Abstract
Purpose
To evaluate the safety and efficacy of favipiravir, which is prescribed for the treatment of patients with mild-to-moderate coronavirus disease 2019 (COVID-19) in India.
Patients and Methods
This was a prospective, open-label, multicenter, single-arm postmarketing study conducted in India. Patients with mild-to-moderate COVID-19 received favipiravir (3600 mg [1800 mg orally twice daily] on the first day, followed by 800 mg orally twice daily, up to a maximum of 14 days) as a part of their treatment. The primary endpoints were to evaluate the safety of favipiravir by assessing the number of adverse events (AEs) and treatment-related AEs. The secondary endpoints were to evaluate the efficacy of favipiravir by assessing time to clinical cure, rate of clinical cure, time to pyrexia resolution, rate of oxygen requirement, and all-cause mortality.
Results
A total of 1083 patients were enrolled in this study from December 2020 to June 2021. Adverse events were reported in 129 patients (11.9%), 116 (10.7%) of whom had mild AEs. Dose modification or withdrawal of favipiravir treatment was reported in four patients (0.37%). The median time to clinical cure and pyrexia resolution was 7 and 4 days, respectively. A total of 1036 patients (95.8%) exhibited clinical cure by day 14. Oxygen support was required by 15 patients (1.4%). One death was reported, which was unrelated to favipiravir.
Conclusion
In the real-world setting, favipiravir was well-tolerated, and no new safety signals were detected.
Keywords: antiviral, coronavirus, COVID-19, favipiravir, SARS-CoV-2
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a serious threat to public health worldwide.1 As on 6 December 2021, there have been 265,194,191 confirmed cases of COVID-19 and 5,254,116 deaths globally as per the World Health Organization (WHO).2 Multiple therapeutic options, including antiviral agents, are being explored to contain the COVID-19 pandemic. Favipiravir, a ribonucleic acid (RNA)-dependent RNA polymerase inhibitor, exhibits antiviral activity against a wide range of RNA viruses such as SARS-CoV-2.3,4 The WHO has listed favipiravir as a broad-spectrum experimental antiviral drug for the treatment for COVID-19.5
In India, a Phase 3 randomized controlled trial (RCT) that evaluated the effects of favipiravir in patients with mild-to-moderate COVID-19 reported that a majority of the adverse events were mild to moderate, and the most common side effect was an asymptomatic, transient rise in the levels of serum uric acid and liver enzymes. Furthermore, very few instances of gastrointestinal disorders were reported in the participants.6 Favipiravir has been well-tolerated among Indian patients with COVID-19.7,8 Favipiravir was approved in India in June 2020 (under restricted emergency use) for the treatment of mild-to-moderate COVID-19 in adults.9 This postmarketing study was conducted to evaluate the safety and efficacy of favipiravir in a large Indian population in a real-world setting, as mandated by the Indian drug regulator (Central Drugs Standard Control Organisation). To our knowledge, this is the first and largest postmarketing study conducted on favipiravir in India.
Patients and Methods
Study Design
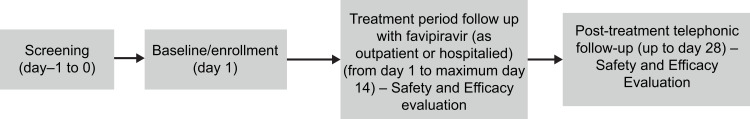
This was a prospective, open-label, multicenter, single-arm, postmarketing study (Clinical Trials Registry, India [CTRI] number: CTRI/2020/11/029263; registered on: 20/11/2020) to assess the safety and efficacy of favipiravir for the management of mild-to-moderate COVID-19 in adult Indian patients. The patients were screened the day preceding (day −1) the day of enrollment (day 1) (Figure 1). The details of the study design are provided in the Supplementary Methods.
Figure 1.
Study design.
Inclusion and Exclusion Criteria
Patients aged ≥18 years with mild-to-moderate COVID-19 and those with a respiratory rate ≤30 breaths/minute, peripheral capillary oxygen saturation (SpO2) ≥90% on room air, pyrexia (defined as axillary temperature ≥99°F or oral temperature ≥100°F), and at least one of the following symptoms—cough, fatigue, dyspnea, expectoration, myalgia, rhinorrhea, sore throat, diarrhea, new loss of taste or smell, myalgia, and headache or fatigue on admission and were prescribed favipiravir were included in the study. Patients on any other antiviral treatment for COVID-19, with hepatic or renal impairment, and requiring intensive care unit management, noninvasive ventilation (NIV), mechanical ventilation (MV), or extracorporeal membrane oxygenation (ECMO) at baseline were excluded from the study.
