Abstract
Mice infected in the left hind footpad with 5 log10 acid-fast bacilli of Mycobacterium ulcerans were divided into an untreated control group and 17 treatment groups that received one of the following regimens for 4 weeks (all doses in milligrams per kilogram): 100 mg of azithromycin (AZM), 100 mg of clarithromycin (CLR), or 50 mg of AZM for a duration of 5 days a week (daily), three times a week, or once weekly. In addition, the following regimens were administered daily: 100 mg of telithromycin (TLM), sparfloxacin (SPX), or moxifloxacin (MOX); 200 mg of levofloxacin (LVX); 100 mg of streptomycin (STR) or amikacin (AMK); 10 mg of rifampin (RIF); and the combination of 10 mg of RIF and 100 mg of AMK (RIF+AMK). After completion of treatment, mice were observed for 30 weeks. The effectiveness of treatment regimens was assessed in terms of the delay in median time to footpad swelling in treated mice compared with that in the untreated controls. Clear-cut bactericidal activity, i.e., an observed delay in footpad swelling that exceeded the period of treatment, was observed in the STR-, AMK-, and RIF+AMK-treated mice. However, all mice treated with either AMK or STR alone had swollen footpads before the end of the 30-week observation period, suggesting regrowth of M. ulcerans. In contrast, 50% of the mice treated with the RIF+AMK combination exhibited no lesion even after 30 weeks, suggesting cure. The remaining regimens could be assigned to one of three groups: (i) no activity (50 mg of AZM, 100 mg of AZM thrice weekly, TLM, and LVX); (ii) bacteriostatic activity, i.e., a delay in footpad swelling shorter than the 4-week treatment duration (100 mg of AZM daily or once weekly, CLR thrice or once weekly, and MOX); or (iii) weak bactericidal activity (CLR daily and SPX). The RIF+AMK combination and possibly RIF+STR warrant further study for the treatment of M. ulcerans infection in humans.
Mycobacterium ulcerans, the only toxin-producing mycobacterial species (5) is responsible for Buruli ulcer (BU), the third most prevalent mycobacterial disease throughout the world (20). Over the past 10 years, an increasing incidence of BU has been reported in several West African countries (6, 8) and Australia (4) and is now reported in most of sub-Saharan Africa, Central and South America, India, Southeast Asia, and Papua New Guinea (20).
In vitro, M. ulcerans is susceptible to several antimicrobials, including rifampin (RIF), clarithromycin (CLR), streptomycin (STR), amikacin (AMK), sparfloxacin (SPX), and clofazimine (1, 11, 13, 14, 19). However, the clinical response of humans with BU to various antimicrobial regimens containing these drugs have not confirmed in vitro studies so that, to date, BU treatment still relies on extensive surgical excision of the lesions followed by skin grafting (20).
In a previous study, the mouse footpad model of Fenner (3) was used to assess the in vivo activity of several drugs against M. ulcerans (2). CLR, for which in vitro activity against M. ulcerans has been demonstrated (11), exhibited only bacteriostatic activity in the mouse. Azithromycin (AZM), a macrolide derivative with long half-life (7), and telithromycin (HMR 3647) (TLM), a semisynthetic ketolide with demonstrated in vitro activity (12), were considered of potential interest for the treatment of BU. Therefore, the in vivo activity of these two drugs against M. ulcerans in mice was compared with that of the reference macrolide CLR, with three fluoroquinolones, and with rifampin (RIF), streptomycin (STR), and amikacin (AMK), all drugs with demonstrated in vivo experimental activity against M. ulcerans (2). The antimicrobial activities of the drugs were measured by the ability of each drug regimen given at different time intervals over 4 weeks to prevent or delay swelling of mouse footpads infected with M. ulcerans.
MATERIALS AND METHODS
Antimicrobial agents.
AZM was generously provided by Pfizer, Orsay, France; CLR by Abbott, Rungis, France; AMK by Bristol-Myers-Squibb, Paris, France; RIF, TLM, LVX, and SPX by Aventis, Antony, France; MOX by Bayer, Puteaux, France; and STR by the hospital pharmacy.
