Abstract
Recoveries of cobalt and lithium metals from spent lithium-ion batteries are very important for prevention of environmental pollution and alleviation of resource shortage. In this study, a hydrometallurgical route for the recovery of lithium fluoride was proposed. Lithium and cobalt could be first selectively leached into solution using formic acid and hydrogen peroxide. By investigating the effects of leaching temperature, time, stoichiometric ratio, H2O2 concentration and solid-to-liquid ratio, the leaching efficiency of Li and Co could reach 99.90% and 99.96%, respectively. Meanwhile, the evaluation of leaching kinetics and calculation of apparent activation energies revealed that the leaching process fitted chemical control satisfactorily. After further fractional precipitation, a high purity of 99.0% lithium fluoride could be finally obtained, thus achieving the effective recovery of spent material from the lithium-ion battery.
Recoveries of cobalt and lithium metals from spent lithium-ion batteries are very important for prevention of environmental pollution and alleviation of resource shortage.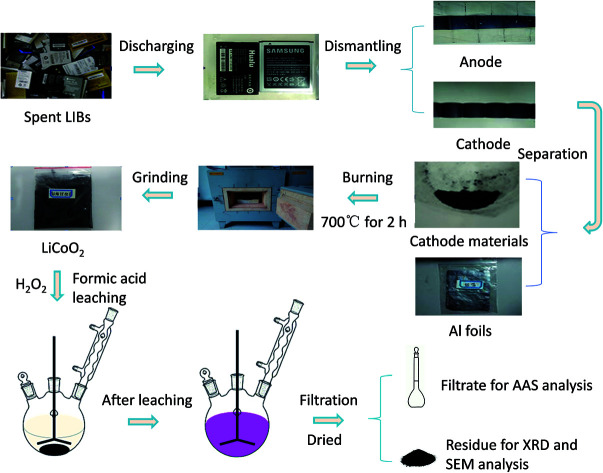
1. Introduction
Nowadays, lithium-ion batteries (LIBs) have been broadly used in various electrical equipments such as mobile devices, personal computers, electric meters, vehicles etc. It has been reported that the quantity of LIBs reached 7.8 billion in 2016 all over the world, which has grown by 40% as compared to 5.6 billion in 2015. As we know, the use of large quantities of LIBs is bound to cause a large amount of discarded batteries. A potential risk to environment and human health may develop due to a large number of potential resources containing heavy metals and poisonous organics from spent LIBs.1,2 Therefore, it is very crucial to find an advanced method for recycling LIBs.
Currently, several methods have been proposed to treat spent LIBs to recycle their valuable metals such as lithium, cobalt, nickel and manganese. The metal recovery of spent LIBs mainly includes pyro-metallurgical and hydro-metallurgical processes.3 The pyro-metallurgical process has several disadvantages, such as exhaust emissions, dust emissions, metal loss, and high energy consumption, which present a threat to our ecosystem and human health. Conversely, hydro-metallurgical process usually produces non-poisonous gases and residues, and it usually transforms toxic substances into purer forms such as salts, hydroxides and metals.4 Moreover, the hydro-metallurgical process has a bright prospect of development because of the low cost, variety of leaching liquids and increasing efficiency of leaching.
According to the literature, the leaching liquids in hydro-metallurgical processes can be classified as mineral acids, alkalis and organic acids. It has been reported that valuable metals such as cobalt and lithium can be leached out from spent LIBs using mineral acids such as H2SO4, H3PO4, and HCl and various organic acids such as succinic acid, ascorbic acid, and citric acid in the presence of reducing agents such as hydrogen peroxide (H2O2).4–7,9,11,12,14Table 1 briefly summarizes the different leaching liquids used for recycling valuable metals from spent LIBs in the last 5 years. Chen et al. published a leading paper where they have reported using dimethylglyoxime reagent to recover nickel selectively, ammonium oxalate solution to precipitate cobalt as CoC2O4·H2O and saturated sodium carbonate solution to precipitate lithium as Li2CO3 under optimized experimental conditions.4 Chen et al. used phosphoric acid and H2O2 to research leaching kinetics, and it fits the logarithmic rate kinetics model of cobalt and lithium well.6 Pagnanelli et al. utilized sulfuric acid and glucose to extract 96% of cobalt and 86% of lithium from spent LIBs.8 Ku et al. treated the waste cathode active materials, which are composites of LiCoxMnyNizO2, LiMn2O4, Al2O3 and C, with ammonia, ammonium sulfite and ammonium carbonate.10 Li et al. used succinic acid as a leaching agent and H2O2 as a reductant, and the results showed that 100% of cobalt and 96% of lithium were recovered under optimized conditions.11 Meshram et al. used an acid mixture (H2SO4 and HNO3) with glucose to recover 93.2% lithium, 90.52% cobalt, 82.8% nickel, and 77.7% manganese.13 Santana et al. used cobalt and lithium recovered from spent LIBs to form a sol–gel material, which can be used as a promising candidate for heterogeneous catalysis.14 It is clear from the above discussion that organic acids, which may effectively alleviate environment pollution during leaching, have similar performance as mineral acids.
