Abstract
Purpose:
We report stone comminution in the first 19 human subjects by burst wave lithotripsy (BWL), which is the transcutaneous application of focused, cyclic ultrasound pulses.
Materials and Methods:
This was a prospective multi-institutional feasibility study recruiting subjects undergoing clinical ureteroscopy (URS) for at least 1 stone ≤12 mm as measured on computerized tomography. During the planned URS, either before or after ureteroscope insertion, BWL was administered with a handheld transducer, and any stone fragmentation and tissue injury were observed. Up to 3 stones per subject were targeted, each for a maximum of 10 minutes. The primary effectiveness outcome was the volume percent comminution of the stone into fragments ≤2 mm. The primary safety outcome was the independent, blinded visual scoring of tissue injury from the URS video.
Results:
Overall, median stone comminution was 90% (IQR 20, 100) of stone volume with 21 of 23 (91%) stones fragmented. Complete fragmentation (all fragments ≤2 mm) within 10 minutes of BWL occurred in 9 of 23 stones (39%). Of the 6 least comminuted stones, likely causative factors for decreased effectiveness included stones that were larger than the BWL beamwidth, smaller than the BWL wavelength or the introduction of air bubbles from the ureteroscope. Mild reddening of the papilla and hematuria emanating from the papilla were observed ureteroscopically.
Conclusions:
The first study of BWL in human subjects resulted in a median of 90% comminution of the total stone volume into fragments ≤2 mm within 10 minutes of BWL exposure with only mild tissue injury.
Keywords: lithotripsy, kidney calculi, ureteral calculi, ultrasonography, ureteroscopy
Kidney stones afflict 1 in 11 Americans at an annual cost of care of $10 billion, and prevalence is rising.1,2 Natural or medically aided stone expulsion therapy is associated with anxiety and pain. The Urologic Diseases in America project found 65% of kidney stone patients filled an opioid prescription and 40% utilized the emergency department (ED), including 10% who used the ED more than once, to deal with their pain while waiting for definitive management.3 That management includes ionizing radiation for monitoring even asymptomatic stones with 70% of stone patients receiving imaging each year and the majority of these receiving more than 1 scan.3 Management is not definitive and 30% of surgeries, including shock wave lithotripsy (SWL), need to be repeated.3 The inefficiencies of ED care, repeat imaging and repeat surgeries make urinary stone disease the costliest nonmalignant urological disease,3 and the annual cost of care of a stone patient is twice that of patients without stone disease.4 The ability to noninvasively break stones and expel the fragments in awake patients at first presentation in the ED or clinic has the potential to provide just-in-time treatment leading to a reduction of the overall pain, cost and resource burden associated with a stone event.
We addressed this unmet need by developing an office-based ultrasound system to image, break and reposition stone fragments to facilitate their natural clearance.5 A stepwise progression of development and human trials has ensued,6-10 and this paper reports on the first human feasibility study of our technology called burst wave lithotripsy (BWL)11 to fragment stones. BWL’s short harmonic bursts of ultrasound from a handheld therapy and imaging transducer have been well tolerated with minimal hematuria by awake patients.9,10
As a precursor to a clinical effectiveness study, the study reported here was designed to directly observe how well BWL fragmented stones and any tissue injury associated with the procedure. As such, we recruited subjects already undergoing ureteroscopy (URS), and while the subjects were anesthetized, we applied 10 minutes of BWL and then used the ureteroscopic video to assess for fragmentation and injury.
METHODS
This was a prospective, multicenter feasibility trial conducted by University of Washington and Indiana University with approval from the WCG-IRB Institutional Review Board and the U.S. Food and Drug Administration through an investigational device exemption (clinicaltrials.gov identifier NCT03873259). Although our ultimate goal is an office-based system, the specific design of the trial described in this paper was to opportunistically recruit subjects who were already undergoing ureteroscopic treatment of a stone.
Preoperative subjects were recruited who had a stone ≤ 12 mm in largest dimension as measured on computerized tomography (CT) imaging obtained within 6 months. Informed consent was obtained from the research subjects prior to surgery. A screening ultrasound with the device was performed to ensure the stone could be visualized and aligned within the BWL target focus in the dorsal lithotomy position. Exclusion criteria included members of a vulnerable group, non-English speakers and those on anticoagulant medications at the time of the procedure. The x-ray density of the stone was measured from the 4 times zoomed axial slices taking the maximum value with the smallest possible region of interest.