Outcomes
The primary objective was to evaluate the safety of favipiravir by assessing the endpoints of the number of adverse events (AEs), serious AEs (SAEs), and the number of treatment-emergent AEs (TEAEs) from the day of study enrollment up to 28 days. The secondary objective was to evaluate the efficacy of favipiravir by assessing endpoints of time to clinical cure (up to 14 days; defined as resolution of baseline clinical signs and symptoms of COVID-19 based on clinical assessment by the investigator), rate of clinical cure at day 7 and day 14, time to resolution of pyrexia (up to 14 days), percentage of patients requiring oxygen support (auxiliary oxygen therapy [AOT]/noninvasive ventilation [NIV]/mechanical ventilation [MV]/extracorporeal membrane oxygenation [ECMO]) support at day 7 and day 14, time to hospital discharge for hospitalized patients (up to 14 days), and all-cause mortality rate at day 7, day 14, and day 28. In addition, time to event endpoints were assessed at day 4 and day 10 as a part of the analysis. Outcome evaluation was by a daily clinical assessment for resolution of baseline clinical signs and symptoms of COVID-19 by an investigator for hospitalized patients or a patient’s diary for outpatients. Assessment of the resolution of pyrexia was objective and part of the clinical cure, and was defined as axillary temperature <99°F or oral temperature <100°F for at least 48 hours. Details on the definitions of outcomes and subgroups are provided in the Supplementary Methods.
Statistical Analysis
The safety analysis set comprised of all the patients who had received at least one dose of favipiravir. A sample size of 1000 patients was estimated to be sufficient for a 95% probability to detect at least one AE with an incidence of 3/1000 (binomial distribution). Data analyses were conducted using SAS® software version 9.4. Additional details are provided in the Supplementary Methods.
Ethical Approval
This study was designed and monitored in accordance with sponsor procedures, which comply with the ethical principles of Good Clinical Practice, as required by the applicable regulatory authorities and in accordance with the Declaration of Helsinki. Ethics committee approvals from Institutional Ethics Committee (IEC) were obtained from each participating center before the initiation of the study at their centers. All patients provided signed written informed consent for their participation in the study.
Results
Baseline Characteristics
A total of 1083 patients were screened and enrolled in the present study. All 1083 (100.0%) patients were included in the Safety Analysis Set, whereas 1081 (99.8%) patients were included in the mITT analysis set. The mean±standard deviation (SD) age and weight of patients were 40.59 years (±13.22) and 66.03 kg (±8.93), respectively (Table 1). The majority of the patients were male (n=647 [59.7%]). Hypertension (HTN) (n = 121 [11.2%]) and diabetes mellitus (DM) (n 84 [7.8%]) were the most commonly reported comorbidities. A large proportion of patients (n=1055 [97.4%]) had mild COVID-19. Moderate COVID-19 was reported in 28 patients (2.6%). Fever was present in all patients at baseline; moreover, cough was observed in 876 patients (81%), fatigue was observed in 500 patients (46.2%), and a new loss of taste/smell was observed in 442 patients (41%) patients. Dyspnea was present in 155 patients (14.3%).
Table 1.
Demographics and Baseline Characteristics
| Parameter | Favipiravir |
|---|---|
| N=1083 | |
| Age (years) | |
| Mean (±SD) | 40.58 (±13.21) |
| Median | 38.13 |
| Minimum | 17.52 |
| Maximum | 85.67 |
| Age-group (years), n (%) | |
| <30 | 270 (24.9) |
| ≥30 and <45 | 457 (42.2) |
| ≥45 and <65 | 302 (27.9) |
| ≥65 | 54 (5.0) |
| Weight (kg) | |
| Mean (±SD) | 66.03 (±8.92) |
| Median | 66 |
| Minimum | 33 |
| Maximum | 103 |
| Sex, n (%) | |
| Female | 436 (40.3) |
| Male | 647 (59.7) |
| Clinical symptoms, n (%) | |
| No | 0 |
| Yes | 1083 (100) |
| SpO2 (%), n (%) | |
| <94 | 20 (1.8) |
| ≥94 | 1063 (98.2) |
| Baseline disease severity | |
| Mild | 1055 (97.4) |
| Moderate | 28 (2.6) |
| Comorbidities, n (%) | |
| DM | 84 (7.8) |
| HTN | 121 (11.2) |
| OAD (asthma/COPD) | 6 (0.6) |
| Obesity | 1 (0.1) |
| Others | 0 |
Notes: Percentages were based on the safety analysis set. SpO2 (%): <94 = moderate, ≥94 = mild. Patients with any comorbidities, such as DM, HTN, OAD (asthma/COPD), and obesity at baseline, were counted under the “yes” category.