Stock solutions of the drugs at the desired concentrations were prepared weekly and stored at 4°C before use.
M. ulcerans strain.
The Cu001 strain of M. ulcerans used in the present study was isolated from a BU excised from a patient in Adzopé, Côte d'Ivoire, in June 1996. The strain has been maintained by successive subcultures in footpads of BALB/c mice.
Mouse model, infection, and treatment.
Six-week-old female BALB/c mice (Janvier Breeding Center, Le Genest-Saint-Isle, France), weighing 18 to 20 g were housed in the animal facility for 1 week before infection. Then, each mouse was infected in the left hind footpad with 5 log10 acid-fast bacilli (AFB) of M. ulcerans contained in a volume of 0.03 ml (2, 15) and started on a 4-week treatment regimen 1 week after infection. The short duration of treatment was chosen in order to rapidly discriminate bactericidal drugs from bacteriostatic drugs that may be useful as second-line drugs or in combination with bactericidal drugs.
The 220 infected mice were allocated randomly to an untreated control group of 30 mice or to one of 17 treatment groups. The first two treatment groups of 20 mice each were given 100 mg (all doses are per kilogram) of either CLR or AZM 5 days a week (daily). Treatment groups 3 to 6, each with 10 mice, were also given 100 mg of CLR or AZM, but delivered either thrice or once weekly. Ten mice in each of treatment groups 7 to 9 were given 50 mg of AZM administered either 5 days a week or thrice or once weekly. The remaining eight groups, of 10 mice each, were given daily 100 mg of either TLM, SPX, or MOX; 200 mg of LVX; 100 mg of either STR or AMK; 10 mg of RIF; and the combination of 10 mg of RIF and 100 mg of AMK (RIF+AMK).
AMK and STR were injected subcutaneously. All other drugs were administered orally with an esophageal canula (gavage). On completion of treatment, 10 mice treated daily with 100 mg of either CLR or AZM were sacrificed to determine AFB and CFU counts in the inoculated footpads. All other mice were kept under observation for 30 additional weeks.
Assessment of M. ulcerans growth and treatment activity.
The growth of M. ulcerans in the inoculated footpads was defined as occurrence of footpad swelling combined with an AFB count equal or superior to 5 log10 per footpad.
The infected footpads of 10 untreated control mice were harvested for baseline and CFU counts 1 week after infection, i.e., the day of treatment onset. Four weeks later, on completion of treatment, AFB and CFU counts were performed on infected footpads from 10 untreated control mice and 10 mice treated daily with 100 mg of either CLR or AZM. All other mice were examined for swelling of the footpad on a weekly basis. As soon as the footpad became swollen, the mouse was sacrificed for AFB and CFU counts.
AFB counts were performed microscopically according to the method developed by Shepard and McRae for enumerating Mycobacterium leprae in the footpad of the mouse (17). CFU counts were performed by plating appropriate dilutions of the harvested footpad onto Löwenstein-Jensen medium incubated at 32°C for 4 months (2).
Because of bacterial contamination of cultures in a previous experiment (2), footpads were carefully disinfected with chlorhexidine before harvesting M. ulcerans for AFB and CFU counts.
The activity of each treatment was assessed in terms of delay in median time to footpad swelling (and AFB count ≥5 log10) in treated mice compared with untreated control mice, using the kinetic method developed by Shepard for assessing activity of antileprosy drugs (16). A drug was defined as having no antimicrobial activity if the time to footpad swelling in treated mice was not significantly different from that in untreated control mice. A drug was defined as bacteriostatic if the additional time to footpad swelling induced by the treatment was greater than that in the control mice but less than the duration of treatment (i.e., 4 weeks). A drug, alone or in combination, was labeled bactericidal if the additional time to footpad swelling was significantly longer than the 4-week duration of treatment, i.e., the longer the additional time, the more bactericidal the drug.
Statistical analysis.
Survival analysis, with the swollen footpad as the measurement, was done by the Kaplan-Meier (KM) method (10). The log rank test was used to determine the level of statistical significance when comparing survival curves of the different groups. P values are two-tailed, and P ≤ 0.05 was considered statistically significant.
