Abstract
The new 8-methoxyquinolone moxifloxacin was tested against two ciprofloxacin-susceptible Staphylococcus aureus strains (strains P8 and COL) and two ciprofloxacin-resistant derivatives of strain P8 carrying a single grlA mutation (strain P8-4) and double grlA and gyrA mutations (strain P8-128). All strains were resistant to methicillin. The MICs of ciprofloxacin and moxifloxacin were 0.5 and 0.125 mg/liter, respectively, for P8; 0.25 and 0.125 mg/liter, respectively, for COL; 8 and 0.25 mg/liter, respectively, for P8-4; and ≥128 and 2 mg/liter, respectively, for P8-128. In vitro, the rate of spontaneous resistance of P8 and COL was 10−7 on agar plates containing ciprofloxacin at two times the MIC, whereas it was ≤10−10 on agar plates containing moxifloxacin at two times the MIC. Rats with experimental aortic endocarditis were treated with doses of drugs that simulate the kinetics in humans: moxifloxacin, 400 mg orally once a day; ciprofloxacin, 750 mg orally twice a day; or vancomycin, 1 g intravenously twice a day. Treatment was started either 12 or 24 h after infection and lasted for 3 days. Moxifloxacin treatment resulted in culture-negative vegetations in a total of 20 of 21 (95%) rats infected with P8, 10 of 11 (91%) rats infected with COL, and 19 of 24 (79%) rats infected with P8-4 (P < 0.05 compared to the results for the controls). In contrast, ciprofloxacin treatment sterilized zero of nine (0%) vegetations infected with first-level resistant mutant P8-4. Vancomycin sterilized only 8 of 15 (53%), 6 of 11 (54%), and 12 of 23 (52%) of the vegetations, respectively. No moxifloxacin-resistant derivative emerged among these organisms. However, moxifloxacin treatment of highly ciprofloxacin-resistant mutant P8-128 failed and selected for variants for which the MIC increased two times in 2 of 10 animals. Thus, while oral moxifloxacin might deserve consideration as treatment for staphylococcal infections in humans, caution related to its use against strains for which MICs are borderline is warranted.
In recent years, major efforts have been made to improve the spectra of activity of quinolones against gram-positive organisms. In the case of levofloxacin, the amelioration consisted of the selection of the most active stereoisomer from the previous racemic mixture, ofloxacin (8). For most other compounds, such as sparfloxacin (22), trovafloxacin (4), moxifloxacin (7), and others, the improvement involved more profound alterations of the quinolone nucleus.
Importantly, while these modifications substantially enhanced the activities of the drugs against gram-positive bacteria, they only moderately affected their effect against gram-negative organisms (2, 19). For instance, the MICs of these newer agents for quinolone-susceptible Staphylococcus aureus and streptococci were at least 10 times lower than those of ciprofloxacin (i.e., ca. 0.05 and 0.5 to 2 mg/liter, respectively). In comparison, their MICs for Escherichia coli and most other gram-negative pathogens were only two to four times greater than those of older compounds.
However, despite their improved activities, newer quinolones still carry the risk of resistance selection, particularly in gram-positive pathogens that have already acquired intermediate resistance (MICs, 2 to 8 mg/liter) or high levels of resistance (MICs, >8 mg/liter) to ciprofloxacin, as described previously (30). This is a genuine clinical problem, especially with methicillin-resistant S. aureus (MRSA) isolates, which are widely resistant to ciprofloxacin (12). Therefore, newer quinolones should be evaluated not only for their toxicities but also for their propensity to select for resistance to the new drug and their intrinsic efficacies against organisms resistant to this drug family.
Quinolone resistance in S. aureus has been well studied. It can result from a combination of a variety of mechanisms, including overexpression of the efflux pump NorA (33) and structural mutations in the topoisomerase IV (grlA and grlB) and gyrase (gyrA and gyrB) genes (15, 17). Ciprofloxacin and other older quinolones readily select for such alterations, yielding highly resistant organisms after only a few serial exposures to the drug (13, 14). Newer quinolones are less susceptible to NorA-mediated drug efflux (26) and have better affinities for their topoisomerase and gyrase targets (25, 29). However, despite their improved activities, newer quinolones do suffer from increased MICs for ciprofloxacin-resistant bacteria (21, 30). While such increases may remain within the susceptible range for the newer compounds, they are a matter of concern because they may put the newer drugs on the brink of clinical efficacy.
In the study described here, we studied the efficacy of the new 8-methoxy quinolone moxifloxacin compared to those of ciprofloxacin and vancomycin against such organisms. The test bacteria included a set of well-characterized ciprofloxacin-susceptible and ciprofloxacin-resistant isogenic MRSA isolates carrying various mutations in their topoisomerase IV and gyrase genes. The risk of resistance selection and the therapeutic efficacy were determined both in vitro and in a rat model of experimental endocarditis.
(A part of these results were presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy [J. M. Entenza, M. Giddey, M. P. Glauser, P. Moreillon, Abstr. 38th Intersci Conf. Antimicrob Agents Chemother., abstr. B-78, 1998], San Diego, Calif., 24 to 27 September 1998.)
MATERIALS AND METHODS
Microorganisms, growth conditions, and antibiotics.
