Abstract
Early warning system (EWS) for vector-borne diseases is incredibly complex due to numerous factors originating from human, environmental, vector and the disease itself. Dengue EWS aims to collect data that leads to prompt decision-making processes that trigger disease intervention strategies to minimize the impact on a specific population. Dengue EWS may have a similar structural design, functions, and analytical approaches but different performance and ability to predict outbreaks. Hence, this review aims to summarise and discuss the evidence of different EWSs, their performance, and their ability to predict dengue outbreaks. A systematic literature search was performed of four primary databases: Scopus, Web of Science, Ovid MEDLINE, and EBSCOhost. Eligible articles were evaluated using a checklist for assessing the quality of the studies. A total of 17 studies were included in this systematic review. All EWS models demonstrated reasonably good predictive abilities to predict dengue outbreaks. However, the accuracy of their predictions varied greatly depending on the model used and the data quality. The reported sensitivity ranged from 50 to 100%, while specificity was 74 to 94.7%. A range between 70 to 96.3% was reported for prediction model accuracy and 43 to 86% for PPV. Overall, meteorological alarm indicators (temperatures and rainfall) were the most frequently used and displayed the best performing indicator. Other potential alarm indicators are entomology (female mosquito infection rate), epidemiology, population and socioeconomic factors. EWS is an essential tool to support district health managers and national health planners to mitigate or prevent disease outbreaks. This systematic review highlights the benefits of integrating several epidemiological tools focusing on incorporating climatic, environmental, epidemiological and socioeconomic factors to create an early warning system. The early warning system relies heavily on the country surveillance system. The lack of timely and high-quality data is critical for developing an effective EWS.
Keywords: dengue early warning system, performance, predictive abilities, alarm indicator, dengue prediction
Introduction
Dengue represents a major public health burden worldwide. Since the 1970s, the frequency and magnitude of dengue epidemics have increased globally. According to the World Health Organization, only nine countries had major dengue epidemics before 1970. However, the disease has now spread to over 100 nations worldwide. The Asia region represents the highest global dengue burden, around 70%.1 Globally, dengue cases increased from 23 million in 1990 to 104 million in 2017. For the past two decades, the trends of dengue incidence, mortality and disability-adjusted life years (DALYs) has increased globally with potential for further spread.2 Few studies indicate that these epidemics will intensify and reach new geographical areas such as Europe and South America throughout the 21st century.3,4
Dengue is a vector-borne disease transmitted by two main vectors, Aedes aegypti and Ae. albopictus. Many factors influencing dengue transmission include climatic and environmental changes,5 globalization, urbanization,6,7 vector activity and human behaviour change.8 The fundamental approach to controlling or preventing dengue transmission is through effective vector control. In most cases, the required vector control measures to prevent transmission are not met, and outbreaks have become more prevalent.9,10 One of the technical elements in the “Western Pacific Regional Action Plan for Dengue Prevention and Control (2016)” is countries should strengthen surveillance systems at the national and regional level. More robust surveillance systems will effectively guide timely decision-making to control dengue.11
Several surveillance system methods exist and serve as an early warning system (EWS). The primary goal of an early warning system is to collect data that leads to prompt decision-making processes that trigger disease intervention strategies to minimize the impact on a specific population.11 EWS aimed at supporting district health managers and national health planners to mitigate or prevent disease outbreaks, ideally using integrated tools in the national surveillance programs.12 EWS for vector-borne diseases is incredibly complex due to the involvement of numerous factors originating from the human, environmental and vector as well the disease itself.11 Several EWSs have been developed for dengue. The ability of EWS to be an effective risk reduction tool has been used in various ways to improve the public health surveillance system.13 They may have a similar structural design, functions, and analytical approaches but different performance and ability to predict dengue outbreaks.14–16
There are a growing number of research reports on dengue outbreak prediction tools.17–21 However, studies summarising the tools’ performance and predictive ability are scarce. A previous study by Racloz et al highlights the advantages of combining various epidemiological tools such as mapping and mathematical models to create an EWS. Ideally, studies should incorporate both spatial and temporal aspects of analysis to enhance the outbreak prediction ability.11 However, this study did not focus on significant predictors in generating dengue EWS. Another study by Louis et al focus on risk-mapping for dengue and has excluded any models dealing only with a temporal component of dengue risk.22 Hence, this review intended to summarise the latest literature and discuss the evidence of different EWSs, their performance, and their ability to predict dengue outbreaks.
Materials and Methods
This systematic review is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement.23
Research Question Formulation
The research question was developed using PICo, a useful tool that assists the construction of a relevant research question for a systematic review. PICo concept combines three essential elements (population or problem, interest, and context).24 Based on PICo, the three main components in this review were dengue (Problem), early warning system performance (Interest) and outbreak prediction (Context). This concept guided the formulation of the research question: “What is the evidence of Dengue Early Warning System and their performance to predict outbreaks?”
Systematic Searching Strategies
Systematic searching strategies include identification, screening, and eligibility process.
Identification
In the identification stage, synonyms and variations were used to enrich the keywords, then applied in the search process. The search string was created and generated using Boolean operators and keyword search, as illustrated in Supplementary Material Table S1. A systematic literature search was performed of four primary databases: Scopus, Web of Science, Ovid MEDLINE, and EBSCOhost, and identified a total of 442 relevant records. There were 136 duplicate records found and removed, leaving 306 records for title screening. All potential records were further exported from the databases and arranged for an Excel sheet for a title and abstract screening.
Screening
Two authors screened the title and abstract based on the developed review question and specific inclusion and exclusion criteria. The inclusion criteria were primary research in a peer-reviewed journal and English-language article. No restriction was applied for the period of publication. We excluded systematic review articles, conference proceedings, book chapters, and reports. The screening process removed 245 articles, leaving 61 for full-text retrieval for further assessment and eligibility screening.
Eligibility
A total of 56 full-text articles were successfully retrieved for eligibility. Two authors independently reviewed all full-text articles for eligibility. All studies found unrelated to the interest and intended outcome were excluded. The reasons for the article exclusion were recorded. There were 39 articles excluded due to: (1) Not related with Dengue EWS (n=18), (2) Absence of prediction model (n=10), (3) Absence of model performance reporting (n=6), (4) EWS of other diseases (n=5). The remaining 17 eligible articles were resumed for a quality appraisal process.
