Abstract
Purpose
Sepsis is the main cause of death in intensive care unit. Maladaptive cytokine storm and T-cell lymphopenia are critical prognosis predictors of sepsis. Electroacupuncture (EA) is expected to be an effective intervention to prevent sepsis. This study aims to determine the potential of EA at ST36 (Zusanli) to prevent experimental septic mice.
Methods
Mice were randomly assigned into PBS, LPS, or EA+LPS group. EA (0.1 mA, continuous wave, 10 Hz) was performed stimulating the ST36 for 30 min, once a day for 3 days. After the third day, all mice were challenged with PBS or LPS (4 mg/kg) simultaneously. Mice were evaluated for survival, ear temperature, and other clinical symptoms. Lung and small intestine tissue injuries were analyzed by hematoxylin and eosin staining. Bio-Plex cytokine assay was used to analyze the concentration of cytokines. T lymphocytes were analyzed by flow cytometry and Western blot assays. The role of T cells in preventing sepsis by EA was analyzed by using nude mice lacking T lymphocytes.
Results
EA at ST36 improved survival, symptom scores, and ear temperature of endotoxemic mice. EA also improved dramatically pulmonary and intestinal injury by over 50% as compared to untreated mice. EA blunted the inflammatory cytokine storm by inducing a lasting inhibition of the production of major inflammatory factors (TNF-α, IL-1β, IL-5, IL-6, IL-10, IL-17A, eotaxin, IFN-γ, MIP-1β and KC). Flow cytometry and Western blot analyses showed EA significantly reduced T-lymphocyte apoptosis and pyroptosis. Furthermore, T lymphocytes were critical for the effects of EA at ST36 stimulation blunted serum TNF-α levels in wild-type but not in nude mice.
Conclusion
EA halted systemic inflammation and improved survival in endotoxemic mice. These effects are associated with the protective effect of EA on T lymphocytes, and T cells are required in the anti-inflammatory effects of EA in sepsis.
Keywords: electroacupuncture, sepsis, inflammation, cytokine storm, lymphopenia
Introduction
Sepsis is an organ dysfunction syndrome caused by dysregulated responses to infection.1 Despite the major advances in the intensive care unit (ICU), sepsis still has high incidence and it is the most common disease and cause of death in hospitals.2 The Global Burden of Disease Study reported approximately 49 million cases of sepsis globally in 2017, with a case fatality rate of nearly 20%.3 In addition to bacterial infections, sepsis may also be one of the main causes of death in coronavirus disease 2019 (COVID-19). The incidence of sepsis in patients with COVID-19 is 59%, and almost all patients who died had sepsis.4,5 In the United States, the annual economic loss caused by sepsis is as high as US$24 billion.6 As an important global health problem, sepsis is not only a clinical and scientific challenge but also a heavy burden to society.
The early cytokine storm and the later immunosuppression characterized by lymphopenia have a dramatic impact on the prognosis of sepsis.7 Patients with severe sepsis usually have an overzealous production of pro-inflammatory factors, leading to lymphocyte exhaustion and lymphopenia.8,9 In turn, lymphopenia disrupts physiologic regulation of cytokine production to further exacerbate the inflammatory responses.10,11 The cytokine storm and lymphopenia are responsible for patients malaise and the most significant symptoms such as hypothermia (core temperature <36°C), fever (core temperature >38°C), cognitive dysfunction, and lethal multiple organ dysfunction syndrome (MODS).12 Current sepsis treatment mainly focuses on controlling the infection by antibiotics, optimize hemodynamics, and support organ care.13 New generation of antibiotics are getting more effective in controlling infections, but sepsis still characterized by high mortality due to the unregulated inflammatory responses causing organ damage. Previous anti-inflammatory clinical trials in sepsis have focused on the inhibition of specific inflammatory factors such as tumor necrosis factor (TNF-α) and interleukin (IL)-1 have failed in part because they targeted single cytokine but sepsis is characterized by the complex production of multiple inflammatory factors.14–16 Thus, there is an urgent unmet need to develop more comprehensive anti-inflammatory therapies that can modulate multiple factors and prevent the multiple detrimental inflammatory processes.
One beneficial strategy for clinical management is the design of early prediction and intervention making sepsis treatment more amenable.17 Screening tools such as national early warning score (NEWS), systemic inflammatory response syndrome (SIRS) criteria, or modified early warning score (MEWS) are usually used in clinic for early identification and screening of patients with high-risk of sepsis.18 To date, the main strategies to prevent sepsis are preventing infection and organ dysfunction, such as using preventive antibiotics, and supplemental enteral nutrition.19,20 New scientific and clinical studies are suggesting that nerve stimulation with electroacupuncture (EA) can be a promising affordable early intervention for patients with high-risk of sepsis.21
As an important part of traditional medicine, acupuncture has significant effects in preventing and treating diseases. For instance, acupuncture treatment can prevent the prevalence of delirium in ICUs cardiovascular patients. The prevalence of delirium in the treatment group is only 1/6 of that of the control group.22 Acupuncture at P6 (Neiguan) acupoint significantly reduced the Visual Analog Scale pain score within 24–48 h in patients after craniotomy, reduced the incidence of vomiting within 24 h (the incidence rate in the acupuncture group is only 1/2 of that of the sham acupuncture group), and effectively prevented postoperative pain.23 The study found that EA pretreatment at bilateral ST36 (Zusanli) acupoints significantly improved the intestinal function (the injury score of the EA pretreatment group is only 1/2 of that of the sepsis group) and survival rate (EA+Sepsis vs Sepsis: 75% vs 25%) in septic rats.24 A randomized controlled trial showed that conventional therapies combined with EA at ST36 and ST37 (Shangjuxu) acupoints significantly reduced plasma level of TNF-α (from baseline 94.33±29.87 pg/mL to 19.68±11.70 pg/mL) and traditional Chinese medicine quantitative scores of intestinal dysfunction (from baseline 9.8±2.7 to 6.9±2.5) on day 7 in septic patients, and the effect of EA treatment group was better than that of conventional treatment alone.25 Low-intensity EA at unilateral ST36 acupoint can significantly reduce serum TNF-α and IL-6 concentrations in septic mice, inhibit systemic inflammation, and increase survival rate (EA group vs sham EA group: 70% vs 35%).26 However, the prophylactic potential of acupuncture on sepsis are unknown. This study aims to investigate the preventive and protective effects of EA in experimental septic mice and explore the possible mechanism of action.
Materials and Methods
Experimental Mice
Male BALB/c wild-type mice and nude mice (6–8 weeks old, weighing 25.0 ± 5.0 g) were purchased from Beijing Charles River Laboratory Animal Technology Co. Ltd. The animals were housed under normal laboratory conditions without pathogens (temperature at 21 ± 1°C, 50 ± 5% air humidity) and had free access to food and water during a regular 12 h light-dark cycles. Mice were randomly assigned into three groups: (1) control mice treated with phosphate-buffered saline (PBS) intraperitoneally (i.p.); (2) endotoxemic mice treated with lipopolysaccharide (LPS; 4 mg/kg; i.p.); (3) the experimental EA treatment of endotoxemic mice (EA+LPS). Wild-type and nude mice were distinguished by morphological observation and flow cytometry. All animal procedures were reviewed and approved by the IACUC of the Shanghai University of Traditional Chinese Medicine in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” as well as the guidelines of the Animal Welfare Act (No. PZSHUTCM190308020).
Experimental Sepsis Model by LPS
Mice were acclimatized in the animal facility for 1 week before use and LPS induced sepsis model was performed following previous publications.27,28 Briefly, LPS (E. coli 0111:B4; Sigma-Aldrich, USA) was dissolved in sterile pyrogen-free PBS (Gibco: Thermo Fisher, USA), formulated as a 1 mg/mL solution before use and stored at 4°C. By comparing different LPS doses (2, 4, 6, 12 mg/kg) and different mouse ages (6 and 8 weeks old), it was finally determined that 4 mg/kg dose of LPS was intraperitoneally injected into 8-week-old mice to ensure that all mice in LPS group died within 24–48 h after LPS challenge.