RESULTS
Mortality.
Among all mice, there was a single unexplained spontaneous death before footpad swelling in the group of mice treated daily with AZM at 50 mg/kg.
Footpad lesions. (i) Control mice
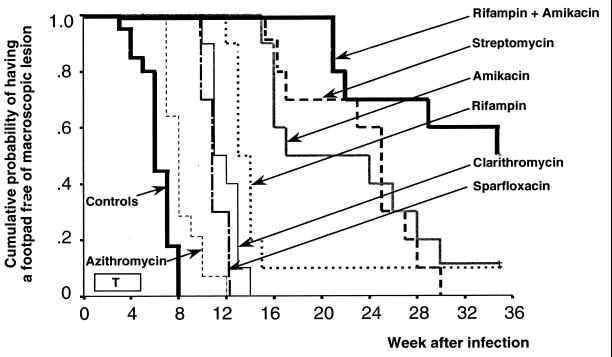
No footpad swelling occurred before the sacrifice of 10 mice 1 week after inoculation. The first lesions appeared at the third week (W3). At W5, four mice had swollen footpads before the sacrifice of 10 additional mice. All 10 remaining mice had swollen footpads by W10. Swelling of 50% of the inoculated footpads (Fig. 1) occurred by 6 weeks (95% confidence interval [CI95], 5 to 7).
FIG. 1.
Cumulative probability (KM method) of having no swollen footpad in mice infected with 5 log10 M. ulcerans in untreated mice and mice treated with different antibiotic regimens from week 1 to week 5 (T = treatment for 5 days a week; CLR, AZM, AMK, STR, and SPX given at 100 mg/kg; RIF given at 10 mg/kg).
(ii) Treated mice.
Swelling of footpads (Table 1) began at W5 in mice treated with once weekly AZM at 50 mg/kg, thrice weekly AZM at 100 mg/kg, and daily TLM at 100 mg/kg. It began at W6 in mice treated with either thrice weekly or daily AZM at 50 mg/kg, once weekly AZM at 100 mg/kg, once weekly CLR at 100 mg/kg, and daily MOX at 100 mg/kg. Swelling began at W7 in mice treated with daily AZM at 100 mg/kg and LVX at 200 mg/kg, and at W10 in mice treated with either thrice weekly or daily CLR at 100 mg/kg, daily SPX at 100 mg/kg, or TLM at 100 mg/kg. The footpad swelling began much later in the remaining groups: W12 in mice treated with daily RIF, W15 in mice treated with daily AMK or STR, and W21 in mice treated with the combination RIF+AMK.
TABLE 1.
Median time to footpad lesion occurrence in mice treated from weeks 1 to 5 after M. ulcerans inoculation
| Treatment regimen and dose (mg/kg) | No. of doses/wk | Median time to lesion occurrence, wk (range) | CI95 (wk) | Pa |
|---|---|---|---|---|
| None (control group) | 6 (1 to 8) | 5–7 | ||
| AZM | ||||
| 100 | 5 | 8 (7 to 12) | 7–9 | <0.001 |
| 100 | 3 | 7 (5 to 8) | 7–7 | 0.85 |
| 100 | 1 | 7 (6 to 11) | 6–8 | 0.03 |
| 50 | 5 | 7 (6 to 8) | 6–8 | 0.06 |
| 50 | 3 | 7 (6 to 8) | 7–7 | 0.29 |
| 50 | 1 | 7 (5 to 9) | 7–8 | 0.25 |
| CLR | ||||
| 100 | 5 | 11 (10 to 14) | 10–12 | <0.001 |
| 100 | 3 | 10 (10 to 13) | 10–12 | <0.001 |
| 100 | 1 | 8 (6 to 9) | 8–8 | 0.007 |
| TLM, 100 | 5 | 6 (5 to 9) | 5–7 | 0.60 |
| LVX, 200 | 5 | 7 (7 to 8) | 7–7 | 0.10 |
| MOX, 100 | 5 | 8 (6 to 9) | 7–9 | 0.01 |
| SPX, 100 | 5 | 11 (10 to 12) | 10–14 | <0.001 |
| RIF, 10 | 5 | 13 (12 to >35) | 12–14 | <0.001 |
| AMK, 100 | 5 | 17 (15 to >35) | 5–29 | <0.001 |
| STR, 100 | 5 | 25 (15 to 30) | 23–27 | <0.001 |
| RIF + AMK, 10 + 100 | 5 | 35 (21 to >35) | NCb | <0.001 |
P calculated from log rank test between control and treated mice.