Procedure
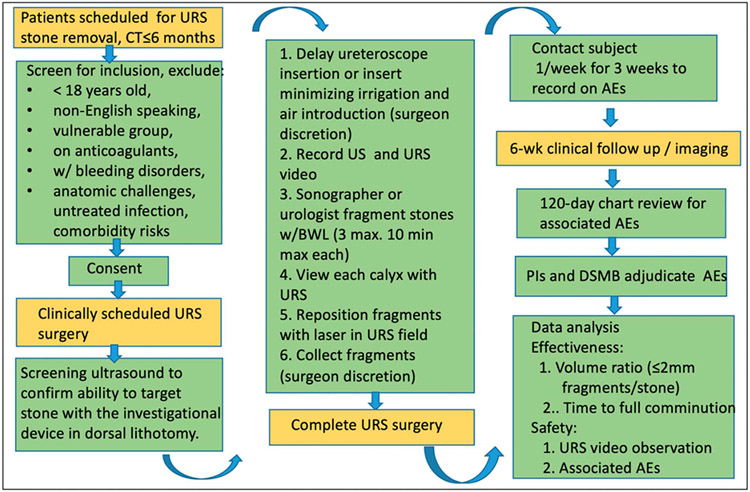
Figure 1 shows a procedure flowchart. In the operating room, subjects were anesthetized and placed in the dorsal lithotomy position with rolled towels under the hip and rib to allow working space for the transducer. The sonographer or urologist operator then attempted to break the stone with transcutaneous BWL during the ureteroscopic procedure. As many as 3 stones were targeted for up to 10 minutes each. Based on prior studies, we expect most stones will require more than 10 minutes of BWL therapy; however, 10 minutes was selected for this study as a compromise to minimize prolonged case duration.
Figure 1.
Procedure flowchart. The BWL exposure took place at the beginning of a scheduled URS. Yellow color indicates standard of care procedures, and green indicates research procedures. DSMB, data safety monitoring board. Pls, principal investigators. US, ultrasound.
Following BWL, the ureteroscope was used to record video of the ureter and collecting system, including the targeted calyx and fragments. Surgeons moved the fragments to visualize 3 dimensions. The laser fiber or basket was maintained in the image to serve as a reference for post processing measurement of fragment size.
For 8 of the 27 stone targets, BWL was initiated before inserting the ureteroscope, while for 19 of 27 stone targets the ureteroscope was inserted before BWL was initiated based on surgeon preference. This strategy allowed a balance between capturing video of the BWL procedure and altering the normal urine condition by potentially introducing air and/or irrigation fluid. Special attention was made to avoid introducing macroscopic air bubbles and to minimize use of irrigation fluid. In particular, introduction of air (even air-saturated irrigation fluid) can obstruct delivery of acoustic energy to the stone and increases the likelihood of tissue injury through cavitation.12-14 Real-time ultrasound imaging with the investigational device allowed detection of cavitation.12,15 When cavitation was detected in the renal papilla, the procedure was paused for 1 minute to allow the cavitation bubbles to dissipate, the imaging angle was adjusted, and the applied peak pressure and/or delivery rate were reduced.
Subjects received a weekly followup phone call for 3 weeks and received clinical followup imaging within 120 days. The followup image modality—CT, plain film x-ray or ultrasound—and exact timing were at the discretion of the surgeon. Chart review occurred after 120 days to identify any safety concerns related to the stone surgery. The Principal Investigators and Data Safety Monitoring Board then adjudicated if any adverse events (AEs) were related to the investigational procedure.