EA Treatment Procedure
Mice were anesthetized with 0.5–1.5% isoflurane and a thermal blanket was used to maintain body temperature. Stainless-steel acupuncture needles (0.3 × 13 mm; Suzhou Medical Supplies Factory Co. Ltd, China) connected to an electronic acupuncture treatment instrument (SDZ-IIB; Suzhou Medical Supplies Factory Co. Ltd, China) were used for EA treatment. According to previous experimental and literature research,27,29 the EA was performed with a frequency of 10 Hz, continuous wave. It has been showed that low-intensity electrical stimulation is sufficient to drive vagal-adrenal anti-inflammatory axis so the electrical current was set to 0.1 mA in accordance with the standard that was appropriate for the slight tremor of the lower limbs of the mouse and minimized damage to mice.21 The needle was inserted into ST36 acupoint with the depth of 3.5 mm, which was about 4 mm below the knee joint and about 2 mm outside the anterior tibial tubercle. ST36 acupoint is located at the confluence of the three neural branches from the sciatic nerve, namely the tibial, sural and peroneal nerves. EA stimulation was given 2 days before establishment of the LPS-induced sepsis model and on the day of modeling, 30 min a day for a total of 3 days (Figure 1A).
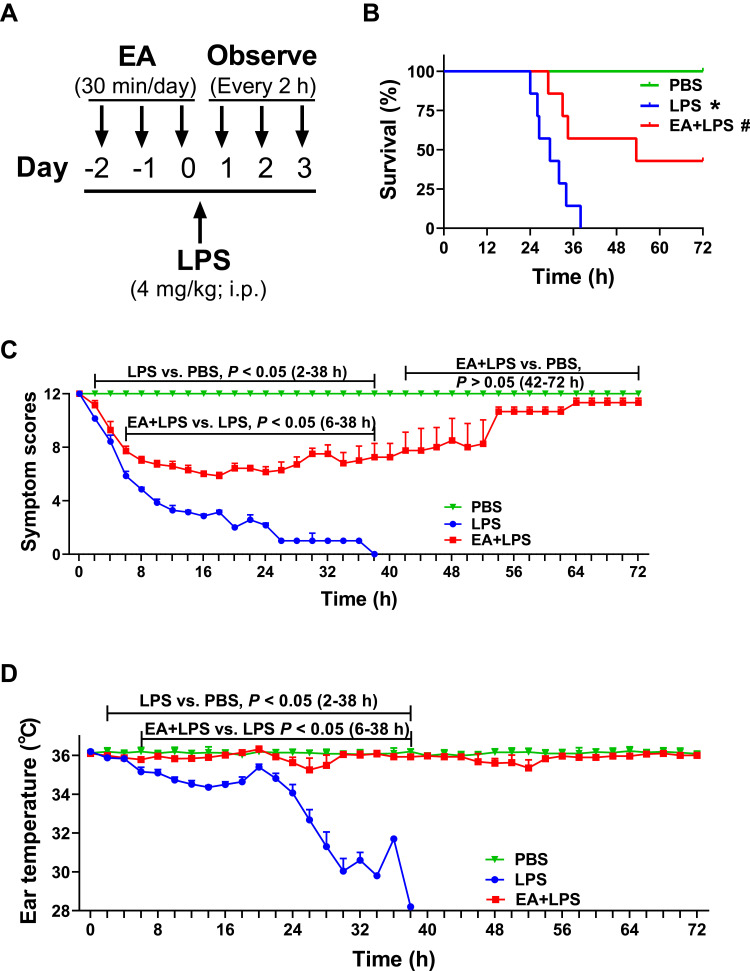
Figure 1.
Survival, symptom scores and ear temperature in mice. (A) 8-week-old BALB/c mice with or without EA (30 min/day for 3 days, 0.1 mA, 10 Hz) and then injected LPS (4 mg/kg; i.p.) or PBS. (B) Survival is represented in a Kaplan-Meier graph. (n = 7/group, *P < 0.05, compared to PBS control mice; #P < 0.05, compared to endotoxemic mice.) (C) Scoring every 2 h according to the symptom scoring system in Supplementary Table 1. (D) Mouse ear temperature was measured every 2 h using an ear thermometer.
Survival Rate, Symptom Scoring and Ear Temperature Monitoring
After i.p. injection of LPS or PBS was given to each group, survival, symptom scores and ear temperature of the mice were observed and recorded every 2 h for a total of 72 h. Each mouse was scored for symptoms according to existing methods and the experimental process was completed by two researchers.30 The detailed scoring rules are shown in Supplementary Table 1. The ear temperature of each mouse was measured with an electronic ear thermometer (FT65; Braun, Germany). The measurement was performed 3 times each time, and the average value was recorded.
Histological Analyses
After 12 h of i.p. injection of PBS or LPS, the mice were anesthetized with 1% sodium pentobarbital (10 mL/kg; Sigma-Aldrich, USA). The mice were dissected and the spleens were removed for subsequent detection of T lymphocyte apoptosis and pyroptosis. Then, the lung and small intestine were removed and immersed in 10% neutral formalin for fixation. The fixed intestine and lung tissues were cut into 3-mm blocks. Sections were cut at a thickness of 4 μm with a microtome (HistoCore BIOCUT, Leica Microsystems Inc., Germany). Sections were deparaffinized with xylene and rehydrated by ethanol-water washes, and then stained with hematoxylin (WH1144-1; Wellbio, China) and eosin (C0109; Beyotime, China). According to the existing methods, lung tissue congestion, exudation, inflammatory cell infiltration, and alveolar septal rupture were scored.31 Pathological score standard: 0 point, normal; 1 point, very mild (<25%); 2 points, mild (25–50%); 3 points, moderate (50–75%); 4 points, severe (>75%). According to the methods provided in the reference, the pathology of the small intestine tissue injury was scored.32 Pathological score standard: 0 point, normal; 1 point (mild), focal edema and necrosis; 2 points (moderate), diffuse swelling, atrophy and necrosis of villi; 3 points (severe), diffuse atrophy and necrosis of villi, inflammatory cell infiltration and/or bleeding in the villi and/or submucosa.
Cytokine Analyses
After 2 h or 12 h of i.p. injection of PBS or LPS, the mice were anesthetized with 1% sodium pentobarbital. Centrifuge to collect the supernatant after the blood from the heart was drawn. The Bio-Plex mouse cytokine assay (M60009RDPD; Bio-Rad, USA) was performed according to the standard experimental procedure to detect the following cytokine concentrations in the serum: TNF-α, IL-1β, IL-4, IL-5, IL-6, IL-9, IL-10, IL-17A, eotaxin, interferon (IFN)-γ, macrophage inflammatory protein (MIP)-1β and keratinocyte-derived chemokine (KC). To verify the role of T lymphocytes in the anti-inflammatory action of EA, enzyme linked immunosorbent assay (ELISA) (430904; BioLegend, USA) was used to detect serum TNF-α concentrations in wild-type mice and lymphocyte-deficient nude mice after 2 h of i.p. injection of PBS or LPS.
Flow Cytometry of T Lymphocyte Apoptosis
The methods of obtaining single cell suspension of mouse spleen and detecting T lymphocyte apoptosis with flow cytometry have been described by other researches and are discussed briefly.33 Cut and gently grind the spleen under sterile conditions, washed with RPMI-1640 medium (10-040-CVR; Corning, USA) to obtain a cell suspension. The red blood cell lysis buffer was added after cell centrifugation (1500 rpm, 5min) and then washed with PBS. The splenocytes could be obtained after another centrifugation. Take 1 × 106 splenocytes for flow cytometry according to the protocol of APC Annexin V Apoptosis Detection Kit with PI (640932; BioLegend, USA). The percentage of apoptosis was analyzed using Flowjo software.