A brief summary of different leaching systems in the last five years.
| Types | Reagents | Materials | Conditions | Efficiency |
|---|---|---|---|---|
| Mineral acid | H2SO4 & H2O2 | LiNi1/3Co1/3Mn1/3O2 | 2 M H2SO4, 2 vol% H2O2, S/L = 50 g L−1, 80 °C, 60 min | 81% Li, 98.2% Co, 98.7% Ni, 97.1% Mn |
| H3PO4 & H2O2 | LiCoO2 | 2 vol% H3PO4, 2 vol% H2O2, S/L = 8 g L−1, 90 °C, 60 min | 88% Li, 99% Co | |
| HCl | LiCoO2 | 3 M HCl, S/L = 20 g L−1, 80 °C, 90 min | 99.4% Li | |
| Alkali | Ammonia | LiNi1/3Co1/3Mn1/3O2 | 4 M NH3, 1.5 M (NH4)2SO4, 0.5 M Na2SO3, S/L = 10 g L−1, 80 °C, 300 min | 95.3% Li, 80.7% Co, 89.8% Ni, 4.3% Mn |
| Organic acid | Succinic acid & H2O2 | LiCoO2 | 1.5 mol L−1, 4 vol% H2O2, S/L = 15 g L−1, 70 °C, 40 min | 96% Li, 100% Co |
| Ascorbic acid | LiCoO2 | 1.25 mol L−1, S/L = 25 g L−1, 70 °C, 20 min | 98.5% Li, 94.8% Co | |
| Citric acid & H2O2 | LiCoO2 | 2.0 mol L−1, 3 vol% H2O2, S/L = 20 g L−1, 80 °C, 90 min | >90% Li, >90% Co |
Although many relevant literatures have been reported for leaching metals under various conditions, the kinetics of the leaching process are less studied, and the mechanism of the leaching process is unclear.15–17 Meshram et al. reported that the leaching kinetics of lithium and cobalt follow a logarithmic rate law and chemical control mechanism.16 Golmohammadzadeh et al. studied the kinetics of cobalt using different organic acids (acetic acid, oxalic acid, dl-malic acid and citric acid), and the results showed that the kinetics of cobalt leaching is governed by surface layer diffusion of the leachant.17
Hence, in our study, formic acid (HCOOH) was selected as the leaching liquid to treat spent LIBs for its low cost (when compared with ascorbic acid, citric acid) and non-polluting and efficient leaching properties. The effects of reaction time, temperature, solid–liquid ratio, H2O2 concentration and LiCoO2/HCOOH ratio were studied to optimize the conditions of leaching. The kinetics of HCOOH leaching of valuable metals from spent LIBs with a reducing agent were particularly stressed, while establishing the model of leaching kinetics by characterizing waste metals and leaching residues using the XRD and SEM/EDX characterization. Meanwhile, lithium fluoride, as a commonly used industrial lithium salt, is an important raw material for the synthesis of other lithium resources, but is very expensive among the lithium salts. In this study, lithium fluoride was finally obtained by the fractional precipitation of the liquor after leaching, and the physicochemical properties of the recycled products were characterized.
2. Experimental
2.1. Materials and reagents
LIBs used in mobiles phones from different manufacturers were collected from Hubei University. HCOOH as a leaching reagent and H2O2 as a reducing reagent were purchased from the Taicang Lushi Reagent Co., Ltd of China. In the pretreatment step, N-methyl-2-pyrrolidone (NMP) was used to separate the cathode material from the aluminum foil.18 All the solutions were prepared in distilled water, and all the chemical reagents were of analytical grade.
2.2. Selective leaching of spent cathode material
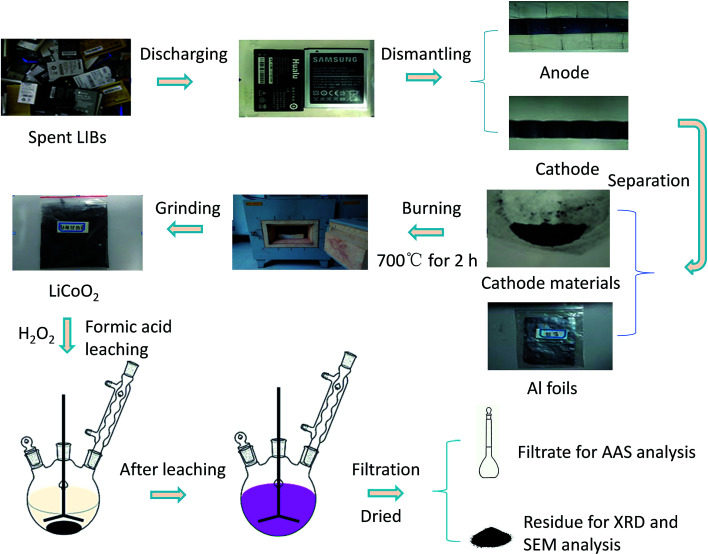
The whole pretreatment and leaching process of spent cathode material with HCOOH is shown in Fig. 1. First, all spent LIBs were discharged before being dismantled to avoid short-circuit and self-ignition, and the cathode material was scraped from the Al foil and ground to less than 0.1 mm diameter. Then, the powder was placed in a muffle oven at 700 °C for 2 h, aiming to remove the carbon and binder polyvinylidene fluoride (PVDF) in the cathode material. Finally, the leaching experiments were performed in a 500 mL three-necked round-bottom flask installed with a reflux condenser to prevent loss by evaporation. A heating bath with a magnetic stirrer was used to ensure optimized kinetics conditions and temperature control. After reacting for a preset period, the mixture was filtered immediately. The effects of several factors including the temperature, solid-to-liquid ratio, and reductant concentration were investigated.