Outcomes and Measurement
The primary effectiveness outcome was the volume of residual stones ≤2 mm normalized by the initial stone volume. The residual volume was determined by analysis of the URS video by a reviewer blinded to exposure conditions. A custom MATLAB® program was used to measure fragment size using the 273 μm laser fiber or 90 μm single arm of the basket itself for scale. When fragments were able to be extracted, the volume and size of extracted fragments were measured by micro CT, and fragment composition was determined by micro CT and Fourier transformed infrared spectroscopy.16 Original stone volume was calculated by measuring 3 orthogonal stone dimensions on CT and estimating the volume as an ellipsoid. Complete fragmentation is defined as all residual fragments ≤2 mm. As an exploratory analysis, a secondary effectiveness outcome was the linearly extrapolated time to full comminution (all fragments ≤2 mm) based on the normalized residual volume.
The primary safety outcome was tissue injury observed on the URS video and described and scored by an experienced kidney tissue anatomist blinded to the exposure conditions. Scoring was done of the targeted papilla, several nontargeted papillae and the ureter. The scores were 0—within normal limits, 1—mild injury including petechial hemorrhage or erythema expected to resolve without consequence, 2—moderate injury, including loss of surface epithelium that may cause papillary scarring, and 3—substantial injury including loss of epithelial and deeper tissue layers or extensive bleeding that would indicate breakage of larger blood vessels. The secondary safety outcome included all associated AEs.
Statistics
Descriptive statistics were used to tabulate the results.
Investigational Device and Exposure Parameters
The investigational device, Propulse 1 (University of Washington, Seattle, Washington), and handheld, water-filled, combined imaging and therapy transducer, SC-60, that was coupled with gel to the skin, allow for simultaneous visualization and comminution or propulsion of kidney stones. The system has previously been used to effectively comminute stones 3—7 mm in size ex vivo.17
Table 1 shows the BWL exposure parameters. BWL was delivered at a frequency of 390 kHz to break stones consistently into ≤2 mm fragments.16 The stone was aligned within an axial target oval indicated on the screen that was 3 cm long starting 4 cm from the transducer aperture (centered at 5.5 cm depth) and 6 mm wide. The oval length and axial depth corresponded to the region of >5 MPa negative pressure in situ based on standard derating for tissue attenuation (0.5 dB/MHz/cm). The in situ peak negative pressure was 6.2 MPa at 6 cm depth assuming no obstruction of the beam. Water tank measurements with water-soaked cadaver ribs indicated a 10% drop in peak negative pressure transmitting through 1 rib.16
Table 1.
BWL acoustic output parameters
| Frequency | Max Peak Neg Pressure (free-field) |
Max Burst Repetition Rate |
No. Cycles (duration) |
Full-Width Half Max of Beam |
|---|---|---|---|---|
| 390 kHz | 7 MPa | 10 Hz | 20 (51 μs) or 50 (127 μs) | 6 mm |
RESULTS
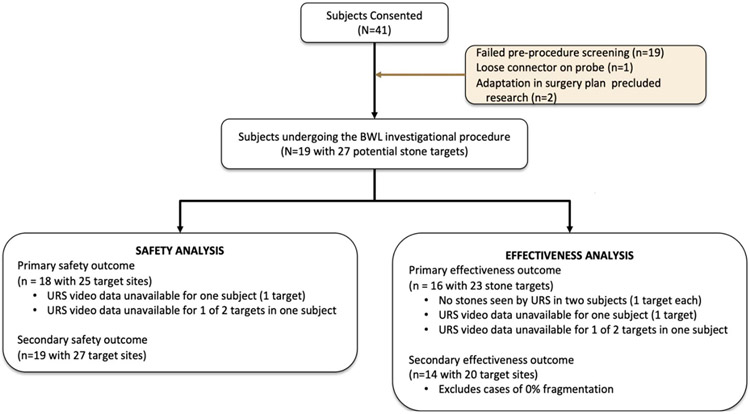
Figure 2 shows the flow diagram of the study. Forty-one subjects were consented and enrolled. Nineteen subjects failed the screening ultrasound because the stone was not visible, too small, too deep, or obstructed by rib or bowel, 2 had surgery plans change, and another did not undergo the investigational procedure because of a loose connector. Nineteen subjects underwent the investigational procedure. The URS video failed to record for subject 6’s stone and subject 8’s first of 2 stones (ureter stone), and therefore subject 6 and these 2 sites were not included in the analysis of effectiveness and primary safety outcomes. The targeted stone was not seen by URS (probably submucosal stones) in subjects 10 and 11; therefore, 2 subjects lacked data to be included in the effectiveness analysis.
