Abstract
Aortic valve calcium scoring by cardiac computed tomographic (CT) has been recommended as an alternative to classify the AS (aortic stenosis) severity, but it is unclear that whether CT findings would have additional value to discriminate significant AS subtypes including high gradient severe AS, classic low-flow, low gradient (LF-LG) AS, paradoxical LF-LG AS, and moderate AS. In this study, we examined the preoperative clinical and cardiac CT findings of different subtypes of AS in patients with surgical aortic valve replacement (AVR) and evaluated the subtype classification as a factor affecting post-surgical outcomes. This study included 511 (66.9 ± 8.8 years, 55% men) consecutive patients with severe AS who underwent surgical AVR. Aortic valve area (AVA) was obtained by echocardiography (AVAecho) and by CT (AVACT) using each modalities measurement of the left ventricular outflow tract. Patients with AS were classified as (1) high-gradient severe (n = 438), (2) classic LF-LG (n = 18), and (3) paradoxical LF-LG (n = 55) based on echocardiography. In all patients, 455 (89.0%) patients were categorized as severe AS according to the AVACT. However, 56 patients were re-classified as moderate AS (43 [9.8%] high-gradient severe AS, 5 [27.8%] classic LF-LG AS, and 8 [14.5%] paradoxical LF-LG AS) by AVACT. The classic LF-LG AS group presented larger AVACT and aortic annulus than those in high-gradient severe AS group and one third of them had AVACT ≥ 1.2 cm2. After multivariable adjustment, old age (hazard ratio [HR], 1.04, P = 0.049), high B-type natriuretic peptide (BNP) (HR, 1.005; P < 0.001), preoperative atrial fibrillation (HR, 2.75; P = 0.003), classic LF-LG AS (HR, 5.53, P = 0.004), and small aortic annulus on CT (HR, 0.57; P = 0.002) were independently associated with major adverse cardiac and cerebrovascular events (MACCE) after surgical AVR.
Subject terms: Cardiology, Diseases, Cardiovascular diseases
Introduction
Evaluation of the severity of aortic valve stenosis (AS) is crucial for stratifying patient management and decision making for the timing of surgical intervention, especially in patients who suspected having significant AS. Echocardiography is the main modality to assess the degree of AS by measuring transaortic peak velocity, mean pressure gradient, and calculating aortic valve area (AVA). However, constant measurement using echocardiography is sometimes difficult, especially in low-flow, low-gradient (LF-LG) conditions. Classic LF-LG severe AS is defined by a small aortic valve (AV) area on echocardiography (AVAecho < 1 cm2), a low mean pressure gradient (PG < 40 mmHg), and low flow (stroke volume [SV] < 35 mL/m2). The condition is characterised by low cardiac output due to a reduced left ventricular ejection fraction (LVEF < 50%)1. Conversely, LF-LG AS may occur despite preserved LVEF and is classified as paradoxical LF-LG AS. Since LF-LG AS presents fewer potential benefits from AV replacement (AVR) and considerable operation risks compared to true-severe AS, a classification for AS is important2.
Although the planimetry of the AVA using three-dimensional transoesophageal echocardiography has been reported to be more accurate than transthoracic echocardiography3, measurement issues still remain unresolved. Even in patients with normal systolic LV function, the grading of AS on echocardiography is inconsistent, and this is partly due to reduced SV4,5. In patients with low-flow state, AS severity may be underestimated due to lower mean PG, while incomplete opening of the AV may overestimate stenosis severity because of the reduced opening forces to the AV6. In patients with low-flow state, there can be a discrepancy between the effective orifice area and the PG. Moreover, the continuity equation assumes circular LV outflow tract (LVOT) which is elliptical shape, and echocardiography may underestimate LVOT. Additional diagnostic tests, dobutamine stress echocardiography (DSE)7,8 and AV calcium score (AVC) obtained by computed tomography (CT) scan9,10, have been used for the confirmation of severity and therapeutic guidance, and there is a chance that the patients with severe AS may be reclassified into the moderate range. However, reference standards used in these studies consisted of subjective assessment of the valve severity by cardiac surgeons and the AVC on CT images, which do not reflect hemodynamic severity.
Cardiac CT is recommended as an alternative to assess AS severity when DSE is inconclusive11. However, discrepancies have been reported between the measured AVA on cardiac CT (AVACT) and AVAecho12,13. AVACT was significantly greater than the AVAecho calculated by continuity equation, and suggested cut-off of AVACT for severe AS was < 1.2 cm2. Moreover, CT findings of different subtypes of AS and whether imaging has prognostic values remain undefined. Thus, we sought to (i) examine the preoperative CT characteristics of different subtypes of AS, and (ii) evaluate prognostic factors including CT findings affecting major adverse cardiovascular and cerebrovascular events (MACCE) after AVR.
Results
Patient characteristics
High-gradient severe AS (85.7% [438/511]) was most common among patients, followed by paradoxical LF-LG AS (10.8% [55/511]) and classic LF-LG AS (3.5% [18/511]) (Table 1). Half of the patients had tricuspid valves (48.1% [246/511]) and bicuspid valves were detected in the remaining patients. The median follow-up period for all patients was 4.12 (interquartile range [IQR], 3.19–5.50) years.
Table 1.
Clinical and imaging characteristics of high-gradient severe, classic LF-LG, paradoxical LF-LG AS groups (n = 511).
