Abstract
Moxifloxacin, an 8-methoxyquinolone with broad-spectrum activity in vitro, was studied in the rabbit model of Escherichia coli meningitis. The purposes of this study were to evaluate the bactericidal effectiveness and the pharmacodynamic profile of moxifloxacin in cerebrospinal fluid (CSF) and to compare the bactericidal activity with that of ceftriaxone and meropenem therapy. After induction of meningitis, animals were given single doses of 10, 20, and 40 mg/kg or divided-dose regimens of 5, 10, and 20 mg/kg twice, separated by 6 h. After single doses, the penetration of moxifloxacin into purulent CSF, measured as percentage of the area under the concentration-time curve (AUC) in CSF relative to the AUC in plasma, was approximately 50%. After single doses of 10, 20, and 40 mg/kg, the maximum CSF concentration (Cmax) values were 1.8, 4.2, and 4.9 μg/ml, respectively; the AUC values (total drug) were 13.4, 25.4, and 27.1 μg/ml · h, respectively, and the half-life values (t½) were 6.7, 6.6, and 4.7 h, respectively. The bacterial killing in CSF for moxifloxacin, calculated as the Δlog10 CFU per milliliter per hour, at 3, 6, and 12 h after single doses of 10, 20, and 40 mg/kg were −5.70, −6.62, and −7.02; −7.37, −7.37, and −6.87; and −6.62, −6.62, and −6.62, respectively, whereas those of ceftriaxone and meropenem were −4.18, −5.24, and −4.43, and −3.64, −3.59, and −4.12, respectively. The CSF pharmacodynamic indices of AUC/MBC and Cmax/MBC were interrelated (r = 0.81); there was less correlation with T > MBC (r = 0.74). In this model, therapy with moxifloxacin appears to be at least as effective as ceftriaxone and more effective than meropenem therapy in eradicating E. coli from CSF.
Despite the use of broad-spectrum antibiotic therapy, gram-negative bacillary meningitis continues to have a poor prognosis. The case fatality rate of gram-negative bacillary meningitis in newborn infants is from 15 to 30%, and sequelae are found in approximately one-third of survivors (8, 14). In adults the disease occurs principally in neurosurgical patients and in elderly or debilitated patients, and the case fatality rates can be as high as 40% (7). Because of broad antibacterial activity and favorable penetration into cerebrospinal fluid (CSF), the new fluoroquinolones are potentially useful single agents for treatment of gram-negative meningitis (10).
Moxifloxacin (BAY 12-8039), an advanced-generation 8-methoxy fluoroquinolone, has excellent activity against gram-negative enteric bacilli (1; K. Rolston, B. LeBlanc, M. Balakrishnan, and D. H. Ho, Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., 2000, abstr. 2324). Moxifloxacin exhibits bactericidal activity and a significant postantibiotic effect (2). In experimental pneumococcal meningitis, the penetration of moxifloxacin into CSF was approximately 30 to 70% (12, 16). Because the pharmacokinetic-pharmacodynamic properties and effectiveness of moxifloxacin in experimental gram-negative meningitis have not been described, we evaluated these properties in a rabbit model of Escherichia coli meningitis. The specific purposes of this investigation were to determine the pharmacodynamic profile of moxifloxacin in CSF of rabbits with experimental E. coli meningitis and to compare the antibacterial effect of moxifloxacin therapy with those of ceftriaxone and meropenem therapy.
(This study was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 26–29 September 1999.)
MATERIALS AND METHODS
Bacterial strain.
E. coli 77-436, a K1:O18 strain (β-lactamase positive) originally isolated from a neonate with bacterial meningitis, was used for induction of meningitis in the rabbit model. After intrathecal passage in rabbits, the strain was grown overnight on blood agar plates. The plates were washed with endotoxin-free phosphate-buffered saline (PBS), and aliquots of the resultant suspension were frozen at −70°C. For preparation of the inoculum, aliquots were thawed and diluted in PBS to a concentration of approximately 1 × 105 to 5 × 105 CFU/ml, of which 250 μl was injected into the cisterna magna of each rabbit. The inoculum size was confirmed by quantitative cultures in each experiment.