Quality Assessment
The quality of the studies was assessed using quality assessment criteria described in TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis).25 The TRIPOD Statement is a checklist of 22 items, considered essential for good reporting of studies developing or validating multivariable prediction models.26 TRIPOD explicitly covers the development and validation of prediction models for diagnosis and prognosis for all medical domains and types of predictors. Two authors independently conduct the quality assessment. Scores for reporting levels were obtained by assigning a single point for each reported item relevant to the study. Total scores were converted to percentages based on the maximum possible score. Finally, a total of 17 articles (with percentages score >70%) were included in this review. Supplementary Material Table S2 present the scores and percentages of each quality assessment adapted from TRIPOD checklist.25
Data Extraction and Synthesis
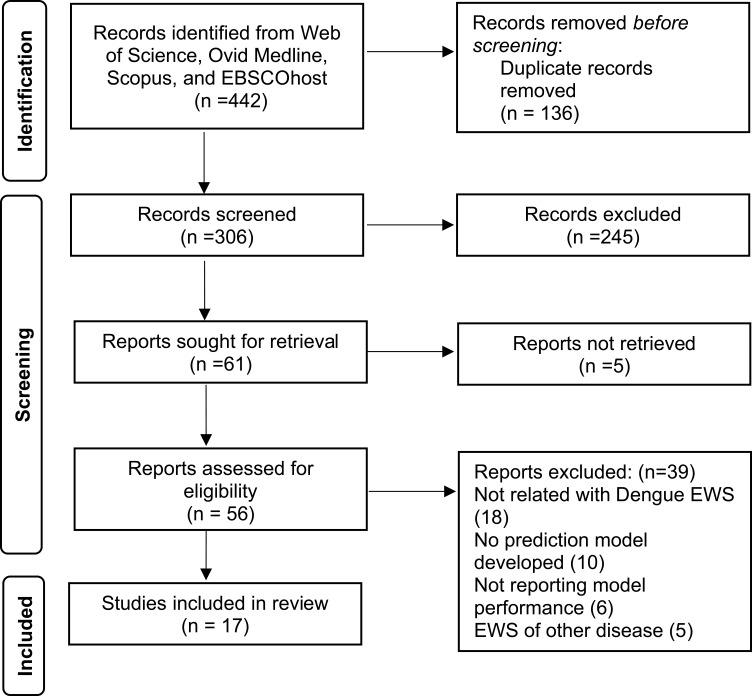
The authors extracted the data independently using a standardized data extraction form and organized it in a standard Microsoft Excel 2019 spreadsheet. The information collected included: (1) authors, (2) publication year, (3) country, (4) study design (cohort, cross-sectional, retrospective analysis of surveillance data, time series analysis), (5) type of surveillance system (indicator-based surveillance (IBS), event-based surveillance (EBS), (6) type of EWS (alarm-informed EWS, case-informed EWS), (7) outbreak indicator, (8) coverage of EWS (district, province, state, national), (9) alarm indicator, (10) data sources, (11) type of models/statistics used, and (12) information of performance (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV)). The PRISMA flow diagram is present in Figure 1.
Figure 1.
The four databases identified 442 potentially relevant records. After 136 records were removed, the title and abstract were screened based on inclusion and exclusion criteria. This screening process had excluded 245 articles. There were five reports not retrieved. The remaining 56 articles were assessed for eligibility. Thirty-nine articles were excluded in view that it is not related to Dengue EWS (18), no prediction model was developed (10), no reporting model performance (6) and include EWS of other diseases (5). A total of 17 studies were included in the final review.
Results
Characteristics of Eligible Studies
A total of 17 studies fulfilled the eligibility criteria and were included in this systematic review. Of the 17 studies, 14 (82.3%) were conducted in Asia, one (5.9%) article was conducted in South America, and two studies were conducted in a combination of countries in two continents which is North America and Asia (n=1) and South America and Asia (n=1). Malaysia was the country with the most eligible studies (n=4),27–30 followed by Thailand (n=2)31,32 and Singapore (n=2).33,34 Other studies were carried out in the Philippines,35 Vietnam,36 Taiwan,37 China,38 India,39 Sri Lanka40 and Colombia.41 Two studies were conducted in Brazil, Dominican Republic, Mexico, Malaysia and Vietnam42 and Brazil, Malaysia and Mexico.43
Most of the eligible studies used a retrospective study design. Of these, 15 studies were retrospective analyses of surveillance data. One is a retrospective cohort study43 and a cross-sectional study design.31 All 17 studies were published between 2014 and 2021, nine between 2018 and 2022. More than half of the studies (53%) applied a six to 10 years data time frame. The most extended surveillance data time frame was 19 years,36 followed by 14 years.43 Ten studies used weekly as a data unit, while the rest used monthly, daily and season as a data unit. The characteristics of included studies are summarised in Table 1. The details for characteristics in each study were presented in Supplementary Material Table S3. The PRISMA checklist is provided in Supplementary Material Table S4.
Table 1.
The Characteristics of Included Studies
| Characteristic | Frequencies |
|---|---|
| Continent | |
| Asia | 14 (82.3%) |
| South Americas | 1 (5.9%) |
| North & South America, Asia | 2 (11.8%) |
| Publication year | |
| 2014–2017 | 8 (47%) |
| 2018–2021 | 9 (53%) |
| Time frame | |
| ≤5 years | 6 (35.5%) |
| 6–10 years | 9 (53%) |
| ≥ 11 years | 2 (11.7%) |
| Data unit | |
| Daily | 1 (5.9%) |
| Weekly | 10 (58.8%) |
| Monthly | 3 (17.6%) |
| Seasons | 2 (11.8%) |
| Not available | 1 (5.9%) |
Characteristic of Dengue EWS
All included studies relied on indicator-based surveillance (IBS). Two studies were case informed EWS,29,34 which is early outbreak detection that relies entirely on the past case trends for outbreaks. Another 15 studies were categorized as alarm informed EWS that used at least one alarm indicator as the independent variable to predict outbreaks. The alarm indicator was classified into meteorological, epidemiological, entomology, population and socioeconomic and others. Other categories include enhanced vegetation index, education, urbanization and search query data such as “Baidu index website”. Almost all studies used laboratory-confirmed cases as outbreak indicators, but two studies used a combination of probable dengue cases and hospitalized dengue cases as an outbreak indicator.42,43 The coverage of EWS varies from city level,37 District,28,29,40,43 province,31,35,36,38 state27,30,39 and national level.33,34,41,42 In terms of data source, all dengue cases were obtained directly from the national surveillance system from the Ministry of Health. However, one study used the reports published by the Ministry of Health as a source of data.30 The characteristic of Dengue EWS for individual studies are presented in Table 2.