| Characteristic | High-gradient severe AS | Classic LF-LG AS | Paradoxical LF-LG AS | P-value |
|---|---|---|---|---|
| No. of patients (%) | 438 (85.7) | 18 (3.5) | 55 (10.8) | |
| Age, years | 66.8 ± 8.8 | 66.8 ± 6.2 | 67.3 ± 9.4 | 0.93 |
| Male | 236 (53.9) | 13 (72.2) | 31 (56.4) | 0.30 |
| BSA, m2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.2 | 0.56 |
| Hypertension | 231 (52.7) | 12 (66.7) | 30 (54.5) | 0.50 |
| Atrial fibrillation | 65 (14.8) | 4 (22.2) | 4 (7.3) | 0.20 |
| PCI or CABG | 94 (21.5) | 9 (50.0)* | 14 (25.5) | 0.02 |
| BNP, pg/mL | 99.5 (43.0–280.5) | 944.5 (304.8–3066.0)* | 70.0 (35.0–190.0) | < 0.001 |
| lnBNP | 4.6 (3.8–5.6) | 6.8 (5.7–8.0)* | 4.2 (3.6–5.2) | < 0.001 |
| Echocardiography | ||||
| LVEF, % | 60.3 ± 10.0 | 36.0 ± 10.3* | 62.6 ± 5.3 | < 0.001 |
| Peak velocity, m/s | 5.2 ± 0.7 | 3.6 ± 0.5* | 3.5 ± 0.5† | < 0.001 |
| Peak PG, mmHg | 108.5 ± 30.5 | 52.0 ± 13.1* | 50.1 ± 16.0† | < 0.001 |
| Mean PG, mmHg | 66.5 ± 19.4 | 29.8 ± 7.9* | 28.0 ± 9.5† | < 0.001 |
| LVMI, g/m2 | 135.4 ± 35.8 | 149.6 ± 31.1 | 124.9 ± 36.2 | 0.03 |
| AV VTI, cm | 124.6 ± 26.1 | 94.9 ± 33.3* | 112.0 ± 27.7† | < 0.001 |
| LVOT VTI, cm | 21.4 ± 4.1 | 16.0 ± 4.4* | 21.2 ± 3.7 | < 0.001 |
| LVOT diameter, mm | 21.0 ± 1.5 | 21.8 ± 1.9 | 21.2 ± 1.6 | 0.06 |
| LVOT diameter/BSA | 12.9 ± 1.3 | 13.1 ± 1.6 | 13.0 ± 1.3 | 0.55 |
| AVAecho, mm2 | 61.3 ± 14.7 | 66.9 ± 15.1 | 69.5 ± 13.8† | < 0.001 |
| ESVI, mL/m2 | 27.7 ± 16.8 | 63.4 ± 27.0* | 24.5 ± 11.4 | < 0.001 |
| EDVI, mL/m2 | 66.8 ± 23.7 | 96.3 ± 29.3* | 64.3 ± 24.5 | < 0.001 |
| SAC, mL/m2/mmHg | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.28 |
| Zva, mmHg/mL/m2 | 5.3 ± 1.6 | 5.0 ± 1.9 | 4.4 ± 1.4† | < 0.001 |
| CT findings | ||||
| Valve morphology | 0.04 | |||
| Tricuspid | 204 (46.6) | 14 (77.8) | 28 (50.9) | |
| Bicuspid with raphe | 106 (24.2) | 3 (16.7) | 17 (30.9) | |
| Bicuspid without raphe | 128 (29.2) | 1 (5.6) | 10 (18.2) | |
| AVC, Agatston unit | 3027.2 ± 1872.0 | 2895.5 ± 1624.5 | 2363.1 ± 1605.8† | 0.04 |
| AVCratio‡ | 1.8 ± 1.0 | 1.6 ± 0.8 | 1.4 ± 0.8† | 0.006 |
| LVOT mean diameter | 24.8 ± 2.9 | 27.1 ± 2.7* | 24.7 ± 2.6 | 0.003 |
| AVACT, mm2 | 84.9 ± 23.4 | 100.8 ± 22.7* | 94.2 ± 25.0† | 0.001 |
| AVAplani, mm2 | 87.2 ± 23.2 | 99.7 ± 25.5* | 97.5 ± 27.1† | 0.003 |
| Aortic annulus | ||||
| Circularity, % | 81.6 ± 7.6 | 77.4 ± 6.6 | 82.3 ± 6.0 | 0.05 |
| Maximal dimeter, mm | 27.5 ± 3.2 | 30.4 ± 3.5* | 27.4 ± 2.8 | 0.001 |
| Mean diameter, mm | 24.9 ± 2.6 | 26.9 ± 2.5* | 25.0 ± 2.6 | 0.005 |
| Perimeter, mm | 79.5 ± 8.4 | 85.4 ± 7.7* | 80.0 ± 8.3 | 0.02 |
| Area, mm2 | 481.8 ± 102.4 | 554.8 ± 101.0* | 489.5 ± 99.9 | 0.01 |
| Sinus of Valsalva, mm | 36.6 ± 4.5 | 38.4 ± 4.7 | 36.8 ± 5.0 | 0.25 |
| Sinotubular junction, mm | 30.9 ± 4.6 | 31.7 ± 3.3 | 31.5 ± 5.9 | 0.58 |
| Ascending aorta tubular portion, mm | 40.7 ± 6.3 | 38.4 ± 4.6 | 40.4 ± 7.7 | 0.31 |
| Normalized to BSA | ||||
| AVACT, mm2 | 51.7 ± 13.6 | 60.7 ± 15.8* | 58.0 ± 15.6† | < 0.001 |
| AVAplani, mm2 | 52.6 ± 13.5 | 59.5 ± 17.5* | 57.8 ± 15.2† | 0.005 |
| Aortic annulus | ||||
| Maximal dimeter, mm | 16.8 ± 2.0 | 18.2 ± 2.6* | 16.9 ± 1.7 | 0.01 |
| Mean diameter, mm | 15.2 ± 1.6 | 16.1 ± 1.9 | 15.4 ± 1.6 | 0.06 |
| Perimeter, mm | 48.7 ± 5.2 | 51.1 ± 5.8 | 49.2 ± 5.3 | 0.12 |
| Area, mm2 | 293.4 ± 55.5 | 331.0 ± 56.6* | 299.7 ± 55.9 | 0.02 |
| Sinus of Valsalva, mm | 22.4 ± 2.9 | 23.0 ± 2.8 | 22.6 ± 2.9 | 0.65 |
| Sinotubular junction diameter, mm | 18.9 ± 2.9 | 18.9 ± 2.1 | 19.3 ± 3.2 | 0.69 |
| Ascending aorta tubular portion, mm | 25.0 ± 4.3 | 23.0 ± 2.9 | 24.8 ± 4.6 | 0.15 |
| Surgical valve size, mm | 22.1 ± 2.1 | 23.0 ± 2.1 | 22.3 ± 2.0 | 0.18 |
| Surgical valve type | N/A | |||
| CE Magna | 144 | 10 | 18 | |
| ATSAP | 82 | 4 | 8 | |
| Hancock | 82 | 1 | 13 | |
| St. Jude Regent | 80 | 3 | 11 | |
| Others | 50 | 0 | 5 | |
| Operator | 0.47 | |||
| Operator 1 | 170 | 5 | 18 | |
| Operator 2 | 95 | 2 | 12 | |
| Operator 3 | 81 | 4 | 15 | |
| Operator 4 | 64 | 4 | 7 | |
| Operator 5 | 28 | 3 | 3 | |
| MACCE | 34 (7.8) | 5 (27.8)* | 4 (7.3) | 0.01 |
| All-cause mortality | 57 (13.0) | 6 (33.3) | 8 (14.5) | 0.05 |
| Follow-up duration, d | 1517.5 (1188.8–2026.5) | 1134.0 (26.0–1682.0) | 1455.0 (1112.0–1944.0) | 0.007 |
Values are means ± standard deviations or numbers and percentages in parentheses.