Fig. 1. Flowchart of pretreatment and leaching process of spent cathode material with HCOOH.
2.3. Fractional precipitation of Li and Co
After leaching, the leachate and sodium hydroxide solution (2 M) were simultaneously pumped into the reactor with a speed of 2 mL min−1 under the protection of nitrogen at 60 °C with a stirring speed of 1000 rpm. The pH was first adjusted and maintained at 11 for 24 h to obtain the cobalt hydroxide precipitate (precipitate I). Then, the raffinate was further adjusted to a neutral pH with formic acid, and a stoichiometric amount of saturated NaF was subsequently added to the raffinate at 60 °C with a speed of 1 mL min−1. The precipitated LiF (precipitate II) was washed with distilled water and dried in a vacuum oven for 12 h.
2.4. Material characterization
To determine the lithium and cobalt concentration after grinding, calcination and leaching, the spent material was dissolved using nitric acid and tested using an Atomic Absorption Spectrophotometer (AAS, AAnalyst 400, PerkinElmer). The phase components and morphology of the spent cathode active material were characterized using X-ray diffraction (XRD, D8 ADVANCE, BRUKER) with Cu Kα radiation (1.54 Å) at a voltage of 40 kV, 300 mA and scanning electron microscopy (SEM, S4700, TOSHIBA), respectively, before and after leaching. The thermo-stability of the spent cathode material was tested using a thermal gravimetric analyzer (TGA, TGA2, METTLER TOLEDO) before leaching.
3. Results and discussion
3.1. Mechanism of leaching
HCOOH is a medium strong acid among organic acids, and its ionization equation in 25 °C water can be explained as:
| HCOOH = H+ + COOH− | 1 |
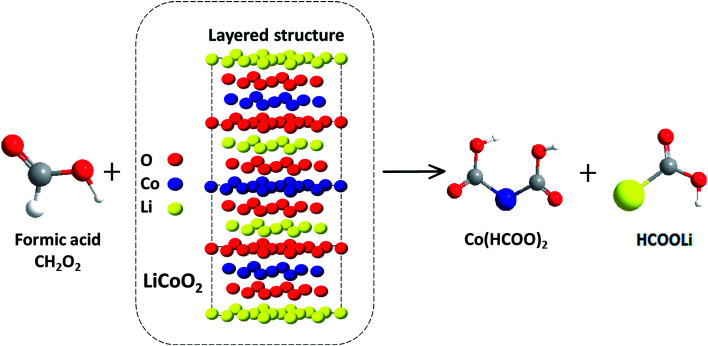
Its Ka is 1.8 × 10−4, which indicates that it can provide enough H+ ions for the leaching reaction. Moreover the carbon atom of HCOOH molecule with the assistance of H2O2 can act as a reductant in aqueous solution.19,20 First, with the help of H2O2, the cobalt in the cathode materials is reduced from high valence Co (LiCoO2, Co3O4 and trace Co2O3) to Co2+, which makes it more stable and dissolvable in HCOOH aqueous solution.21,22 Then, Li+ and Co2+ react with COOH+ during the whole leaching process. After calcination, the product of CoO may be generated in the powder, whereas CoO can be directly leached out. Fig. 2 shows the main leaching reaction of spent materials and HCOOH and theoretically, LiCOOH and Co(COOH)2 are the two main leaching products as shown in the following equation:
| 6HCOOH + 2LiCoO2 + H2O2 = 2Co(COOH)2 + 2LiCOOH + 4H2O + 2O2 | 2 |
| 4HCOOH + 2CoO = 2Co(COOH)2 + 2H2O | 3 |
Fig. 2. Leaching mechanism and main products of spent LiCoO2 with HCOOH.
