Abstract
Purpose
Postoperative cognitive decline (POCD) is highly prevalent in elderly patients who received surgery. The systemic immune-inflammation index (SII) has been shown to be an independent predictor of many diseases associated with inflammation, but the relationship between the SII and POCD is unknown. We aimed to investigate the association between POCD and SII levels to examine the potential of SII in predicting POCD in elderly patients.
Patients and Methods
The present study was carried out among elderly patients who underwent elective orthopedics operation in our hospital, and SII, neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR) were calculated from the admission blood sample. POCD was measured by Mini-mental State Examination (MMSE) in elderly patients. The association between SII levels and POCD was analyzed by binary logistic regression analysis.
Results
Finally, 19 (25%) of 76 patients were diagnosed with POCD. Compared with Non-POCD patients, POCD patients showed significantly higher levels of NLR, MLR, SII, especially SII at admission. SII was independently associated with the occurrence of POCD through the logistic regression analysis. Receiver operating characteristic curve analysis indicated that preoperative SII was a significant predictor for POCD, and the area under the curve was 0.909.
Conclusion
Our data suggest that preoperative NLR, MLR, SII levels in the blood are related to the occurrence of POCD. Preoperative SII level is a prognostic biomarker of POCD in elderly patients after orthopedics operation. More clinical studies are needed to further verify the value of SII in POCD.
Keywords: systemic immune-inflammation index, postoperative cognitive decline, geriatric, neutrophil to lymphocyte ratio, monocyte to lymphocyte ratio
Introduction
Cognitive decline after surgery can have an important impact on patients’ health, function and prognosis, and the incidence of postoperative cognitive decline (POCD) is approximately 5% to 40%.1 POCD is characterized by acute or persistent impairment of concentration, attention, learning and memory after surgery and which has been merged into a new term “perioperative neurocognitive disorders (PND)”.2 POCD delays patients’ hospitalization time with high medical expenses and increases perioperative mortality,3 which urges us to find an easily available and valid strategy to predict POCD among elderly patients.
Previous clinical and animal studies have shown that acute peripheral inflammation was caused by anesthesia or surgery, followed by neuro-inflammation and synaptic disorder, which can lead to hippocampus-dependent cognitive damages.4–6 A great many of studies have been reported that numerous pro-inflammatory cytokines, for instance, TNF-α, IL-1β, and IL-6 in the peripheral body fluid of patients and animals after surgery were increased significantly.7,8 The pro-inflammatory cytokines can destroy the blood–brain barrier by entering the central nervous system and lead to activation of microglia.9 The activation of microglia may produce inflammatory cytokines and induce the generation of free radicals, which result in oxidative stress and damage neurocognitive function.10
Although pathological mechanisms have been demonstrated to be associated with PND,11,12 there are no valid clinical biomarkers to predict occurrence of PND. SII is a derivative and new inflammatory biomarker, derived from neutrophil, lymphocyte and platelet counts, which was used to evaluate the outcome of patients who were suffered from solid cancers and coronary heart disease.13,14 However, the value of SII in POCD of elderly patients is still unclear. Therefore, this study aimed to investigate the association between POCD and SII levels in order to examine the ability of SII in predicting POCD.
Materials and Methods
Study Population and Setting
We selected 76 aged patients over 65 years old who underwent elective orthopedics operation in the Faculty of Anesthesiology of Changhai Hospital for a prospective clinical study. The study was approved by the Ethics Committee of Shanghai Changhai Hospital (Shanghai, China) (CHEC2018-133) and published in Clinicaltrials.gov (number: NCT03765840) and obtained the written informed consent of all patients.
Patients underwent orthopedics surgery under spinal anesthesia and received postoperative patient-controlled analgesia (PCA). The inclusion criteria included: over 65 years old, orthopedics, American Society of Anesthesiologists (ASA) physical status I–III, the educational level of primary school or above, a negative Clock drawing experiment, and MMSE scores≥22 points. The exclusion criteria included: over 85 years old, a history of nervous system diseases, severe psychiatric diseases, autoimmune-illness, persistent infectious diseases, acute inflammation or malignancies.