*Significant difference between patients with high-gradient severe aortic stenosis and patients with classic LF-LG AS groups.
†Significant difference between high-gradient severe aortic stenosis and paradoxical LF-LG AS groups.
‡Value divided by sex-specific thresholds (Male, 2000; Female, 1250).
AS aortic stenosis, AV aortic valve, AVA aortic valve area, AVC aortic valve calcium score, BNP B-type natriuretic peptide, CABG coronary artery bypass graft, EDVI end-diastolic volume index, ESVI end-systolic volume index, LF-LG low-flow and low-gradient, lnBNP log-transformed B-type natriuretic peptide, LVEF left ventricular ejection fraction, LVMI left ventricular mass index, LVOT left ventricular outflow tract, MACCE major adverse cardiac and cerebrovascular event, N/A not available, PCI percutaneous coronary artery intervention, PG pressure gradient, SAC systemic arterial compliance, VTI velocity time integral, Zva valvulo-arterial impedance.
Among the groups with high-gradient severe AS, classic LF-LG AS, and paradoxical LF-LG AS, the age of patients was not statistically different (P = 0.93) (Table 1). The number of concurrent percutaneous coronary artery intervention or coronary artery bypass graft with AVR was highest in classic LF-LG AS group (50%, P = 0.02). B-type natriuretic peptide (BNP) was highest in classic LF-LG AS (median 944.5 pg/mL, P < 0.001). MACCE was more common in the classic LF-LG AS than in the high-gradient severe AS (27.8 vs. 7.8%, P = 0.01).
Echocardiography
LVEF, transaortic peak velocity and PG were lower in the classic LF-LG AS group and reflected the characteristics of LF-LG AS (P < 0.001, for all) (Table 1). The end-systolic volume index (ESVI) (63.4 vs. 27.7 mL/m2, P < 0.001) and end-diastolic volume index (EDVI) (96.3 vs. 66.8 mL/m2, P < 0.001) were significantly larger in classic LF-LG AS, compared to the high-gradient severe AS group. Systemic arterial compliance was not different among the groups (P = 0.28), although valvulo-arterial impedence (Zva) was lower in paradoxical LF-LG AS compared to others (P < 0.001).
We found that AVACT ≥ 1.2 cm2 was noted in 9.8% (43/438) of the patients with high-gradient severe AS, 27.8% (5/18) of with classic LF-LG AS, and 14.5% of paradoxical LF-LG AS (Fig. 1). In high-gradient severe AS, patients with AVACT ≥ 1.2 cm2 also showed larger AVAecho (80.4 vs. 59.0 mm2, P < 0.001) with higher LVOT velocity time integral (VTI) (22.6 vs. 21.3 cm, P = 0.04) and lower AV VTI (101.0 vs. 127.2 cm, P < 0.001) than those of with AVACT < 1.2 cm2. The LVOT diameter had no significant different between two groups (21.4 vs. 21.0, P = 0.10) (Table 2). In classic LF-LG AS, AVAecho was larger in patients with AVACT ≥ 1.2 cm2 than those with AVACT < 1.2 cm2 (61.1 vs. 81.9 mm2, P = 0.005). However, other echocardiography parameters such as LVEF, peak velocity, and PG were not statistically different between subgroups with AVACT < 1.2 cm2 and AVACT ≥ 1.2 cm2 (P > 0.05, for all). In patients with paradoxical LF-LG AS, AVAecho was larger in AVACT ≥ 1.2 cm2 group (68.2 vs. 77.3 mm2, P = 0.08), but without statistical significance.
Figure 1.
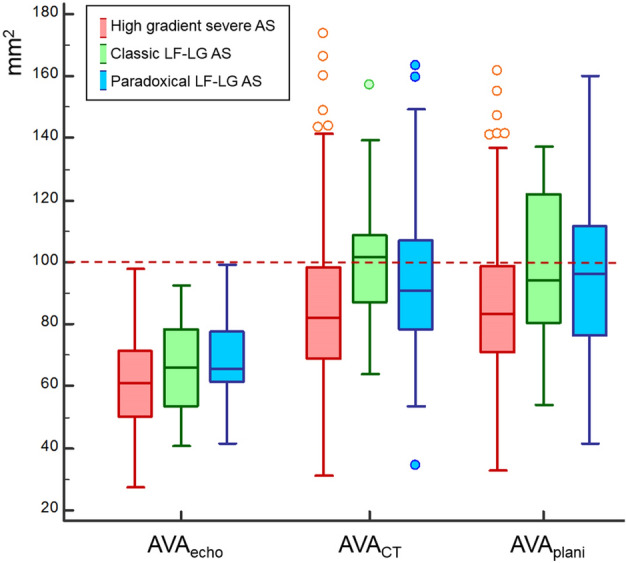
Box plot to demonstrate the distribution of AVAecho, AVACT, and AVAplani according to categories of AS. AS aortic stenosis, AVA aortic valve area, LF-LG low-flow and low-gradient.
Table 2.
Subgroups of LF-LG AS according to AVACT.