Table 2.
Characteristic Dengue EWS in 17 Included Studies
| First Author, Year | Type of EWS (Alarm-Informed/ Case-Informed) | IBS/EBS | Outbreak Indicator | Coverage of the Tool | Alarm Indicator | Data Sources |
|---|---|---|---|---|---|---|
| Withanage, 201840 | Alarm | IBS | Monthly dengue incidence | District | Meteorological (T, RH, WS) Epidemiological (dengue incidence) |
Regional surveillance system, Department of Meteorology |
| Bowman, 201642 | Alarm | IBS | Weekly probable and hospitalized dengue cases | National | Meteorological (T, R, RH) Epidemiological (mean age, circulating serotype) Entomological (Breteau Index, House Index, Ovitrap Index) |
National surveillance system, Department of Meteorology |
| Siriyasatien, 201631 | Alarm | IBS | Season Incidences of DHF | Province | Meteorological (T, RH, WS) Epidemiological (dengue cases) Entomological (Aedes aegypti infection rate, female and male mosquito infection rate) Others (Total population) |
National surveillance system, Thai Meteorology Department, Parasitology Department |
| Chen, 202034 | Case | IBS | Weekly dengue incidences | National | Epidemiological (dengue incidence) | National surveillance system |
| Hussain-Alkhateeb, 201843 | Alarm | IBS | Weekly probable and laboratory-confirmed dengue cases and hospitalized dengue cases | District | Meteorological (T, R, RH) Epidemiological (mean age, circulating serotype) Entomological (Ovitrap Index) |
National surveillance system, Department of Meteorology |
| Nejad, 202130 | Alarm | IBS | Weekly dengue fever incident and confirmed cases | State | Meteorological (T, R, RH) Epidemiological (dengue cases) |
Reports from MOH, Meteorological Department |
| Salim, 202128 | Alarm | IBS | Weekly dengue cases | District | Meteorological (T, R, RH, Southern Oscillation Index) Epidemiological (dengue cases) |
National surveillance system, Department of Meteorology |
| Buczak, 201435 | Alarm | IBS | Weekly dengue incidence | Province | Meteorological (T, R, WS, Sea Surf. Temp. Anomaly) Epidemiological (dengue cases) Others (Socio-economic, NDVI, EVI) |
NASA Global Change Mastery Directory, Unisys Weather, USGS Land Processes, Philippines National Statistics Office |
| Colo ´n-Gonza ´lez, 202136 | Alarm | IBS | Monthly dengue cases | Province | Meteorological (T, R, RH) Epidemiological (dengue cases) Others (urbanization) |
National surveillance system, Department of Meteorology, Socioeconomic Data and Applications Center (SEDAC) Gridded Population |
| Chang,201537 | Alarm | IBS | Daily confirmed dengue cases | City | Meteorological (T, R, RH) Epidemiological (dengue cases) Entomological (Breteau Index, House Index, Adult aedes index) |
National Notifiable Disease Surveillance System of the Taiwan Centers for Disease Control (Taiwan-CDC) National surveillance system, Environmental Protection Administration (EPA) |
| Shi, 201633 | Alarm | IBS | Weekly dengue cases | National | Meteorological (T, R, RH) Epidemiological (dengue cases) Entomological (reading percentage) Others (population density) |
Singapore’s Ministry of Health Singapore Department of Statistics Meteorological Services Singapore |
| Patil, 202139 | Alarm | IBS | Monthly dengue cases | State | Meteorological (T, RH, WS) Epidemiological (dengue cases) |
National Vector Borne Disease Control Program Indian Meteorological Department (IMD) |
| Zhao, 202041 | Alarm | IBS | Weekly dengue cases | National | Meteorological (T, R) Epidemiological (dengue cases) Others (Population, GINI index, education coverage) |
SIVIGILA (national surveillance program of Colombia) MOD11C2 from NASA’s LP DAAC Colombian National Administrative Department of Statistics |
| Nordin, 202029 | Case | IBS | NA | District | Meteorological (T, R) Epidemiological (dengue cases) |
National surveillance system, Department of Meteorology |
| Guo, 201738 | Alarm | IBS | Weekly dengue cases | Province | Meteorological (T, R, RH) Epidemiological (dengue cases) Others (Search query-Baidu index website, population) |
China National Notifiable Disease Surveillance System Statistics Bureau of Guangdong Province Baidu Index website (https://index.baidu.com/) |
| Jaafar, 201627 | Alarm | IBS | Weekly dengue cases | State | Meteorological (T, R, RH) Epidemiological (dengue cases) Others (population density) |
Ministry of Health Malaysia, Malaysian Meteorological Department and Ministry of Rural and Regional Development. |
| Kesorn, 201532 | Alarm | IBS | Season dengue cases | Region | Meteorological (T, R, RH, WS) Epidemiological (dengue cases) Entomological (female and male mosquito infection rate) Others (Population density) |
National surveillance system, Thai Meteorology Department, Parasitology Department, Ministry of Interior |
Abbreviations: EBS, event-based surveillance; IBS, indicator-based surveillance; MOH, Ministry of Health; NA, not applicable; R, rainfall; RH, relative humidity; T, temperature; WS, wind speed.
Alarm-Indicators for Dengue EWS
All studies include dengue cases as one of the alarm-indicator. Meteorological alarm indicators, particularly temperature and rainfalls, were the most applied in 15 studies. Other meteorological variables used to build the prediction models were humidity (13 studies) and wind (5 studies). One study uses other climate parameters such as sea surface temperature anomalies and southern oscillation index as alarm indicators in EWS. All studies reported using meteorological data from the local meteorological station, except one study used the international data sources from the National Aeronautics and Space Administration, US Geological Survey and Unisys Weather.35
Six studies utilized entomology alarm indicators31–33,37,42,43 such as Breteau index (BI), House index (HI), Ovitrap index, Male and female mosquito infection rate, larvae infection rate, adult Aedes index (AI) and breeding percentage. The population and socioeconomic alarm indicators were used in six studies.27,31–33,35,41 It includes population density, midyear population size, poverty index, GINI index, electricity access, drinking water access, sanitation index, and education coverage. Two studies used enhanced vegetation data as an alarm indicator.35,41 Only one study utilizes search query data such as “Baidu index website” as a predictor in dengue EWS.38 Table 3 presents the alarm indicator frequency used to develop the Dengue EWS model.