NC, not calculable.
Swelling of 50% of the footpads (Fig. 1) occurred by 7 weeks in mice treated with daily LVX at 200 mg/kg and in all AZM-treated mice except those treated with daily AZM at 100 mg/kg, in which it occurred by 8 weeks (CI95, 7 to 9). Swelling also occurred by 8 weeks in mice treated with once weekly CLR and with MOX, by 10 weeks in mice treated with thrice weekly CLR, and 11 weeks in mice treated with daily CLR or SPX. Swelling of 50% of the footpads occurred later in other treatment groups: 13 weeks in RIF-treated mice; 17 weeks in AMK-treated mice; 25 weeks in STR-treated mice; and 35 weeks in RIF+AMK-treated mice. Among the four latter groups, a single mouse remained free of lesions by the end of the study in the RIF- and AMK-treated mice, and five in the mice treated with RIF+AMK.
Enumeration of AFB in footpads.
In the control mice, the mean AFB count performed 1 week after inoculation was 5.7 log10, and 5.5 log10 4 weeks later. AFB counts were equal or superior to 5.2 log10 (median, 6.0; range, 5.2 to 6.8) in all treated mice with swollen footpads but one (AFB count, 4.7 for the once a week AZM at 50 mg/kg treatment group).
Enumeration of CFU in footpads.
Despite disinfection before handling, all cultures were superinfected with Staphylococcus aureus or enterobacteria. As a result, CFU counts could not be assessed.
Comparative activity of different drug regimens.
There was no statistically significant difference between KM curves drawn from the control mice and from mice treated with AZM at 50 mg/kg given once, three times, or five times a week (Table 1). Similarly, there was no statistically significant difference between KM curves drawn from the control mice and from the TLM-treated and LVX-treated mice.
In mice treated with 100 mg/kg of AZM once a week and 5 days a week, the median time to M. ulcerans growth (i.e., swelling plus AFB count >5 log10) was 1 week longer than in control mice. Although both KM curves were significantly different from control mice (P < 0.001), the activities of both regimens were considered poorly bacteriostatic. Similar conclusion can be drawn for MOX-treated mice, because the median time to M. ulcerans growth was only delayed by 2 weeks compared to control mice. The activities of CLR given once or thrice weekly and of daily LVX were slightly superior but still bacteriostatic, as the median time to swelling was only 3 or 4 weeks longer than in control mice. On the other hand, the activities of daily CLR and SPX regimens were considered only slightly bactericidal because the median time to M. ulcerans growth was 5 weeks longer than in control mice, i.e., 1 week longer than the duration of therapy.
Finally, only RIF, AMK, STR, and, above all, the combination AMK+RIF had clear-cut bactericidal activities (Fig. 1). However, the KM curve drawn from the AMK+RIF-treated mice was significantly more favorable than those drawn from mice treated with RIF (P = 0.001), AMK, or STR alone (P = 0.02). In addition, there was no statistically significant difference between AMK and STR curves (P = 0.79).
DISCUSSION
CLR and AZM exhibited limited activity against M. ulcerans when tested by the kinetic method developed by Shepard (16) to assess the activity of antimicrobial drugs against M. leprae in the mouse footpad model. At a daily dose of 100 mg/kg, equipotent to 15 mg/kg in humans (21), CLR was weakly bactericidal, and AZM was bacteriostatic. These findings confirm previous data on CLR activity against M. ulcerans in the mouse (2). They also strongly suggest that AZM, a long-lasting macrolide of interest in the preventive therapy of Mycobacterium avium complex infection (9), cannot substitute for CLR in the curative treatment of M. ulcerans infection. Therefore, it is not possible to propose an easier-to-implement intermittent treatment with an AZM-containing regimen for the treatment of M. ulcerans infections.