| Characteristic | High-gradient severe AS (n = 438) | Classic LF-LG AS (n = 18) | Paradoxical LF-LG AS (n = 55) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AVACT < 1.2 cm2 | AVACT ≥ 1.2 cm2 | P-value | AVACT < 1.2 cm2 | AVACT ≥ 1.2 cm2 | P-value | AVACT < 1.2 cm2 | AVACT ≥ 1.2 cm2 | P-value | |
| No. of patients (%) | 395 (90.2) | 43 (9.8) | 13 (72.2) | 5 (27.8) | 47 (85.5) | 8 (14.5) | |||
| Age, years | 66.8 ± 8.9 | 67.0 ± 8.4 | 0.92 | 66.1 ± 5.8 | 68.6 ± 7.7 | 0.46 | 67.3 ± 9.2 | 67.3 ± 10.9 | 0.99 |
| Male | 201 (50.9) | 35 (81.4) | < 0.001 | 9 (69.2) | 4 (80.0) | 1.00 | 25 (53.2) | 6 (75.0) | 0.72 |
| BSA, m2 | 1.6 ± 0.2 | 1.7 ± 0.2 | < 0.001 | 1.7 ± 0.2 | 1.6 ± 0.2 | 0.12 | 1.6 ± 0.2 | 1.7 ± 0.1 | 0.34 |
| Hypertension | 208 (52.7) | 23 (53.5) | 1.00 | 7 (53.8) | 5 (100.0) | 0.11 | 26 (55.3) | 4 (50.0) | 1.00 |
| Atrial fibrillation | 59 (14.9) | 6 (14.0) | 1.00 | 3 (23.1) | 1 (20.0) | 1.00 | 2 (4.3) | 2 (25.0) | 0.10 |
| PCI or CABG | 88 (22.3) | 6 (14.0) | 0.29 | 6 (46.2) | 3 (60.0) | 1.00 | 14 (25.5) | 0 (0.0) | 0.10 |
| Rheumatic valvular disease | 37 (9.4) | 1 (2.3) | 0.12 | 2 (15.4) | 0 (0.0) | 1.00 | 6 (12.8) | 2 (25.0) | 0.33 |
| B-type natriuretic peptide, pg/mL | 106.0 (49.0 –282.5) | 51.0 (31.0 –190.5) | 0.05 | 1118.0 (304.8–3066.0) | 738.5 (239.3–3227.5) | 0.80 | 59.0 (32.5–180.5) | 200.5 (59.0–305.3) | 0.38 |
| lnBNP | 4.7 (3.9–5.6) | 3.9 (3.4–5.2) | 0.05 | 7.0 (5.7–8.0) | 6.6 (5.0–7.9) | 0.60 | 4.1 (3.5–5.2) | 5.2 (4.1–5.7) | 0.12 |
| Blood urea nitrogen, mg/dL | 17.7 ± 7.2 | 20.4 ± 10.2 | 0.10 | 25.2 ± 16.7 | 18.6 ± 4.7 | 0.22 | 18.8 ± 8.5 | 18.0 ± 4.7 | 0.81 |
| Creatinine, mg/dL | 0.9 ± 0.6 | 1.4 ± 1.9 | 0.09 | 2.1 ± 2.3 | 1.9 ± 1.3 | 0.76 | 1.0 ± 0.4 | 0.9 ± 0.1 | 0.46 |
| Echocardiography | |||||||||
| LVEF, % | 60.3 ± 10.1 | 60.8 ± 8.9 | 0.72 | 36.9 ± 11.2 | 33.8 ± 8.0 | 0.59 | 62.8 ± 5.4 | 61.5 ± 4.6 | 0.53 |
| Peak velocity, m/s | 5.2 ± 0.7 | 4.9 ± 0.6 | 0.006 | 3.6 ± 0.4 | 3.4 ± 0.7 | 0.37 | 3.5 ± 0.5 | 3.6 ± 0.6 | 0.67 |
| Peak PG, mmHg | 109.8 ± 31.0 | 96.9 ± 22.4 | 0.001 | 53.5 ± 11.7 | 48.0 ± 17.0 | 0.44 | 49.5 ± 16.0 | 53.4 ± 16.9 | 0.53 |
| Mean PG, mmHg | 67.3 ± 19.7 | 58.5 ± 14.7 | 0.001 | 30.8 ± 6.8 | 27.2 ± 10.6 | 0.41 | 27.7 ± 9.4 | 29.8 ± 10.2 | 0.59 |
| LVMI, g/m2 | 135.4 ± 35.8 | 134.8 ± 36.5 | 0.91 | 151.5 ± 33.5 | 144.7 ± 26.3 | 0.69 | 121.5 ± 32.7 | 144.6 ± 50.6 | 0.10 |
| AV VTI, cm | 127.2 ± 25.7 | 101.0 ± 16.5 | < 0.001 | 101.3 ± 34.2 | 78.2 ± 26.9 | 0.20 | 114.2 ± 28.2 | 98.9 ± 21.3 | 0.15 |
| LVOT VTI, cm | 21.3 ± 4.1 | 22.6 ± 3.6 | 0.04 | 16.0 ± 4.8 | 16.2 ± 3.8 | 0.92 | 21.4 ± 3.6 | 19.7 ± 3.7 | 0.24 |
| LVOT diameter, mm | 21.0 ± 1.5 | 21.4 ± 1.5 | 0.10 | 22.1 ± 2.2 | 21.2 ± 0.2 | 0.22 | 21.0 ± 1.4 | 22.2 ± 2.2 | 0.18 |
| LVOT diameter/BSA | 12.9 ± 1.3 | 12.3 ± 1.0 | < 0.001 | 12.9 ± 1.6 | 13.6 ± 1.4 | 0.40 | 13.0 ± 1.4 | 13.2 ± 1.2 | 0.75 |
| AVAecho, mm2 | 59.0 ± 13.6 | 80.4 ± 8.8 | < 0.001 | 61.1 ± 12.8 | 81.9 ± 9.1 | 0.005 | 68.2 ± 13.6 | 77.3 ± 12.7 | 0.08 |
| ESVI | 27.7 ± 16.9 | 28.0 ± 16.4 | 0.92 | 63.0 ± 30.0 | 64.4 ± 20.0 | 0.92 | 23.4 ± 10.6 | 30.7 ± 14.5 | 0.10 |
| EDVI | 66.6 ± 23.4 | 68.7 ± 26.7 | 0.57 | 96.4 ± 32.6 | 95.9 ± 21.3 | 0.98 | 62.0 ± 22.6 | 78.4 ± 31.5 | 0.08 |
| SAC, mL/m2/mmHg | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.11 | 0.7 ± 0.3 | 0.6 ± 0.1 | 0.52 | 0.7 ± 0.3 | 1.0 ± 0.5 | 0.21 |
| Zva, mmHg/mL/m2 | 5.4 ± 1.6 | 5.2 ± 1.7 | 0.51 | 4.7 ± 1.7 | 5.8 ± 2.2 | 0.82 | 4.5 ± 1.4 | 3.8 ± 1.5 | 0.21 |
| CT findings | |||||||||
| Valve morphology | 0.04 | 0.28 | 0.14 | ||||||
| Tricuspid | 177 (44.8) | 27 (62.8) | 9 (69.2) | 5 (100.0) | 26 (55.3) | 2 (25.0) | |||
| Bicuspid | 218 (55.2) | 16 (37.2) | 4 (30.8) | 0 (0.0) | 21 (44.7) | 6 (75.0) | |||