Abbreviations: COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; OAD, obstructive airway disease; SD, standard deviation; SpO2, saturation of peripheral oxygen.
Treatment-Emergent Adverse Events
All reported AEs were TEAEs in the present study. A total of 129 patients (11.9%) reported at least one TEAE, of which 116 (10.7%) had TEAEs with mild intensity. The most commonly reported TEAEs were gastrointestinal (GI) disorders [n=72 (6.65%), includes nausea (n=22, 2.03%), vomiting (n=20, 1.85%), diarrhea (n=12, 1.11%), upper abdominal pain (n=8, 0.74%), gastroesophageal reflux disease (n=6, 0.55%), hyperchlorhydria (n=5, 0.46%), gastritis (n=3, 0.28%) and mouth ulceration and toothache (n=2, 0.18% for each TEAE)] followed by general disorders [n= 22, 2.03% (includes asthenia: n=19, 1.75% and pyrexia: n=2, 0.18%)] (Table 2). Eleven patients (1.0%) had TEAEs related to favipiravir. The TEAEs related to favipiravir included gastritis (n=3, 0.3%), arthralgia (n=2, 0.2%), myalgia (n=2, 0.2%), vomiting (n=2, 0.2%), hyperchlorhydria (n=1, 0.1%), mouth ulceration (n=1, 0.1%), nausea (n=1, 0.1%), and elevated alanine aminotransferase (n=1, 0.1%). One (0.1%) patient reported a treatment-emergent SAE unrelated to favipiravir. Additional details are provided in the Supplementary Results).
Table 2.
Treatment-Emergent Adverse Events
| SOC/PT | Favipiravir |
|---|---|
| N=1083 | |
| n (%) (E) | |
| Number of patients with at least one event | 129 (11.91) (195) |
| Ear and labyrinth disorders | 1 (0.09) (1) |
| Vertigo | 1 (0.09) (1) |
| Eye disorders | 12 (1.11) (13) |
| Eye irritation | 1 (0.09) (1) |
| Ocular hyperemia | 12 (1.11) (12) |
| Gastrointestinal disorders | 72 (6.65) (90) |
| Abdominal distension | 2 (0.18) (2) |
| Abdominal pain lower | 4 (0.37) (4) |
| Abdominal pain upper | 8 (0.74) (8) |
| Diarrhea | 12 (1.11) (12) |
| Gastritis | 3 (0.28) (3) |
| Gastroesophageal reflux disease | 6 (0.55) (6) |
| Hyperchlorhydria | 5 (0.46) (5) |
| Mouth ulceration | 2 (0.18) (2) |
| Nausea | 22 (2.03) (25) |
| Toothache | 2 (0.18) (2) |
| Vomiting | 20 (1.85) (21) |
| General disorders | 22 (2.03) (22) |
| Asthenia | 19 (1.75) (19) |
| Fatigue | 1 (0.09) (1) |
| Pyrexia | 2 (0.18) (2) |
| Hepatobiliary disorders | 1 (0.09) (1) |
| Hepatomegaly | 1 (0.09) (1) |
| Infections and infestations | 1 (0.09) (1) |
| Nasopharyngitis | 1 (0.09) (1) |
| Investigations | 5 (0.46) (11) |
| Alanine aminotransferase increased | 3 (0.28) (3) |
| Aspartate aminotransferase increased | 2 (0.18) (2) |
| Blood glucose increased | 1 (0.09) (1) |
| Coma scale abnormal | 1 (0.09) (2) |
| Neutrophil count increased | 1 (0.09) (1) |
| Oxygen saturation decreased | 1 (0.09) (1) |
| White blood cell count increased | 1 (0.09) (1) |
| Metabolism and nutrition disorders | 1 (0.09) (1) |
| Decreased appetite | 1 (0.09) (1) |
| Musculoskeletal and connective tissue disorders | 12 (1.11) (19) |
| Arthralgia | 8 (0.74) (8) |
| Muscle spasms | 2 (0.18) (2) |
| Myalgia | 9 (0.83) (9) |
| Nervous system disorders | 19 (1.75) (20) |
| Ageusia | 1 (0.09) (1) |
| Dizziness | 4 (0.37) (4) |
| Headache | 15 (1.39) (15) |
| Psychiatric disorders | 3 (0.28) (3) |
| Anxiety | 3 (0.28) (3) |
| Renal and urinary disorders | 1 (0.09) (1) |
| Dysuria | 1 (0.09) (1) |
| Respiratory, thoracic, and mediastinal disorders | 2 (0.18) (3) |
| Pneumonia aspiration | 1 (0.09) (2) |
| Rales | 1 (0.09) (1) |
| Skin and subcutaneous tissue disorders | 9 (0.83) (9) |
| Rash | 7 (0.65) (7) |
| Skin irritation | 2 (0.18) (2) |
Notes: A patient with multiple AEs was counted only once in the “at least one event” row. At each level of summarization, a patient with multiple AEs within SOC/PT was counted only once. SOCs were presented alphabetically; PTs were sorted within the primary SOC by descending frequency. Percentages were based on the number of patients in the safety analysis set.