The bacteria used in the animal experiments are listed in Table 1. They included two ciprofloxacin-susceptible clinical isolates of MRSA (strains COL and P8) previously used for experimental endocarditis (13, 14) and two ciprofloxacin-resistant laboratory derivatives of strain P8 (strains P8-4 and P8-128), selected by serial exposure of the P8 parent to this drug (12; J. M. Entenza, M. Blatter, P. Francioli, M. P. Glauser, and P. Moreillon, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B9, p. 23, 1996). In addition, the quinolone MICs were determined for a panel of 20 ciprofloxacin-susceptible clinical strains of staphylococci (10 methicillin-susceptible S. aureus strains and 10 MRSA strains) and 12 ciprofloxacin-resistant MRSA strains from our strain collection (13, 14). Bacteria were grown at 35°C either in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) with aeration in a shaking incubator at 120 rpm or on Columbia agar plates (Becton Dickinson Microbiology Systems, Cockeysville, Md.). Bacterial stocks were kept at −70°C in Mueller-Hinton broth supplemented with 10% (vol/vol) glycerol.
TABLE 1.
MICs of various antibiotics for the S. aureus isolates tested in animals with experimental endocarditis and mutations identified in the QRDRs of the grlA and gyrA genes
| MRSA strain | MIC (mg/liter)
|
Mutation identified in QRDRs of grlA and gyrA genes
|
|||
|---|---|---|---|---|---|
| Ciprofloxacin | Moxifloxacin | Vancomycin | grlA | gyrA | |
| COL | 0.25 | 0.12 | 2 | —a | — |
| P8 | 0.5 | 0.12 | 1 | — | — |
| P8-4 | 8 | 0.25 | 1 | Ser-80→Phe | — |
| P8-128 | 128 | 2 | 1 | Ser-80→Phe | Ser-85→Pro |
—, no change.
Moxifloxacin and ciprofloxacin were provided by Bayer AG (Wuppertal, Germany), and vancomycin was purchased from Eli Lilly (Geneva, Switzerland). Before being diluted to the desired concentrations, moxifloxacin was solubilized in 1 M sodium hydroxide and ciprofloxacin and vancomycin were solubilized in sterile water.
Susceptibility testing and time-kill curves.
The MICs were determined by a previously described broth macrodilution method (13, 23), with a final inoculum of 105 to 106 CFU/ml. For time-kill curve studies, series of flasks containing fresh prewarmed medium were inoculated with ca. 106 CFU/ml (final concentration) from an overnight culture of bacteria, and the bacteria were further incubated at 35°C with aeration. Antibiotics were added at various times during normal bacterial growth at final concentrations that approximate the peak levels achieved in the serum of humans during standard therapy (see Results section). The concentrations were (i) 3 mg/liter for moxifloxacin (27, 28; H. Kubitza, H. Stass, W. Wingender, and J. Kuhlmann, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F25, p. 104, 1996), (ii) 2 mg/liter for ciprofloxacin (9), and (iii) 40 mg/liter for vancomycin (3). Viable counts were determined at various times before and after drug addition by subculturing on agar plates. Antibiotic carryover was avoided as described previously (13, 14). All determinations were done in triplicate.
Nucleotide sequences of quinolone target genes.
Preparation of chromosomal DNA and selection of oligonucleotide primers and PCR conditions for amplification of the quinolone resistance-determining regions (QRDRs) of the grlA and gyrA genes were as described previously (15, 20, 24). Nucleotide sequencing was carried out in both directions with a Perkin-Elmer automatic sequencer (ABI Prism 377 DNA sequencer).
Production and treatment of experimental endocarditis.
The production of catheter-induced aortic vegetations in the rats and the installation of the programmable infusion pump (Pump 44; Harvard Apparatus, Inc., South Natick, Mass.) for the delivery of the antibiotics were performed as described previously (16, 18). Bacterial endocarditis was induced 24 h after catheterization by intravenous (i.v.) challenge of the animals with 0.5 ml of saline containing 105 CFU of either test MRSA isolate. This inoculum was 10 times larger than the minimum inoculum that produces endocarditis in 90% of the untreated rats.
Treatment was started either early (12 h) or relatively late (24 h) after bacterial challenge and lasted for 3 days. Antibiotics were delivered at changing flow rates and at doses that simulated their kinetics in the serum of humans, as follows: (i) 400 mg of moxifloxacin given orally every 24 h (27, 28; Kubitza et al., 36th ICAAC), (ii) 750 mg of ciprofloxacin given orally every 12 h (8), or (iii) 1 g of vancomycin given i.v. every 12 h (2). This required total drug amounts (in milligrams per kilogram of body weight per 24 h) of 133 mg of moxifloxacin, 63.2 mg of ciprofloxacin, and 109.6 mg of vancomycin.
Pharmacokinetic studies.
The concentrations of antibiotics in serum were determined on day 2 of therapy for groups of three to six uninfected or infected rats. The levels in the sera of infected animals were determined by use of internal controls for the therapeutic experiments, in which the adequacy of drug delivery was tested routinely. Blood was drawn by puncturing the periorbital sinuses of the animals (one puncture per animal) at several time points during and after antibiotic administration. Antibiotic concentrations were determined by an agar diffusion bioassay with antibiotic medium 1 (Difco Laboratories) and Bacillus subtilis ATCC 6633 as the indicator organism. The diluent was pooled rat serum. The limits of detection of the assays were 0.15 mg/liter for moxifloxacin, 0.12 mg/liter for ciprofloxacin, and 0.6 mg/liter for vancomycin. The linearity of the standard curves was assessed by a regression coefficient of ≥0.994, and intraplate and interplate variations were ≤10%. The area under the serum concentration-time curve (AUC) from time zero to 24 h (AUC0–24) was calculated by the trapezoidal summation method only for moxifloxacin and ciprofloxacin.