| AVC, Agatston unit | 2834.2 (1605.2–4178.4) | 2426.7 (1616.1–3808.9) | 0.57 | 3912.2 (2247.0–4496.1) | 1360.1 (960.1–2108.6) | 0.002 | 2002.4 (1192.2–2841.6) | 2584.3 (1306.5–5172.0) | 0.12 |
| AVACT | 80.9 ± 18.8 | 136.6 ± 29.6 | < 0.001 | 92.4 ± 15.5 | 122.7 ± 25.2 | 0.007 | 90.2 ± 22.4 | 117.7 ± 27.9 | 0.003 |
| AVAplani, mm2 | 85.2 ± 22.1 | 105.0 ± 26.1 | < 0.001 | 88.2 ± 20.0 | 129.9 ± 5.4 | < 0.001 | 90.1 ± 21.2 | 140.9 ± 13.8 | < 0.001 |
| LVOT area | 346.8 ± 50.0 | 359.6 ± 51.3 | 0.11 | 587.5 ± 129.7 | 572.3 ± 79.9 | 0.81 | 468.9 ± 94.5 | 581.3 ± 94.9 | 0.003 |
| Aortic annulus | |||||||||
| Maximum diameter, mm | 27.1 ± 3.0 | 30.6 ± 3.2 | < 0.001 | 30.6 ± 3.9 | 30.0 ± 2.1 | 0.77 | 27.1 ± 2.6 | 29.0 ± 3.6 | 0.05 |
| Circularity, % | 80.0 ± 1.0 | 80.0 ± 1.0 | 0.22 | 77.6 ± 7.5 | 76.9 ± 4.4 | 0.84 | 82.0 ± 5.7 | 83.8 ± 7.6 | 0.18 |
| Mean diameter, mm | 24.6 ± 2.5 | 27.5 ± 2.6 | < 0.001 | 27.1 ± 2.9 | 26.5 ± 1.4 | 0.70 | 24.7 ± 2.3 | 27.0 ± 3.0 | 0.01 |
| Perimeter, mm | 78.7 ± 8.0 | 87.3 ± 8.6 | < 0.001 | 86.0 ± 8.9 | 83.8 ± 3.7 | 0.46 | 78.7 ± 7.4 | 87.3 ± 10.0 | 0.01 |
| Area, mm2 | 471.4 ± 95.0 | 576.8 ± 119.4 | < 0.001 | 563.3 ± 116.1 | 532.8 ± 45.9 | 0.44 | 475.2 ± 91.3 | 572.9 ± 113.2 | 0.009 |
| Sinus of Valsalva, mm | 36.2 ± 4.5 | 39.4 ± 3.8 | < 0.001 | 39.3 ± 4.9 | 36.1 ± 3.4 | 0.20 | 35.9 ± 4.6 | 42.0 ± 4.2 | 0.001 |
| Sinotubular junction, mm | 30.7 ± 4.6 | 32.8 ± 4.4 | 0.004 | 32.1 ± 3.5 | 30.7 ± 2.8 | 0.44 | 30.6 ± 5.1 | 36.7 ± 7.7 | 0.006 |
| Ascending aorta, mm | 40.7 ± 6.3 | 41.0 ± 5.8 | 0.74 | 39.5 ± 4.4 | 35.5 ± 4.2 | 0.10 | 39.6 ± 7.1 | 45.1 ± 9.8 | 0.06 |
| Normalized to BSA | |||||||||
| AVACT | 51.8 ± 13.0 | 59.7 ± 15.7 | < 0.001 | 53.9 ± 9.4 | 78.3 ± 16.1 | 0.001 | 55.9 ± 14.3 | 70.3 ± 17.9 | 0.01 |
| AVAplani, mm2 | 52.4 ± 13.5 | 60.9 ± 16.4 | < 0.001 | 51.5 ± 12.3 | 82.7 ± 6.0 | < 0.001 | 55.7 ± 12.7 | 84.1 ± 10.4 | < 0.001 |
| LVOT area, mm2 | 294.1 ± 61.4 | 322.3 ± 62.4 | 0.005 | 341.2 ± 69.4 | 365.5 ± 54.6 | 0.50 | 289.3 ± 55.3 | 345.5 ± 54.4 | 0.01 |
| Aortic annulus | |||||||||
| Maximal dimeter, mm | 16.7 ± 1.9 | 17.7 ± 2.0 | 0.002 | 17.9 ± 2.8 | 19.2 ± 2.0 | 0.35 | 16.8 ± 1.7 | 17.4 ± 1.6 | 0.37 |
| Mean diameter, mm | 15.2 ± 1.6 | 15.9 ± 1.6 | 0.006 | 15.8 ± 2.0 | 17.0 ± 1.7 | 0.27 | 15.3 ± 1.6 | 16.1 ± 1.6 | 0.20 |
| Perimeter, mm | 48.5 ± 5.2 | 50.4 ± 5.1 | 0.02 | 50.2 ± 6.0 | 53.6 ± 5.0 | 0.28 | 48.7 ± 4.9 | 52.0 ± 6.8 | 0.10 |
| Area, mm2 | 289.3 ± 53.4 | 331.3 ± 60.0 | < 0.001 | 327.6 ± 65.3 | 339.7 ± 26.4 | 0.70 | 292.7 ± 50.4 | 341.4 ± 71.4 | 0.02 |
| Sinus of Valsalva | 36.2 ± 4.5 | 39.4 ± 3.8 | < 0.001 | 22.9 ± 3.0 | 23.0 ± 2.4 | 0.95 | 22.1 ± 2.6 | 25.1 ± 3.5 | 0.007 |
| Sinotubular junction diameter | 30.7 ± 4.6 | 32.8 ± 4.4 | 0.004 | 18.7 ± 1.9 | 19.6 ± 2.5 | 0.40 | 18.9 ± 2.5 | 21.9 ± 5.3 | 0.01 |
| Ascending aorta tubular portion | 40.7 ± 6.3 | 41.0 ± 5.8 | 0.74 | 23.1 ± 3.0 | 22.6 ± 2.9 | 0.78 | 24.5 ± 4.3 | 26.9 ± 5.7 | 0.18 |
| Surgical valve size, mm | 21.9 ± 2.1 | 23.5 ± 1.8 | < 0.001 | 23.2 ± 2.3 | 22.6 ± 1.7 | 0.63 | 22.1 ± 2.1 | 23.3 ± 1.7 | 0.14 |
| MACCE (cardiovascular death) | 30 (7.6) | 4 (9.3) | 0.92 | 2 (15.4) | 3 (60.0) | 0.10 | 4 (8.5) | 0 (0.0) | 1.00 |
| All-cause mortality | 52 (13.2) | 5 (11.6) | 0.96 | 4 (30.8) | 2 (40.0) | 1.00 | 7 (14.9) | 1 (12.5) | 1.00 |
AS, aortic stenosis; AVA, aortic valve area; AVACT, AVA measured on CT; AVC, aortic valve calcium score; BSA, body surface area; CABG, coronary artery bypass graft; EDVI, end-diastolic volume index; ESVI, end-systolic volume index; LF-LG, low-flow and low-gradient; lnBNP, log-transformed B-type natriuretic peptide; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; LVOT, left ventricular outflow tract; MACCE, major adverse cardiac and cerebrovascular event; PCI, percutaneous coronary artery intervention; VTI, velocity time integral.