Susceptibility tests.
The MICs and MBCs of different antibiotics were measured by the standard microdilution method (11).
Meningitis model.
The rabbit meningitis model, modified from the original description by Dacey and Sande, was used (4). Young male New Zealand White rabbits weighing 2 to 2.5 kg were anesthetized with intramuscular ketamine (50 mg/kg of body weight) and acepromazine (4 mg/kg) before every procedure and frequently while the animals were immobilized in the frames. Flunixin meglumine (1.1 mg/kg) was administered intramuscularly every 12 h for analgesia. Meningitis was induced by inoculation of 250 μl of an E. coli suspension containing 1 × 105 to 5 × 105 CFU/ml into the cisterna magna. Once meningitis was established (16 h later), an initial collection of CSF was withdrawn (0 h) for quantification of initial bacterial concentration, and antibiotic therapy was initiated via a marginal ear vein. Animals were restrained in stereotactic frames, and a spinal needle remained in the cisterna magna for the first 3 h to ensure nontraumatic collection of CSF. The rate of removal of CSF did not exceed the rate of CSF formation, which is approximately 0.4 ml/h (18). Blood samples were taken from a central ear artery. The rabbits were euthanized with intravenous injection of pentobarbital (120 mg/kg) at the end of each experiment or earlier if they appeared to be severely lethargic.
Antimicrobial therapy.
Moxifloxacin (Bayer Corporation, Pharmaceutical Division, West Haven, Conn.), meropenem (Zeneca, Wilmington, Del.), and ceftriaxone (Roche, Nutley, N.J.) were prepared according to the manufacturers' instructions. Antibiotics were initiated 16 h after inoculation and were given intravenously (i.v.) over 3 to 5 min. In the first experiment, moxifloxacin was given as single injections of 10 mg/kg (n = 7), 20 mg/kg (n = 6), and 40 mg/kg (n = 8). In the second, two doses each of 5 mg/kg (n = 8), 10 mg/kg (n = 5), and 20 mg/kg (n = 8) were given at a 6-h interval. Moxifloxacin given at 20 mg/kg as a single dose was tested in uninfected rabbits (n = 5). Moxifloxacin therapy was compared with meropenem at 75 mg/kg given every 6 h for two doses (n = 5), and ceftriaxone at 125 mg/kg as a single dose (n = 5). Antibiotic dosages were chosen to simulate serum and CSF maximum concentrations achieved in humans. Six untreated rabbits were used as controls.
Sample collection and processing.
For single-dose studies, 150 μl of CSF was collected at 30 min and 1, 2, 3, 6, 12, and 24 h, and 700 μl of blood was collected at 15 and 30 min and 1, 2, 3, 6, 12, and 24 h after initiation of therapy. For divided-dose experiments, blood and CSF samples were also withdrawn at 7 h (1 h post-second dose). The last time point observed was 24 h after the first dose for both single- and divided-dose regimens. An additional 100 to 150 μl of CSF was collected for quantification of bacterial concentrations before the initiation of therapy (0 h) and at 3, 6, 12, and 24 h. Bacterial concentrations were determined by plating undiluted and serial dilutions of CSF on sheep blood agar and incubating the plates at 35°C for 24 h. The lowest bacterial concentration detectable by this method was 10 CFU/ml. For purposes of analysis, specimens with <10 CFU/ml were assigned a value of 1 (0 log10) CFU/ml. Bacterial killing rates (BKR) were calculated as the difference between bacterial concentrations at the start of therapy and at 3, 6, 12, and 24 h divided by time. The remaining CSF and blood samples were centrifuged, and the supernatants were immediately stored at −70°C for subsequent analysis.
Antibiotic bioassay.
Moxifloxacin concentrations were determined by a disk diffusion microbioassay with Bacillus subtilis ATCC 6633 (17). For plasma and CSF samples, different standard curves were constructed with rabbit serum and CSF. Standards were stored according to the manufacturer's specifications. Standard curves were created using concentrations from 10 to 0.2 μg/ml for plasma samples and from 6 to 0.1 μg/ml for CSF samples. The lower limit of detection was 0.2 μg/ml for plasma and 0.1 μg/ml for CSF specimens. Some samples demonstrated zones of inhibition that could be extrapolated to calculate concentrations lower than these values. The lowest extrapolated values for plasma and for CSF were 0.07 and 0.02 μg/ml, respectively. Values lower than the limit of detection were not used for the pharmacokinetic calculations. The intra- and interassay coefficients of variation were 2.5 and 1.5% for plasma and 3.1 and 1.9% for CSF, respectively.