Clinical Measurement and Laboratory Tests
Patients’ demographic characteristics (such as age, gender, education) and vascular risk factors (such as hypertension, diabetes mellitus, hyperlipidaemia, heart disease) were recorded in this study. Cognitive function was determined by the MMSE scores before surgery, on the first day and third day after surgery. As depicted in previous studies, POCD was diagnosed when the MMSE score was lower than 1.5 standard deviations (SD) compared with the baseline score.15
On the next morning after admission, the trained nurses collected all blood samples by heparinized tubes and the samples were tested by clinical laboratory technicians in a hospital. Neutrophil (N), lymphocyte (L), monocyte (M), platelet (P) were recorded and NLR was calculated as neutrophil count/lymphocyte count, MLR was calculated as monocyte count/lymphocyte count, and SII was calculated as platelet count × neutrophil count/lymphocyte count.16
Statistical Analyses
All statistical analysis was performed by SPSS 23.0. Student’s t-test was used to compare continuous variables in normally distributed data described as mean (SD) while Mann–Whitney U-test was used to compare continuous variables in non-normal distribution data described as medians (quartiles). Categorical variables represented as frequency and percentages were compared using χ2 test or Fisher’s exact test. After adjusting for confounding factors such as age, gender, body mass index (BMI), preoperative MMSE scores and education, the relationship between NLR, SII, MLR and POCD was analyzed by binary logistic regression analysis, P<0.05 was considered statistically significant.
Results
Patients’ Characteristics and Inflammatory Parameters
We collected demographic data for all patients in Table 1. 19 patients who underwent elective orthopedics operation developed POCD and were defined as the POCD group. The rest consists of the Non-POCD group. There was no difference between the two groups in ASA Physical Status Classification, cardiovascular risk factors, gender, preoperative MMSE scores, age, education, duration of operation, BMI. POCD group had significantly lower postoperative MMSE scores compared with Non-POCD group. And POCD group had significantly higher levels of SII, NLR and MLR compared with Non-POCD group.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | POCD Group (n=19) | Non-POCD Group (n=57) | P |
|---|---|---|---|
| Age (years) | 73.89±4.16 | 71.89±5.49 | 0.15 |
| Male(%) | 10(33.3%) | 20(66.7%) | 0.28 |
| ASA (I/II/III) | 4/12/3 | 14/40/3 | 0.39 |
| BMI(kg/m2) | 23.32±3.43 | 24.49±3.04 | 0.16 |
| Education (years) | 8.05±3.61 | 8.47±3.06 | 0.62 |
| MMSE scores | |||
| Preoperative | 26.63±1.92 | 27.26±1.62 | 0.17 |
| postoperative day 1 | 23.58±1.98 | 25.95±1.36 | <0.01 |
| postoperative day 3 | 25.84±1.64 | 26.84±1.65 | 0.03 |
| Operation duration (h) | 1.69±0.74 | 1.68±0.73 | 0.95 |
| Diabetes | 4 | 9 | 0.86 |
| Hypertension | 5 | 25 | 0.28 |
| Hyperlipemia | 0 | 5 | 0.32 |
| Heart disease | 1 | 5 | 0.62 |
| SII(109) | 934 (643–1065) | 388 (270–531) | <0.01 |
| NLR | 5.14 (3.41–7.49) | 2.12 (1.45–2.86) | <0.01 |
| MLR | 0.47 (0.39–0.66) | 0.27 (0.20–0.35) | <0.01 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; MMSE, Mini-Mental State Examination; SII, systemic immune-inflammation index; NLR, neutrophil/lymphocyte ratio; MLR, monocyte/lymphocyte ratio; POCD, postoperative cognitive decline.