Evaluation of infection.
Control rats were killed at various times after inoculation. To determine the rate of intravegetation growth, groups of three rats were killed at various time points over 48 h postinfection. Controls for therapeutic experiments were killed at the start of treatment to assess the frequency and severity of valve infection at that time. Treated rats were killed 8 h after the trough level in serum after administration of the last antibiotic dose was achieved. At that time, no residual antibiotic was detected in serum.
Vegetations, blood, and spleens were cultured as described previously (13, 14). Few animals died before the end of treatment due to either complications of the operation itself (such as possible catheter-induced arrhythmia) or the infection process, or both. Only rats that received at least two-thirds of the treatment were considered for determination of bacterial counts in the vegetations. Bacterial densities in the organs were expressed as log10 CFU per gram of tissue. The minimum detection level was ≥2 log10 CFU/g of vegetation. For statistical comparisons, culture-negative vegetations were considered to contain 2 log10 CFU/g.
Selection of antibiotic resistance in vivo and in vitro.
The emergence of resistance to moxifloxacin and ciprofloxacin during therapy was determined by plating 0.1-ml portions from each undiluted vegetation homogenate on agar plates supplemented with the drugs at two to four times the MICs. For vancomycin, standard MICs were determined for colonies (approximately 1 of 100) randomly picked from the plates of positive valve cultures.
The propensities of the test quinolones to select for resistance in vitro were determined by previously described methods (13, 14). First, large inocula (ca. 1010 CFU) were spread onto agar plates containing the drugs at 2 to 16 times the MICs for the test organisms, and the plates were incubated for 48 h at 35°C before the colonies were counted. Colonies growing on antibiotic-containing plates were retested for the new, increased MICs for the colonies. Second, selection for resistance was also performed in broth cultures by serial exposure of the bacteria to twofold stepwise increasing antibiotic concentrations. The stabilities of resistant derivatives were assessed by subculturing the organisms for up to five passages on drug-free medium.
Statistical analysis.
The incidences of valve infection were compared by Fisher's exact test with Bonferroni's correction for multiple group comparisons. The Mann-Whitney rank sum test for comparison of differences between residual bacterial titers in the vegetations was used in certain analyses. Differences were considered significant when P was ≤0.05 by use of two-tailed significance levels.
RESULTS
Antibiotic susceptibility and resistance mutations in grlA and gyrA genes.
The MICs of the antibiotics for the four isolates tested in animals are presented in Table 1. All isolates were susceptible to vancomycin. With regard to quinolones, on the other hand, the susceptibilities varied as a function of both the type of drugs and the QRDR mutations. For the two ciprofloxacin-susceptible isolates, the moxifloxacin MICs were two to fourfold lower than those of ciprofloxacin. This magnitude of difference was also observed for 20 additional ciprofloxacin-susceptible, methicillin-susceptible S. aureus and MRSA isolates, for which the median MIC at which 90% of bacteria were inhibited (MIC90) by moxifloxacin and ciprofloxacin were 0.06 mg/liter (range, 0.06 to 0.12 mg/liter) and 0.5 mg/liter (range, 0.25 to 1 mg/liter), respectively.
Moxifloxacin retained its superior activity against the ciprofloxacin-resistant derivatives. Indeed, while a single grlA mutation (Ser-80 to Phe) increased the MIC of ciprofloxacin 16 times (from 0.5 to 8 mg/liter), it increased the MIC of moxifloxacin only 2 times (from 0.12 to 0.25 mg/liter). Likewise, a double grlA and gyrA mutation (Ser-80 to Phe and Ser-85 to Pro, respectively) increased the MIC of ciprofloxacin 258 times but increased the MIC of moxifloxacin only 16 times. This improved efficacy was again confirmed against 12 clinical isolates of ciprofloxacin-resistant MRSA, for which the MIC90 of moxifloxacin was 16 mg/liter (range, 0.25 to 32 mg/liter), whereas the ciprofloxacin MIC90 was >128 mg/liter (range, 4 to >128 mg/liter). It is noteworthy, however, that the clinical isolates of ciprofloxacin-resistant staphylococci tended to be less susceptible to moxifloxacin than the well-characterized laboratory mutants, an observation that was also reported for other new quinolones such as trovafloxacin (14).
Time-kill experiments.
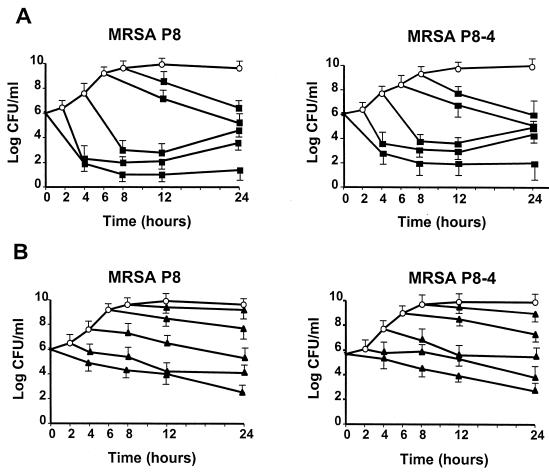
Figure 1 indicates that moxifloxacin at simulated peak concentrations in serum (3 mg/liter) was rapidly bactericidal for both the ciprofloxacin-susceptible parent strain (strain P8) and its first-level resistant derivative (strain P8-4). This was observed during both the exponential and the postexponential phases of growth. It underlines the activity of the drug against rapidly growing as well as slowly growing organisms. In some experiments, late bacterial regrowth was observed. This was not due to the emergence of resistance but, rather, to slow drug degradation.