Pharmacokinetic and pharmacodynamic indices.
Pharmacokinetic analysis was performed with the computer program TopFit V2 (Karl Thomae, Boehringer, Ingelheim, Germany). A two-compartment model was considered for calculations of plasma pharmacokinetic indices, and a noncompartmental model was used for calculation of CSF indices. The time course of moxifloxacin concentrations in plasma and CSF was evaluated for each rabbit. Plasma and CSF maximum concentrations (Cmax) of moxifloxacin were the highest measured values. The half-life (t½) and the area under the concentration-time curve (AUC) for plasma and CSF were calculated from 0 to 24 h for each animal that finished the experiment at 24 h. The areas under the concentration-time curves (AUC0–24) for serum and CSF were estimated from 0 h (initial antibiotic therapy) to the last quantifiable concentration (24 h) using the linear trapezoidal rule and the logarithmic trapezoidal rule, respectively. The log-trapezoidal method was used for CSF values because it is believed to have less distortion of the AUC computation at both the ascending and descending parts of the concentration-time curve (6).
The percentage of time during which the CSF concentration of moxifloxacin was above the MBC (T > MBC) was calculated as described by Turnidge (19). The ratios of CSF Cmax to MBC (Cmax/MBC) and CSF AUC over MBC (AUC/MBC) were obtained. The MBC of the antibiotics was used in the calculation of the pharmacodynamic indices. The relationships between the three pharmacodynamic indices (T > MBC, Cmax/MBC, and AUC/MBC) and the BKR were fitted to a sigmoid Emax model with the computer program WinNonlin version 1.5 (Scientific Consulting, Inc.). The following formula was used: E = (Emax × Cγ)/(Cγ + ECγ50), where E is the estimated bacterial killing rate, Emax is the maximum BKR, C is the mean Cmax/MBC or AUC/MBC, EC50 is the C producing half-maximal BKR, and γ is the Hill coefficient indicating the slope of the sigmoid curve. Linear regression analysis was used to express the relationship between T > MBC and BKR. Penetration of moxifloxacin into CSF (expressed as a percentage) was calculated as the ratio of CSF to plasma AUC0–24 (AUCCSF/AUCplasma).
Statistical analysis.
Comparisons between two groups were performed by t test or Mann-Whitney test if normally distributed or not, respectively. Comparisons among three or more groups were performed by Kruskal-Wallis test followed by Dunn's multiple-comparisons test among groups when they were significantly different. A P value of <0.05 was considered significant. Data are expressed as mean ± 1 standard deviation (SD).
RESULTS
In vitro susceptibility.
For the E. coli strain used, the MICs and MBCs of moxifloxacin, ceftriaxone, and meropenem were 0.06 and 0.06 μg/ml, 0.125 and 0.125 μg/ml, and 0.03 and 0.06 μg/ml, respectively.
Single-dose pharmacokinetics.
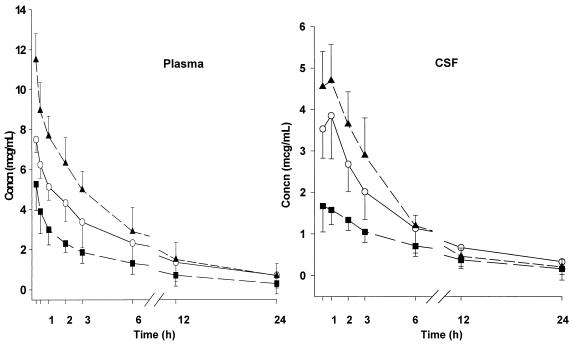
The concentration-time curves of moxifloxacin in plasma and CSF after single-dose regimens are shown in Fig. 1. Pharmacokinetic indices in infected animals are summarized in Table 1. Maximum concentrations in the CSF of individual animals occurred within 30 min to 1 h after administration of the initial dose. The t½ in CSF was 0.8- to 1.2-fold greater than in plasma for the 10- and 20-mg/kg groups. The mean penetration of moxifloxacin into the CSF, expressed as AUCCSF/AUCplasma, was 50% in infected animals. When calculated as the CSF to plasma Cmax ratio, it was 45% (range, 34 to 57%). Among infected animals, a linear correlation was found between the single-dose regimens and CSF Cmax values (r = 0.81; P < 0.001) and AUC (r = 0.66; P = 0.001).