Table 3.
Frequency of Alarm Indicator Used to Develop Dengue EWS Model
| Author, Year, Country | Epidemiological | Meteorological | Entomological | Population and Socioeconomic | Others | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dengue Cases | Circulating Serotype | Temperature | Rainfall | Humidity | Wind | Breteau Index | House Index | Ovitrap Index | Male Mosquito Infection Rate | Female Mosquito Infection Rate | 0thers | |||
| Withanage, 201840 | / | / | / | / | / | |||||||||
| Bowman, 201642 | / | / | / | / | / | / | / | / | ||||||
| Siriyasatien, 201631 | / | / | / | / | / | / | / | Larvae infection rate | / | |||||
| Chen, 202034 | / | |||||||||||||
| Hussain-Alkhateeb, 201843 | / | / | / | / | / | / | ||||||||
| Nejad, 202130 | / | / | / | / | ||||||||||
| Salim, 202128 | / | / | / | / | / | |||||||||
| Buczak, 201435 | / | / | / | / | Vegetation data, sea surface temperature anomalies, Southern Oscillation Index | |||||||||
| Colo ´n-Gonza ´lez, 202136 | / | / | / | / | Urbanization | |||||||||
| Chang, 201537 | / | / | / | / | / | / | Container index, Adult aedes index | |||||||
| Shi, 201633 | / | / | / | / | Breeding percentage | / | ||||||||
| Patil, 202139 | / | / | / | / | / | |||||||||
| Zhao, 202041 | / | / | / | / | Enhance vegetation index, GINI Index, education | |||||||||
| Nordin, 202029 | / | |||||||||||||
| Guo, 201738 | / | / | / | / | Search query data (Baidu index website) | |||||||||
| Jaafar, 201627 | / | / | / | / | / | |||||||||
| Kesorn, 201532 | / | / | / | / | / | / | / | / | ||||||
| Total | 17 | 2 | 15 | 15 | 13 | 5 | 2 | 2 | 2 | 2 | 2 | 3 | 6 | 8 |
Dengue EWS Model Approaches and Performance
A range of modelling approaches, such as statistical and machine learning (ML) methods for Dengue EWS, has been used in all included studies. The details of the modelling approach, main findings, limitations, and conclusion of each included study were presented in Supplementary Material Table S2. Out of 17 studies, four used statistical analysis approaches such as the general additive mixed (GAM) model,34 time-series regression40 and Poisson regression models.31,37 On the other hand, the ML approach was the most frequent approach applied in the included studies. Five studies solely used ML approaches such as Support Vector Machine (SVM) with RBF kernel Function,29,32 Fuzzy Association Rule Mining techniques,35 a combination of SVM, Bayes network (BN) models, decision table and naïve Baye30 and combination of SVM, Decision Trees (CART), Artificial Neural Network (ANN) and BN.28 Some studies applied a combination of ML with statistical analysis approaches such as time-series forecasting33,39,41 and negative binomial regression model.38 Other method approaches were the Shewhart method and Endemic Channel,42,43 system dynamic modelling27 and bayesian spatiotemporal model with modification in the superensemble specification.36
In terms of performance, most of the studies used sensitivity, specificity, and PPV as indicators of the models’ validity. The reported sensitivity ranged from 50 to 100%, while specificity was 74 to 94.7%. The included studies have reported between 43 to 86% for PPV. A range between 70 to 96.3% was reported for prediction model accuracy. Besides, some studies reported the performance of forecasting using the lowest mean absolute error (MAE),29,32,39,41 mean absolute percentage error (MAPE),31,33 mean square error (MSE),27 Pierce skill score (PSS)40 and continuous rank probability score (CRPS).36 Furthermore, depending on the model approach, there were also studies that reported the model performance using the R squared (R2),39 the most common performance measure for continuous outcomes.
Generally, all EWS models in the included studies demonstrated reasonably good predictive abilities subject to different alarm indicators as a predictor in the model. Overall, meteorological alarm indicators were the most frequently used and displayed the best performing indicator.28,30,33,35,36,39,41,43 But, among studies that used a combination of meteorological and epidemiological indicators, two studies demonstrated previous dengue cases significantly influenced the present dengue cases.40,42 However, Chen et al suggested incorporating other covariates, such as weather factors, could more accurately estimate their prediction model based on previous dengue case trends.34 On the other hand, the infection rates of the Ae. aegypti female mosquitoes and larvae improved forecasting efficiency better than the climate parameters used in traditional frameworks.31,32 The same goes for using the adult Aedes index, Breteau index, container index, and house index, which also improved the forecasting efficiency.37
There were seven studies conducted to compare different model approaches. Four studies evaluated the best ML model to predict dengue outbreaks.28–30,38 The BN model showed the highest accuracy with temperature, rain factor of 92.35% compared to SVM, radial basis function (RBF) tree, decision table and naive Bayes.30 Two studies showed the SVM exhibited the best prediction performance superior to other ML models. However, Salim et al28 demonstrated SVM with the linear kernel as the best prediction model while Nordin et al29 displayed SVM with RBF kernel function enhanced prediction accuracy and performance. Guo et al confirmed that the support vector regression (SVR) model achieves superior performance in comparison with other forecasting techniques such as gradient boosted regression tree algorithm (GBM), negative binomial regression model (NBM) and least absolute shrinkage and selection operator (LASSO).38
Three studies compared an ML model with alternative time-series forecasting models such as holt’s forecasting, autoregressive, moving average (ARIMA), seasonal autoregressive integrated moving average (SARIMA) and Facebook prophet. Out of three studies, two confirmed that Random Forest regression is the best-fit regression model, superior to time-series forecasting models.39,41 Besides, Patil and Pandya found that vector regression and Facebook prophet models were also best to fit the series forecasting models for two cities and six cities, respectively.39 Another outstanding performance model was using the LASSO approach. Shi et al evaluated the LASSO, step-down linear regression and SARIMA method and found that the LASSO approach provided more accurate forecasts (smaller MAPE) than the SARIMA model.33
Discussion
The present systematic review aimed to summarise and discuss the evidence of different EWSs, their performance, and their ability to predict dengue outbreaks. This review demonstrates that dengue prediction studies have become a research attention topic, particularly in Asia, where 94.1% of the included research were performed. This trend is expected as the Asia region represents the highest global dengue burden, around 70%.1 Besides, there were also studies performed in Pan American Health Organization (PAHO), as countries such as Brazil, Colombia, and Mexico reported most dengue cases.44