FIG. 1.
Time-kill experiments performed with peak levels of moxifloxacin (3 mg/liter) (A) and vancomycin (40 mg/liter) (B) in serum against ciprofloxacin-susceptible MRSA isolate P8 and ciprofloxacin-resistant MRSA derivative P8-4 (a grlA mutant). Antibiotics were added to the cultures at various times of bacterial growth, and the number of surviving organisms over time was determined by counting the colonies on agar plates. The results are the means ± standard deviations of three independent experiments.
In comparison, the peak concentration of ciprofloxacin (2 mg/liter) showed slightly decreased bactericidal activity against the parent strain and was ineffective against P8-4 (data not presented). Vancomycin (40 mg/liter) was only slowly bactericidal.
Moxifloxacin behaved similarly against ciprofloxacin-susceptible isolate COL (Table 1). However, it failed to kill the highly resistant derivative, strain P8-128 (data not shown).
Pharmacokinetic studies.
The concentrations of antibiotics in the serum of rats simulated the kinetics in human serum during standard therapy (see Materials and Methods). The peak and trough concentrations (means ± standard deviations for 6 to 16 individual determinations for each drug) were as follows: (i) for moxifloxacin, 3.04 ± 0.6 and 0.71 ± 0.18 mg/liter at 1.5 and 24 h, respectively; (ii) for ciprofloxacin, 2.47 ± 0.05 and 0.28 ± 0.08 mg/liter at 1.2 and 12 h, respectively; and (iii) for vancomycin, 55.9 ± 8.7 and 11.1 ± 2.4 mg/liter at 1 and 12 h, respectively. The AUC0–24 values for moxifloxacin and ciprofloxacin were 27.3 and 10.7 mg · h/liter, respectively; i.e., they were close to the reported values for these drugs (9, 27, 28).
Therapy of experimental endocarditis due to ciprofloxacin-susceptible isolates.
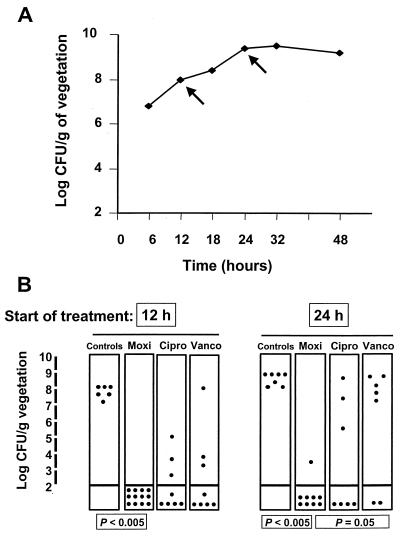
In a first series of experiments, rats with catheter-induced vegetations were inoculated with ciprofloxacin-susceptible MRSA strain P8 and treatment was started either 12 or 24 h after bacterial challenge. Figure 2A indicates that these two time points corresponded to the exponential and postexponential growth phases, respectively, in the vegetation milieu. Figure 2B indicates that moxifloxacin was very effective in both of these conditions. In contrast, ciprofloxacin and vancomycin tended to be less effective, especially when therapy was started after 24 h. In vancomycin-treated animals, the difference between early and late treatment was at the verge of statistical significance (P = 0.054 when the results were compared by the Mann-Whitney test). Moreover, animals treated with vancomycin after 24 h did significantly less well than those treated with moxifloxacin (P < 0.05). No treatment failures were due to drug-resistant derivatives.
FIG. 2.
Intravegetation growth curve (A) and therapeutic results (B) for experimental endocarditis due to ciprofloxacin-susceptible MRSA strain P8. Rats were treated for 3 days with moxifloxacin (Moxi; 400 mg orally once a day), ciprofloxacin (Cipro; 750 mg orally every 12 h), or vancomycin (Vanco; 1 g i.v. every 12 h) at doses that resulted in kinetics similar to those achieved in humans. Therapy was started either early (12 h) or relatively late (24 h) after bacterial challenge (arrows on the growth curve). Each dot represents the bacterial density in the vegetation of a single animal. P values of ≤0.05 indicate statistically significant differences when groups were compared by Fisher's exact test with Bonferroni's correction for multiple group comparisons.
The good efficacy of moxifloxacin against ciprofloxacin-susceptible S. aureus was also demonstrated against MRSA COL. In an early-therapy experiment (at 12 h) with this bacterium, moxifloxacin cured 10 of 11 (91%) rats, whereas all 6 of 6 (100%) untreated controls were infected (P < 0.005). In comparison, ciprofloxacin cured only 5 of 8 (62%) rats and vancomycin cured 6 of 11 (54.5%) rats. However, this lower efficacy was only a trend and did not reach statistical significance compared to the results of moxifloxacin treatment (P > 0.05).
Therapy of experimental endocarditis due to ciprofloxacin-resistant isolates.