Abbreviations: AE, adverse event; E, the total number of events in the safety analysis set; PT, preferred term; SOC, system organ class.
TEAEs Leading to Dose Modification or Discontinuation of Treatment
TEAE that led to dose modification or discontinuation of treatment were reported by four patients (0.37%). As presented in Table 3, two (0.18%) patients developed gastritis, whereas two patients showed elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). A total of three patients discontinued the treatment with favipiravir (two due to gastritis and one due to an increase in AST). The dose of favipiravir was reduced to 400 mg twice a day for 3 days in one patient due to an increase in AST/ALT levels.
Table 3.
Treatment-Emergent Adverse Events Leading to Dose Modification/Discontinuation of Treatment
| Treatment-Emergent Adverse Events | Favipiravir |
|---|---|
| N=1083 | |
| n (%) (E) | |
| Number of patients with at least one TEAE | 4 (0.37) (6) |
| Gastrointestinal disorders | 2 (0.18) (2) |
| Gastritis | 2 (0.18) (2) |
| Investigations | 2 (0.18) (4) |
| Alanine aminotransferase increased | 2 (0.18) (2) |
| Aspartate aminotransferase increased | 2 (0.18) (2) |
Notes: A patient with multiple AEs was counted only once in the “at least one event” row. At each level of summarization, a patient with multiple AEs within SOC/PT was counted only once. SOCs were presented alphabetically; PTs were sorted within the primary SOC by descending frequency. Percentages were based on the number of patients in the safety analysis set. AEs with a start date on or after the date of the first study treatment administration were counted.
Abbreviations: AE, adverse event; E, the total number of events in the safety analysis set; PT, preferred term; SOC, system organ class; TEAE, treatment-emergent adverse event.
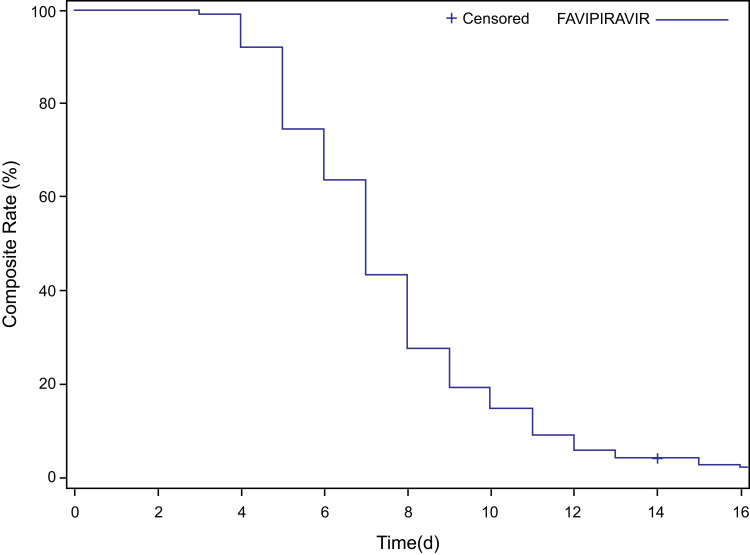
Time to Clinical Cure
Out of 1081 patients in the mITT population, 1048 (96.9%) patients achieved a clinical cure. The median time to clinical cure was 7 days (95% confidence interval [CI]=7.0, 7.0). Figure 2 represents the Kaplan–Meier survival curve for time to clinical cure. Additional details on subgroups are provided in the Supplementary Results.
Figure 2.
Time to clinical cure.