FIG. 1.
Concentration-time curves of moxifloxacin in plasma (left) and CSF (right) after single-dose regimens for experimental E. coli meningitis. Drug concentrations are expressed as means ± SD. Moxifloxacin doses of 10 mg/kg (■), 20 mg/kg (○), or 40 mg/kg (▴) were given i.v. 16 h after inoculation (0 h). Plasma concentrations were obtained at 15 and 30 min and 1, 2, 3, 6, 12, and 24 h; CSF concentrations were determined at 30 min and 1, 2, 3, 6, 12, and 24 h.
TABLE 1.
Pharmacokinetic indices over 24 h after single-dose regimens of moxifloxacin therapy for E. coli meningitis
| Dose mg/kg (n) | CSF indices (mean ± SD)
|
Plasma indices (mean ± SD)
|
|||||
|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | AUC0–24a (μg/ml · h) | t½ (h) | Cmax (μg/ml) | AUC0–24 (μg/ml · h) | t½ (h) | AUCCSF/AUCplasma (%) | |
| 10 (7) | 1.8 ± 0.6 | 13.4 ± 4.3 | 6.7 ± 1.9 | 5.3 ± 1.3 | 26.2 ± 16.1 | 5.9 ± 3.0 | 50 |
| 20 (6) | 4.2 ± 0.7 | 25.4 ± 11.1 | 6.6 ± 3.9 | 7.5 ± 0.7 | 36.9 ± 10.7 | 5.4 ± 1.2 | 52 |
| 40 (8) | 4.9 ± 0.9 | 27.1 ± 6.4 | 4.7 ± 1.3 | 11.1 ± 1.6 | 56.6 ± 24.3 | 4.7 ± 2.2 | 51 |
AUC0–24 calculated as total drug.
The CSF pharmacokinetic indices of moxifloxacin at 20 mg/kg in uninfected animals were as follows: Cmax was 1.3 ± 0.2 μg/ml, AUC0–24 was 5.8 ± 1.0 μg/ml · h, and t½ was 4.1 ± 0.4 h. The penetration of moxifloxacin through uninflamed meninges (AUCCSF /AUCplasma) was 23%.
Pharmacodynamics and bacteriologic effectiveness of single-dose therapy.
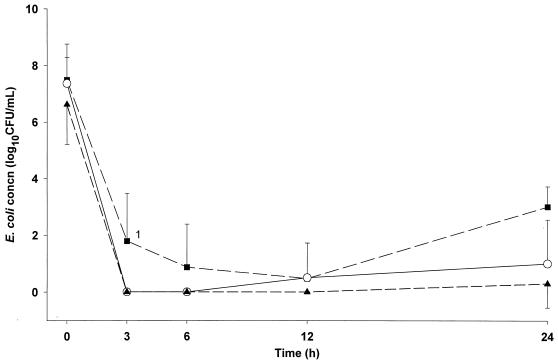
Pharmacokinetic-pharmacodynamic indices in CSF after single-dose regimens and bacteriologic effectiveness are summarized in Table 2 and Table 3, respectively. Mean concentrations of E. coli in CSF determined in animals treated with single-dose regimens are shown in Fig. 2. No significant differences in bacterial concentrations were observed among single-dose, multiple-dose, and comparison (meropenem and ceftriaxone) groups at the start of antibiotic therapy. The bactericidal effectiveness of moxifloxacin was concentration dependent for 10- and 20-mg/kg doses. However, there was no significant difference in the bacterial killing rate between the 20- and the 40-mg/kg dosage groups. Bacterial regrowth occurred after 12 h in all animals treated with 10 mg/kg, in two of six animals treated with 20 mg/kg, and in one of eight animals receiving 40 mg/kg. Moxifloxacin concentrations in CSF were above the MBC at all time points except at 24 h in two animals given 10 mg/kg and in one each for the 20- and 40-mg/kg doses. At 12 h in single-dose experiments, moxifloxacin concentrations in CSF were 6- to 11-fold greater than the MBC, and at 24 h they were 2- to 5-fold greater. The maximal BKR0–3 of −2.4 CFU/ml/h was reached with AUC/MBC (total drug) of 422.6, AUC/MBC (free drug) of 295.8, Cmax/MBC of 70.8, and T > MBC values of 99% in animals given 20 mg/kg. Free moxifloxacin values were calculated using a protein binding of 30%. This value was derived from data with pooled human sera by Woodcock et al. (21) and with rabbit sera by O/stergaard et al. (12). The CSF AUC/MBC and Cmax/MBC were interrelated (r = 0.84, P < 0.001), whereas the T > MBC was less well correlated with the other values (r = 0.74).