Figure 2.
Flow diagram. The denominator in the different analyses changes as described in this diagram.
Demographics and Clinical Characteristics
BWL was used in 19 subjects to target 25 confirmed stones. Subject characteristics (table 2) included males and females, and a variety of ages, stone sizes, locations and stone types. We also included stone targets with a wide range of densities to assess the technology, considering the limitation in SWL to effectively fragment harder stones (>1,000 HU).18-20 As the BWL transducer has a fixed treatment depth of 4 to 7 cm, the upper pole was avoided and body mass index (BMI) fell over a relatively narrow range (mean±SD BMI 24±5 kg/m2) to maintain acceptable skin-to-stone distance.
Table 2.
Demographics and clinical characteristics
| Subject | Sex | Age (yrs) | BMI | Side | Location | Size (mm) | Density (HU) | Composition* |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 36 | 25.8 | Lt | Lower pole | 7.0 | 696 | Unknown |
| 2 | Female | 46 | 23.4 | Rt | Distal ureter | 5.8 | 1,058 | 71% calcium oxalate monohydrate |
| 3 | Female | 70 | 37.4 | Rt | Lower pole, lower pole | 10.3, 3.8 | 699, 242 | Unknown |
| 4 | Female | 36 | 20.3 | Rt | Lower pole | 7.0 | 973 | 80% apatite |
| 5 | Male | 76 | 21.5 | Rt | Lower pole, lower pole | 4.1, 3.3 | 257, 205 | 100% calcium oxalate monohydrate |
| 6 | Female | 43 | 30.0 | Lt | Lower pole | 6.9 | 1,600 | 94% brushite |
| 7 | Female | 72 | 19.7 | Lt | Lower pole, lower pole | 7.0, 7.0 | 1,200, 1,200 | 74% calcium oxalate monohydrate |
| 8 | Male | 52 | 20.7 | Rt | Distal ureter, lower pole | 6.0, 6.0 | 1,900, 1,366 | 100% calcium oxalate monohydrate |
| 9 | Female | 54 | 24.3 | Rt | Lower pole | 6.0 | 1,200 | 80% brushite |
| 10 | Female | 38 | 19.6 | Lt | Mid pole | Not applicable | Not applicable | Not applicable |
| 11 | Male | 84 | 23.6 | Lt | Lower pole | Not applicable | Not applicable | Not applicable |
| 12 | Male | 72 | 20.0 | Rt | Lower pole | 4.5 | 870 | 85% calcium oxalate monohydrate |
| 13 | Male | 63 | 26.1 | Rt | Mid pole | 5.9 | 2,000 | 55% calcium oxalate dihydrate |
| 14 | Male | 71 | 26.0 | Lt | Lower pole | 3.5 | 427 | 72% calcium oxalate monohydrate |
| 15 | Female | 46 | N/A | Lt | Lower pole, lower pole | 6.1, 5.5 | 620, 730 | Unknown, No. 2 was a thin crystal growth attached to plaque |
| 16 | Male | 65 | 22.1 | Rt | Lower pole | 4.4 | 623 | 100% calcium oxalate monohydrate |
| 17 | Male | 68 | 26.3 | Rt | Lower pole, mid pole | 6.3, 7.0 | 741, 800 | Unknown |
| 18 | Female | 65 | 31.2 | Lt | Lower pole, lower pole, lower pole | 7.9, 4.7, 2.8 | 931, 213, 328 | 100% struvite |
| 19 | Male | 37 | 19.2 | Rt | Mid pole | 4.0 | 731 | 74% apatite |
| Total/overall | 10 Male, 9 female | Mean (SD) 58±15 | Mean (SD) 24±5 | 11 Rt, 8 lt | 21 lower pole, 4 mid pole, 2 ureter | Mean (SD) 5.7±1.7 | Mean (SD) 864±495 | 10 calcium oxalate monohydrate, 3 struvite, 2 apatite, 2 brushite, 1 calcium oxalate dihydrate |
Effectiveness Outcomes: Comminution and Projected Complete Comminution Time
Table 3 shows the effectiveness outcomes. Overall, a median of 90% (IQR 20, 100) of the stone volume was completely comminuted in 10 minutes. Complete fragmentation occurred in 39% (9 of 23) of stone targets, partial comminution occurred in 52% (12 of 23) of stone targets, and no comminution occurred in 9% (2 of 23) of stone targets. In exploratory analysis, we linearly extrapolated the time for full fragmentation for the 12 stones that were only partially comminuted. The median projected time to full comminution, including those fully comminuted within 10 minutes, was 10 minutes (10, 14.9). Subjects 2, 15 (stone 2), 18 (stone 3) and 19 did not receive the complete 10-minute treatment, and although comminution was incomplete, the projected time was in these 3 cases less than 10 minutes. In subject 2, BWL was applied for 4 minutes before the ureter stone passed to the bladder. Subject 16 was treated with the transducer on 1 rib and correspondingly lower in situ pressure.