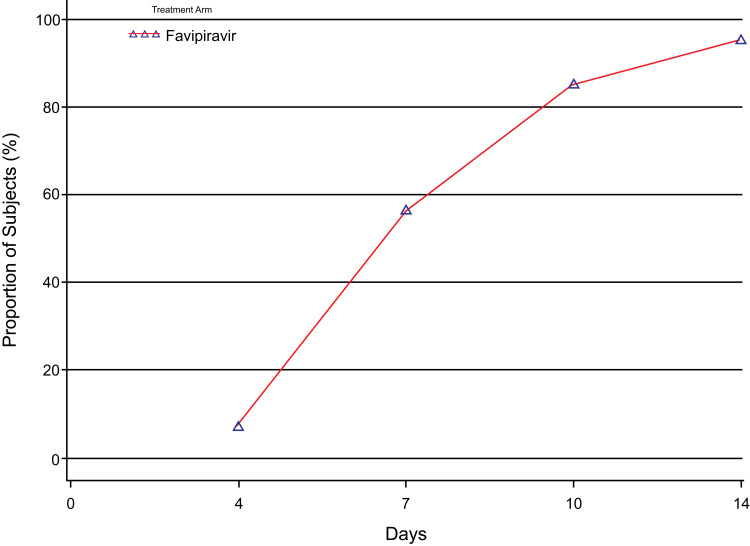
Rate of Clinical Cure
Clinical cure by day 7 was achieved by more than half (56.7%) of the patients of the mITT population (Table S1). By day 10 and day 14, 924 (85.5%) and 1036 (95.8%) patients achieved clinical cure, respectively. The rate of clinical cure on day 4, day 7, day 10, and day 14 in the mITT population is depicted in Figure 3.
Figure 3.
Rate of clinical cure at days 4, 7, 10, and 14.
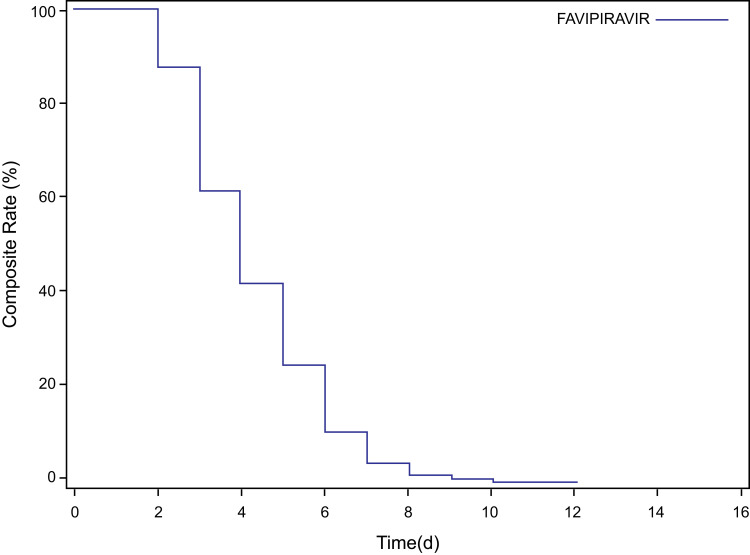
Time to Resolution of Pyrexia
The median time to resolution of pyrexia in the present study was 4 days. The Kaplan–Meier survival curve for time to resolution of pyrexia in the mITT population is presented in Figure 4. Additional details on subgroups are provided in the Supplementary Results.
Figure 4.
Time to resolution of pyrexia.
Requirement of Oxygen Support
Out of 1081 patients in the mITT population, 15 patients (1.4%) required some form of oxygen support (Table 4). Auxiliary oxygen therapy, NIV, and MV were required by 1.0%, 0.6%, and 0.1% of patients, respectively. A sequential form of oxygen therapy as AOT→NIV→MV and AOT→NIV was required by two patients. In the present study, ECMO was not required by any of the patients.
Table 4.
Percentage of Patients Requiring AOT/NIV/MV/ECMO Support by Visit
| Parameter | Visit | Statistics | Favipiravir |
|---|---|---|---|
| N=1081 | |||
| n (%) | |||
| Overall oxygen therapy | Day 4 | n (%) | 7 (0.6) |
| 95% CI | (0.3, 1.3) | ||
| Day 7 | n (%) | 14 (1.3) | |
| 95% CI | (0.7, 2.2) | ||
| Day 10 | n (%) | 15 (1) | |
| 95% CI | (0.8, 2.3) | ||
| Day 14 | n (%) | 15 (1.4) | |
| 95% CI | (0.8, 2.3) | ||
| AOT | Day 4 | n (%) | 4 (0.4) |
| Day 7 | n (%) | 10 (0.9) | |
| Day 10 | n (%) | 11 (1.0) | |
| Day 14 | n (%) | 11 (1.0) | |
| NIV | Day 4 | n (%) | 3 (0.3) |
| Day 7 | n (%) | 5 (0.5) | |
| Day 10 | n (%) | 6 (0.6) | |
| Day 14 | n (%) | 6 (0.6) | |
| MV | Day 4 | n (%) | 0 |
| Day 7 | n (%) | 0 | |
| Day 10 | n (%) | 1 (0.1) | |
| Day 14 | n (%) | 1 (0.1) | |
| ECMO | Day 4 | n (%) | 0 |
| Day 7 | n (%) | 0 | |
| Day 10 | n (%) | 0 | |
| Day 14 | n (%) | 0 |
Notes: Percentages were based on the total number of patients in the mITT population. 95% Confidence interval was calculated using binomial proportion (exact method). Patients requiring more than oxygen support were counted only once under overall oxygen therapy.