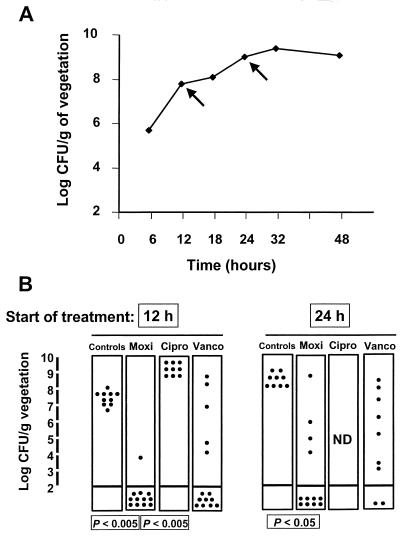
The therapeutic experiments starting 12 and 24 h after bacterial challenge were repeated with the first-level ciprofloxacin-resistant derivative, strain P8-4. Figure 3 indicates that moxifloxacin remained very effective against this organism. In contrast, vancomycin was generally less efficacious and ciprofloxacin was totally inactive. As described above, the lower level of efficacy with a delay in treatment tended to be more pronounced with vancomycin than with moxifloxacin.
FIG. 3.
Intravegetation growth curve (A) and therapeutic results (B) for experimental endocarditis due to ciprofloxacin-resistant MRSA isolate P8-4 (grlA mutant). Therapy was started either early (12 h) or relatively late (24 h) after bacterial challenge (arrows on the growth curve). Details are as described in the legend to Fig. 2.
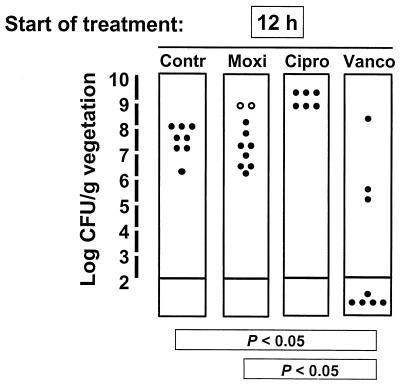
A few explorative experiments were performed with highly ciprofloxacin-resistant derivative P8-128. Figure 4 indicates that despite the relatively low moxifloxacin MIC for the organism, moxifloxacin treatment failed and the emergence of staphylococcal variants with decreased susceptibility to this antibiotic was observed in 2 of 10 treatment failures. Importantly, this could be predicted from the pharmacodynamic parameters presented in Table 2, thus supporting the importance of consideration of these characteristics for determination of the adequacy of antibiotic therapy.
FIG. 4.
Therapeutic results for experimental endocarditis due to ciprofloxacin-resistant MRSA isolate P8-128 carrying double grlA and gyrA mutations. Therapy was started early (12 h) after bacterial challenge. Open dots indicate the vegetations which grew staphylococcal derivatives for which the moxifloxacin MIC had increased twofold after 3 days of therapy. Details are as described in the legend to Fig. 2.
TABLE 2.
Relation between pharmacodynamic parameters, therapeutic efficacy, and selection for resistance in experimental endocarditisa
| MRSA strain | Drugb | Cmax/MIC ratio | AUC/MIC ratio | % Sterile vegetations | Selection of resistance |
|---|---|---|---|---|---|
| COL | CIP | >8 | <100 | 63 | Noc |
| MXF | >8 | ≥100 | 100 | No | |
| P8 | CIP | <8 | <100 | 63 | Noc |
| MXF | >8 | ≥100 | 100 | No | |
| P8-4 | CIP | <8 | <100 | 0 | NDd |
| MXF | >8 | ≥100 | 92 | No | |
| P8-128 | CIP | <8 | <100 | 0 | NDc |
| MXF | <8 | <100 | 0 | Yes |
The in vivo results were compared with the parameters recently proposed by Andes and Craig (1) for prediction of fluoroquinolone treatment success (Cmax/MIC and AUC/MIC ratios of 8 and ≥ 100, respectively) in experimental models of endocarditis.
CIP, ciprofloxacin; MXF, moxifloxacin.
ND, not done.
Blood and spleen cultures.
Organ cultures reflected the observations in the vegetations. The results presented in Fig. 2 and 3 were analyzed together. While all (32 of 32) untreated controls had positive blood and spleen cultures, among the animals in the treated groups, only rats with ≥5.6 log CFU/g of vegetation grew organisms from the spleens (but not the blood). This represented only 2 of 45 (4%) animals treated with moxifloxacin, whereas it represented 11 of 24 (46%) of rats treated with ciprofloxacin (P < 0.05) and 13 of 38 (34%) of rats treated with vancomycin (P < 0.05). These data validated the generally good performance of moxifloxacin against the susceptible organisms. On the other hand, blood and spleen cultures were all positive for the resistant isolate P8-128, as presented in Fig. 4.
Relation between pharmacodynamic parameters, therapeutic efficacy, and selection for resistance in vivo.
Table 2 compares the in vivo results with the pharmacodynamic parameters recently proposed by Andes and Craig (1) for prediction of fluoroquinolone treatment success in experimental endocarditis. Those investigators suggested that maximum concentration of drug in serum (Cmax)/MIC and AUC/MIC ratios of >8 and ≥100, respectively, are needed for treatment efficacy. It can be seen that these values predicted treatment success in the present experiments as well. Importantly, all animals for which both values were above the predictive limits (ratios of 8 and 100, respectively) responded to therapy. In contrast, for animals for which either one or both of these values were below the limits, the result was suboptimal therapy and/or resistance selection.
Selection for moxifloxacin and ciprofloxacin resistance in vitro.