Western Blot Analysis of T Lymphocyte Pyroptosis
Splenic T lymphocytes were purified from the remaining splenocytes using a Pan T Cell Isolation Kit II (130-095-130; Miltenyi Biotech, Germany). The T lymphocytes protein extract was prepared with cell lysis buffer for western and IP (P0013J; Beyotime Biotech, China) containing protease inhibitor (04693124001; Roche, USA) and centrifuged at 12,000 rpm for 10 min at 4°C. Protein concentration was analyzed using the bicinchoninic acid (BCA) protein concentration kit (P0012; Beyotime Biotech, China). Total proteins were separated by 12% SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred onto the PVDF membranes (1620177; Bio-Rad; USA). The membranes were blocked with 5% BSA-TBST for 30 min and incubated with antibody to cleaved caspase-1 (1:1000, 89332; Cell Signaling, USA) or anti–β-actin (1:1000, 4970; Cell Signaling, USA) overnight at 4°C. After washing five times for 5 min with TBST, the membranes were incubated with HRP-linked antibody to rabbit IgG (1:2000, 7074S, Cell Signaling, USA) at room temperature for 1 h and were washed again with TBST. The membranes were then exposed to chemiluminescence and imaged on an Amersham Imager 600 (GE Healthcare, USA). Determination of protein bands densities were performed using NIH Image software.
Statistical Analyses
All data were expressed as mean ± s.e.m. Survival rates were expressed using Kaplan–Meier curves, and analyses of survival curves were performed with the Log rank test. Statistical analyses were performed by one-way ANOVA, followed by the least significant difference method or Mann–Whitney non-parametric test (non-normal distribution) for multiple pairwise comparisons. Student’s t-test was used to compare mean values between two groups. P value less than 0.05 (P < 0.05) was considered statistically significant.
Results
EA Improves Survival, Symptom Score and Ear Temperature in Endotoxemic Mice
Mice underwent PBS, LPS, or EA+LPS treatment and survival, symptom scores, and ear temperature were recorded every 2 h. The lethal dose of LPS killed mice within 48 h. EA treatment significantly improved survival of endotoxemic mice inducing a lasting effect and did not merely delay the pathogenesis. All endotoxemic mice died at 38 h post-LPS, while 57% of the EA+LPS mice are still alive. Mice were observed by two weeks with a final 42.9% survival rate of the endotoxemic mice with EA (Figure 1B).
Endotoxemic mice showed a wide spectrum of symptoms ranging down from 12 with a progressive evolution toward death (symptom score = 0). LPS induced already a significant effect on the symptom score at 2 h post injection (P < 0.05). EA induced a significant protection and improved the symptom score after 4 h post-LPS (P < 0.05). Endotoxemic mice with EA received the worse symptom scores at 16–24 h post-LPS, and gradually recovered showing a score similar to PBS mice by 42 h post-LPS (P > 0.05) (Figure 1C).
Endotoxemic mice started to have a significantly lower ear temperature at 2 h post-LPS and this effect was statistically significant as compared to control mice (P < 0.05). EA treatment protected endotoxemic mice from hypothermia and this effect was statistically significant after 6 h post-LPS as compared to endotoxemic mice without EA (P < 0.05). Again, endotoxemic mice with EA gradually recovered normal ear temperature and showed a temperature similar to control mice after 38 h post-LPS (P > 0.05). The results showed that EA maintained the normal body temperature of the sepsis mouse model and avoided death due to hypothermia (Figure 1D).
EA Ameliorates Lung and Small Intestinal Tissues Damage in Endotoxemic Mice
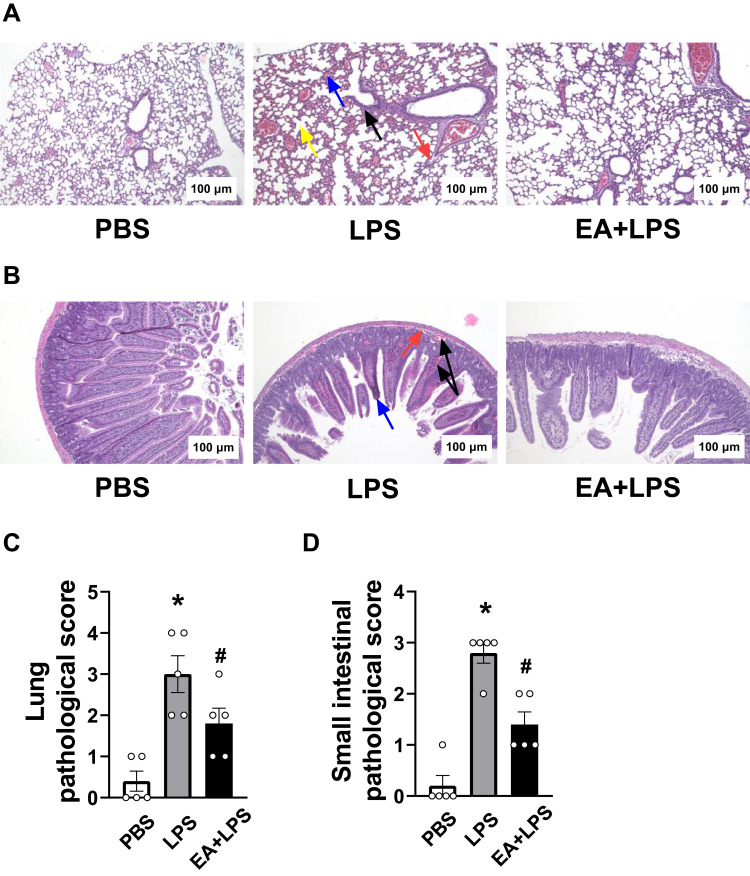
The protective effects of EA on organ injury were determined by histological analyses of the lung and the small intestine. Histopathological analysis was performed at 12 h post-LPS because septic mice reached the most severe stage before mice start to die. Hematoxylin and eosin (H&E) staining showed normal lung tissue organization in control mice treated with PBS. Endotoxemic mice showed inflammatory cell infiltration, congestion in the alveoli with fluid exudation, and rupture of the alveolar septa. EA protected endotoxemic mice from lung injury as the intra-alveolar hemorrhage was significantly lower (Figure 2A). Moreover, the intestinal villi of the control mice were arranged neatly and there was no obvious pathological damage as compared to the atrophy and bleeding observed in the submucosa and villi of the endotoxemic mice. EA improved the histological scores, decreasing the submucosal edema and preventing the intestinal villus hemorrhage (Figure 2B). According to the pathological score criteria of lung damage, 40% (2/5) of the endotoxemic mice had severe damage, and 20% (1/5) and 40% (2/5) of the mice had moderate and mild damage, respectively. By contrast, EA treatment prevented lung damage and only 20% (1/5) and 40% (2/5) of the EA+LPS mice had moderate and mild damage, respectively (Figure 2C). Intestinal histological analyses showed similar protective effects of EA. 80% (4/5) and 20% (1/5) of the endotoxemic mice had severe and moderate intestinal damage, respectively. Only 40% (2/5) of the EA+LPS mice had moderate damage and the rest 60% (3/5) showed only mild damage (Figure 2D). The pathological scores in lung and small intestine tissues were statistically significantly higher in endotoxemic mice as compared to control mice (P < 0.05). The protection induced by EA was also statistically significant as compared to endotoxemic mice (P < 0.05). These results suggested that EA effectively ameliorated the histological injuries of lung and small intestine tissues in endotoxemic mice.
Figure 2.
H&E staining (100X) of lung and small intestine and tissue pathological scores. (A) Changes of lung histology in PBS group, LPS group and EA+LPS group after 12 h of i.p. injection of LPS or PBS. (Black arrow, inflammatory cell infiltration; Blue arrow, alveolar congestion; Red arrow, exudation of fluid in the alveoli; Yellow arrow, alveolar septa rupture.) (B) Changes of small intestine histology in PBS group, LPS group and EA+LPS group after 12 h of i.p. injection of LPS or PBS. (Black arrow, intestinal submucosal or villi congestion; Blue arrow, intestinal villi atrophy; Red arrow, intestinal submucosal edema.) (C) Tissue pathological scores of lungs in each group. (n = 5/group, *P < 0.05, compared to PBS control mice; #P < 0.05, compared to endotoxemic mice.) (D) Tissue pathological scores of small intestines in each group. (n = 5/group, *P < 0.05, compared to PBS control mice; #P < 0.05, compared to endotoxemic mice).