Abbreviations: AOT, auxiliary oxygen therapy; ECMO, extracorporeal membrane oxygenation; mITT, modified intent-to-treat; MV, mechanical ventilation; N, number of patients in mITT; NIV, noninvasive ventilation.
Time to Hospital Discharge
Out of 1081 patients in the mITT population, 83 patients (7.7%) required hospitalization. The median time to hospital discharge was 10 days (95% CI=9.0, 11.0). Additional details on subgroups are provided in the Supplementary Results.
All-Cause Mortality Rate at Day 7, 14, and 28
In the present study, no deaths were reported on day 7 and 14. Death was reported in only one patient (0.1%) by day 28 (95% CI=0.0, 0.5), and it was unrelated to favipiravir.
Discussion
Several clinical trials are ongoing for repurposing different antiviral agents such as favipiravir for the treatment of COVID-19.10,11 The present study was a postmarketing study for the evaluation of the safety and efficacy of favipiravir in adult Indian patients with mild-to-moderate COVID-19 in a real-world setting. A majority of the patients (97.4%) in the present study had mild COVID-19, which was contrary to the Japanese observational study where only 45.2% of the patients had mild COVID-19.12 This may be attributed to a variation in the definition of COVID-19 severity across countries. The majority of the patients (59.7%) in the present study were males, which was comparable with the Japanese observational study (67.1%).(12) HTN was the most common comorbidity observed in the present study (11.2%), whereas the Japanese observational study had reported DM to be the most predominant comorbidity (24.4%).(12) Clinical symptoms at baseline were reported by all patients in the present study, as opposed to 70% of patients that had clinical symptoms at baseline in an open-label study conducted in China.13
All AEs were TEAEs in the present study with 11.9% of patients reporting at least one TEAE, which was comparable to the findings from the Chinese RCT (11.4%),13 but was less than that of a Japanese observational study (24.6%)(12), a Thai observational study (61.9%),14 an RCT by Chen et al (31.9%),15 and an Indian phase 3 trial (28.8%).6 Favipiravir has been reported to be well-tolerated in Indian patients with mild COVID-19 in real-world settings, wherein elevated liver transaminases (5%) and diarrhea (1.8%) were the most frequently reported TEAEs.7 A retrospective cohort study in Indian COVID-19 patients with mortality-associated risk factors reported that favipiravir was well, with diarrhea being the most common TEAE.8 In the present study, asthenia, headache, gastritis, diarrhea, nausea, and vomiting were the most commonly reported AEs, which was contrary to other studies on favipiravir, wherein asymptomatic, transient increases in the levels of uric acid and liver enzymes were the common AEs.6,12,15 The low prevalence of asymptomatic, transient elevation in laboratory parameters in the present study might be due to the non-interventional and real-world nature of the study. Another reason could be the fact that as the treating physicians may not have deemed laboratory investigations necessary as the patients had mild COVID-19 (97.4% of patients had mild COVID-19).
A majority of the patients reported AEs with mild intensity, whereas TEAEs leading to dose modification or discontinuation of treatment were reported by a very small proportion of patients (0.37%) in the present study. Our finding corroborates the good tolerability of favipiravir in the population. Overall, there were no new safety signals reported with regard to the safety of favipiravir in the present postmarketing study.16
The median time to clinical cure with favipiravir was 7 days in the present study, which was greater compared to the Indian phase 3 trial (3 days) and a retrospective Indian cohort study (5 days).6,7 In the present study, the median time to clinical cure did not differ with disease severity (ie, mild vs moderate). The Indian phase 3 trial had reported a median time to clinical cure of 3.5 days in patients with moderate COVID-19.6 Possible reasons that could have contributed to a time to clinical cure of 7 days were: a more symptomatic population, patients in the outpatient department or undergoing home management, and outcomes were patients reported, which were contrary to the phase 3 Indian study.6
In a recent RCT of an antibody cocktail therapy involving casirivimab and imdevimab (REGEN-COV) in patients with mild-to-moderate COVID-19, the median time to symptom resolution was 10 days with the cocktail therapy compared to 14 days with placebo.16 In another RCT with ivermectin in patients with mild COVID-19, the time to clinical cure was 10 days (9–13 days) in the ivermectin group and 12 days (9–13 days) in the placebo group.17 In the indirect comparison, favipiravir appears to facilitate rapid clinical improvement. A single center study in Bangladesh reported that favipiravir in combination with remdesivir and plasma was effective in critically ill patients with COVID-19.18
The clinical cure rate with favipiravir at day 7 was 56.7% in the present study, which was similar to that of the Chinese RCT (61.2%),15 and lower compared to that of the Japanese observational study (70.2%),12 Indian phase 3 trial (≈70%),6 and Indian retrospective cohort study (90.5%).7 The clinical cure rate at day 14 in the present study (95.8%), which was similar to that of the Indian retrospective cohort study (94.7%),7 but higher compared to that of the Japanese observational study (86.1%)12 and the Indian phase 3 trial (≈89%).6 Real-world evidence in Indian patients with COVID-19 show that the rate of clinical improvement was >90% by day 10.19 Subjective assessment of symptoms by patients to determine clinical improvement is likely to be the important reason for a slight delay in the rate of clinical cure in the present study compared to that of other studies.