Comparison of AVA measured by echocardiography and CT
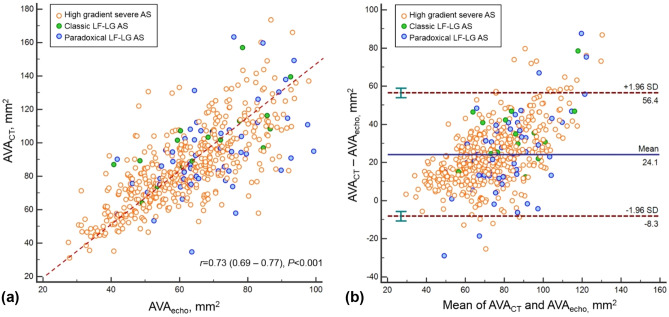
Interobserver agreements for aortic root measurement on CT are high with the range of intra-class correlation coefficient (ICC) from 89.2 to 97.0 (Supplementary Table 2). The Pearson correlation coefficient for AVAecho and AVACT was good (r = 0.73, P < 0.001). AVACT is larger than AVAecho and the mean difference between AVAecho and AVACT was 24.1 mm2 (95% confidence interval [CI], − 8.3 to 56.4 mm2, P < 0.001) (Fig. 2A,B). Comparison of AVAecho and AVAplani is presented in Supplementary Fig. 2.
Figure 2.
(a) Pearson correlation analysis result and (b) Bland–Altman plot to comparison of AVACT and AVAecho. AS aortic stenosis, AVA aortic valve area, LF-LG low-flow and low-gradient.
CT findings according to AS subtypes
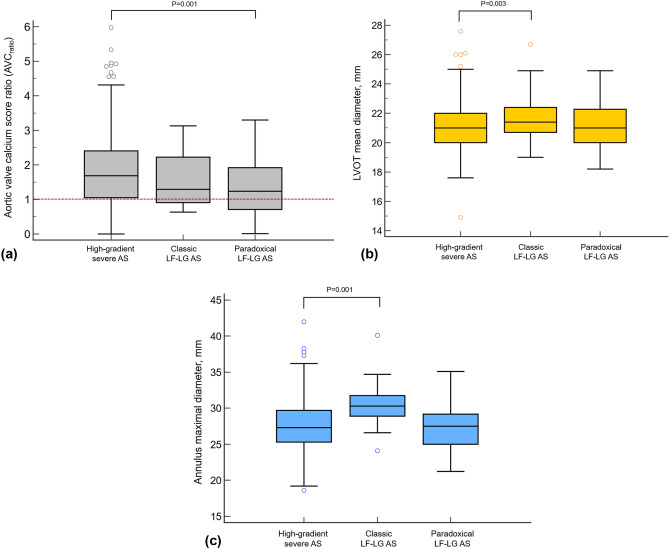
AVC was highest in patients with high-gradient severe AS, and statistically lower in paradoxical LF-LG AS (P = 0.04). When adjusted to sex-specific threshold, AVCratio was lowest in patients with paradoxical LF-LG AS and lower than that of high-gradient severe AS (1.8 vs. 1.4, P = 0.006, Fig. 3A). LVOT mean dimeter measured on CT was largest in LF-LG AS group and larger than that in high-gradient severe AS (24.8 vs. 27.1 mm, P = 0.003, Fig. 3B). The maximal diameter of aortic annulus largest in classic LF-LG AS group and larger than that of high-gradient severe AS (27.5 vs. 30.4 mm, P = 0.001, Fig. 3C). The mean AVACT was larger in the classic LF-LG AS group, compared to the high-gradient severe AS group (100.8 vs. 84.9 mm2, P = 0.001).
Figure 3.
Box plot to demonstrate the distribution of AVCratio, LVOT mean diameter, and maximal diameter of aortic annulus according to subtypes. (a) AVCratio was calculated by dividing AVC with sex-specific thresholds (Male, 2000; Female, 1250). The score above the red-dotted line represents AVC above the sex-specific threshold, and consequently, severe aortic stenosis. The score below the red-dotted line represents the AVC below the sex-specific threshold and nonsevere calcification. The mean of AVCratio was significantly lower in paradoxical LF-LG AS than that of high gradient severe AS (P = 0.001). In addition, the proportion of nonsevere calcification was most frequent in paradoxical LF-LG AS patients. Both (b) LVOT mean diameter and (c) maximal diameter of aortic annulus were lowest in LF-LG AS patients among the three subtypes and significantly larger than those of high-gradient severe AS patients. AS aortic stenosis, AVC aortic valve calcium score, LF-LG low-flow and low-gradient, LVOT left ventricular outflow tract.
With cut-off value of AVACT < 1.2 cm2, 455 of 511 (89.0%) patients were categorized as severe AS. However, 56 patients were re-classified as moderate AS (43 [9.8%] high-gradient severe AS, 5 [27.8%] classic LF-LG AS, and 8 [14.5%] paradoxical LF-LG AS). In high-gradient severe AS group, the re-classified moderate AS patients had larger normalized measurements of annulus than those in concordant severe AS (P < 0.05, for all). Normalized LVOT area was also larger in re-classified moderate AS (322.3 vs. 294.1 mm2, P = 0.005) (Table 2). In classic LF-LG AS group, severe AS patients who showed concordance between echocardiography and CT (AVACT < 1.2 cm2) had higher mean AVC (3912.2 vs. 1360.1, P = 0.002) and smaller AVAplani (88.2 vs. 129.9 mm2, P < 0.001) than those in re-classified moderate AS patients (AVACT ≥ 1.2 cm2). The normalized annulus sizes and aortic root diameters on CT were not statistically different between concordant severe AS and re-classified moderate AS groups (P < 0.05, for all). In paradoxical LF-LG AS group, LVOT area normalized to body surface area (BSA) (289.3 vs. 345.5 mm2, P = 0.01), normalized sizes of aortic annulus area (292.7 vs. 341.4 mm2, P = 0.02), sinus of Valsalva (22.1 vs. 25.2 mm, P = 0.003) and ST junction (30.6 vs. 36.7 mm, P = 0.006) were larger in concordant severe AS patients, compared to those measured in re-classified moderate AS patients.