3.2. Characterization of LiCoO2 in spent LIBs
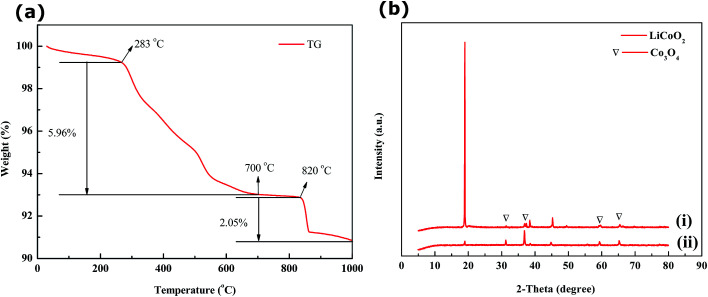
The spent LiCoO2 powder was heated from 30 °C to 1000 °C using TGA in an air atmosphere at a heating rate of 10 °C min−1. In Fig. 3a, the TGA results indicate that a weight loss of 1.02 wt% was found from 30 °C to 283 °C due to the loss of bound water. From 283 °C to 700 °C, there was a weight loss of 5.96 wt%, possibly resulting from the burning of the powdered carbon and the decomposition of PVDF. When the temperature was between 820 °C and 1000 °C in the TG curve, a weight loss of 2.05 wt% illustrated the phase change of LiCoO2 and the decomposition of Co3O4 to CoO.
Fig. 3. (a) TG curve for spent cathode materials heated from 30 °C to 1000 °C in an air atmosphere with a heating rate of 10 °C min−1; (b) XRD patterns of (i) spent cathode materials after calcination and (ii) black residues after leaching.
The spent cathodic materials after calcination and the black residues after HCOOH leaching were analyzed. From the XRD patterns in Fig. 3b, it is clear that the residues had a small amount of LiCoO2 and insoluble Co3O4 as compared to the spent cathodic materials. This could be due to large amounts of LiCoO2 of the spent cathodic materials reacting with HCOOH and Co3O4 being partially leached by HCOOH. The carbon peaks were not detected as can be concluded from Fig. 3b, indicating that a large amount of carbon was burnt off in the calcination process.
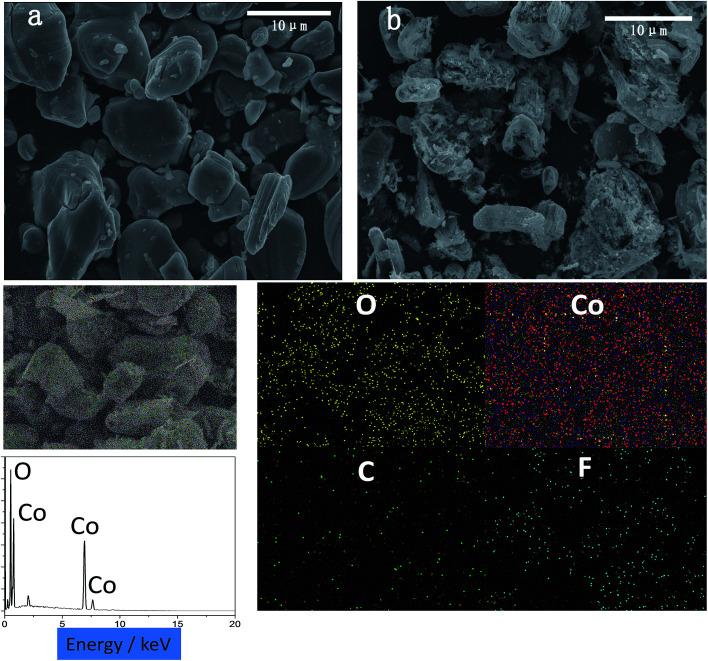
The SEM images of the spent cathode active materials after calcination and the residues after HCOOH leaching are shown in Fig. 4, which presents the spent cathode materials, mostly LiCoO2, whose particle diameters are in the range of 5–11 μm. Moreover, the LiCoO2 particles are irregular and agglomerated because of the repeated usage of LIBs. The leaching residues show a small particle size of about 3–6 μm in Fig. 4b. The smaller particles are caused because the larger agglomerate particles are broken up in the HCOOH leaching stirring reaction. From EDS spectroscopy, it is determined that most of the Co and O elements can be found in the leaching residues from which we inferred that the residues had a some amount of Co3O4 and traces of CoO. A small amount of F and C elements in the EDS image indicates that plenty of carbon and binder (PVDF) was burnt off during the calcination process.
Fig. 4. SEM micrographs of (a) spent cathode materials after calcination and (b) black residues after leaching; EDS spectroscopy of leaching residues.