TABLE 2.
CSF pharmacodynamic indices over 24 h after different regimens of moxifloxacin therapy for E. coli meningitis
| Regimen and dose, mg/kg (no. of rabbits) | Cmax/MBC ratio | AUC/MBC ratioa | T > MBC (h) | t½ (h) | AUCCSF/AUCpl (%) |
|---|---|---|---|---|---|
| Single dose | |||||
| 10 (7) | 29.61 ± 9.35 | 156.40 ± 49.89 | 23.2 ± 0.8 | 6.7 ± 1.9 | 50 |
| 20 (6) | 70.89 ± 11.70 | 295.83 ± 129.79 | 23.8 ± 0.2 | 6.6 ± 3.9 | 52 |
| 40 (8) | 81.16 ± 15.35 | 316.15 ± 74.48 | 23.1 ± 0.9 | 4.7 ± 1.3 | 51 |
| Divided dose | |||||
| 5 (8) | 9.88 ± 2.38 | 58.39 ± 11.90 | 19.6 ± 2.4 | 5.8 ± 0.9 | 75 |
| 10 (5) | 17.70 ± 4.40 | 162.14 ± 43.10 | 23.7 ± 0.3 | 8.7 ± 4.4 | 79 |
| 20 (8) | 60.72 ± 12.31 | 504.00 ± 88.79 | 24 | 9.9 ± 3.4 | 85 |
AUC0–24 calculated as free drug.
TABLE 3.
Bacterial killing in CSF over 24 h after different dosing regimens of moxifloxacin therapy for experimental E. coli meningitis
| Regimen and dose, mg/kg (no. of rabbits) | BKR0–3 (CFU/ml/h) | Δlog10 CFU/ml after:
|
|
|---|---|---|---|
| 3 h | 12 h | ||
| Single dose | |||
| 10 (7) | −1.9 ± 0.6 | −5.70 ± 1.78 | −7.02 ± 0.65 |
| 20 (6) | −2.4 ± 0.4 | −7.37 ± 1.39 | −6.87 ± 1.33 |
| 40 (8) | −2.2 ± 0.5 | −6.62 ± 1.41 | −6.62 ± 1.41 |
| Divided dose | |||
| 5 (8) | −1.7 ± 0.3 | −5.13 ± 0.95 | −5.02 ± 1.10 |
| 10 (5) | −1.9 ± 0.5 | −5.76 ± 1.42 | −5.76 ± 1.42 |
| 20 (8) | −1.9 ± 0.5 | −6.14 ± 1.43 | −6.14 ± 1.38 |
FIG. 2.
Mean bacterial concentrations (±SD) in CSF after single-dose regimens of moxifloxacin for experimental E. coli meningitis. Animals were treated with moxifloxacin at 10 mg/kg (■), 20 mg/kg (○), or 40 mg/kg (▴). 1, P < 0.05 compared to 20-mg/kg and 40-mg/kg groups.