Table 3.
Effectiveness outcomes
| Subject | Vol Fraction ≤ 2 mm (%) | Projected Time to 100% |
|---|---|---|
| 1 | 100 | ≤ 10 mins |
| 2 | 73 | ≤ 10 mins |
| 3 | 100 | ≤ 10 mins |
| 20 | 50:00 | |
| 4 | 90 | 11:06 |
| 5 | 100 | ≤ 10 mins |
| 100 | ≤ 10 mins | |
| 6 | 67 | 14:56 |
| 7 | 20 | 50:00 |
| 8 | 124:20 | |
| 8 | 100 | ≤ 10 mins |
| 12 | 0 | Not applicable |
| 13 | 5 | 200:00 |
| 14 | 0 | Not applicable |
| 15 | 26 | 36:00 |
| 1 | 200:00 | |
| 16 | 100 | ≤ 10 mins |
| 17 | 100 | ≤ 10 mins |
| 98 | 10:14 | |
| 18 | 100 | ≤ 10 mins |
| 100 | ≤ 10 mins | |
| 95 | ≤ 10 mins | |
| 19 | 85 | ≤ 10 mins |
| Median (IQR 25%, 75%) | 90 (20, 100) | 10 mins (10, 14.9) |
Figure 3 shows representative examples of partial and complete (all residual stones ≤2 mm) fragmentation. Here, the partially fragmented stone had only 20% of volume ≤2 mm; however, the stone was broken into at least 17 pieces. Since stone sizes were measured in different ways depending on whether the surgeon basket extracted stone fragments or not, we compared stone size measured from the URS video to the actual stones size for 7 basket extracted fragments including the approximately 2 mm fragment in figure 3, a. All 7 fragments passed through a 2 mm sieve, but the largest dimension ranged from 1.8 to 2.3 mm with 5 of the 7 fragments having a dimension larger than 2.0 mm. The URS measurement overestimated the fragment size with an average volume error of 9.6%±6.1%, which worst case would have meant underestimation to 12%, not 20%, breakage for subject 7 stone 2 and proportionately less underestimation in cases of better comminution. The largest possible measurement error would have been if subject 19’s largest stone dimension had been measured as 2.0 mm not 2.2 mm. The 85% comminution would have been 100% comminution.
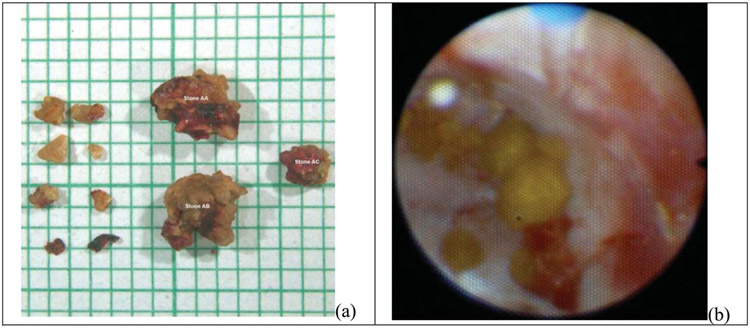
Figure 3.
Photograph of a 20% fragmented stone (a) and a 100% comminuted stone (b). In part a of figure, the largest 11 of 17 observed fragments were extracted and are shown on mm scale graph paper. The 2 largest fragments are approximately 4 mm in size. The stone was projected to fragment completely within 50 minutes. In part b of figure, all fragments were <2 mm, and mild clotting as well as bruising on the papilla is shown. This was scored as grade 1 injury.