Predictors of the Occurrence of POCD
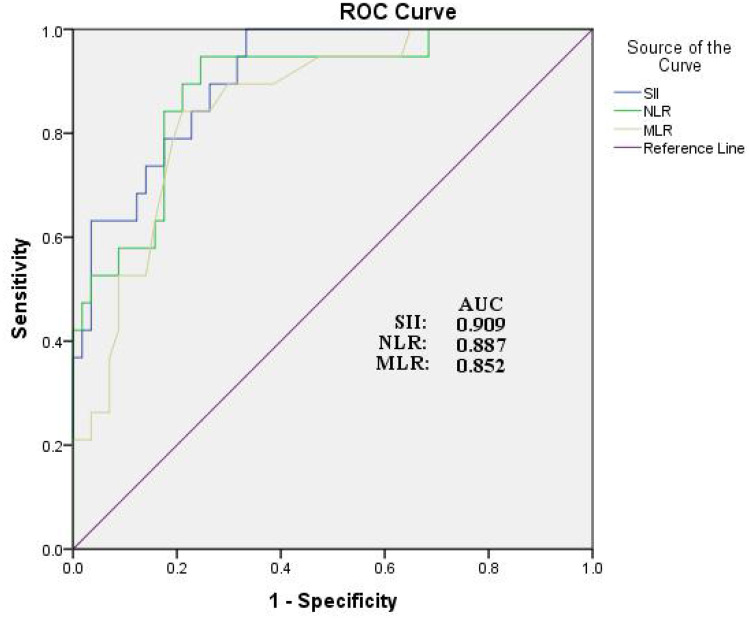
We performed univariate and multivariate logistic regression analyses to determine the independent predictors of the presence of POCD. The SII, NLR and MLR correlated with POCD. After adjusting for age, gender, BMI, preoperative MMSE scores, education, high SII levels were an independent risk factor for the presence of POCD (Table 2). Receiver operator characteristics curve (ROC) analysis revealed that the SII had the best predictive power for POCD among the inflammatory parameters and the area under the curve (AUC) was 0.909 (95% CI, 0.842–0.975, P<0.01) (Figure 1). In addition, we added age, gender, education and BMI to the ROC analyses and found that the predictive power of SII in POCD was unchanged (Supplementary Figure 1).
Table 2.
Univariate and Multivariate Logistic Regression Analysis Showing the Independent Predictors of POCD
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| OR | Lower | Upper | P | OR | Lower | Upper | P | |
| Age | 1.078 | 0.973 | 1.196 | 0.151 | 1.112 | 0.939 | 1.317 | 0.217 |
| Gender | 0.486 | 0.17 | 1.393 | 0.179 | 1.907 | 0.34 | 10.694 | 0.463 |
| BMI | 0.884 | 0.743 | 1.051 | 0.163 | 0.992 | 0.755 | 1.304 | 0.954 |
| Education | 0.958 | 0.808 | 1.134 | 0.616 | 0.937 | 0.715 | 1.23 | 0.641 |
| Preoperative MMSE scores | 0.805 | 0.593 | 1.094 | 0.166 | 0.928 | 0.569 | 1.515 | 0.766 |
| SII | 1.006 | 1.003 | 1.009 | <0.01 | 1.005 | 1.001 | 1.009 | 0.026 |
| NLR | 2.572 | 1.623 | 4.075 | <0.01 | 1.517 | 0.643 | 3.582 | 0.342 |
| MLR×10 | 2.023 | 1.396 | 2.932 | <0.01 | 1.142 | 0.595 | 2.191 | 0.689 |
Abbreviations: BMI, body mass index; MMSE, Mini-Mental State Examination; SII, systemic immune-inflammation index; NLR, neutrophil/lymphocyte ratio; MLR, monocyte/lymphocyte ratio; POCD, postoperative cognitive decline; OR, odds ratio; CI, confidence interval.
Figure 1.
Receiver operating characteristic (ROC) curve for systemic immune-inflammation index as a predictor of POCD.