EA Halts the Production of Pro-Inflammatory Cytokines and Prevents Detrimental Systemic Inflammation
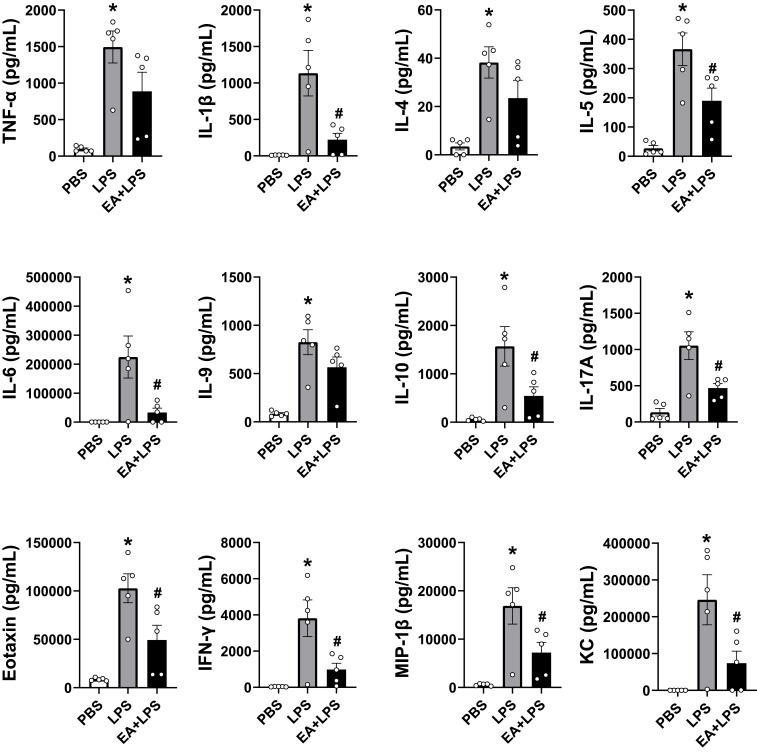
Bio-Plex mouse cytokine arrays were used to analyze systemic inflammation and serum cytokines at 12 h post-LPS as discussed above and to correlate the results with the histological results. The cytokines we tested include TNF-α, interleukins (IL-1β, IL-4, IL-5, IL-6, IL-9, IL-10, IL-17A), interferons (IFN-γ), and chemokines (eotaxin, MIP-1β and KC). Control mice had very low levels of inflammatory cytokines in the serum, whereas endotoxemic mice had a statistically significant increase in the production of all 12 inflammatory factors analyzed (Figure 3). EA prevented systemic inflammation and significantly attenuated the serum levels of most of these inflammatory factors including IL-1β, IL-5, IL-6, IL-10, IL-17A, eotaxin, IFN-γ, MIP-1β and KC (P < 0.05), and the concentrations of TNF-α, IL-4 and IL-9 also had a downward trend.
Figure 3.
Detection of the 12 cytokine concentrations at 12 h after i.p. injection by Bio-Plex mouse cytokine arrays. (n = 5/group, *P < 0.05, compared to PBS control mice; #P < 0.05, compared to endotoxemic mice).
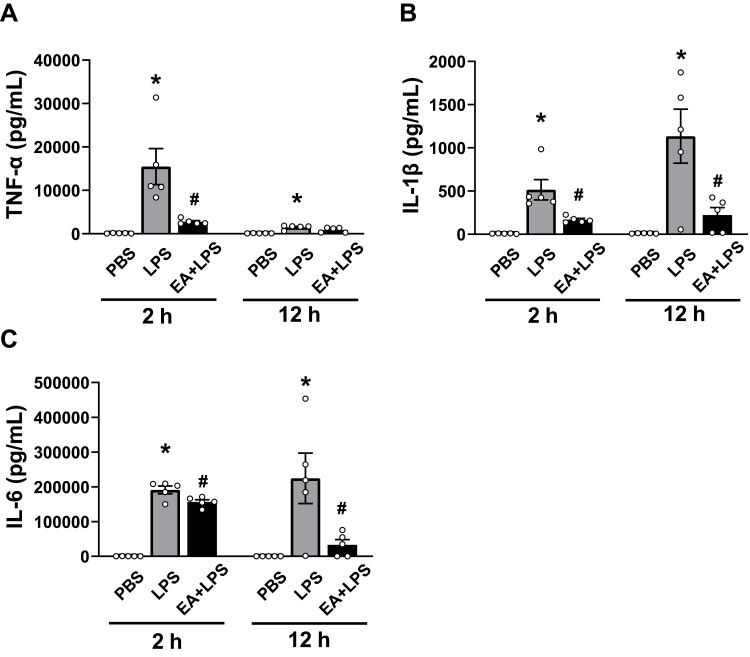
We speculated that the decreased levels of inflammatory factors in EA+LPS mice might be related to early pro-inflammatory cytokines, so we analyzed the concentrations of two typical early pro-inflammatory cytokines TNF-α and IL-1β, and a key secondary cytokine IL-6 at 2 h after i.p. injection of LPS. Serum TNF-α, IL-1β, and IL-6 levels were all significantly higher in the endotoxemic mice than in control mice (P < 0.05). EA statistically decreased the concentrations of these 3 cytokines (P < 0.05). Serum TNF-α levels were reduced by about 82% in EA+LPS mice as compared to endotoxemic mice (Figure 4A). EA also induced a significant decrease of IL-1β both at 2 and 12 h post-LPS (Figure 4B). By contrast, EA did not induce a significant decrease of early IL-6 levels at 2 h but the effect was very significant at 12 h post-LPS (Figure 4C). These results suggested that early EA treatment can dramatically decrease of production of critical early factors and thereby halt detrimental inflammatory responses. It is indicated that pretreatment of EA effectively prevented the occurrence of inflammatory cytokine storms in endotoxemic mice by inhibiting the production of key, early inflammatory factors.
Figure 4.
Detection of the cytokine concentrations at 2h and 12 h after i.p. injection. (A) TNF-α, (B) IL-1β, (C) IL-6. (n = 5/group, *P < 0.05, compared to PBS control mice; #P < 0.05, compared to endotoxemic mice).
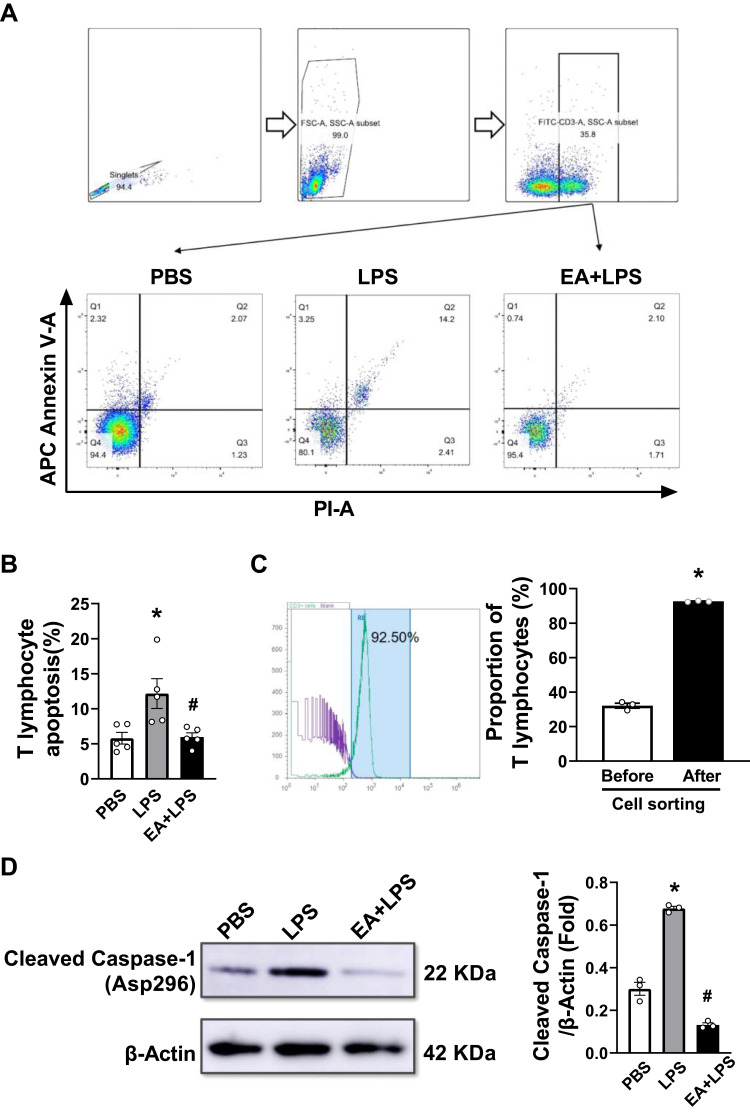
EA Reduces T Lymphocyte Apoptosis and Pyroptosis in Septic Mice
We prepared mouse spleen single cell suspension to detect the effect of EA on the apoptosis and pyroptosis of splenic T lymphocytes. Flow cytometry was used to detect Annexin V and propidium iodide (PI) positive cells in splenic T lymphocytes (Figure 5A). The results indicated that LPS induced apoptosis of splenic T lymphocytes (P < 0.05), while EA significantly reduced T cell apoptosis induced by LPS (P < 0.05) (Figure 5B). We used the immunomagnetic beads to isolate T lymphocytes from the single cell suspension of splenocytes for purity determination. The purity of T lymphocytes was higher than 90% through flow cytometry and met the requirements of subsequent experiments (Figure 5C). After extracting the protein of T lymphocytes, Western blot assays were performed to detect the cleaved caspase-1 protein related to pyroptosis. The results showed that LPS challenge significantly increased caspase-1 protein cleavage (P < 0.05), and EA significantly prevented this effect (P < 0.05). Thus, EA can significantly prevent lymphopenia and T lymphocytes pyroptosis in endotoxemic mice (Figure 5D).