Most eligible studies used retrospective analysis of surveillance data as a study design and relied on IBS. IBS is a surveillance approach that regularly collects and analyses data from pre-defined sources, such as healthcare delivery institutions. Besides contributing to an early warning function, IBS also effectively establishes transmission patterns, such as seasonality, risk groups, and disease burden.45 Since all eligible studies relied on IBS; thus, these requirements necessitated regular and prompt access to surveillance data, which could compromise the effectiveness of early warning systems. However, even though the IBS is routinely collected from health-based formal sources, it is often delayed and incomplete.46 Therefore, as suggested in the revised International Health Regulations (IHR) 2005, all member states are urged to develop the capacities of their surveillance systems to meet the early warning and alert requirements comprehensively.47
The Indicator Used in Dengue EWS
Meteorological data, particularly temperature and rainfall, are important predictors, but they are sometimes unavailable in a timely manner for health care providers dealing with the EWS. Compared to countries with fewer data points, Bowman et al discovered that countries with better meteorological records (Mexico and Brazil) provided higher performance metrics.42 Thus, integration with the local meteorological department on real-time meteorological data will improve access to meteorological information and benefit end-users in early outbreak detection. One study utilized climate data from international data sources.35 Therefore, highly skilled and trained users with up-to-date technology will be required for data acquisition and processing. Therefore, human resource development should be a crucial component of EWAR implementation to build a sensitive and reliable dengue EWS.46 Only one study utilizes another climate factor, such as the sea surface temperature anomalies index.35 Hence, the uses of this climatic factor can be further explored as evidence showed an association between the sea surface temperature anomalies index and the number of reported dengue cases.19,20
Despite the importance of vector surveillance in dengue prevention and control, this review also highlighted the underutilization of entomological data, which has been exploited only in 5 of the 17 included studies. Although the rate of dengue virus infection in mosquitoes is an efficient predictor of dengue outbreaks, the approach is expensive and time-consuming; hence it has rarely been used to assess dengue outbreaks in previous studies.31 The combination of surveillance data with data mining tools, such as social media or travel information, has emerged as a new source of real-time high-resolution geospatial data on a wide scale.21 However, this review showed minimal evidence of studies exercising such potential alarm indicators, limiting their contribution to outbreak preparedness and response planning.
Indicator with Predictive Potential
Generally, meteorological variables demonstrated the most potential predictive alarm indicator in Dengue EWS. Meteorological variables such as mean temperature,38,42,43 minimum temperature,30,40 maximum temperature,28,39 rainfall,30,37,38,40,41,43 humidity,36,39,43 relative humidity,37,38,40 wind speed,36 sea surface temperature anomalies,36 season31 and Typhon status35 were important input parameter in the development of dengue outbreak prediction model. Temperature demonstrated the most frequently good predictive capacity of the meteorological variables studied in this review. In Sri Lanka, the minimum temperature was a significant predictor in the best dengue forecasting model, where receiver operating characteristic and sensitivity were 86% and 92%, respectively.40 In Brazil and Mexico, mean temperature displayed as a reliable meteorological variable where Hussain-Alkhateeb et al reported sensitivity and PPV were 91% and 65% for Brazil, while 81% and 72% for Mexico.43 Besides, rainfall plays an important role in the dengue prediction model. Few studies identified rainfall as an indicator in the dengue outbreak prediction model with a sensitivity range from 87–92%.40,43
In dengue-endemic countries such as Malaysia, temperature is also a reliable alarm indicator for Dengue EWS in few studies.27,28,30,43 Mean temperature was a reliable predictor in Malaysia, where sensitivity and PPV were 99%/80%.43 A study conducted in Selangor state, Malaysia, reveals that maximum temperature was the most crucial variable for CART and ANN models, with accuracy, specificity and sensitivity of 63%, 86%, 12% and 66%, 95%, 14%, respectively. However, due to overfitting problems for the CART and ANN, the SVM linear kernel model displays the best prediction performance model in the study.28 Another recent study from Malaysia identified a new significant risk factor contributing to dengue outbreak prediction, namely TempeRain factor (TRF). TRF combines the average minimum temperature of 5 weeks plus the current week and cumulative rainfall for two weeks before the current week. Prediction models with TRF variable reveal higher accuracies compared to those without TRF. The BN model obtained the highest accuracy with TRF with an accuracy of 92.35%.30 Additionally, another potential predictive alarm indicator in Malaysia is the predominant dengue virus serotype. This indicator indicates a change of predominant serotype in which the sensitivity and PPV were 50% and 71%, respectively.43
An entomological parameter also exhibits a potential predictive alarm indicator in Dengue EWS. In Mexico, the proportion of positive ovitraps and the mean number of eggs per block of houses showed promising findings with sensitivity and PPVs of 79%, 60% for ovitraps and 78%, 50% for the mean number of eggs per block of houses, respectively.43 In Thailand, two studies reveal the importance of entomological parameters in predicting dengue outbreaks. Siriyasatien et al demonstrated that female mosquito infection rates and season are directly correlated with the number of dengue cases, thus significantly beneficial for the dengue forecasting model.31 Similarly, Kesorn et al reported the use of Ae. aegypti female mosquito and larva infection rates as a predictor in the forecasting model could effectively signal the outbreak risk to local authorities in Thailand.32 Furthermore, Chang et al found the combination of meteorological factors (relative humidity and mean rainfall) with entomological indices (AI, BI, CI and HI) was found to improve dengue prediction model performance with an accuracy range from 84% to 89%.37
As with other vector-borne diseases, the relationship between meteorological variables and dengue have been recognized. Previous systematic reviews have shown that ambient temperature and precipitation are the most important meteorological risk factors for dengue fever.48–50 Temperature may raise the risk of dengue fever by altering the mosquito’s life cycle and development rate,51 as well as affecting mosquitoes’ general activity and host-seeking behaviour.52 Generally, female Ae. aegypti could fly in the temperature range of 15 to 32°C sustainably. Meanwhile, at extreme temperatures such as 10°C and 35°C, mosquitoes’ flight was feasible but only for a limited duration. In terms of time and distance, the optimal flight temperature for mosquitoes was at 21°C.53 Moreover, previous studies proved that increased temperatures would decrease the extrinsic incubation period (EIP). For DENV-2 and DENV-4 serotypes in Ae. aegypti, at 26°C and 28°C, the virus first detected at day 9, while at 30°C, the virus can be seen at day 5.54 Similarly, for DENV-2 serotypes in Ae. albopictus, the EIP will gradually shorten when the temperature increases.55 These findings might explained the “change of predominant serotype” as a reliable predictive alarm indicator of a dengue prediction model.