Discussion
POCD is one of the most common postoperative complications in older patients, which caused remarkable clinical, social and economic impacts on patients and their family.17 As there are few treatments for POCD, it is essential to identify high-risk patients. Therefore, it is very important to find a biomarker that can easily predict the presence of POCD. In the present study, the association of increased SII and POCD was demonstrated for the first time. Additionally, the SII, NLR, and MLR correlated with POCD. More importantly, high SII levels were an independent risk factor for the presence of POCD.
More and more evidence, mainly from animal models of PND shows that the innate immune system is involved in the peripheral inflammatory response caused by trauma, and the circulating pro-inflammatory cytokines enter the central nervous system through a disrupted blood–brain barrier resulting in neuro-inflammation.9 Neutrophils, lymphocytes and monocytes are subtype of white blood cell in the blood. These cells have been shown to play an important role in mediating the peripheral and central inflammatory response, and to be involved in the regulation of immunity and inflammation resulting in cognitive dysfunction.18–20 Platelets have an important role in the regulation of immunity and inflammation, which may contribute to cognitive disorder.21 As mentioned above, derivatives of neutrophils, lymphocytes, monocytes and platelets, NLR, MLR and SII may be a better predictive parameter of immunity and inflammatory diseases.
Previous studies have demonstrated the NLR, MLR and SII were useful in predicting the inflammatory process in heart diseases and solid cancers.13,14 In clinical studies, it has been found that increased SII was an independent predictor of survival in patients with invasive vulvar cancer.22 However, although the relationship between inflammation and the pathogenesis of PND has been discussed for a long time, there are few studies related to PND on this topic. In our study, we found that POCD group had higher NLR, MLR and SII compared with Non-POCD group. Moreover, we found that the preoperative SII levels had the best predictive power for POCD among the inflammatory parameters.
Our study mainly demonstrated that patients with higher SII were more prone to developing POCD. In order to diagnose and treat POCD, it was easy and cost-effective for POCD screening and management to monitor their dynamic changes of NLR, MLR, especially SII. Higher preoperative SII could provide some diagnostic and prognostic clues for anesthesiologists to identify early POCD in patients undergoing elective orthopedics surgery, so as to better prevent and treat it. This will also help study how the immune-inflammatory response participates in psychiatric illness, so as to promote our understanding of the pathogenesis of these neurodegenerative diseases.
The current research also has some unavoidable limitations. Firstly, this is a small-sample single-center prospective study, and patients who had received orthopedics surgery were only included in our study. Secondly, we only recorded the NLR, MLR and SII once at admission. The association between POCD and dynamic changes in blood index after surgery requires further study. Thirdly, our study did not set a control group, and we also failed to collect the values of SII, NLR and MLR in patients before they became ill. Therefore, the normal range of them was unknown and it was unclear whether the SII, NLR and MLR of the Non-POCD group were normal or high, which requires further studies. Lastly, in order to determine whether the present risk factor has high predictive power, multi-center clinical large samples and observational studies need to be done.
Conclusion
Our study found that high NLR, MLR and SII level were related to cognitive decline among the patients receiving orthopedics operation, and preoperative SII was an independent risk factor for POCD in elderly patients. Therefore, anesthesiologists, surgeons and nurses should be more cautious about patients with high preoperative SII level. In conclusion, the SII, a cheap, universal and easily evaluable marker in the peripheral blood, may be useful predictor for POCD. More investigations, especially large-scale clinical studies, are needed to elucidate our views and clarify the role and mechanism of SII in patients with POCD.
Acknowledgments
We thank all those who support and participate in the study, including our staff, patients and their family members.
Funding Statement
This study was funded by the National Natural Science Foundation of China (81871579 to Jinjun Bian, and 81701062 to Xiya Yu) and Innovative Program of First Affiliated Hospital of Naval Medical University (2020YXK013 to Xiya Yu).
Data Sharing Statement
The data supporting the findings of this study can be obtained from the corresponding author according to reasonable request, and the corresponding author/s can be directly contacted for further inquiry.