Safety Outcomes: Tissue Injury and AEs
Table 4 shows the safety outcomes. In all cases, tissue injury was within normal limits (grade 0) or mild (grade 1) and consisted of mild hematuria. Figure 3, b illustrates mild injury seen in subject 1. No other AEs associated with the device or procedure were identified. Five subjects (subjects 4, 7, 11, 12 and 15) experienced mild redness of the skin that resolved within 24 hours; this was anticipated from the manipulation of the transducer against the skin and consistent with that which can occur with diagnostic ultrasound imaging. Cavitation, extending into the tissue and warranting three 1-minute pauses was detected in subjects 7 and 19. Both subjects had mild injury consistent with cases where no cavitation was observed. In both subjects, cavitation bubbles were observed by ultrasound to be introduced with the ureteroscope and its irrigation. Subject 7’s stones were 5% and 20% comminuted. Subject 19’s stone was 85% comminuted in the first 90 seconds before treatment was paused based on observation of echogenicity within the tissue. Minimal further comminution was observed by the ureteroscope with the resumption of BWL.
Table 4.
Safety outcomes
| Subject | Visual Score |
Visual Observation |
|---|---|---|
| 1 | 1 | Small blood clots between fragments, erythema along calyx wall |
| 2 | 0 | No injury |
| 3 | 1 | Petechial hemorrhage along some papillae, mild to negligible bruising of urothelium |
| 4 | 1 | Mild to negligible urothelium bruising |
| 5 | 1 | Small blood clots between fragments, mild to negligible urothelium bruising |
| 6 | 0 | No injury |
| 7 | 0 | No injury |
| 8 | Not applicable | Treated papilla obscured by blood clot |
| 9 | Not applicable | There were no data to score since ureteroscope video did not record |
| 10 | 1 | Small blood clots attached to heavy plaque at papilla tip |
| 11 | 0 | No injury observed |
| 12 | 0 | No injury observed |
| 13 | 1 | Tiny sites of bleeding at papilla tip |
| 14 | 1 | Tiny red spots on papilla tip |
| 15 | 1 | Small bleed for fornix region |
| 16 | 1 | Small blood clots, some petechiae |
| 17 | 1 | Small blood clots, some petechiae |
| 18 | 1 | Thin streams of blood from tiny sites of injury |
| 19 | 1 | Multiple but tiny sites of bleeding on papilla |
DISCUSSION
New BWL technology successfully fragmented stones of a variety of sizes, locations and densities to under 2 mm fragments within 10 minutes with negligible tissue injury. BWL subjects experienced few associated AEs. Our goal is a noninvasive, 30-minute treatment in the clinic without anesthesia. Despite the abbreviated treatment duration in this study, 39% of the stones were completely broken to fragments ≤2 mm. In contrast, existing SWL procedures fragment approximately 60% of stones to under 4 mm fragments within about an hour.19,21 While this is not a comparison study, better performance than SWL by BWL is possible in theory because BWL delivers more pulses, and therefore more energy per minute than SWL, and is calculated to cause amplification of the applied pressure within the stone not seen with SWL.22-24 In addition, our previous work has shown BWL has been well tolerated by awake subjects, even those with obstructing stones in the ED.8,9 Stones can be observed to fragment by the real-time image guidance,9,11 and ultrasonic propulsion may help determine when stones are completely fragmented.17
Three of the partially comminuted stones received less than 10 minutes of BWL exposure. A stone was also completely comminuted with the BWL beam partially obstructed by a rib. Of the 7 least comminuted stones (those projected to require 50 minutes or more for full comminution), 1 was larger than the transducer beamwidth (subject 3, stone 2), 2 were smaller than the BWL wavelength (subjects 14 and 15, stone 2) and insertion of the ureteroscope introduced air bubbles around another 3 (subjects 7 and 19), all of which would be expected to have reduced effectiveness. In the 2 subjects where tissue cavitation was observed with the iatrogenic introduction of air bubbles with the ureteroscope, countermeasures were employed to reduce the cavitation and as a result, injury was not increased, although comminution was diminished.