Model Employed and Performance of Dengue EWS
Generally, all EWS models in the included studies demonstrated reasonably good predictive abilities. However, the accuracy of their predictions varied greatly depending on the model used and the data quality. The most commonly used statistical modelling techniques in dengue studies are Poisson Regression, Negative Binomial Regression, ARIMA and GAM. GAM and ARIMA are the standard reference models for associating environmental factors towards disease outcome and a tool for time series prediction analysis.56,57 In recent years, data-driven techniques based on ML algorithms such as Decision Tree, SVM, Naïve Bayes and Random Forest have demonstrated promising outcomes in predictive analytics for classification problems.28 More than half of the included studies relied on machine learning methods, mainly supervised learning models, to assess conventional and novel data streams. The supervised learning model is defined by its use of labeled datasets to train algorithms to classify data or accurately predict outcomes.58 The disparity between health scientists who prefer “conventional” modelling assessment measures and information technology scientists who focus on information retrieval metrics may explain the diverse methodological choices.59
Evaluation metrics assist in determining if the data acquisition is appropriate for the required goal (dengue surveillance and prediction), as well as assessing data quality and bias.60 While most of the included prediction studies used at least one of the gold standard metrics for performance evaluation, such as positive predictive value, sensitivity and specificity, a few articles only used error-based metrics, such as root mean square error and mean absolute error. Some studies used R2 as an evaluation metric since considered to be a useful summary of the predictive information in linear, logistic, and Cox regression models.61 A study used CRPS as evaluation metrics to report the forecasting model’s performance. The CRPS has an advantage over the mean absolute error (MAE), or root mean squared error (RMSE) in that it does not focus on a single point of the forecasts’ probability distribution but rather on the distribution as a whole.36
Study Limitation
The exclusion criteria of non-English language articles could be one of the limitations of this review. Most of the included studies were from Asia, which comprises of non-English speaking countries. Therefore, this review might miss the wealth of related literature published in other languages. On the other hand, including studies published in languages other than English may need more resources in terms of cost, effort, and non-English language proficiency. The second limitation is that since the inclusion criteria were primary research in a peer-reviewed journal, we exclude preprints and grey literature such as conference abstracts, committee and government reports. Due to limitations in time and resources, we did not search for other evidence that is not published in the commercial publication, such as theses and dissertation. Ideally, despite the difficulties of looking for evidence in grey literature, it is beneficial to include grey literature in the systematic reviews since it has the ability to provide a balanced assessment of the evidence and reduce the publication bias.62
Conclusion
EWS is an essential tool to support district health managers and national health planners to mitigate or prevent disease outbreaks. This systematic review demonstrated evidence of dengue EWS related to the structural, statistical and performance of EWS to predict outbreaks. It highlights the benefits of integrating several epidemiological tools focusing on incorporating climatic, environmental, epidemiological and socioeconomic factors to create an early warning system. The early warning system relies heavily on the country surveillance system. The lack of timely and high-quality data is critical for developing an effective EWS.
Acknowledgments
This review is a part of research by Ministry of Higher Education Malaysia under Long-term Research Grant Scheme project 3, grant number LRGS/1/2020/UKM–UKM/01/6/3 which is under the program of LRGS/1/2020/UKM–UKM/01/6.
Funding Statement
The authors received no specific funding for this work.
Abbreviations
ANN, Artificial Neural Network; ARIMA, Autoregressive Moving Average; BN, Bayes Network; CRPS, Continuous Rank Probability Score; DALYs, Disability-Adjusted Life Years; EBS, Event-Based Surveillance; EWS, Early Warning System; GAM, General Additive Mixed; EIP, Extrinsic Incubation Period; GBM, Gradient Boosted Regression Tree Algorithm; IBS, Indicator-Based Surveillance; LASSO, Least Absolute Shrinkage And Selection Operator; ML, Machine Learning; MAE, Mean Absolute Error; MSE, Mean Square Error; MOH, Ministry Of Health; NA, Not Applicable; NBM, Negative Binomial Regression Model; NPV, Negative Predictive Value, PAHO, Pan American Health Organization; MAPE, Percentage Error; PSS, Pierce Skill Score; PICo, Population Or Problem, Interest, And Context; PPV, Positive Predictive Value; PRISMA, Preferred Reporting Items for Systematic Reviews And Meta-Analyses; R2, R Squared; RBF, Radial Basis Function; RMSE, Root Mean Squared Error; SARIMA, Seasonal Autoregressive Integrated Moving Average; SVM, Support Vector Machine; SVR, Support Vector Regression; TRIPOD, Transparent Reporting Of A Multivariable Prediction Model For Individual Prognosis Or Diagnosis; TRF, TempeRain factor; WS, Wind Speed.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization. Dengue and Severe Dengue; 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue#:~:text=Global%20burden&text=One%20modelling%20estimate%20indicates%20390,with%20any%20severity%20of%20disease. Accessed January 12, 2022.