There was a direct correlation between the bacterial killing rate at all time points and AUC/MBC (r = 0.76), Cmax/MBC (r = 0.58), and T > MBC (r = 0.57). The relation between AUC/MBC or Cmax/MBC and bacterial killing rate was best described by the sigmoid Emax model. The T > MBC correlated best with BKR by linear regression. The highest Emax was achieved with the CSF AUC/MBC, followed by Cmax/MBC and by T > MBC.
The pharmacodynamic data in five uninfected animals given moxifloxacin at 20 mg/kg were Cmax/MBC, 21.5 ± 3.7; AUC/MBC (total drug), 97.3 ± 16.8; AUC/MBC (free drug), 68.1 ± 11.7; and T > MBC, 73.9%.
Antibacterial effect and pharmacodynamics with divided-dose regimens.
The pharmacodynamic indices in CSF after divided-dose regimens and the bacteriologic effectiveness are summarized in Tables 2 and 3. The bacterial killing rate of moxifloxacin was concentration dependent at 3 h (P = 0.04) and 6 h (P = 0.04) comparing the 5-, 10-, and 20-mg/kg two-dose regimens. However, there was not a significant difference in bacterial clearance from CSF for the 10-mg/kg and 20-mg/kg two-dose regimens. The bacterial killing in CSF for moxifloxacin, at 3, 6, and 12 h after divided-dose regimens of 5, 10, and 20 mg/kg twice, separated by 6 h, were −5.13, −5.54, and −5.02; −5.76, −5.76, and −5.76; and −6.14, −5.39, and −6.14 Δlog10 CFU/ml, respectively. At 24 h the divided-dose regimens demonstrated superior bacterial clearance compared with the corresponding single-dose regimens (18 of 21 animals versus 11 of 21 animals, respectively) (P = 0.050). The treatment that exhibited maximal bacterial clearance during the study period was 20 mg/kg given twice.
Moxifloxacin in divided-dose regimens showed greater penetration into the CSF than after single doses. The AUCCSF/AUCplasma values were 75, 79, and 85% with 5-, 10-, and 20-mg/kg two-dose regimens, respectively.
Divided-dose treatments resulted in 25 to 74% lower maximum CSF concentrations but similar T > MBC compared with those for the same total dosage given as a single-dose. The AUC/MBC values were 34 to 55% lower for multiple-dose than for single-dose regimens except with the 40-mg/kg regimen, which showed a 63% superior AUC/MBC ratio for divided doses than for the single-dose regimen.
Comparison of moxifloxacin with other antibiotics.
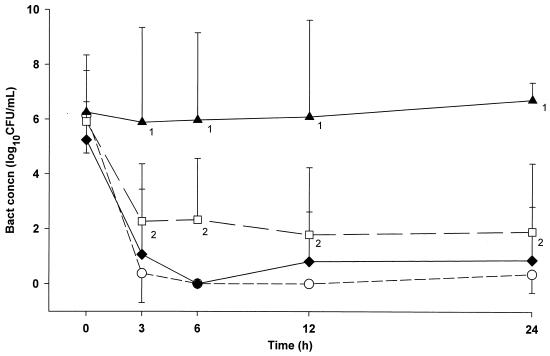
The antibacterial effects of moxifloxacin, ceftriaxone, and meropenem are shown in Fig. 3. All antibiotic-treated groups had significantly higher reductions in CSF bacterial concentrations than the untreated group (P < 0.05). The bacterial killing rates achieved for animals treated with all dosing regimens of moxifloxacin were greater than those of ceftriaxone, but these were not statistically significant. The antibacterial effectiveness of moxifloxacin was superior to that of meropenem (P < 0.05).
FIG. 3.
Mean bacterial concentrations (±SD) in CSF after moxifloxacin given 20 mg/kg twice (○), ceftriaxone given as a 125-mg/kg single dose (⧫), and meropenem given as 75 mg/kg twice (□) for E. coli meningitis. The following differences were significant (P < 0.05): 1, control animals (▴) compared to all antibiotic groups, and 2, moxifloxacin group versus meropenem group.
