7:47366. doi: 10.1039/C7RA09516C. [DOI] [Google Scholar]
- (a) Zhang C. Tang C. Jiao N. Chem. Soc. Rev. 2012;41:3464. doi: 10.1039/C2CS15323H. [DOI] [PubMed] [Google Scholar]; (b) Sambiagio C. Marsden S. P. Blacker A. J. McGowan P. C. Chem. Soc. Rev. 2014;43:3525. doi: 10.1039/C3CS60289C. [DOI] [PubMed] [Google Scholar]; (c) Guo X.-X. Gu D.-W. Wu Z. Zhang W. Chem. Rev. 2015;115:1622. doi: 10.1021/cr500410y. [DOI] [PubMed] [Google Scholar]
- (a) Patil R. D. Adimurthy S. Adv. Synth. Catal. 2011;353:1695. doi: 10.1002/adsc.201100100. [DOI] [Google Scholar]; (b) Patil R. D. Adimurthy S. RSC Adv. 2012;2:5119. doi: 10.1039/C2RA20339A. [DOI] [Google Scholar]
- (a) Clark R. A. Parker D. C. J. Am. Chem. Soc. 1971;93:7257. doi: 10.1021/ja00755a022. [DOI] [Google Scholar]; (b) Eisch J. J. Gadek F. J. J. Org. Chem. 1971;36:2065. doi: 10.1021/jo00814a008. [DOI] [Google Scholar]
- (a) Liu T. Liang W. Tu G. Planta Med. 1988;54:472. doi: 10.1055/s-2006-962513. [DOI] [PubMed] [Google Scholar]; (b) Su B.-N. Chang L. C. Park E. J. Guendet M. Santarsiero B. D. Mesecar A. D. Mehta R. G. Fong H. H. S. Pezzuto J. M. Kinghorn A. D. Planta Med. 2002;68:730. doi: 10.1055/s-2002-33798. [DOI] [PubMed] [Google Scholar]; (c) Jeffreys J. A. D. J. Chem. Soc. C. 1970:1091. doi: 10.1039/J39700001091. [DOI] [Google Scholar]; (d) Nakatsuka S.-i. Feng B.-n. Goto T. Kihara K. Tetrahedron Lett. 1986;27:3399. doi: 10.1016/S0040-4039(00)84806-6. [DOI] [Google Scholar]; (e) Han B. H. Park M. H. Han Y. N. Woo L. K. Arch. Pharmacal Res. 1986;9:21. doi: 10.1007/BF02857702. [DOI] [Google Scholar]; (f) Han B. H. Park J. H. Park M. H. Park Y. N. Arch. Pharmacal Res. 1985;8:249. doi: 10.1007/BF02856498. [DOI] [Google Scholar]
- (a) Wu N. Song F. Yan L. Li J. You J. Chem. – Eur. J. 2014;20:3408. doi: 10.1002/chem.201304613. [DOI] [PubMed] [Google Scholar]; (b) Dassonneville B. Witulski B. Detert H. Eur. J. Org. Chem. 2011:2836. doi: 10.1002/ejoc.201100121. [DOI] [Google Scholar]; (c) Gessner W. P. Brossi A. Arch. Pharm. 1988;321:95. doi: 10.1002/ardp.19883210212. [DOI] [PubMed] [Google Scholar]; (d) Bracher F. Hildebrand D. Liebigs Ann. Chem. 1992:1315. doi: 10.1002/jlac.1992199201216. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.