The time to resolution of pyrexia in the present study was 4 days, whereas it was 6 days in a Chinese RCT15 and 2 days in a Russian RCT.20 Fever is one of the cardinal symptoms in patients with COVID-19. Multivariate logistic analysis showed that body temperature (>99.5°F) in patients with mild disease has higher odds (odds ratio = 1.24, p = 0.002) for progression to the severe stage.21 Early control of fever as a surrogate measure for disease progression in this study has supported the effectiveness of favipiravir in COVID-19.
The requirement of supplemental oxygen is one of the surrogate markers for severe illness or disease progression.22 The present study reported that only 1.4% of patients required supplemental oxygen, which was lower than other studies. In the study conducted in Thailand, approximately 12.7% of patients on favipiravir needed ECMO or invasive MV,14 whereas the Chinese RCT reported a requirement of AOT and noninvasive MV in 18.0% of patients in the favipiravir group.15 In the Indian phase 3 study, <10% of patients required supplemental oxygen with a statistically significant delay in the need for first oxygen in the favipiravir group, compared to that of the control group.6 None of the patients in a multicenter, randomized study in Egypt and in the Chinese RCT required oxygen support.23,24
The median time to hospital discharge was 10 days in the present study, which was comparable to that of the Indian phase 3 trial, which reported a median time of 9 days.12 The reason for an extended time to hospital discharge compared to time to clinical cure was variations in discharge policies across different states in India. A multicenter randomized study from Egypt reported a mean (±SD) duration of hospital stay of 13.29 (±5.86) days in patients in the favipiravir group.23 In the present study, no deaths were reported on day 7 and day 14. Death was reported in only one patient by day 28, which was not related to favipiravir. A multicenter observational study in Thailand reported three deaths on day 28, with superimposed infection being the main cause of death.14 A retrospective, single center study in Bangladesh reported that mortality was lower in patients with COVID-19 receiving favipiravir compared with the those receiving remdesivir.25
There were several limitations to this postmarketing study. First, it was an open-label, observational study with no control group. Second, the subjective definition of clinical cure may have contributed to assessment bias. However, the key strength of the present study was its large sample size and the inclusion of a heterogeneous population with comorbidities, which simulates a real-world scenario and evaluates the safety and efficacy of favipiravir.
Conclusion
The present study showed that favipiravir is a safe antiviral drug for the treatment of mild-to-moderate COVID-19. No new safety signals or concerns were observed, other than the known adverse drug reactions. Based on our results and previous clinical studies, favipiravir promises to serve as a therapeutic agent for the management of mild-to-moderate COVID-19.
Acknowledgments
We would like to extend our thanks to Rajesh Gaikwad, Harshani Sawant, Nikhil Khanwilkar, Vishakha Utekar, Sanjay Jankar, Megha Gupta, Komal Sonawane, Prafulla Shetty, Pankaj Khaire, Roshan More, Shubhangi Kalamkar, Urmila Gupta, Sagar Borle, Rujuta Gadkari and Dr Rahul Bhagat for their kind support with the protocol writing, study conduct, study documentation, data management, clinical study report.
Funding Statement
This study was sponsored and funded by Glenmark Pharmaceuticals Limited, India.
Medical Writing, Editorial, and Other Assistance
We would like to thank BioQuest Solutions for their support with editorial assistance.
Data Sharing Statement
Individual participant data that underlie the reported results are not available. Additional data will be provided upon request.