DISCUSSION
In this E. coli meningitis model, moxifloxacin demonstrated excellent penetration into the CSF and antibacterial effectiveness. Like other fluoroquinolones (3), moxifloxacin exhibited concentration-dependent bacterial killing. We demonstrated that the antibacterial effectiveness of moxifloxacin was concentration dependent during the first 6 h after doses of 5, 10, and 20 mg/kg. However, increasing the dosage beyond 20 mg/kg did not enhance CSF sterilization because the CSF was sterile in the first 6 h of therapy. Therefore, the previously described paradoxical decrease in the killing rate above a certain fluoroquinolone dosage was not observed (9). As one of six animals receiving a single dose of 20 mg/kg had a positive CSF culture at 12 h, divided-dose regimens were tested to define the optimal regimen for moxifloxacin. We showed that the 20-mg/kg two-dose regimen exhibited maximal bacterial clearance during the study period. The majority of CSF concentrations of moxifloxacin in single-dose regimens and all in divided-dose regimens were above the MBC for the entire treatment period; consequently, a postantibiotic effect of moxifloxacin could not be characterized.
Because the protein binding of moxifloxacin is approximately 30%, the presence of serum has little effect on the MICs of moxifloxacin. The low degree of protein binding influences the penetration across the blood-brain barrier, explaining why moxifloxacin achieved excellent penetration into CSF in experimental E. coli meningitis. The penetration was 50 to 85%, which is similar to those obtained in other experimental meningitis studies. Schmidt et al. (16) obtained a penetration based on the ratio of concentration in CSF to serum of 44% ± 8% in animals without the coadministration of dexamethasone and 34% ± 15% with the addition of dexamethasone. Østergaard et al. (12) demonstrated a mean percent penetration into CSF based on an AUC ratio of CSF to blood of 78% ± 9% and 50% ± 2% for rabbits with and without meningitis, respectively.
As with other fluoroquinolones, the AUC/MBC was the principal pharmacodynamic value that correlated with antibacterial effectiveness of moxifloxacin. We studied this relation using an Emax model that correlated AUC0–24/MBC values with the bacterial killing rate at 24 h. The BKR of moxifloxacin was better correlated with AUC/MBC than with Cmax/MBC or T > MBC. The MBC value was used in this study because bactericidal activity is critical for clearance of organisms from CSF (15). Our findings indicate that a single dose of moxifloxacin was not as effective as a daily two-dose regimen in rabbits with experimental E. coli meningitis. After analysis of animal models of pneumonia, peritonitis, and sepsis in mice, rats, and guinea pigs, Craig observed that 24-h AUC/MIC ratios of ≥100 were associated with almost 100% survival (3). Forrest et al. conducted a retrospective clinical trial with 74 acutely ill patients treated with intravenous ciprofloxacin in different dosages. They demonstrated that the 24-h AUC/MIC ratio was the most significant parameter for probability of both microbiologic and clinical cures and that a value below 125 was predictive of failure (5). Preston et al., in a multicenter open-label trial of 313 patients, showed that both clinical and microbiological outcomes were likely to be favorable if the ratio of peak plasma concentration to MIC was greater than 12.2 (13). These pharmacodynamic correlations were based on total-drug values. Combining single- and divided-dose regimens, we obtained effective bacterial killing with Cmax/MBC values from 17 to 80 and AUC/MBC ratios from 220 to 720 for total drug and 160 to 500 for free drug. By contrast, the regimen that was less microbiologically effective (5 mg/kg in two doses) had a Cmax/MBC ratio of 9.8 and AUC/MBC ratio of 83.
Although no human data are available on the CSF pharmacokinetic profile of moxifloxacin, a potential regimen for therapy of E. coli meningitis in humans may be extrapolated based on our results and on available human serum pharmacokinetic data (20). The CSF t½ approximates that in serum (0.8- to 1.2-fold in our study). Because the plasma t½ in humans receiving an i.v. or oral single 400-mg dose is approximately 8 h (range, 6.6 to 11.2 h), the predictive CSF t½ in humans would be about 7 to 10 h. Assuming that the CSF penetration of moxifloxacin in humans and in rabbits is comparable (approximately 30%), we suggest that a loading dose of moxifloxacin followed by a 12-h regimen would be adequate in humans to ensure high CSF AUC and concentrations above MBC as therapy for E. coli meningitis. These assumptions would need to be validated in humans before a trial could be undertaken.