3.3. Leaching of LiCoO2 with HCOOH
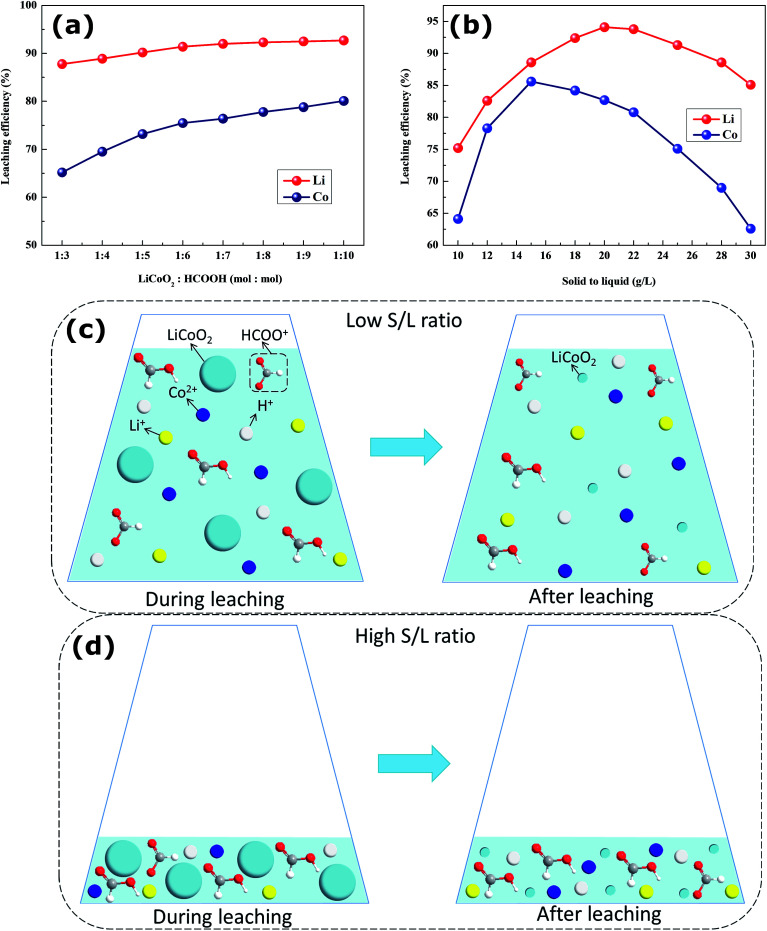
The LiCoO2/HCOOH ratio plays a vital role in improving the leaching efficiency of cobalt and lithium from spent cathode materials. The LiCoO2/HCOOH ratio was varied from 1 : 3 to 1 : 10 at a reaction temperature of 60 °C, reaction time of 20 min, solid-to-liquid (S/L) ratio of 20 g L−1, and H2O2 concentration of 6 vol%. Fig. 5a shows that when the dosage of HCOOH was increased, the leaching efficiency of cobalt and lithium, especially the cobalt, improved. Initially the leaching efficiency was about 65% for cobalt and 88% for lithium with a LiCoO2/HCOOH ratio at 1 : 3 and then, it increased to 81% for cobalt and 94% for lithium at 1 : 10 mole ratio. Although the highest leaching efficiency of 81% for cobalt and 94% for lithium was obtained at 1 : 10 mole ratio, increasing HCOOH had little effect on the improvement of the leaching efficiency. These phenomena can be interpreted as follows: when HCOOH was increased, sufficient ionized H+ species were generated to react with LiCoO2 powder in the solid/liquid interphase, and when the interfacial H+ concentration was reduced, the additional ionized H+ species could diffuse outward.23–25Fig. 2 shows that the theoretical LiCoO2/HCOOH reactive ratio was 1 : 3. However, the virtual optimal LiCoO2/HCOOH reactive ratio was 1 : 10, resulting from incomplete ionization and gasification loss of HCOOH.
Fig. 5. Effects of (a) LiCoO2/HCOOH ratio and S/L ratio on the leaching of Co and Li from spent LiCoO2 batteries; (b) possible multiphase leaching reactions of LiCoO2 particles and HCOOH solutions at (c) low S/L ratio and (d) high S/L ratio.
To investigate the effect of S/L, the experiment was performed at S/L ratios from 10 to 30 g L−1 with the following conditions: LiCoO2/HCOOH ratio of 1 : 10, leaching temperature of 60 °C, leaching time of 20 min, and H2O2 concentration of 6 vol%. From Fig. 5b, it can be observed that the leaching efficiency of cobalt and lithium from the spent LiCoO2 powder appeared to first increase and then decrease with increasing S/L ratio. The highest leaching efficiency was 85% for cobalt and 93% for lithium; however, the leaching efficiency was significantly decreased to 62% for cobalt and 86% for lithium at an S/L ratio of 30 g L−1. As shown in Fig. 5c and d, at a low S/L ratio, the leaching solution included abundant water molecules so that HCOOH had a strong ionized capacity and low concentration. With the S/L ratio increasing, the ionized capacity of HCOOH became increasingly weak, and the concentration of HCOOH became increasingly high. At a high S/L ratio, some water molecules led to a weak ionized capacity and high concentration of HCOOH. Thus, the leaching efficiency of Co and Li increased initially and decreased afterwards. The S/L ratio of 20 g L−1 was chosen to be optimal for leaching valuable metals.