Figure 5.
Analyses of apoptosis and pyrolysis by flow cytometry and Western-blots. (A) Annexin V and propidium iodide (PI) markers were used to identify apoptotic T lymphocytes in spleen cells. The cells in the upper right quadrant were the apoptotic cells. The flow cytometry circle gate strategy was shown in the figure. (B) The percentage of apoptosis of mouse splenic T lymphocytes of the three groups (n = 5/group, *P < 0.05, compared to PBS control mice; #P < 0.05, compared to endotoxemic mice.) (C) T lymphocytes were labeled with FITC anti-mouse CD3 and sorted with immunomagnetic beads. Diagram of flow cytometry shows the proportion of T lymphocytes after separation. Purity of T lymphocyte after sorting was 92.7 ± 0.2% (n = 3/group, *P < 0.05, compared to before cell sorting.) (D) Western blot data showed the expression of protein cleaved caspase-1 in each group. β-actin was set as the loading control. (n = 3/group, *P < 0.05, compared to PBS control mice; #P < 0.05, compared to endotoxemic mice).
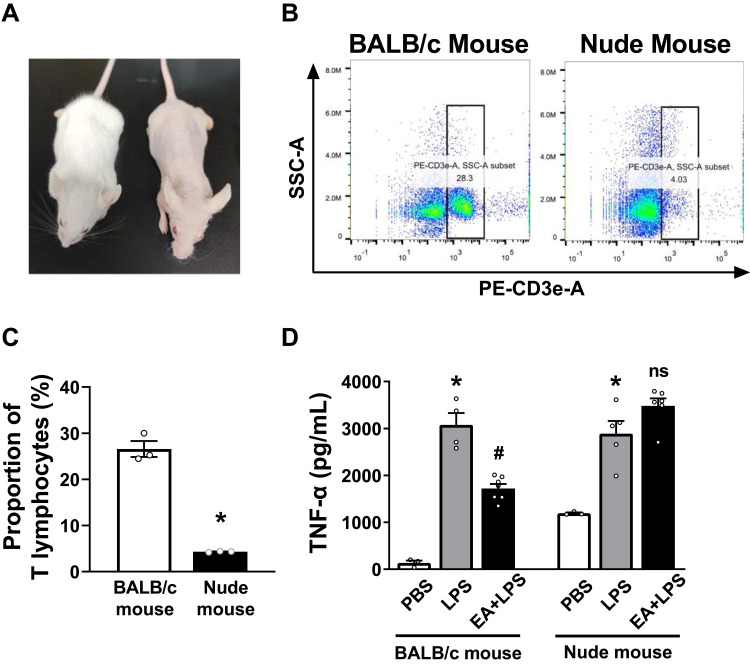
T Lymphocytes are Required in the Anti-Inflammatory Action of EA
Nude mice lacking T cells were used to determine the role of T lymphocytes in the prevention of sepsis by EA. According to morphological observation, BALB/c nude mice were hairless and athymic as compared with BALB/c wild-type mice (Figure 6A). Flow cytometry analyses were used to analyze CD3+ T cells in the splenocytes of BALB/c wild-type and nude mice (Figure 6B). Over 26.6 ± 1.7% of the splenocytes were CD3+ lymphocytes in the wild-type mice as compared to 4.3 ± 0.1% of the splenocytes in the nude mice (Figure 6C). According to the previously described experimental protocol, BALB/c mice and nude mice were pretreated with EA. The results of ELISA experiments showed that EA significantly reduced serum TNF-α concentrations in wild-type (P < 0.05), but not in lymphocyte-deficient nude mice (P > 0.05) (Figure 6D).
Figure 6.
Identification of nude mice lacking T lymphocytes and ELISA detection of serum TNF-α concentration. (A) Morphological observation of BALB/c mice and BALB/c nude mice. (B) The anti-mouse CD3-PE antibody was used to identify T lymphocytes in splenocytes of BALB/c mice and nude mice. The cells in the right box were the CD3+ T lymphocytes. (C) The percentage of CD3+ T lymphocytes of the BALB/c mice and nude mice (n = 3/group, *P < 0.05, compared to BALB/c mice.) (D) Serum was collected 2 h after i.p. injection of PBS or LPS in BALB/c mice and nude mice, and the serum TNF-α concentration in each group was detected by ELISA. (n = 5/group, *P < 0.05, compared to PBS control mice; #P < 0.05, compared to endotoxemic mice).
Abbreviation: ns, no significance.
Discussion
Sepsis is the main cause of death in ICU patients worldwide.4 Antibiotics and glucocorticoids have major side effects that limit their clinical implications in sepsis.34 By contrast, acupuncture has minimal side effects and significant therapeutic potential that can provide beneficial effects on sepsis patients. Studies have shown that EA at ST36 acupoint stimulates neurons expressing Prokr2 protein on the sciatic nerve, uploads nerve signals to the central nervous system, thereby stimulating the adrenal glands to release glucocorticoids and dopamine, inhibiting the secretion of cytokines, and controlling systemic inflammation.26,27 At the same time, nerve signals from the sciatic nerve further activate the vagus nerve, regulate the physiological and pathological functions of organs such as the lungs, intestines, and adrenal glands, and even regulate T lymphocytes in the spleen to control inflammation.35 Our data suggested that unilateral EA at ST36 significantly decreased the clinical signs of detrimental inflammatory responses, maintained the normal body temperature, and reduced the mortality of septic mice. EA significantly improved lung and small intestinal tissues injury induced by LPS. The protective effects of EA preconditioning on the endotoxemic mice may be through controlling the cytokine storms and reducing apoptosis and pyrolysis of T lymphocytes.
Body temperature is one of the most vital signs in critically ill patients. Hypothermia (core temperature <36°C) is associated with organ failure and increased mortality in sepsis, and is considered an independent predictor of mortality in septic patients.36,37 In the emergency department of the United States, about 30% of patients with severe sepsis have hypothermia.38 The mortality rate of hypothermia patients is twice that of those having fever, and hypothermia patients have worse prognosis.39 When encountering major infections, rodents are more prone to hypothermia than humans.40 Our research found that the body temperature of septic mice induced by LPS decreased significantly. Hypothermia is thought to be related to the host’s immune dysfunction. In severe septic patients in the ICU, the serum TNF-α level in hypothermic patients was 2.7 times that of febrile patients, and IL-6 levels were 5-fold higher in hypothermia than those found in febrile patients.41 Hypothermia in experimental sepsis animals is associated with high levels of IL-6.40 In addition, persistent lymphopenia (including T and B cells) is a feature of sepsis-induced immunosuppression and a predictor of mortality in septic patients. Sepsis patients with hypothermia were found to be more prone to persistent lymphopenia.42 Therefore, we speculate that EA maintained normal body temperature in mice may be achieved by regulating inflammatory cytokines and preventing lymphopenia.