Compliance with Ethics Guidelines
The protocol and informed consent form were approved by institutional/independent ethics committees (Ethics Committee of Bangalore Medical College and Research Institute; IEC NIMS; IEC, Dr Vasantrao Pawar Medical College, Nashik; Institutional Ethics Committee - Care Hospital; Institutional Ethics Committee Human Research Lokmanya Tilak Municipal Medical College and General Hospital; Institutional Ethics Committee Topiwala National College and BYL Nair CH. Hospital; Institutional Ethics Committee, GGMC, Mumbai; Institutional Ethics Committee, Govt Medical College, Nagpur; Institutional Ethics Committee, Neelima Hospitals; Shree Hospital Ethics Committee; Siddhi Hospital Institutional Ethics Committee; Institutional Ethics Committee, Aster Prime Hospital, Hyderabad) and the Drugs Controller General of India. The study was undertaken in accordance with current guidelines of the Central Drugs Standard Control Organization, which is the National Regulatory Authority in India, the International Conference on Harmonization Good Clinical Practice, and the Declaration of Helsinki. All patients provided written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
SP, SB, PD, AP, WW, SR, HB are full time employee of Glenmark Pharmaceuticals Ltd. PKR reports grants from Glenmark, during the conduct of the study. MK reports personal fees from Glenmark limited, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382(8):760–762. doi: 10.1056/NEJMe2001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/. Accessed October 25, 2021.
- 3.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO R&D Blueprint COVID 19 Experimental Treatments. COVID classification of treatment types; 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/covid-classification-of-treatment-types-rev.pdf?sfvrsn=5b90b2f2_1&download=true. Accessed August 16, 2021.
- 6.Udwadia ZF, Singh P, Barkate H, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. doi: 10.1016/j.ijid.2020.11.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panchal S, Venugopal K, Vora A, et al. P5-93: therapeutic effectiveness and tolerability of favipiravir in mild COVID-19: real world experience from India. Respirology. 2021;26(S3):194. doi: 10.1111/resp.14150_300 [DOI] [Google Scholar]
- 8.Panchal S, Venugopal K, Vora A, et al. P5-64: therapeutic effectiveness & safety of favipiravir in COVID-19 patients with risk factors for mortality: clinical practice experience from India. Respirology. 2021;26(S3):182. doi: 10.1111/resp.14150_271 [DOI] [Google Scholar]
- 9.Central Drugs Standard Control Organisation, India. List of new drugs approved in the year 2020. Available from: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadApprovalNewDrugs/newdrugs%20approval%20july2020.pdf. Accessed August 16, 2021.
- 10.Nasir M, Perveen RA, Saha SK, Talha KA, Selina F, Islam MA. Systematic review on repurposing use of favipiravir against SARS-CoV-2. Mymensingh Med J. 2020;29(3):747–754. [PubMed] [Google Scholar]
- 11.Perveen RA, Nasir M, Talha KA, Selina F, Islam MA. Systematic review on current antiviral therapy in COVID-19 pandemic. Med J Malaysia. 2020;75(6):710–716. [PubMed] [Google Scholar]
- 12.Favipiravir Observational Study Group. Preliminary report of the favipiravir observational study in Japan; 2020. Available from: https://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_en_200529.pdf. Accessed August 13, 2021.
- 13.Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rattanaumpawan P, Jirajariyavej S, Lerdlamyong K, Palavutitotai N, Saiyarin J. Real-world experience with favipiravir for treatment of COVID-19 in Thailand: results from a multicenter observational study. medRxiv. 2020;2020:2020.06.24.20133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020. doi: 10.3389/fphar.2021.683296 [DOI] [Google Scholar]
- 16.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasir MPR, Murshed M, Nazneen R, Talha KA. Survival and biomarkers of COVID-19 patients treated with remdesivir and favipiravir in ICU during the peak of pandemic: a single center study in Bangladesh. J Pharma Res Int. 2020;32(45):14–22. [Google Scholar]
- 19.Panchal S, Venugopal K, Vora A, et al. P5-70: effectiveness of favipiravir in COVID-19 patients with multiple (≥2) or less (<2) comorbidities: a real word experience from India. Respirology. 2021;26(S3):184. [Google Scholar]
- 20.Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73(3):531–534. doi: 10.1093/cid/ciaa1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang MC, Park YK, Kim BO, Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis. 2020;20(1):445. doi: 10.1186/s12879-020-05144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turcotte JJ, Meisenberg BR, MacDonald JH, et al. Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital. PLoS One. 2020;15(8):e0237558. doi: 10.1371/journal.pone.0237558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabbous HM, Abd-Elsalam S, El-Sayed MH, et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch Virol. 2021;166(3):949–954. doi: 10.1007/s00705-021-04956-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Lou Y, Liu L, Yao H, et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 2021;157:105631. doi: 10.1016/j.ejps.2020.105631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ara Perveen R, Nasir M, Murshed MM, Naznin R, Ahmed SN. Remdesivir and favipiravir changes hepato-renal profile in COVID-19 patients: a cross sectional observation in Bangladesh. ijmsci. 2021;8(01):5196–5201. doi: 10.18535/ijmsci/v8i01.03 [DOI] [Google Scholar]