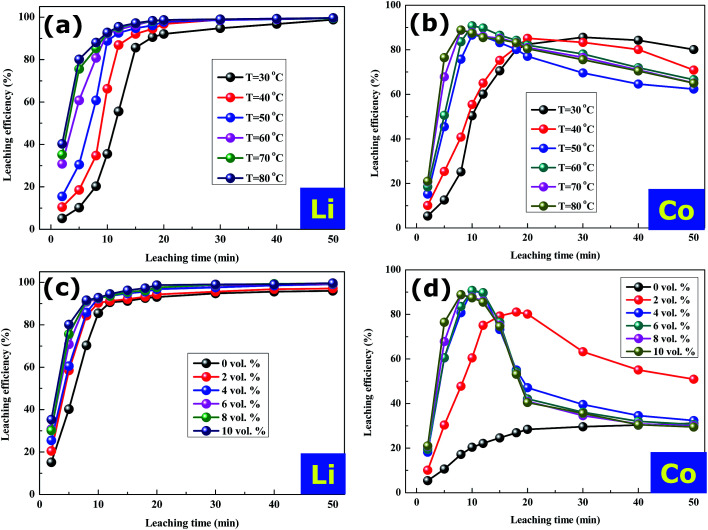
The effect of the leaching temperature on the leaching efficiency of lithium and cobalt was studied under the condition of the LiCoO2/HCOOH ratio of 1 : 10, H2O2 concentration of 6 vol%, and S/L ratio of 20 g L−1. With the temperature increasing from 30 to 60 °C, the slopes of the leaching curves for Li and Co metals both significantly increased (Fig. 6a and b), indicating that the leaching process was an endothermic reaction. However, the leaching behavior of Li was found to be very different from that of Co, that is, the leaching efficiency of Li rapidly reached over 90% within 20 minutes in the early stage of leaching and remained stable afterwards. The leaching efficiency of Co first increased and then decreased with the increase in temperature, especially at high temperatures (60 to 80 °C), which could be attributed to the decomposition of hydrogen peroxide when the reaction temperature is higher than 60 °C. Therefore, the leaching efficiency was not significantly improved after 60 °C by further increasing the leaching temperature.
Fig. 6. Effects of (a, b) leaching temperature and (c, d) LiCoO2/H2O2 ratio on the leaching of Li and Co from spent LiCoO2 batteries.
The effect of concentration of H2O2 as the reducing agent on the leaching efficiency was investigated using a LiCoO2/HCOOH ratio of 1 : 10, S/L ratio of 20 g L−1 and temperature of 60 °C for 20 min. The results, observed in Fig. 6c and d, indicate that the leaching efficiency of Li was not obviously affected by the concentration of H2O2 and increased significantly, reaching almost 100% within 10 min. On the contrary, the leaching efficiency of Co under any H2O2 concentration increased rapidly within 10 min and then decreased steadily before reaching a certain low level. It must be pointed out that Co3+ was reduced to Co2+ by H2O2 to be more easily reactive with HCOOH, that is to say, H2O2 as a reductant helped dissolve cobalt in the crystals of LiCoO2. Meanwhile, when the concentration and dosage of H2O2 was increased, the molecules of H2O2 disintegrated sharply due to the excess H2O2, according to the following equation and thus, the best concentration of H2O2 was chosen to be 6 vol%.
| H2O2 (aq) = H2O (aq) + O2 (g) | 4 |
Therefore, on the basis of the previous experimental results, the global recovery efficiency of Li and Co could reach 99.90% and 99.96%, respectively, at the following optimal leaching conditions: LiCoO2/HCOOH ratio of 1 : 10, S/L ratio of 20 g L−1, and H2O2 concentration of 6 vol% at a temperature of 60 °C for 20 min. Co metal was recovered in the form of cobalt hydroxide precipitate, whereas Li metal was recycled in the form of LiF.
3.4. Kinetics of leaching
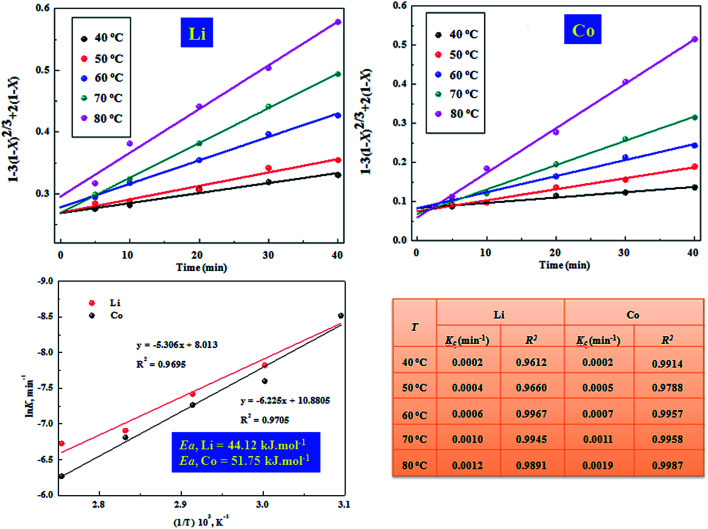
To further explore the mechanism of the reaction between the LiCoO2 powder and leachant, the kinetics of leaching were studied in the HCOOH solution for different leaching temperatures (40–80 °C) and times (5–40 min) under the following condition: LiCoO2/HCOOH ratio of 1 : 10, S/L ratio of 20 g L−1, and H2O2 concentration of 6 vol%. From the details of Fig. 7, an approach was adopted to fit the kinetic data satisfactorily using the empirical model based on the surface chemical control, described by eqn (5):
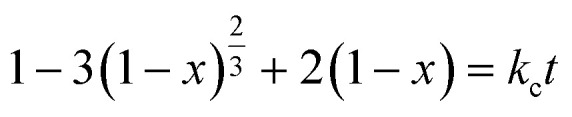 |
5 |
here, x is the fraction of metals leached in time t (minutes) and kc is the reaction rate constant (min−1). The plots of ‘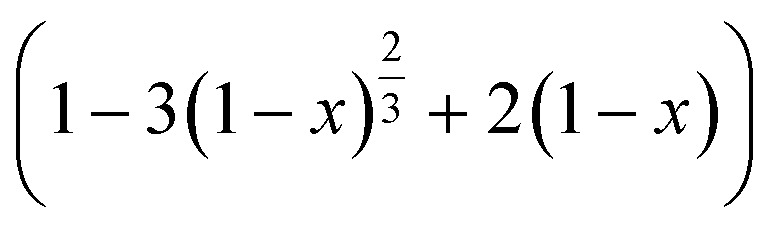 vs. t’ at different temperatures showed that the kinetic data fitted well to this kinetic model, which had high correlation coefficients (R2 > 0.96) ranging from 0.96 to 0.99 for Li and 0.97 to 0.99 for Co.