MODS usually occurs at the late stage of sepsis, which usually involves multiple organs such as the lung, gastrointestinal tract, liver, kidney, heart, and brain.1 Our research showed that the lung and intestine were seriously damaged in endotoxemic mice. EA effectively prevented lung and intestine tissue damage, and reduced the pathological scores of both. The mechanisms of the protective effect of EA on organs are complicated. Lung is one of the most vulnerable organs susceptible to sepsis.43 Once acute lung injury caused by sepsis develops into acute respiratory distress syndrome, MODS will follow. Oxidative stress or the dysfunction of the antioxidant system can cause inflammation and lead to lung damage. EA at ST36 acupoints can reduce the biosynthesis of nitric oxide (NO) by reducing the expression of inducible nitric oxide synthase (iNOS) in the lung, thereby reducing the degree of acute lung injury in rats caused by LPS.44 In addition, EA on ST36 and BL13 (Feishu) acupoints for 5 days can reduce the lung injury of septic rabbits by activating the antioxidant regulatory pathway, Nrf2/ARE pathway, and up-regulating the expression of heme oxygenase (HO)-1 in lung tissue.45 Gut damage is also a key factor that promotes MODS.46 Due to intestinal barrier damage, LPS and bacteria enter the systemic circulation and eventually lead to MODS and sepsis.47 Previous studies have found that the protective effects of EA on the intestinal barrier are mainly related to cholinergic anti-inflammatory pathways and the reduction of inflammatory cytokines. EA may replace vagus nerve (VN) stimulation or cholinergic receptor agonists to protect the intestinal tract.48,49 Furthermore, studies have also confirmed that EA at bilateral ST36 acupoints improves the intestinal mucosal immune barrier of rats with polymicrobial peritonitis by increasing the sIgA content and the percentage of T lymphocytes in the intestinal mucosa.24
The cytokine storm and lymphopenia, the two major factors worsening the prognosis of sepsis, also seem to be related to COVID-19.50,51 Therefore, we studied the effect of EA pretreatment on cytokines and T lymphocytes in septic mice. The cytokine storm is a cascade of cytokine caused by an uncontrolled immune response.9 Pathogens first induce the production of early cytokines, and then these cytokines with the help of chemokines recruit more inflammatory cells to release secondary cytokines, thereby amplifying the cytokine storm.52 TNF-α and IL-1β are critical, early pro-inflammatory cytokines in sepsis, and their serum levels play a key role in the release of other cytokines and the systemic inflammatory responses.53 TNF-α and IL-1β activate macrophages to secrete other pro-inflammatory cytokines (IL-6, IL-8, etc.), gradually amplify the inflammatory cascade, and ultimately lead to sepsis-induced MODS.54 In addition, TNF-α also participates in the recruitment of inflammatory cells such as monocytes, neutrophils and NK cells by inducing chemokines and adhesion molecules.55 TNF-α starts to be produced within a few minutes after sepsis infection, and the secretion stops after reaching a peak around at 3–4 h post infection.15 Our results confirmed significantly higher serum TNF-α levels at 2 h were than at 12 h in endotoxemic mice, and EA attenuated early serum TNF-α levels. Likewise, IL-1β, is another inflammatory factor produced in early responses to bacterial endotoxin.53 IL-1β production is thought to reduce myocardial contractility and participate in sepsis-induced cardiomyopathy.56 IL-1β also mediates sepsis-induced lung injury by down-regulating cAMP and the expression of VE-cadherin.57 IL-6 is one of the most studied factor among the secondary cytokines and is considered to be the center of the cytokine storm.58 Serum IL-6 levels have been considered as an independent risk factor for 28-day mortality of sepsis, and can be used as a biomarker for the diagnosis and prognosis of sepsis and COVID-19.58,59 IL-6 is the main activator of signal transducer and activator of transcription (STAT) 3 in inflammation.60 The activation of the IL-6-induced STAT3 pathway and TNF-α-related nuclear factor kappa B (NF-κB) pathway in non-immune cells will lead to the cytokine storm, which can cause MODS and other fatal symptoms.61,62 IL-6 is expected to become a new target for treating sepsis due to its course of expression and potential therapeutic time window.63 Our study showed that EA effectively prevented the production of early pro-inflammatory cytokines such as TNF-α and IL-1β, thus significantly reduced IL-6 levels and other secondary cytokines, and eventually prevented the detrimental cytokine storm in septic mice. Previous studies have found that vaccination of cytokines such as TNF-α, IL-1β or IL-6 may prevent the development of sepsis and other infections, but the clinical results are not promising.64 We speculate that it may be difficult to block early cytokines like TNF-α in time; or the inhibition of a single cytokine maybe insufficient to control the cytokine storms. EA controls the production of multiple rather than a single inflammatory cytokine at the beginning, and can effectively attenuate the detrimental cytokine storm.
In the late stage of immunosuppression, the lymphocyte function is impaired by the continuous infiltration of inflammatory cytokines and eventually leads to lymphocyte exhaustion.8 Lymphocyte apoptosis and pyroptosis are the two main causes of lymphopenia, which is key for the immunosuppressive stage of sepsis.65 Apoptosis is the process of autonomous and orderly cell death to regulate immune homeostasis. However, cell apoptosis in sepsis may be harmful, especially the apoptosis of T lymphocytes has been proven to be one of the reasons for the depletion of immune cells and weakened immune function.7 T cell apoptosis (mainly the apoptosis of CD4+ T cells) will lead to the decrease in the differentiation of T cell subsets and inflammatory cytokines. As a result, memory T cells (prevent secondary infection), Th17 cells (clear bacterial and fungal infections), CD8+ cytotoxic T cells (clear infections) and B cells (produce antibodies) will also decrease.66 T lymphocyte apoptosis also provides an opportunity for secondary infections and potential viral reactivation.67 The toll-like receptor (TLR) 4/NF-κB signaling pathway is considered the main signal transduction signal involved in apoptosis.68,69 Studies have found that inhibition of TLR4 increases LPS-induced death of CD8 lymphocytes and monocytes.70 In addition, TNF-α also participates in the immunosuppression of sepsis by increasing cell apoptosis. TNF-α and its receptors directly lead to cell apoptosis by activating the caspase signaling pathway.71 Pyroptosis is a kind of pro-inflammatory programmed cell death, which is another key factor in controlling infection in sepsis.72 Moderate pyroptosis can inhibit the replication of pathogens in sepsis and activate immune cells to eliminate pathogens. However, excessive pyroptosis will activate the inflammatory responses of neighboring cells and tissues, to further aggravate inflammatory damage, leading to septic shock or MODS.73 Caspase-1 is an inflammatory protease that specifically mediates pyroptosis, so we analyzed cleaved caspase-1 to detect pyroptosis.65 The activation of caspase-1 not only promotes pyroptosis, but also promotes the secretion of cytokines such as IL-1β and IL-18, aggravating the inflammatory storm.74 Our flow cytometry and Western blot analyses showed that EA significantly reduced the apoptosis and pyroptosis of T lymphocytes in endotoxemic mice. EA may prevent the apoptosis and pyroptosis of T lymphocytes and immunosuppression by inhibiting the production of early inflammatory cytokines and apoptosis/pyroptosis-related proteins and signal pathways. At the same time, EA on endotoxemic nude mice failed to inhibit the inflammatory responses showing that T lymphocytes are required for EA to modulate inflammation.
There are several limitations in our study: 1) Our study is a pilot animal study with a statistically significant but limited sample size that will have to be replicate in clinical studies; 2) Our study focused on the prophylactic potential of EA to manage high-risk septic patients, our results warrant future studies to determine the effects of EA after the septic challenge; 3) This study also explores the effects of EA on T lymphocyte apoptosis and pyroptosis, future studies will be required to determine the effects of EA on different T cell subtypes; 4) Finally, future mechanistic studies will be required to determine the specific physiologic and molecular mechanisms induced by EA in sepsis and similar inflammatory and infectious disorders.75,76
Conclusion
In summary, our study showed that EA at ST36 acupoint with a frequency of 10 Hz significantly improved the symptoms, maintained normal ear temperature, protected organs, and ultimately improved the survival of septic mice. EA effectively prevented the increase of early pro-inflammatory cytokines TNF-α and IL-1β, thus significantly reduced the levels of IL-6 and secondary chemokines such as eotaxin, MIP-1β and KC, and prevented the development of cytokine storm in septic mice. Moreover, EA significantly reduced the T lymphocyte apoptosis and pyroptosis in septic mice. T lymphocytes play an essential role in the prevention and protection of sepsis by EA. This study shows the potential and possible mechanism of EA in the prevention of systemic inflammation in sepsis, thus, provides a promising strategy in the early management of high-risk patients for sepsis.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 81774429, 81973952), Natural Science Foundation of Shanghai (No. 19ZR1451500), National Key R&D Program of China (No. 2018YFC1704600).