- 2.Zeng Z, Zhan J, Chen L, Chen H, Cheng S. Global, regional, and national dengue burden from 1990 to 2017: a systematic analysis based on the global burden of disease study 2017. EClinicalMedicine. 2021;32:100712. doi: 10.1016/j.eclinm.2020.100712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu-Helmersson J, Quam M, Wilder-Smith A, et al. Climate change and Aedes Vectors: 21st century projections for dengue transmission in Europe. EBioMedicine. 2016;7:267–277. doi: 10.1016/j.ebiom.2016.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maz L, Lockwood R. Climate change will increase the vector capacity of the Aedes aegypti in South America: a systematic map. PSU McNair Scholars Online J. 2021;15(1):6. doi: 10.15760/mcnair.2021.15.1.1 [DOI] [Google Scholar]
- 5.Colón-González FJ, Fezzi C, Lake IR, Hunter PR. The effects of weather and climate change on dengue. PLoS Negl Trop Dis. 2013;7(11):e2503. doi: 10.1371/journal.pntd.0002503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu Y, Shi X, Wang Y, Li R, Lu L, Liu Q. Effects of socioeconomic and environmental factors on the spatial heterogeneity of dengue fever investigated at a fine scale. Geospat Health. 2018;13(2). doi: 10.4081/gh.2018.682 [DOI] [PubMed] [Google Scholar]
- 7.Gwee XWS, Chua PEY, Pang J. Global dengue importation: a systematic review. BMC Infect Dis. 2021;21(1):1078. doi: 10.1186/s12879-021-06740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu G, Liu J, Tan Q, Shi B. Inferring the spatio-temporal patterns of dengue transmission from surveillance data in Guangzhou, China. PLoS Negl Trop Dis. 2016;10(4):e0004633. doi: 10.1371/journal.pntd.0004633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu-Helmersson J, Stenlund H, Wilder-Smith A, Rocklöv J. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS One. 2014;9(3):e89783. doi: 10.1371/journal.pone.0089783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Global Vector Control Response 2017–2030. Geneva: World Health Organization; 2017. [Google Scholar]
- 11.Racloz V, Ramsey R, Tong S, Hu W. Surveillance of dengue fever virus: a review of epidemiological models and early warning systems. PLoS Negl Trop Dis. 2012;6(5):e1648. doi: 10.1371/journal.pntd.0001648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Runge-Ranzinger S, Kroeger A, Olliaro P, et al. Dengue contingency planning: from research to policy and practice. PLoS Negl Trop Dis. 2016;10(9):e0004916. doi: 10.1371/journal.pntd.0004916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Research Council (US) Committee on Climate E, Infectious Diseases, and Human Health. Toward the Development of Disease Early Warning Systems. In: Under the Weather: Climate, Ecosystems, and Infectious Disease. Washington (DC): National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 14.Chuang TW, Chaves LF, Chen PJ. Effects of local and regional climatic fluctuations on dengue outbreaks in southern Taiwan. PLoS One. 2017;12(6):e0178698. doi: 10.1371/journal.pone.0178698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Zhu G, Lin L. Research of dengue fever prediction in San Juan, Puerto Rico based on a KNN regression model. In: Intelligent Data Engineering and Automated Learning – IDEAL; Vol. 2017, 2017:146–153. [Google Scholar]
- 16.Chumpu R, Khamsemanan N, Nattee C. The association between dengue incidences and provincial-level weather variables in Thailand from 2001 to 2014. PLoS One. 2019;14(12):e0226945. doi: 10.1371/journal.pone.0226945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baquero OS, Santana LMR, Chiaravalloti-Neto F. Dengue forecasting in São Paulo city with generalized additive models, artificial neural networks and seasonal autoregressive integrated moving average models. PLoS One. 2018;13(4):e0195065. doi: 10.1371/journal.pone.0195065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anno S, Hara T, Kai H, et al. Spatiotemporal dengue fever hotspots associated with climatic factors in Taiwan including outbreak predictions based on machine-learning. Geospat Health. 2019;14(2). doi: 10.4081/gh.2019.771. [DOI] [PubMed] [Google Scholar]
- 19.Ogashawara I, Li L, Moreno-Madriñán MJ. Spatial-temporal assessment of environmental factors related to dengue outbreaks in São Paulo, Brazil. Geohealth. 2019;3(8):202–217. doi: 10.1029/2019GH000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colón-González FJ, Bentham G, Lake IR. Climate variability and dengue fever in warm and humid Mexico. Am J Trop Med Hyg. 2011;84:757–763. doi: 10.4269/ajtmh.2011.10-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluskin RT, Johansson MA, Santillana M, Brownstein JS. Evaluation of internet-based dengue query data: google dengue trends. PLoS Negl Trop Dis. 2014;8(2):e2713. doi: 10.1371/journal.pntd.0002713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis VR, Phalkey R, Horstick O, et al. Modeling tools for dengue risk mapping - a systematic review. Int J Health Geogr. 2014;13(1):50. doi: 10.1186/1476-072X-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockwood C, Munn Z, Porritt K. Qualitative research synthesis: methodological guidance for systematic reviewers utilizing meta-aggregation. Int J Evid Based Healthc. 2015;13(3):179–187. doi: 10.1097/XEB.0000000000000062 [DOI] [PubMed] [Google Scholar]
- 25.Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. doi: 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- 26.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): the TRIPOD Statement. J Br Surg. 2015;162(1):55–63. doi: 10.7326/M14-0697 [DOI] [PubMed] [Google Scholar]
- 27.Jaafar IA, Zainal Abidin N, Mohd Jamil J. Modelling the prediction of dengue outbreak using system dynamics approach. J Teknol. 2016;78(6–4). doi: 10.11113/jt.v78.8984 [DOI] [Google Scholar]
- 28.Salim NAM, Wah YB, Reeves C, et al. Prediction of dengue outbreak in Selangor Malaysia using machine learning techniques. Sci Rep. 2021;11(1):939. doi: 10.1038/s41598-020-79193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordin NI, Mohd Sobri N, Ismail NA, Zulkifli SN, Abd Razak NF, Mahmud M. The classification performance using support vector machine for endemic dengue cases. J Phys Conf Ser. 2020;1496(1):012006. [Google Scholar]