Outcome analysis
Of 511 patients, the all-cause mortality was 13.9% (n = 71) and MACCE occurred 8.4% (n = 43). MACCE were composed of major arrhythmia requiring treatment (n = 6), nonfatal cerebrovascular accident (n = 10), nonfatal myocardial infarction (n = 4), heart failure (n = 4), reoperation (n = 1), and cardiovascular death (n = 18). To identify clinical and radiological factors that affect MACCE, cox-proportional hazard regression analysis was performed (Table 3). In univariate analysis, older age, high BNP, high blood urea nitrogen and creatinine, presence of preoperative atrial fibrillation (AF), tricuspid AV, classic LF-LG AS, small AV VTI and LVOT VTI, and small aortic annulus were factors significantly associated with MACCE (P < 0.05, for all).
Table 3.
Cox proportional hazard regression model for prediction of MACCE.
| Parameter | Univariate | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, years | 1.06 (1.02–1.10) | 0.005 | 1.04 (1.00–1.09) | 0.049 |
| BSA, m2 | 0.49 (0.08–3.15) | 0.45 | ||
| B-type natriuretic peptide, 10 pg/mL | 1.01 (1.003–1.01) | < 0.001 | 1.005 (1.003–1.01) | < 0.001 |
| lnBNP | 1.42 (1.13–1.77) | 0.002 | ||
| Atrial fibrillation, (%) | 3.22 (1.70–6.11) | < 0.001 | 2.75 (1.40–5.40) | 0.003 |
| LVEF, % | 0.98 (0.96–1.01) | 0.20 | ||
| Peak velocity, m/s | 0.80 (0.57–1.12) | 0.19 | ||
| Mean PG, mmHg | 0.99 (0.98–1.01) | 0.21 | ||
| LVMI, g/m2 | 0.99 (0.99–1.00) | 0.18 | ||
| ESVI, mL/m2 | 1.01 (0.99–1.02) | 0.55 | ||
| EDVI, mL/m2 | 1.00 (0.99–1.01) | 0.90 | ||
| SAC, mL/m2/mmHg | 0.57 (0.21–1.53) | 0.27 | ||
| Zva, mmHg/mL/m2 | 1.00 (0.83–1.20) | 0.98 | ||
| Bicuspid aortic valve | 0.40 (0.21–0.77) | 0.006 | ||
| Classic LF-LG AS | 5.04 (1.98–12.84) | 0.001 | 5.53 (1.74–17.56) | 0.004 |
| AVAecho, m2 | 1.01 (0.99–1.03) | 0.60 | ||
| AV VTI, cm | 0.98 (0.96–0.99) | < 0.001 | ||
| LVOT VTI, cm | 0.92 (0.86–0.99) | 0.02 | ||
| lnAVC | 0.89 (0.71–1.09) | 0.26 | ||
| Normalized AVAplani, mm2 | 1.00 (0.98–1.03) | 0.76 | ||
| Normalized AVACT, mm2 | 1.02 (1.00–1.04) | 0.50 | ||
| Annulus circularity, % | 0.34 (0.01–18.69) | 0.59 | ||
| Aortic annulus area, cm2 | 0.73 (0.53–1.01) | 0.06 | 0.57 (0.40–0.81) | 0.002 |
| Surgical valve size, mm | 0.89 (0.77–1.04) | 0.13 | ||
| Surgical valve type | ||||
| CE Magna | 1 | 0.63 | ||
| ATSAP | 0.55 (0.20–1.48) | 0.24 | ||
| Hancock | 1.09 (0.50–2.38) | 0.83 | ||
| St. Jude Regent | 0.63 (0.25–1.59) | 0.32 | ||
| Others | 0.85 (0.31–2.31) | 0.75 | ||
| Operator | ||||
| Operator 1 | 1 | 0.33 | ||
| Operator 2 | 1.63 (0.78–3.38) | 0.19 | ||
| Operator 3 | 0.37 (0.05–2. 76) | 0.33 | ||
| Operator 4 | 0.94 (0.37–2.41) | 0.90 | ||
| Operator 5 | 0.73 (0.30–1.78) | 0.49 | ||
AS aortic stenosis, AVA aortic valve area, AVC aortic valve calcium score, CI confidence interval, EDVI end-diastolic volume index, ESVI end-systolic volume index, HR hazard ratio, LF-LG low-flow and low-gradient, lnBNP log-transformed B-type natriuretic peptide, LVEF left ventricular ejection fraction, LVMI left ventricular mass index, LVOT left ventricular outflow tract, MACCE major adverse cardiac and cerebrovascular event, PG pressure gradient, SAC systemic arterial compliance, VTI velocity time integral, Zva valvulo-arterial impedance.
On multivariable analysis, old age (hazard ratio10, 1.04, 95% CI, 1.00–1.09; P = 0.049), high BNP (HR, 1.005; 95% CI, 1.003–1.01 P < 0.001), AF (HR, 2.75; 95% CI, 1.40–5.40; P = 0.003), classic LF LG AS (HR, 5.53; 95% CI, 1.74–17.56; P = 0.004), and small aortic annulus area (cm2), [HR, 0.57; 95% CI, 0.40–0.81; P = 0.002]) were factors significantly associated with MACCE (Table 3). Normalized aortic annulus area (cm2) (HR, 0.40; 95% CI, 0.22–0.74; P = 0.004) was also a significantly associated factor when the parameter was substitute instead of aortic annulus area in multivariable analysis. When the normalized aortic sinus of Valsalva diameter instead of the aortic annulus size was substituted in the calculation, the weight of the other factors remained almost unchanged, while the normalized aortic sinus of Valsalva diameter (cm) (HR, 0.30; 95% CI, 0.09–0.98; P = 0.04) was also identified as a significant factor.