Abbreviations
ICU, intensive care unit; COVID-19, coronavirus disease 2019; MODS, multiple organ dysfunction syndrome; NEWS, national early warning score; SIRS, systemic inflammatory response syndrome; MEWS, modified early warning score; TNF-α, tumor necrosis factor; IL, interleukin; P6 acupoint, Neiguan acupoint; EA, electroacupuncture; ST36 acupoint, Zusanli acupoint; ST37 acupoint, Shangjuxu acupoint; PBS, phosphate-buffered saline; i.p., intra-peritoneal; LPS, lipopolysaccharide; IFN, interferon; MIP, macrophage inflammatory protein; KC, keratinocyte-derived chemokine; PAGE, polyacrylamide gel electrophoresis; ELISA, enzyme linked immunosorbent assay; H&E, hematoxylin and eosin; PI, propidium iodide; NO, nitric oxide; iNOS, inducible nitric oxide synthase; BL13 acupoint, Feishu acupoint; HO, heme oxygenase; VN, vagus nerve; STAT, signal transducer and activator of transcription; NF-κB, nuclear factor kappa B; TLR, toll-like receptor.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marik PE. Early management of severe sepsis: concepts and controversies. Chest. 2014;145(6):1407–1418. doi: 10.1378/chest.13-2104 [DOI] [PubMed] [Google Scholar]
- 3.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fudim M, Qadri YJ, Ghadimi K, et al. Implications for neuromodulation therapy to control inflammation and related organ dysfunction in COVID-19. J Cardiovasc Transl Res. 2020;13(6):894–899. doi: 10.1007/s12265-020-10031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42(3):625–631. doi: 10.1097/CCM.0000000000000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JS, Kim SJ, Lee SM. Genipin attenuates sepsis-induced immunosuppression through inhibition of T lymphocyte apoptosis. Int Immunopharmacol. 2015;27(1):15–23. doi: 10.1016/j.intimp.2015.04.034 [DOI] [PubMed] [Google Scholar]
- 8.Fathi N, Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol Int. 2020;44(9):1792–1797. doi: 10.1002/cbin.11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pena G, Cai B, Ramos L, Vida G, Deitch EA, Ulloa L. Cholinergic regulatory lymphocytes re-establish neuromodulation of innate immune responses in sepsis. J Immunol. 2011;187(2):718–725. doi: 10.4049/jimmunol.1100013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vida G, Pena G, Kanashiro A, et al. beta2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011;25(12):4476–4485. doi: 10.1096/fj.11-191007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: the evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7:2050312119835043. doi: 10.1177/2050312119835043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashiouris MG, L’Heureux M, Cable CA, Fisher BJ, Leichtle SW, Fowler AA. The emerging role of vitamin C as a treatment for sepsis. Nutrients. 2020;12(2):292. doi: 10.3390/nu12020292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326 [DOI] [PubMed] [Google Scholar]
- 15.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11(2):56–63. doi: 10.1016/j.molmed.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 16.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195–203. doi: 10.1016/j.molmed.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 17.Hassan N, Slight R, Weiand D, et al. Preventing sepsis; how can artificial intelligence inform the clinical decision-making process? A systematic review. Int J Med Inform. 2021;150:104457. doi: 10.1016/j.ijmedinf.2021.104457 [DOI] [PubMed] [Google Scholar]
- 18.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma XY, Tian LX, Liang HP. Early prevention of trauma-related infection/sepsis. Mil Med Res. 2016;3:33. doi: 10.1186/s40779-016-0104-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph B, Shimojo G, Li Z, et al. Glucose activates vagal control of hyperglycemia and inflammation in fasted mice. Sci Rep. 2019;9(1):1012. doi: 10.1038/s41598-018-36298-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulloa L. Electroacupuncture activates neurons to switch off inflammation. Nature. 2021;598(7882):573–574. doi: 10.1038/d41586-021-02714-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto-Miyazaki J, Ushikoshi H, Miyata S, et al. Acupuncture and traditional herbal medicine therapy prevent delirium in patients with cardiovascular disease in intensive care units. Am J Chin Med. 2017;45(2):255–268. doi: 10.1142/S0192415X17500161 [DOI] [PubMed] [Google Scholar]
- 23.Lv JQ, Li PC, Zhou L, Tang WF, Li N. Acupuncture at the P6 acupoint to prevent postoperative pain after craniotomy: a randomized, placebo-controlled study. Evid Based Complement Alternat Med. 2021;2021:6619855. doi: 10.1155/2021/6619855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu MF, Xing X, Lei S, et al. Electroacupuncture at bilateral zusanli points (ST36) protects intestinal mucosal immune barrier in sepsis. Evid Based Complement Alternat Med. 2015;2015:639412. doi: 10.1155/2015/639412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng JB, Jiao YN, Zhang G, et al. Electroacupuncture improves intestinal dysfunction in septic patients: a randomised controlled trial. Biomed Res Int. 2018;2018:8293594. doi: 10.1155/2018/8293594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Wang Z, Su Y, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598(7882):641–645. doi: 10.1038/s41586-021-04001-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres-Rosas R, Yehia G, Pena G, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20(3):291–295. doi: 10.1038/nm.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang CP, Leelahavanichkul A. Over-expression of miR-223 induces M2 macrophage through glycolysis alteration and attenuates LPS-induced sepsis mouse model, the cell-based therapy in sepsis. PLoS One. 2020;15(7):e0236038. doi: 10.1371/journal.pone.0236038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai F, Ren Y, Lai C, et al. Acupuncture at Zusanli (ST36) for experimental sepsis: a systematic review. Evid Based Complement Alternat Med. 2020;2020:3620741. doi: 10.1155/2020/3620741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadl A, Pontiller J, Exner M, Leitinger N. Single bolus injection of bilirubin improves the clinical outcome in a mouse model of endotoxemia. Shock. 2007;28(5):582–588. doi: 10.1097/shk.0b013e31804d41dd [DOI] [PubMed] [Google Scholar]
- 31.Su X, Wang L, Song Y, Bai C. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med. 2004;30(1):133–140. doi: 10.1007/s00134-003-2001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coimbra R, Porcides R, Loomis W, et al. HSPTX protects against hemorrhagic shock resuscitation-induced tissue injury: an attractive alternative to ringer’s lactate. J Trauma. 2006;60(1):41–51. doi: 10.1097/01.ta.0000197417.03460.0a [DOI] [PubMed] [Google Scholar]
- 33.Cai G, Sun K, Wang T, et al. Mechanism and effects of zearalenone on mouse T lymphocytes activation in vitro. Ecotoxicol Environ Saf. 2018;162:208–217. doi: 10.1016/j.ecoenv.2018.06.055 [DOI] [PubMed] [Google Scholar]
- 34.Pan WX, Fan AY, Chen S, Alemi SF. Acupuncture modulates immunity in sepsis: toward a science-based protocol. Auton Neurosci. 2021;232:102793. doi: 10.1016/j.autneu.2021.102793 [DOI] [PubMed] [Google Scholar]
- 35.Chavan SS, Tracey KJ. Regulating innate immunity with dopamine and electroacupuncture. Nat Med. 2014;20(3):239–241. doi: 10.1038/nm.3501 [DOI] [PubMed] [Google Scholar]
- 36.Kushimoto S, Gando S, Ogura H, et al. Complementary role of hypothermia identification to the quick sequential organ failure assessment score in predicting patients with sepsis at high risk of mortality: a retrospective analysis from a multicenter, observational study. J Intensive Care Med. 2020;35(5):502–510. doi: 10.1177/0885066618761637 [DOI] [PubMed] [Google Scholar]