In conclusion, moxifloxacin was effective as a single agent for the therapy of experimental E. coli meningitis. The bacterial killing rate of moxifloxacin was comparable to that of ceftriaxone and superior to that of meropenem. These data suggest that moxifloxacin would be effective for treatment of E. coli meningitis. Clinical trials are needed to confirm these findings.
ACKNOWLEDGMENTS
Violeta Rodriguez-Cerrato is the recipient of a Fellowship grant from the European Society of Paediatric Infectious Diseases (ESPID) sponsored by Wyeth-Lederle Vaccines and Pediatrics. This work was supported in part by a grant from Bayer Corporation, Pharmaceutical Division, West Haven, Conn.
REFERENCES
- 1.Bauernfeind A. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 2.Boswell F J, Andrews J M, Wise R. Pharmacodynamic properties of BAY 12-8039 on gram-positive and gram-negative organisms as demonstrated by studies of time-kill kinetics and postantibiotic effect. Antimicrob Agents Chemother. 1997;41:1377–1379. doi: 10.1128/aac.41.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 4.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrielsson J, Weiner D. Non-compartmental analysis. In: Gabrielsson J, Weiner D, editors. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. 2nd ed. Stockholm, Sweden: Swedish Pharmaceutical Press; 1997. pp. 139–144. [Google Scholar]
- 7.Harder E, Moller K, Skinhoj P. Enterobacteriaceae meningitis in adults: a review of 20 consecutive cases 1977–97. Scand J Infect Dis. 1999;31:287–291. doi: 10.1080/00365549950163590. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan S L, Patrick C C. Cefotaxime and aminoglycoside treatment of meningitis caused by gram-negative enteric organisms. Pediatr Infect Dis J. 1990;9:810–814. doi: 10.1097/00006454-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lode H, Borner K, Koeppe P. Pharmacodynamics of fluoroquinolones. Clin Infect Dis. 1998;27:33–39. doi: 10.1086/514623. [DOI] [PubMed] [Google Scholar]
- 10.Lutsar I, Friedland I R, Jafri H S, Wubbel L, Ng W, Ghaffar F, McCracken G H., Jr Efficacy of gatifloxacin in experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1999;43:1805–1807. doi: 10.1128/aac.43.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 5th ed. NCCLS publication No. M7–A5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 12.Østergaard C, Sorensen T K, Knudsen J D, Frimodt-Moller N. Evaluation of moxifloxacin, a new 8-methoxyquinolone, for treatment of meningitis caused by a penicillin-resistant pneumococcus in rabbits. Antimicrob Agents Chemother. 1998;42:1706–1712. doi: 10.1128/aac.42.7.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxacin: a new paradigma for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 14.Schaad U B, McCracken G H, Jr, Loock C A, Thomas M L. Pharmacokinetics and bacteriological efficacy of moxalactam ( LY127935), netilmicin, and ampicillin in experimental gram-negative enteric bacillary meningitis. Antimicrob Agents Chemother. 1980;17:406–411. doi: 10.1128/aac.17.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheld W M, Sande M A. Bactericidal versus bacteriostatic antibiotic therapy of experimental pneumococcal meningitis in rabbits. J Clin Investig. 1983;71:411–419. doi: 10.1172/JCI110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt H, Dalhoff A, Stuertz K, Trostdorf F, Chen V, Schneider O, Kohlsdorfer C, Brück W, Nau R. Moxifloxacin in the therapy of experimental pneumococcal meningitis. Antimicrob Agents Chemother. 1998;42:1397–1401. doi: 10.1128/aac.42.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon H J, Yin E J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970;19:573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector R, Lorenzo A V. Inhibition of penicillin transport from the cerebrospinal fluid after intracisternal inoculation of bacteria. J Clin Investig. 1974;54:316–325. doi: 10.1172/JCI107767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnidge J D. The pharmacodynamics of β-lactams. Clin Infect Dis. 1998;27:10–22. doi: 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 20.Wise R, Andrews J M, Marshall G, Hartman G. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob Agents Chemother. 1999;43:1508–1510. doi: 10.1128/aac.43.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12–8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]