- 30.Yavari Nejad F, Varathan KD. Identification of significant climatic risk factors and machine learning models in dengue outbreak prediction. BMC Med Inform Decis Mak. 2021;21(1):141. doi: 10.1186/s12911-021-01493-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siriyasatien P, Phumee A, Ongruk P, Jampachaisri K, Kesorn K. Analysis of significant factors for dengue fever incidence prediction. BMC Bioinform. 2016;17:166. doi: 10.1186/s12859-016-1034-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kesorn K, Ongruk P, Chompoosri J, et al. Morbidity rate prediction of Dengue Hemorrhagic Fever (DHF) using the support vector machine and the Aedes aegypti infection rate in similar climates and geographical areas. PLoS One. 2015;10(5):e0125049. doi: 10.1371/journal.pone.0125049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y, Liu X, Kok SY, et al. Three-month real-time dengue forecast models: an early warning system for outbreak alerts and policy decision support in Singapore. Environ Health Perspect. 2016;124(9):1369–1375. doi: 10.1289/ehp.1509981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen P, Fu X, Ma S, et al. Early dengue outbreak detection modeling based on dengue incidences in Singapore during 2012 to 2017. Stat Med. 2020;39(15):2101–2114. doi: 10.1002/sim.8535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buczak AL, Baugher B, Babin SM, et al. Prediction of high incidence of dengue in the Philippines. PLoS Negl Trop Dis. 2014;8(4):e2771. doi: 10.1371/journal.pntd.0002771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colon-Gonzalez FJ, Soares Bastos L, Hofmann B, et al. Probabilistic seasonal dengue forecasting in Vietnam: a modelling study using superensembles. PLoS Med. 2021;18(3):e1003542. doi: 10.1371/journal.pmed.1003542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang FS, Tseng YT, Hsu PS, Chen CD, Lian IB, Chao DY. Re-assess vector indices threshold as an early warning tool for predicting dengue epidemic in a dengue non-endemic country. PLoS Negl Trop Dis. 2015;9(9):e0004043. doi: 10.1371/journal.pntd.0004043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo P, Liu T, Zhang Q, et al. Developing a dengue forecast model using machine learning: a case study in China. PLoS Negl Trop Dis. 2017;11(10):e0005973. doi: 10.1371/journal.pntd.0005973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patil S, Pandya S. Forecasting dengue hotspots associated with variation in meteorological parameters using regression and time series models. Front Public Health. 2021;9:798034. doi: 10.3389/fpubh.2021.798034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Withanage GP, Viswakula SD, Nilmini Silva Gunawardena YI, Hapugoda MD. A forecasting model for dengue incidence in the District of Gampaha, Sri Lanka. Parasit Vectors. 2018;11(1):262. doi: 10.1186/s13071-018-2828-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao N, Charland K, Carabali M, et al. Machine learning and dengue forecasting: comparing random forests and artificial neural networks for predicting dengue burden at national and sub-national scales in Colombia. PLoS Negl Trop Dis. 2020;14(9):e0008056. doi: 10.1371/journal.pntd.0008056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowman LR, Tejeda GS, Coelho GE, et al. Alarm variables for dengue outbreaks: a multi-centre study in Asia and Latin America. PLoS One. 2016;11(6):e0157971. doi: 10.1371/journal.pone.0157971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussain-Alkhateeb L, Kroeger A, Olliaro P, et al. Early warning and response system (EWARS) for dengue outbreaks: recent advancements towards widespread applications in critical settings. PLoS One. 2018;13(5):e0196811. doi: 10.1371/journal.pone.0196811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Centre for Disease Prevention and Control. Geographical distribution of dengue cases reported worldwide, October to December 2021; 2022. Available from: https://www.ecdc.europa.eu/en/dengue-monthly. Accessed January 25, 2022.
- 45.Balajee SA, Arthur R, Mounts AW. Global health security: building capacities for early event detection, epidemiologic workforce, and laboratory response. Health Secur. 2016;14(6):424–432. doi: 10.1089/hs.2015.0062 [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Early Detection, Assessment and Response to Acute Public Health Events: Implementation of Early Warning and Response with a Focus on Event-Based Surveillance: Interim Version. Geneva: World Health Organization; 2014. [Google Scholar]
- 47.World Health Organization. Protocol for Assessing National Surveillance and Response Capacities for the IHR (2005). Geneva: World Health Organization; 2010. [Google Scholar]
- 48.Li Y, Dou Q, Lu Y, Xiang H, Yu X, Liu S. Effects of ambient temperature and precipitation on the risk of dengue fever: a systematic review and updated meta-analysis. Environ Res. 2020;191:110043. [DOI] [PubMed] [Google Scholar]
- 49.Baharom M, Ahmad N, Hod R, Arsad F, Tangang F. The impact of meteorological factors on communicable disease incidence and its projection: a systematic review. Int J Environ Res Public Health. 2021;18:11117. doi: 10.3390/ijerph182111117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan J, Wei W, Bai Z, et al. A systematic review and meta-analysis of dengue risk with temperature change. Int J Environ Res Public Health. 2014;12(1):1–15. doi: 10.3390/ijerph120100001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Couret J, Benedict MQ. A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: culicidae). BMC Ecol. 2014;14(1):3. doi: 10.1186/1472-6785-14-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinhold JM, Lazzari CR, Lahondère C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: a review. Insects. 2018;9(4):158. doi: 10.3390/insects9040158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowley WA, Graham CL. The effect of temperature and relative humidity on the flight performance of female Aedes aegypti. J Insect Physiol. 1968;14(9):1251–1257. doi: 10.1016/0022-1910(68)90018-8 [DOI] [PubMed] [Google Scholar]
- 54.Rohani A, Wong YC, Zamre I, Lee HL, Zurainee MN. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.). Southeast Asian J Trop Med Public Health. 2009;40(5):942–950. [PubMed] [Google Scholar]
- 55.Xiao F-Z, Zhang Y, Deng Y-Q, et al. The effect of temperature on the extrinsic incubation period and infection rate of dengue virus serotype 2 infection in Aedes albopictus. Arch Virol. 2014;159(11):3053–3057. doi: 10.1007/s00705-014-2051-1 [DOI] [PubMed] [Google Scholar]
- 56.Nayak M, Narayan KA. Prediction of dengue outbreaks in Kerala state using disease surveillance and meteorological data. Int J Community Med Public Health. 2019;6:4392. doi: 10.18203/2394-6040.ijcmph20194500 [DOI] [Google Scholar]
- 57.Liu D, Guo S, Zou M, et al. A dengue fever predicting model based on Baidu search index data and climate data in South China. PLoS One. 2019;14:e0226841. doi: 10.1371/journal.pone.0226841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delua J Supervised vs. unsupervised learning: what’s the difference?; 2021. Available from: https://www.studocu.com/row/document/air-university/business-mathematics/supervised-vs-unsupervised-learning-what-s-the-difference-ibm-24062021-035331-pm/19270091. Accessed January 25, 2022.
- 59.Sylvestre E, Joachim C, Cécilia-Joseph E, et al. Data-driven methods for dengue prediction and surveillance using real-world and Big Data: a systematic review. PLoS Negl Trop Dis. 2022;16(1):e0010056–e0010056. doi: 10.1371/journal.pntd.0010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearl J. Causality: Models, Reasoning and Inference. 2nd ed. Cambridge University Press; 2009. [Google Scholar]
- 61.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. doi: 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paez A. Gray literature: an important resource in systematic reviews. J Evid Based Med. 2017;10(3):233–240. doi: 10.1111/jebm.12266 [DOI] [PubMed] [Google Scholar]