In a first series of tests, large bacterial inocula (ca. 1010 CFU) were spread onto antibiotic-containing agar plates. Drug concentrations were used in multiples of the MICs to ensure that the antibiotic pressures applied to the organisms were comparable between the two drugs. In this test, both ciprofloxacin-susceptible MRSA P8 and COL grew colonies on plates containing ciprofloxacin at eight times the MIC at a frequency of 10−7 of the original inoculum. Upon redetermination of the MIC, all these variants were highly resistant to ciprofloxacin (MICs, >32 mg/liter) but were still susceptible to moxifloxacin (MICs, 0.25 to 0.5 mg/liter). The same test performed with moxifloxacin indicated that the rate of spontaneous resistance to this drug was ≤10−10, even when selection was on plates containing the compound at only two times the MIC.
For the ciprofloxacin-resistant derivatives, both strain P8-4 and strain P8-128 grew spontaneous mutants at a frequency of 10−8 on plates containing moxifloxacin at two to four times the MIC. This low increase in resistance was unchanged when the MICs for the derivatives was rested in liquid medium. No organisms grew on plates containing higher drug concentrations.
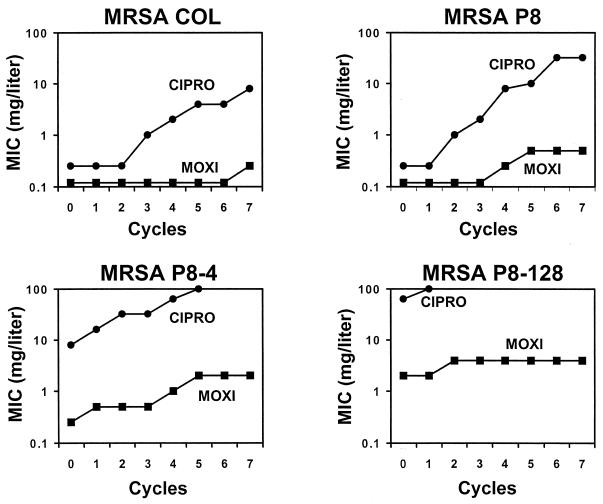
In a second study, the four isolates were repeatedly exposed to twofold stepwise increasing concentrations of ciprofloxacin and moxifloxacin, as described previously (13, 14). Figure 5 indicates that exposure to ciprofloxacin resulted in the rapid emergence of organisms with high levels of resistance. In contrast, moxifloxacin was notably less likely than ciprofloxacin to select for resistance. Most importantly, the ciprofloxacin-resistant laboratory mutants were not more likely than their susceptible parents to develop moxifloxacin resistance after multiple passages. Thus, moxifloxacin was much less likely than ciprofloxacin to select for quinolone resistance in this setting. All derivatives with decreased susceptibilities to quinolones were stable for up to five passages on antibiotic-free plates, as determined in tests with 20 individual derivatives randomly isolated from the plates.
FIG. 5.
Selection of resistance to moxifloxacin (MOXI) and ciprofloxacin (CIPRO) during exposure of ciprofloxacin-susceptible MRSA isolates COL and P8 and ciprofloxacin-resistant derivatives of P8, strains P8-4 and P8-128, to stepwise increasing concentrations of the drugs. Series of tubes containing twofold increasing drug concentrations were inoculated with a final concentration of 106 CFU of the test organisms per ml and were further incubated for 24 h at 35°C, like for the MIC tests. On the next day, the tubes with the highest antibiotic concentration still showing turbidity were used to inoculate a new series of antibiotic-containing tubes. The procedure was repeated and the increase in the MIC was monitored over time.
DISCUSSION
The present results underline the excellent in vitro and in vivo activities of moxifloxacin against both ciprofloxacin-susceptible and first-level ciprofloxacin-resistant S. aureus isolates. The drug was bactericidal during both the exponential and the postexponential growth phases in vitro, as well as with early and relatively late treatment onset in vivo. This suggests that moxifloxacin might overcome, to a certain extent, the intrinsic killing resistance of slowly growing bacteria (referred to as phenotypic tolerance) (31) to several antibiotic classes, including quinolones (6, 11, 31, 32).
In the present work, the effect of the intravegetation growth rate on treatment success was less pronounced for the quinolones than for vancomycin. Enhanced killing of slowly growing bacteria might be of clinical importance, especially for therapy for dormant bacteria infecting implanted foreign materials (32, 34). One issue is whether more prolonged treatment with vancomycin would have improved the results obtained with this compound. This is likely to be the case (5). However, it would not have altered the present observation that moxifloxacin was more rapidly bactericidal than vancomycin in this infection model. Since the present results were obtained with a pharmacokinetic-pharmacodynamic setting mimicking that expected in humans, they might represent one potential advantage of the newer quinolones against infections caused by gram-positive bacteria.
Aside from these positive aspects, two crucial questions must be addressed when studying the activities of newer quinolones against gram-positive pathogens: first, their intrinsic risk of resistance selection and, second, their activities against bacteria already resistant to older quinolones due to previous exposure to this class of molecules.