The limitations of the study are as follows. First, the unique design of the study measured fragmentation and not the more clinically relevant clearance of stones. Our fragmentation criterion, though—under 2 mm—was stringent, being smaller than the 4 mm standard, with smaller fragments being more likely to pass and to pass asymptomatically. The design of the study also required the patient to be under general anesthesia; this made the procedure more challenging for the research team as the subject was unable change position or voluntarily breath hold to improve stone targeting. With the same transducer and device, we have shown that ultrasonic propulsion may be used to reposition and facilitate passage of stones and fragments which would arguably further increase the likelihood of clearance of BWL treated stones.8,10 Second, the design of the study allowed for the insertion of instruments and irrigation fluid, which alters the state of the collecting space, and in turn could impact the safety and effectiveness of BWL. Third, as a compromise, BWL was limited to 10 minutes per stone, and this limited the degree of stone comminution and the proportion of subjects with complete comminution. Because of the short exposure time, linear extrapolation of the fragmentation rate was used to predict time to complete comminution. However, linear extrapolation is an imperfect predictor, as fragmentation is not linear with time. Fourth, the device specifications potentially limited fragmentation effectiveness in select cases. We believe we failed to comminute the smallest stone in this study (2.6 mm) or the thin crystal growth because they were too small or thin to produce sufficient stress to be further comminuted at the selected 390 kHz BWL frequency. This study evaluated stones up to 12 mm, but BWL is most effective when the beamwidth is larger than the stone, which in this case, corresponds to 6 mm.25 The 1 stone larger than 10 mm (subject 3, stone 2) was 20% comminuted. In addition to a transducer modification for a broader beamwidth,26 a modification for an adjustable focal depth or a selection of probes with different focal depths will enable treatment of a larger range of skin-to-stone depths. Lastly, because of our study design, the detection of AEs due to BWL may be masked by the side effects of URS; however, all reported AEs were mild and resolved spontaneously.
Despite the limitations, the median result was 90% of the stone volume was comminuted to ≤2 mm fragments in 10 minutes with mild side effects in the first in human feasibility study of BWL.
CONCLUSIONS
In this first feasibility study of BWL in humans, 21 of 23 (91%) stones showed fragmentation and 9 of 23 stones (39%) were fragmented completely within 10 minutes. Injury was negligible and included mild reddening of the papilla with some hematuria. The results are a step toward an office-based lithotripsy for awake patients.
ACKNOWLEDGMENTS
We appreciate the help of our research coordinators and colleagues.
Support:
This study was supported by NIH Program Project grant P01 DK043881. Related research in the understanding of BWL and ultrasonic propulsion was funded by NIH training grant K01 DK104854 and NASA grants initially through the National Space Biomedical Research Institute.
Abbreviations and Acronyms
- AE
adverse event
- BMI
body mass index
- BWL
burst wave lithotripsy
- CT
computerized tomography
- ED
emergency department
- SWL
shock wave lithotripsy
- URS
ureteroscopy
Footnotes
Conflict of Interest: RMS: CEO of Simagine Health, recipient of U.S. Department of Defense grants (current), recipient of AUA honorarium for course directorship (past). Authors Michael Bailey, Adam Maxwell, Barbrina Dunmire, Bryan Cunitz and Mathew Sorensen have equity in, and consulting agreements with, SonoMotion, Inc., which licensed the reported technology from the University of Washington for commercialization.
Ethics Statement: Study received approval from the WCG-IRB Institutional Review Board (20210905) and the U.S. Food and Drug Administration through an investigational device exemption (clinicaltrials.gov identifier NCT03873259).