Ethics Statement
The studies concerning human participants were reviewed and approved by the ethics committee of Shanghai Changhai Hospital. The patients/participants participated in the study by providing written informed consent. The study project conforms to the ethical guidelines of the Declaration of Helsinki. In order to publish any potentially identifiable images or data contained in this article, written informed consent was obtained from the individual(s).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors state that the study was conducted without any commercial or financial relationships and potential conflicts of interest.
References
- 1.Wei P, Yang F, Zheng Q, Tang W, Li J. The Potential Role of the NLRP3 Inflammasome Activation as a Link Between Mitochondria ROS Generation and Neuroinflammation in Postoperative Cognitive Dysfunction. Front Cell Neurosci. 2019;13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evered L, Silbert B, Knopman D, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skvarc D, Berk M, Byrne L, et al. Post-Operative Cognitive Dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehavioral Rev. 2018;84:116–133. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Wang R, Li S, et al. Methylene blue reduces incidence of early postoperative cognitive disorders in elderly patients undergoing major non-cardiac surgery: an open-label randomized controlled clinical trial. J Clin Anesth. 2021;68:110108. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Sun Y, Huang H, et al. Sirtuin 3 protects against anesthesia/surgery-induced cognitive decline in aged mice by suppressing hippocampal neuroinflammation. J Neuroinflammation. 2021;18(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alam A, Hana Z, Jin Z, Suen K, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J, Feng X, Valdearcos M, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120(3):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Meng B, Li X, Lu B, Wu G, Chen J. NF-κB/P65 signaling pathway: a potential therapeutic target in postoperative cognitive dysfunction after sevoflurane anesthesia. Eur Rev Med Pharmacol Sci. 2017;21(2):394–407. [PubMed] [Google Scholar]
- 9.Wang P, Velagapudi R, Kong C, et al. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimer’s Dementia. 2020;16(5):734–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen C, Xie T, Pan K, et al. Acetate attenuates perioperative neurocognitive disorders in aged mice. Aging. 2020;12(4):3862–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad S. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2(7):e91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. 2020;21(11):1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jomrich G, Paireder M, Kristo I, et al. High Systemic Immune-Inflammation Index is an Adverse Prognostic Factor for Patients With Gastroesophageal Adenocarcinoma. Ann Surg. 2021;273(3):532–541. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Wu C, Hsu P, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Huang H, Le Y. Risk factors and predictive value of perioperative neurocognitive disorders in elderly patients with gastrointestinal tumors. BMC Anesthesiol. 2021;21(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Jung W, Chan Shin Y, et al. The diagnostic and prognostic values of inflammatory markers in intraductal papillary mucinous neoplasm. HPB. 2021;1:w54. [DOI] [PubMed] [Google Scholar]
- 17.Inouye S, Westendorp R, Saczynski J. Delirium in elderly people. Lancet. 2014;383(9920):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R, Zhang X, Gu L, et al. New Insight Into Neutrophils: a Potential Therapeutic Target for Cerebral Ischemia. Front Immunol. 2021;12:692061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukita K, Sakamaki-Tsukita H, Takahashi R. Lower Circulating Lymphocyte Count Predicts ApoE ε4-Related Cognitive Decline in Parkinson’s Disease. Movement Disorder. 2021;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger M, Murdoch D, Staats J, et al. Flow Cytometry Characterization of Cerebrospinal Fluid Monocytes in Patients With Postoperative Cognitive Dysfunction: a Pilot Study. Anesth Analg. 2019;129(5):e150–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Liu Y, He B, et al. Platelet biomarkers for a descending cognitive function: a proteomic approach. Aging Cell. 2021;20(5):e13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartl T, Bekos C, Postl M, et al. The systemic immune-inflammation index (SII) is an independent prognostic parameter of survival in patients with invasive vulvar cancer. J Gynecol Oncol. 2021;32(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]