vs. t’ at different temperatures showed that the kinetic data fitted well to this kinetic model, which had high correlation coefficients (R2 > 0.96) ranging from 0.96 to 0.99 for Li and 0.97 to 0.99 for Co.
Fig. 7. Chemical control model for leaching kinetics of Co and Li at different temperatures; Arrhenius plot for the leaching of Li and Co under the chemical control model.
From the Arrhenius eqn (6), the relation between the reaction rate constant mentioned in eqn (5) and the temperature can be explained, and the activation energy can be calculated by the following equation:
| k = Ae−Ea/RT | 6 |
Here, k is the reaction rate constant, A is the frequency factor, Ea is the apparent activation energy and R is the gas constant (8.314 J K−1 mol−1). From further plotting ‘ln k vs. 1/T’, as shown in Fig. 7, the apparent activation energy is calculated to be 44.12 kJ mol−1 for Li, and 51.75 kJ mol−1 for Co. The values reach the specified activation energies for chemical reaction control (>42 kJ mol−1).22
3.5. Recovery of lithium fluoride
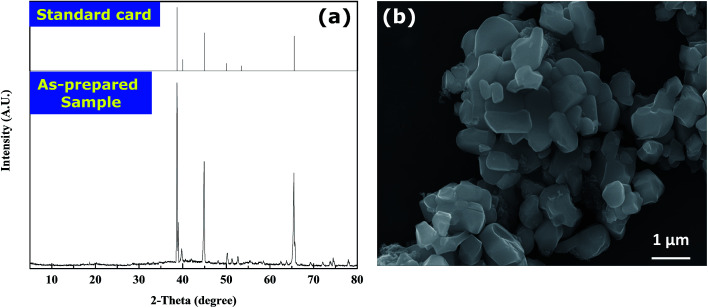
The leaching solution is first processed according to the aforementioned procedures to precipitate Co metal. Subsequently, lithium fluoride can be obtained by adding saturated NaF to the remaining leaching solution. The XRD pattern of the obtained lithium fluoride is shown in Fig. 8a, and it agrees well with the standard patterns of peaks. The SEM image of the precipitated LiF can be also seen in Fig. 8b, and it can be noticed that the precipitated LiF is present as massive agglomerates integrated by numerous primary sheets (estimated to be 1.00 ± 0.50 μm). Meanwhile, to accurately calculate the purity of lithium fluoride, the precipitated LiF is further dissolved by aqua regia and the mass fraction of metals is measured by AAS. After calculation, the mass fraction of lithium is found to be 99.0 wt%.
Fig. 8. (a) XRD patterns of the precipitated LiF and standard powder diffraction peaks of LiF; (b) SEM images of the precipitated LiF.
4. Conclusions
Recycling cobalt and lithium from spent LIBs is considered to be a beneficial and crucial way to prevent environmental pollution and alleviate resource shortage. In this study, we proposed the hydrometallurgical process for recovery of lithium fluoride as the target product. The spent cathode material could be first leached out using formic acid and hydrogen peroxide. To achieve efficient leaching, the molar ratio of formic acid and LiCoO2 at 10 : 1, a leaching temperature of 60 °C, a solid-to-liquid ratio of 20 g L−1 and reaction time of 20 min were used as optimal conditions. With the help of these conditions, more than 99.96% of Co and 99.90% of Li could be recovered from the spent batteries. Meanwhile, by evaluating the leaching kinetics and calculating the apparent activation energies, the leaching process was verified to be controlled by the chemical reaction satisfactorily. After further fractional precipitation, a highly pure (99.0%) lithium fluoride could be finally obtained.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 21706055). The authors would like to thank the Analytical and Testing Center of Hubei University for providing the facilities to complete the experimental measurements. Technical support from Hubei Nuobang Chemical Company Co. Ltd., is also gratefully acknowledged.