REFERENCES
- 1.Scales CD, Tasian GE, Schwaderer AL et al. : Urinary stone disease: advancing knowledge, patient care, and population health. Clin J Am Soc Nephrol 2016; 11: 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwin MS and Saigal CS: Table 14-47: economic impact of urologic disease. In: Urologic Diseases in America. US Department of Health and Human Services, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service. Washington, DC: US Government Printing Office; 2012; NIH publication 12–7865. [Google Scholar]
- 3.Kidney Stones 2017. In: Urologic Diseases in America. Edited by Feinstein L. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2018; NIH Publication 12–7865. [Google Scholar]
- 4.Saigal CS, Joyce G, Timilsina AR et al. : Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 2005; 68: 1808. [DOI] [PubMed] [Google Scholar]
- 5.Simon JC, Dunmire B, Sorensen MD et al. : Developing complete ultrasonic management of kidney stones for spaceflight. J Space Saf Eng 2016; 3: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper JD, Cunitz BW, Dunmire B et al. : First-in-human clinical trial of ultrasonic propulsion of kidney stones. J Urol 2016; 195: 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai JC, Harper JD, Chang HC et al. : Quantitative assessment of effectiveness of ultrasonic propulsion of kidney stones. J Endourol 2019; 33: 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper JD, Metzler IS, Hall MK et al. : First-in-human burst-wave lithotripsy (BWL) for kidney stone comminution. J Endourol 2021; 35: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper JD, Thiel J, Samson PC et al. : Ultrasound to reposition and accelerate passage of distal ureteral stones. J Urol 2021; 206: e320. [Google Scholar]
- 10.Sorensen MD, Dai JC, Chen TT et al. : Randomized control trial of ultrasonic propulsion to facilitate clearance of chronic residual fragments. J Urol 2021; 206: e1172. [Google Scholar]
- 11.Maxwell AD, Cunitz BW, Kreider W et al. : Fragmentation of urinary calculi in vitro by burst wave lithotripsy. J Urol 2015; 193: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May PC, Kreider W, Maxwell AD et al. : Detection and evaluation of renal injury in burst wave lithotripsy using ultrasound and magnetic resonance imaging. J Endourol 2017; 31: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paterson RF, Lifshitz DA, Lingeman JE et al. : Stone fragmentation during shock wave lithotripsy is improved by slowing the shock wave rate: studies with a new animal model. J Urol 2002; 168: 2211. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell AD, Wang YN, Kreider W et al. : Evaluation of renal stone comminution and injury by burst wave lithotripsy in a pig model. J Endourol 2019; 33: 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunitz BW, Dunmire B, Sorensen MD et al. : Quantification of renal stone contrast with ultrasound in human subjects. J Endourol 2017; 31: 1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JC Jr, Lingeman JE, Daudon M et al. : Using micro computed tomographic imaging for analyzing kidney stones. C R Chim 2021; 24: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramesh S, Chen TT, Maxwell AD et al. : In vitro evaluation of urinary stone comminution with a clinical burst wave lithotripsy (BWL) system. J Endourol 2020; 34: 1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaussy C, Brendel W and Schmiedt E: Extra-corporeally induced destruction of kidney stones by shockwaves. Lancet 1980; 2: 1265. [DOI] [PubMed] [Google Scholar]
- 19.Assimos D, Krambeck A, Miller NL et al. : Surgical management of stones: American Urological Association (AUA)/Endourological Society guideline. J Urol 2016; 196: 1161. [DOI] [PubMed] [Google Scholar]
- 20.EAU guidelines on urological infections. Presented at annual EAU congress. Amsterdam, Netherlands, March 18–21, 2020. [Google Scholar]
- 21.Alexander CE, Gowland S, Cadwallader J et al. : Shock wave lithotripsy (SWL): outcomes from a national SWL database in New Zealand. BJU Int 2016; 117: 76. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell AD, MacConaghy B, Bailey MR et al. : An investigation of elastic waves causing stone fracture in burst wave lithotripsy. J Acoust Soc Am 2020; 147: 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapozhnikov OA, Maxwell AD and Bailey MR: Maximizing mechanical stress in small urinary stones during burst wave lithotripsy. J Acoust Soc Am 2021; 150: 4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rassweiler JJ, Knoll T, Khrmann KU et al. : Shock wave technology and application: an update. Eur Urol 2011; 59: 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenmenger W: The mechanisms of stone fragmentation in ESWL. Ultrasound Med Biol 2001; 27: 683. [DOI] [PubMed] [Google Scholar]
- 26.Randad A, Ghanem MA, Bailey MR et al. : Design, fabrication, and characterization of broad beam transducers for fragmenting large renal calculi with burst wave lithotripsy. J Acoust Soc Am 2020; 148: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]