- 37.Shimazui T, Nakada TA, Walley KR, et al. Significance of body temperature in elderly patients with sepsis. Crit Care. 2020;24(1):387. doi: 10.1186/s13054-020-02976-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramgopal S, Horvat CM, Adler MD. Association of triage hypothermia with in-hospital mortality among patients in the emergency department with suspected sepsis. J Crit Care. 2020;60:27–31. doi: 10.1016/j.jcrc.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushimoto S, Gando S, Saitoh D, et al. The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Crit Care. 2013;17(6):R271. doi: 10.1186/cc13106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remick DG, Xioa H. Hypothermia and sepsis. Front Biosci. 2006;11:1006–1013. doi: 10.2741/1858 [DOI] [PubMed] [Google Scholar]
- 41.Arons MM, Wheeler AP, Bernard GR, et al. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med. 1999;27(4):699–707. doi: 10.1097/00003246-199904000-00020 [DOI] [PubMed] [Google Scholar]
- 42.Drewry AM, Fuller BM, Skrupky LP, Hotchkiss RS. The presence of hypothermia within 24 hours of sepsis diagnosis predicts persistent lymphopenia. Crit Care Med. 2015;43(6):1165–1169. doi: 10.1097/CCM.0000000000000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang CL, Tsai PS, Wang TY, Yan LP, Xu HZ, Huang CJ. Acupuncture stimulation of ST36 (Zusanli) attenuates acute renal but not hepatic injury in lipopolysaccharide-stimulated rats. Anesth Analg. 2007;104(3):646–654. doi: 10.1213/01.ane.0000255288.68199.eb [DOI] [PubMed] [Google Scholar]
- 44.Huang CL, Huang CJ, Tsai PS, Yan LP, Xu HZ. Acupuncture stimulation of ST-36 (Zusanli) significantly mitigates acute lung injury in lipopolysaccharide-stimulated rats. Acta Anaesthesiol Scand. 2006;50(6):722–730. doi: 10.1111/j.1399-6576.2006.01029.x [DOI] [PubMed] [Google Scholar]
- 45.Yu JB, Shi J, Gong LR, et al. Role of Nrf2/ARE pathway in protective effect of electroacupuncture against endotoxic shock-induced acute lung injury in rabbits. PLoS One. 2014;9(8):e104924. doi: 10.1371/journal.pone.0104924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Shi Y, Cai D, et al. Effect of electroacupuncture at ST36 on the intestinal mucosal mechanical barrier and expression of occludin in a rat model of sepsis. Acupunct Med. 2018;36(5):333–338. doi: 10.1136/acupmed-2016-011187 [DOI] [PubMed] [Google Scholar]
- 47.MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45(2):223–228. doi: 10.1136/gut.45.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu S, Du MH, Luo HM, et al. Electroacupuncture at Zusanli (ST36) prevents intestinal barrier and remote organ dysfunction following gut ischemia through activating the cholinergic anti-inflammatory-dependent mechanism. Evid Based Complement Alternat Med. 2013;2013:592127. doi: 10.1155/2013/592127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4(8):673–684. doi: 10.1038/nrd1797 [DOI] [PubMed] [Google Scholar]
- 50.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gholami M, Safari S, Ulloa L, Motaghinejad M. Neuropathies and neurological dysfunction induced by coronaviruses. J Neurovirol. 2021;27(3):380–396. doi: 10.1007/s13365-021-00977-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo XJ, Thomas PG. New fronts emerge in the influenza cytokine storm. Semin Immunopathol. 2017;39(5):541–550. doi: 10.1007/s00281-017-0636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgescu AM, Banescu C, Azamfirei R, et al. Evaluation of TNF-alpha genetic polymorphisms as predictors for sepsis susceptibility and progression. BMC Infect Dis. 2020;20(1):221. doi: 10.1186/s12879-020-4910-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87–103. doi: 10.1615/CritRevEukarGeneExpr.v20.i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steeland S, Libert C, Vandenbroucke RE. A new venue of TNF targeting. Int J Mol Sci. 2018;19(5):1442. doi: 10.3390/ijms19051442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Busch K, Kny M, Huang N, et al. Inhibition of the NLRP3/IL-1beta axis protects against sepsis-induced cardiomyopathy. J Cachexia Sarcopenia Muscle. 2021;12(6):1653–1668. doi: 10.1002/jcsm.12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong S, Hong Z, Huang LS, et al. IL-1beta suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J Clin Invest. 2020;130(7):3684–3698. doi: 10.1172/JCI136908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang L, Zhao X, Qi Y, et al. Sepsis-associated severe interleukin-6 storm in critical coronavirus disease 2019. Cell Mol Immunol. 2020;17(10):1092–1094. doi: 10.1038/s41423-020-00522-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song J, Park DW, Moon S, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis. 2019;19(1):968. doi: 10.1186/s12879-019-4618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang X, Hu LF, Ma XJ, Yin J, Liu XY, Li JB. Influence of roflumilast on sepsis mice through the JAK/STAT signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(3):1335–1341. doi: 10.26355/eurrev_201902_17028 [DOI] [PubMed] [Google Scholar]
- 61.Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]
- 63.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020 [DOI] [PubMed] [Google Scholar]
- 64.Guo HL, Shi FD, Zhou Q, et al. Interleukin-1beta protection against experimental sepsis in mice. Inflammation. 2021;44(1):358–370. doi: 10.1007/s10753-020-01341-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinheiro da silva F, Nizet V. Cell death during sepsis: integration of disintegration in the inflammatory response to overwhelming infection. Apoptosis. 2009;14(4):509–521. doi: 10.1007/s10495-009-0320-3 [DOI] [PubMed] [Google Scholar]
- 66.Brady J, Horie S, Laffey JG. Role of the adaptive immune response in sepsis. Intensive Care Med Exp. 2020;8(Suppl 1):20. doi: 10.1186/s40635-020-00309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou Q, Yang M, Yu M, Liu C. Influences of regulation of miR-126 on inflammation, Th17/Treg subpopulation differentiation, and lymphocyte apoptosis through caspase signaling pathway in sepsis. Inflammation. 2020;43(6):2287–2300. doi: 10.1007/s10753-020-01298-7 [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–1221. doi: 10.1038/nm1124 [DOI] [PubMed] [Google Scholar]
- 69.Kanashiro A, Franchin M, Bassi GS, et al. Inhibition of spinal p38 MAPK prevents articular neutrophil infiltration in experimental arthritis via sympathetic activation. Fundam Clin Pharmacol. 2018;32(2):155–162. doi: 10.1111/fcp.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu CM, Chiu LC, Yu CC, et al. Increased death of peripheral blood mononuclear cells after TLR4 inhibition in sepsis is not via TNF/TNF receptor-mediated apoptotic pathway. Mediators Inflamm. 2021;2021:2255017. doi: 10.1155/2021/2255017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kothari N, Bogra J, Abbas H, et al. Tumor necrosis factor gene polymorphism results in high TNF level in sepsis and septic shock. Cytokine. 2013;61(2):676–681. doi: 10.1016/j.cyto.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 72.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi: 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng X, Chen W, Gong F, Chen Y, Chen E. The role and mechanism of pyroptosis and potential therapeutic targets in sepsis: a review. Front Immunol. 2021;12:711939. doi: 10.3389/fimmu.2021.711939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhuo L, Chen X, Sun Y, et al. Rapamycin inhibited pyroptosis and reduced the release of IL-1beta and IL-18 in the septic response. Biomed Res Int. 2020;2020:5960375. doi: 10.1155/2020/5960375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ulloa L, Quiroz-Gonzalez S, Torres-Rosas R. Nerve stimulation: immunomodulation and control of inflammation. Trends Mol Med. 2017;23(12):1103–1120. doi: 10.1016/j.molmed.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bassi GS, Kanashiro A, Coimbra NC, Terrando N, Maixner W, Ulloa L. Anatomical and clinical implications of vagal modulation of the spleen. Neurosci Biobehav Rev. 2020;112:363–373. doi: 10.1016/j.neubiorev.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]