References
- Choubey P. K. Chung K. S. Kim M. S. Lee J. C. Srivastava R. R. Miner. Eng. 2017;110:104–121. doi: 10.1016/j.mineng.2017.04.008. [DOI] [Google Scholar]
- Nayaka G. P. Pai K. V. Manjanna J. Keny S. J. Waste Manage. 2015;51:234–238. doi: 10.1016/j.wasman.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Tesfaye F. Lindberg D. Hamuyuni J. Miner. Eng. 2017;111:209–221. doi: 10.1016/j.mineng.2017.06.018. [DOI] [Google Scholar]
- Chen X. P. Chen Y. B. Zhou T. Liu D. P. Hu H. Waste Manage. 2015;38:349–356. doi: 10.1016/j.wasman.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Pinna E. G. Ruiz M. C. Ojeda M. W. Hydrometallurgy. 2017;167:66–71. doi: 10.1016/j.hydromet.2016.10.024. [DOI] [Google Scholar]
- Chen X. P. Ma H. R. Luo C. B. J. Hazard. Mater. 2016;326:77–86. doi: 10.1016/j.jhazmat.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Guo Y. Li F. Zhu H. C. Li G. M. Huang J. W. Waste Manage. 2015;51:227–233. doi: 10.1016/j.wasman.2015.11.036. [DOI] [PubMed] [Google Scholar]
- Pagnanelli F. Moscardini E. Altimari P. Atia T. A. Toro L. Waste Manage. 2016;60:706–715. doi: 10.1016/j.wasman.2016.11.037. [DOI] [PubMed] [Google Scholar]
- Barik S. P. Prabaharan G. Kumar L. J. Cleaner Prod. 2017;147:37–43. doi: 10.1016/j.jclepro.2017.01.095. [DOI] [Google Scholar]
- Ku H. Jung Y. Jo M. Park S. Kim S. J. Hazard. Mater. 2016;313:138–146. doi: 10.1016/j.jhazmat.2016.03.062. [DOI] [PubMed] [Google Scholar]
- Li L. Qu W. J. Zhang X. X. Lu J. Chen R. J. J. Power Sources. 2015;282:544–551. doi: 10.1016/j.jpowsour.2015.02.073. [DOI] [Google Scholar]
- Li L. Lu J. Ren Y. Zhang X. X. Chen R. J. Wu F. J. Power Sources. 2012;218:21–27. doi: 10.1016/j.jpowsour.2012.06.068. [DOI] [Google Scholar]
- Meshram P. Pandey B. D. Mankhand T. R. J. Ind. Eng. Chem. 2016;43:117–126. doi: 10.1016/j.jiec.2016.07.056. [DOI] [Google Scholar]
- Santana I. L. Moreira T. F. M. Lelis M. F. F. Freitas M. B. J. G. Mater. Chem. Phys. 2017;190:38–44. doi: 10.1016/j.matchemphys.2017.01.003. [DOI] [Google Scholar]
- Zheng X. H. Gao W. F. Zhang X. H. He M. M. Lin X. Waste Manage. 2016;60:680–688. doi: 10.1016/j.wasman.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Meshram P. Pandey B. D. Mankhand T. R. Chem. Eng. J. 2015;281:418–427. doi: 10.1016/j.cej.2015.06.071. [DOI] [Google Scholar]
- Golmohammadzadeh R. Rashchi F. Vahidi E. Vahidi E. Waste Manage. 2017;321:561–578. doi: 10.1016/j.wasman.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Prasad N. S. Moulik S. Bohra S. Rani K. Y. Sridhar S. Carbohydr. Polym. 2015;136:1170–1181. doi: 10.1016/j.carbpol.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Wu H. H. Wang Y. Li H. Huang L. B. Atmos. Environ. 2017;164:61–70. doi: 10.1016/j.atmosenv.2017.05.038. [DOI] [Google Scholar]
- Nowicki L. Siuta D. Godala M. Thermochim. Acta. 2017;653:62–70. doi: 10.1016/j.tca.2017.04.006. [DOI] [Google Scholar]
- Hammouda S. B. Zhao F. P. Safaei Z. Srivastava V. Ramasamy D. L. Iftekhar S. Kalliola S. Appl. Catal., B. 2017;215:60–73. doi: 10.1016/j.apcatb.2017.05.051. [DOI] [Google Scholar]
- Gao W. F. Zhang X. H. Zheng X. H. Lin X. Environ. Sci. Technol. 2017;51:1662–1669. doi: 10.1021/acs.est.6b03320. [DOI] [PubMed] [Google Scholar]
- Cha E. Jeong E. S. Cha S. Lee J. Anal. Chim. Acta. 2017;964:123–133. doi: 10.1016/j.aca.2017.01.058. [DOI] [PubMed] [Google Scholar]
- Pratik S. M. Datta A. J. Phys. Chem. B. 2016;120:7606–7613. doi: 10.1021/acs.jpcb.6b05830. [DOI] [PubMed] [Google Scholar]
- Elmongy H. Ahmed H. Wahbi A. A. Koyi H. Rehim M. A. J. Chromatogr. A. 2015;1418:110–118. doi: 10.1016/j.chroma.2015.09.051. [DOI] [PubMed] [Google Scholar]