The present results indicate that first-level moxifloxacin-resistant variants emerged at a very low rate (<10−10) and that higher-level resistant derivatives (MICs, >2 mg/liter) were not selected by either the plating or the serial passage techniques used in this study. This was very different from the results achieved with ciprofloxacin, which rapidly selected for high-level resistance to itself. This was also different from the results obtained with levofloxacin and trovafloxacin, which both selected for resistance in similar types of experiments (13, 14). Previous studies indicated that ciprofloxacin therapy could select for ciprofloxacin-resistant S. aureus and viridans group streptococci in experimental endocarditis (10, 13, 14). This might reflect both its intrinsic trend to select for resistance in these organisms and its suboptimal AUC/MIC ratio in vivo (Table 2) (1). Therefore, newer quinolones with better pharmacokinetic-pharmacodynamic profiles might be more appropriate than older quinolones against such pathogens.
Importantly, the moxifloxacin MIC for even the grlA and gyrA double mutant, strain P8-128, which was highly resistant to ciprofloxacin, barely increased upon repeated exposures to this drug. This was in contrast to the results obtained with an experimental quinolone, Y-688, with activity against gram-positive bacteria, which readily selected for additional resistance mutations when it was used against ciprofloxacin-resistant MRSA (12). Thus, moxifloxacin demonstrated a very low propensity to select for resistance. The precise reason for this distinct advantage over other newer quinolones remains to be determined.
Yet, despite its improved efficacy, moxifloxacin will probably not be clinically effective against staphylococci carrying more than one topoisomerase mutation. Indeed, while it retained a relatively low MIC (2 mg/liter) for the grlA and gyrA double mutant, strain P8-128, the drug failed to cure experimental endocarditis due to this laboratory isolate. Moreover, the moxifloxacin MIC90 for ciprofloxacin-resistant MRSA isolates from the clinical environment was 16 mg/liter, suggesting that these organisms had collected yet additional alterations that confer on them resistance to the new quinolone. In one of them, this was identified as a second mutation in the grlA gene (J. M. Entenza, unpublished observation).
Taken together, the results show that moxifloxacin is highly bactericidal for S. aureus and has a very low propensity to select for resistance. Moreover, it retains killing activity against slowly growing bacteria both in vitro and in vivo, an observation that should stimulate the study of this drug in models of foreign-body infections. Importantly, however, moxifloxacin will probably not be active against clinical isolates of staphylococci already highly resistant to ciprofloxacin because their susceptibility to the new drug is already out of its therapeutic range.
Such observations apply to all the newer quinolones available (12, 13, 14). They raise two fundamental questions regarding the current antimicrobial strategy. The first is whether the quinolone MIC cutoff should be reevaluated in light of the pharmacokinetic-pharmacodynamic properties of quinolones. Indeed, although moxifloxacin had a relatively low MIC (2 mg/liter) for P8-128, treatment of an infection with moxifloxacin resulted in treatment failure. Such predictable discrepancies should be taken into account when assessing therapeutic guidelines. Second, older quinolones are usually not used to treat staphylococcal infections. Therefore, most existing quinolone-resistant staphylococci were probably selected from nonpathogenic bystanders during quinolone therapy for unrelated infections (e.g., infections of the urinary tact). Hence, whenever quinolone therapy is indicated, one might consider preferential use of one of the newer compounds of this family because they are less likely to select for resistance in the resident flora. At a minimum, such a hypothesis could be tested in vivo.
ACKNOWLEDGMENTS
We thank Marlyse Giddey for outstanding technical work.
This work was partially supported by grant 32-47099.96 from the Swiss National Funds for Scientific Research.
REFERENCES
- 1.Andes D R, Craig W A. Pharmacodynamics of fluoroquinolones in experimental models of endocarditis. Clin Infect Dis. 1998;27:47–50. doi: 10.1086/514624. [DOI] [PubMed] [Google Scholar]
- 2.Bauernfeind A. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 3.Blouin R A, Bauer L A, Miller D D, Record K E, Griffen W O. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21:575–580. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brighty K E, Gootz T D. The chemistry and biological profile and trovafloxacin. J Antimicrob Chemother. 1997;39(Suppl. B):1–14. doi: 10.1093/jac/39.suppl_2.1. [DOI] [PubMed] [Google Scholar]
- 5.Cantoni L, Glauser M P, Bille J. Comparative efficacy of daptomycin, vancomycin, and cloxacillin for the treatment of Staphylococcus aureus endocarditis in rats and role of test conditions in this determination. Antimicrob Agents Chemother. 1990;34:2348–2353. doi: 10.1128/aac.34.12.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremieux A C, Saleh-Mghir A, Vallois J M, Muffat-Joly M, Devine C, Pocidalo J J, Carbon C. Influence of pretreatment duration of infection on the efficacies of various antibiotic regimens in experimental streptococcal endocarditis. J Antimicrob Chemother. 1993;32:843–852. doi: 10.1093/jac/32.6.843. [DOI] [PubMed] [Google Scholar]
- 7.Dalhoff A, Petersen U, Endermann R. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone. Chemotherapy (Basel) 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 8.Davis R, Bryson H M. Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 1994;47:677–700. doi: 10.2165/00003495-199447040-00008. [DOI] [PubMed] [Google Scholar]