Similar conclusions can be drawn for TLM (HMR 3647), a semisynthetic ketolide, as well as LVX and MOX, which have bacteriostatic activities. SPX had weakly bactericidal activity similar to that of CLR, and neither could be recommended as a first-line drug for the treatment of M. ulcerans infections. However, because of the short duration of treatment (4 weeks), we cannot rule out potential usefulness of the latter drugs when given in combination with the more bactericidal drugs.
Among drugs with bactericidal activity, RIF was clearly the least potent. After 4 weeks of treatment with daily RIF, the time to swelling of 50% of the footpads was only 2 weeks longer than that obtained with CLR or SPX. Like these latter two drugs, RIF cannot be considered a major drug against M. ulcerans infection. Actually, when tested with the kinetic method, only STR and AMK appeared to be major bactericidal drugs against M. ulcerans. Compared to that with RIF, the time to swelling of 50% of the footpads induced by the two drugs was delayed 2 weeks for AMK and 6 weeks for STR, the difference between the two drugs being insignificant when the entire curve was considered. However, even though both drugs exhibit clear bactericidal activities, their overall effect in a 4-week treatment regimen would discourage their suggested implementation in the field, since regrowth of M. ulcerans occurred in all animals.
The most effective drug regimen was the apparently synergistic combination of AMK+RIF that delayed the occurrence of lesions by at least 10 weeks compared to STR or AMK alone. In addition, 5 of the 10 mice treated for 4 weeks with the combination regimen were free of lesions 35 weeks after infection, or 31 weeks after treatment completion, suggesting that these mice might have been cured. Such findings are in accordance with the previous observation that RIF or AMK given for 8 weeks prevented footpad swelling in all treated mice up to 4 months after treatment completion (2), and with the observation of Stanford and Phillips (18) that treatment failure in mice treated with RIF alone was related to the duration of treatment, 1 month of treatment resulting in relapse and 3 months producing apparent cure.
This study also suggests that the STR+RIF combination is likely to be as effective as the AMK+RIF combination because STR appeared to be as active as AMK against M. ulcerans. Although not an ideal combination for field use because of the need for injection and the potential of drug toxicity, the STR+RIF and AMK+RIF combinations deserve to be tested in humans with BU, with the following two objectives: to determine the feasibility and acceptability and the minimal treatment duration for effectiveness. A clinical trial with both objectives is under way under the guidance of the World Health Organization Global Buruli Ulcer Initiative.
Acknowledgments
This work was supported by a grant from Pfizer.
We thank Kingsley Asiedu for assistance and George Kubica for critical review of the manuscript.
REFERENCES
- 1.Darie H, Djakeaux S, Cautoclaud A. Approche thérapeutique des infections àMycobacterium ulcerans. Bull Soc Pathol Exp. 1994;87:19–21. [PubMed] [Google Scholar]
- 2.Dega H, Robert J, Bonnafous P, Jarlier V, Grosset J. Activities of several antimicrobials against Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother. 2000;44:2367–2372. doi: 10.1128/aac.44.9.2367-2372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenner F. Pathogenic behavior of Mycobacterium ulcerans and Mycobacterium balnei in mouse and developing chick embryo. Am Rev Tuberc. 1956;73:650–673. doi: 10.1164/artpd.1956.73.5.650. [DOI] [PubMed] [Google Scholar]
- 4.Flood P, Street A, O'Brien P, Hayman J. Mycobacterium ulcerans infection on Phillip Island, Victoria. Med J Aust. 1994;160:160. [PubMed] [Google Scholar]