- 9.Davis R, Markham A, Balfour J A. Ciprofloxacin. An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs. 1996;51:1019–1074. doi: 10.2165/00003495-199651060-00010. [DOI] [PubMed] [Google Scholar]
- 10.Entenza J M, Caldelari I, Glauser M P, Moreillon P. Efficacy of levofloxacin in the treatment of experimental endocarditis caused by viridans streptococci. J Antimicrob Chemother. 1999;44:775–786. doi: 10.1093/jac/44.6.775. [DOI] [PubMed] [Google Scholar]
- 11.Entenza J M, Fluckiger U, Glauser M P, Moreillon P. Antibiotic treatment of experimental endocarditis due to methicillin-resistant Staphylococcus epidermidis. J Infect Dis. 1994;179:100–109. doi: 10.1093/infdis/170.1.100. [DOI] [PubMed] [Google Scholar]
- 12.Entenza J M, Marchetti O, Glauser M P, Moreillon P. Y-688, a new quinolone active against quinolone-resistant Staphylococcus aureus: lack of in vivo efficacy in experimental endocarditis. Antimicrob Agents Chemother. 1998;42:1889–1894. doi: 10.1128/aac.42.8.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Entenza J M, Vouillamoz J, Glauser M P, Moreillon P. Levofloxacin versus ciprofloxacin, flucloxacillin, or vancomycin for treatment of experimental endocarditis due to methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1662–1667. doi: 10.1128/aac.41.8.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Entenza J M, Vouillamoz J, Glauser M P, Moreillon P. Efficacy of trovafloxacin in treatment of experimental staphylococcal and streptococcal endocarditis. Antimicrob Agents Chemother. 1999;43:77–84. doi: 10.1128/aac.43.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 16.Flückiger U, Francioli P, Blaser J, Glauser M P, Moreillon P. Role of amoxicillin serum levels for successful prophylaxis of experimental endocarditis due to tolerant streptococci. J Infect Dis. 1994;169:1397–1400. doi: 10.1093/infdis/169.6.1397. [DOI] [PubMed] [Google Scholar]
- 17.Fournier B, Hooper D C. Mutations in topoisomerase IV and DNA gyrase of Staphylococcus aureus: novel pleiotropic effects on quinolone and coumarin activity. Antimicrob Agents Chemother. 1998;42:121–128. doi: 10.1128/aac.42.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heraief E, Glauser M P, Freedmann L. Natural history of aortic valve endocarditis in rats. Infect Immun. 1982;37:127–131. doi: 10.1128/iai.37.1.127-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoogkamp-Korstanje J A A. In vitro activity of ciprofloxacin, levofloxacin, lomefloxacin, ofloxacin, pefloxacin, sparfloxacin and trovafloxacin against gram-positive and gram-negative pathogens from respiratory tract infections. J Antimicrob Chemother. 1997;40:427–431. doi: 10.1093/jac/40.3.427. [DOI] [PubMed] [Google Scholar]
- 20.Hori S, Ohshita Y, Utsui Y, Hiramatsu K. Sequential acquisition of norfloxacin and ofloxacin resistance by methicillin-resistant and -susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:2278–2284. doi: 10.1128/aac.37.11.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones M E, Visser M R, Klootwijk M, Heisig P, Verhoef J, Schmitz F J. Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin, sparfloxacin, and trovafloxacin and nonquinolones linezolid, quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-resistant and -susceptible Staphylococcus aureus strains. Antimicrob Agents Chemother. 1999;43:421–423. doi: 10.1128/aac.43.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto T, Matsumoto J, Chiba K, Egawa H, Shibamori K, Minamida A, Nishimura Y, Okada H, Kataoka M, Fujita M, Hirose T, Nakano J. Synthesis and structure-activity relationships of 5-substituted 6,8-difluoro-quinolones, including sparfloxacin, a new quinolone antibacterial agent with improved potency. J Med Chem. 1990;33:1645–1656. doi: 10.1021/jm00168a018. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1996. [Google Scholar]
- 24.Sambrook J, Fritsch F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Schedletzky H, Wiedemann B, Heisig P. The effect of moxifloxacin on its target topoisomerases from Escherichia coli and Staphylococcus aureus. J Antimicrob Chemother. 1999;43(Suppl. B):31–37. doi: 10.1093/jac/43.suppl_2.31. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz F J, Fluit A C, Lückefahr M, Engler B, Hofmann B, Verhoef J, Heinz H P, Hadding U, Jones M E. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in-vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1998;42:807–810. doi: 10.1093/jac/42.6.807. [DOI] [PubMed] [Google Scholar]
- 27.Stass H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan J T, Woodruff M, Lettieri J, Agarwal V, Krol G J, Leese P T, Watson S, Heller A H. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob Agents Chemother. 1999;43:2793–2797. doi: 10.1128/aac.43.11.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Onodera Y, Uchida Y, Sato K, Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2362–2366. doi: 10.1128/aac.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson K S, Sanders C C. The effects of increasing levels of quinolone resistance on in-vitro activity of four quinolones. J Antimicrob Chemother. 1998;42:179–187. doi: 10.1093/jac/42.2.179. [DOI] [PubMed] [Google Scholar]
- 31.Tuomanen E. Phenotypic tolerance: the search for β-lactam antibiotics that kill nongrowing bacteria. Rev Infect Dis. 1986;8(Suppl. 3):S279–S291. doi: 10.1093/clinids/8.supplement_3.s279. [DOI] [PubMed] [Google Scholar]
- 32.Widmer A F, Frei R, Rajacic Z, Zimmerli W. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis. 1990;162:96–102. doi: 10.1093/infdis/162.1.96. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerli W, Frei R, Widmer A F, Rajacic Z. Microbiological test to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J Antimicrob Chemother. 1994;33:959–967. doi: 10.1093/jac/33.5.959. [DOI] [PubMed] [Google Scholar]