- 5.George K M, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small P L. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 6.Josse R, Guedenon A, Darie H, Anagonou S, Portaels F, Meyers W M. Les infections cutanées àMycobacterium ulcerans: ulcères de Buruli. Med Trop. 1995;55:363–373. [PubMed] [Google Scholar]
- 7.Lode H, Borner K, Koeppe P, Schaberg T. Azithromycin–review of key chemical, pharmacokinetic and microbiological features. J Antimicrob Chemother. 1996;37(Suppl. C):1–8. doi: 10.1093/jac/37.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- 8.Marston B J, Diallo M O, Horsburgh C R, Jr, Diomande I, Saki M Z, Kanga J M, Patrice G, Lipman H B, Ostroff S M, Good R C. Emergence of Buruli ulcer disease in the Daloa region of Cote d'Ivoire. Am J Trop Med Hyg. 1995;52:219–224. doi: 10.4269/ajtmh.1995.52.219. [DOI] [PubMed] [Google Scholar]
- 9.Oldfield E C, Fessel W J, Dunne M W, Dickinson G, Wallace R M, Byrne W, Chung R, Wagner K F, Paparello S F, Craig D B, Melcher G, Zajdowicz M, Williams R F, Kelly J W, Zelasky M, Heifets L B, Berman J D. Once weekly azithromycin therapy for prevention of Mycobacterium avium complex infection in patients with AIDS: a randomized, double-blind, placebo-controlled multicenter trial. Clin Infect Dis. 1998;26:611–619. doi: 10.1086/514566. [DOI] [PubMed] [Google Scholar]
- 10.Peto R, Pike M C, Armitage P, Breslow N E, Cox D R, Howard S V, Mantel N, McPherson K, Peto J, Smith P G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portaels F, Traore H, De Ridder K, Meyers W M. In vitro susceptibility of Mycobacterium ulcerans to CLR. Antimicrob Agents Chemother. 1998;42:2070–2073. doi: 10.1128/aac.42.8.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rastogi N, Goh K S, Berchel M, Bryskier A. In vitro activities of the ketolides telithromycin (HMR 3647) and HMR 3004 compared to those of CLR against slowly growing mycobacteria at pHs 6.8 and 7.4. Antimicrob Agents Chemother. 2000;44:2848–2852. doi: 10.1128/aac.44.10.2848-2852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Revill W D, Morrow R H, Pike M C, Ateng J. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet. 1973;ii:873–877. doi: 10.1016/s0140-6736(73)92005-9. [DOI] [PubMed] [Google Scholar]
- 14.Schroder K H. Investigation into the relationship of Mycobacterium ulcerans to M. buruli and other mycobacteria. Am Rev Respir Dis. 1975;111:559–562. doi: 10.1164/arrd.1975.111.4.559. [DOI] [PubMed] [Google Scholar]
- 15.Shepard C C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J Exp Med. 1960;112:445–454. doi: 10.1084/jem.112.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepard C C. A kinetic method for the study of the activity of drugs against Mycobacterium leprae. Int J Lepr. 1967;35:429–436. [Google Scholar]
- 17.Shepard C C, Mc Rae D H. A method for counting acid-fast bacteria. Int J Lepr. 1968;36:78–82. [PubMed] [Google Scholar]
- 18.Stanford J L, Phillips I. Rifampicin in experimental Mycobacterium ulcerans infection. J Med Microbiol. 1972;5:39–45. doi: 10.1099/00222615-5-1-39. [DOI] [PubMed] [Google Scholar]
- 19.Thangaraj H S, Adjei O, Allen B W, Portaels F, Evans M R, Banerjee D K, Wansbrough-Jones M H. In vitro activity of ciprofloxacin, sparfloxacin, ofloxacin, amikacin and rifampicin against Ghanaian isolates of Mycobacterium ulcerans. J Antimicrob Chemother. 2000;45:231–233. doi: 10.1093/jac/45.2.231. [DOI] [PubMed] [Google Scholar]
- 20.van der Werf T S, van der Graaf W T, Tappero J W, Asiedu K. Mycobacterium ulcerans infection. Lancet. 1999;354:1013–1018. doi: 10.1016/S0140-6736(99)01156-3. [DOI] [PubMed] [Google Scholar]
- 21.Xiong J H, Ji B, Perani E G, Petinon C, Grosset J H. Further study of the effectiveness of single doses of CLR and minocycline against Mycobacterium leprae in mice. Int J Lepr. 1994;62:37–42. [PubMed] [Google Scholar]