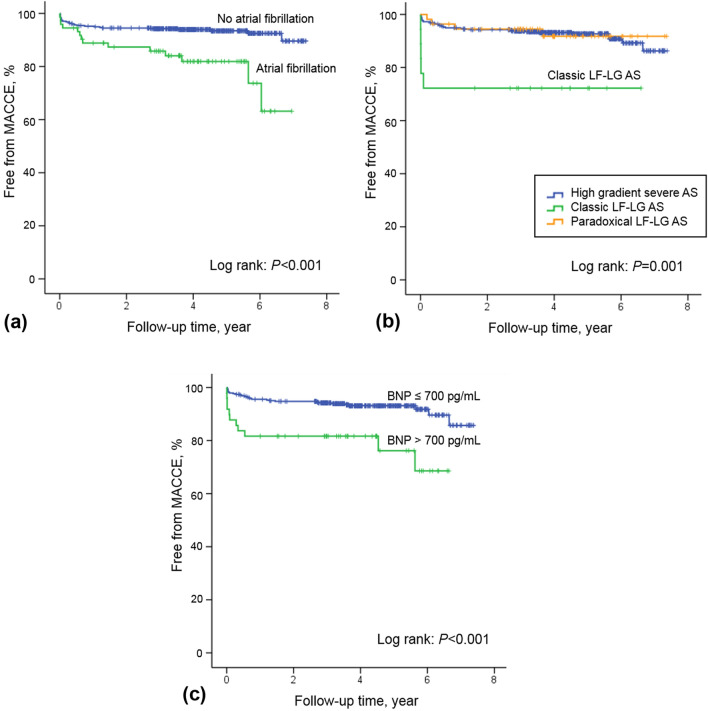
Kaplan–Meier curves indicated significant mortality in the high BNP group (BNP > 700 pg/mL) compared to the low BNP group (P = 0.001) (Fig. 4A). Furthermore, preoperative AF was also associated with significant mortality (Fig. 4B) (P < 0.001), and the outcome of classic LF-LG AS was worse in the cumulative survival curve (Fig. 4C) (P = 0.001).
Figure 4.
Survival according to (a) B-type natriuretic peptide, (b) presence of atrial fibrillation, and (c) categories of aortic stenosis. AS aortic stenosis, LF-LG low-flow and low-gradient.
Discussion
Herein, we described preoperative CT characteristics of different subtypes of AS, compared AVACT with AVAecho, and identified prognostic factors after AVR. AVAecho and AVACT showed high concordance rate (89.0%) to classify severe AS, except 56 patients who were re-classified as moderate AS (43 [9.8%] high-gradient severe AS, 5 [27.8%] classic LF-LG AS, and 8 [14.5%] paradoxical LF-LG AS) by AVACT. The AVACT and aortic annulus were larger in classic LF-LG AS compared to those in high-gradient severe AS. High BNP, preoperative AF, classic LF-LG AS, and smaller aortic root were associated with MACCE after AVR.
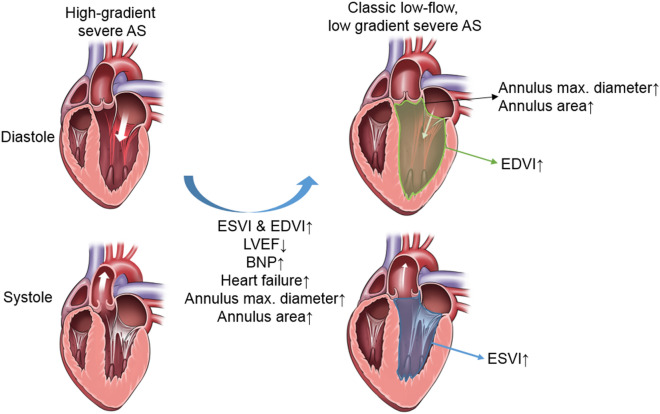
Among the three different subtypes of severe AS, classic LF-LG AS patients demonstrated higher ESVI and EDVI, lower LVEF, larger AVAecho and AVACT, and larger aortic annulus compared to high-gradient severe AS. In a previous study, patients with severe AS had significantly larger aortic annulus and sinotubular (ST) junction diameters compared with those measured in control groups14. Compensatory increment of ESVI and EDVI and subsequent LV dilatation may lead to aortic root remodelling, the dilatation of aortic annulus (Fig. 5). They also had larger LVOT mean diameter and aortic annulus maximal diameter on CT compared to high-gradient severe AS, which could be explained by dilated LV in classic LF-LG AS. Importantly, ESVI, EDVI, and BNP were significantly higher in LF-LG AS than those of high-gradient severe AS. This suggest the adverse remodelling may occur in LF-LG AS and is line with previous description on LF-LG, which shows dilated LV with LV dysfunction2. Classic LF-LG AS may be a compensation failure of high-gradient severe AS whereas paradoxical LF-LG AS presented preserved ESVI, EDVI, and LVEF, although AVAecho and AVACT were larger than in high-gradient severe AS.
Figure 5.
Characteristics of classic LF-LG AS. Classic LF-LG AS patients demonstrated higher ESVI and EDVI, lower LVEF, larger aortic annulus diameter and area compared to high-gradient severe AS. The drawings were prepared using Photoshop 2019 (version 20). AS aortic stenosis, BNP B-type natriuretic peptide, EDVI end-diastolic volume index, ESVI end-systolic volume index, LVEF left ventricular ejection fraction.
In terms of AVC, mean of the AVCratio was lowest in paradoxical LF-LG AS followed by classic LF-LG AS. That means nonsevere calcification is more frequent in LF-LG AS patients than those of high-gradient severe AS and implies that other factors (e.g., different hemodynamics in AS subtypes) rather than calcification burden could affect the decreased AVA in LF-LG. Although the current diagnostic standard for AS grading and classification is echocardiography, these CT-derived parameters could have a supplementary role in classification of severe AS, especially with poor sonic window or high interobserver variability of echocardiography. We hope the findings based on cardiac CT could provide beginning on further study for prognostic implication of CT-derived parameters, which is less flow-dependent. Further study with large portion of LF-LG AS might reveal different outcomes with AS reclassified by AVACT.
In this study, we used cut-off value of AVACT < 1.2 cm2 as this value was suggested for severe AS in a previous study12. In high-gradient severe AS group, approximately 10% (43/438) of patients were re-classified to moderate AS. Different from echocardiography in which the LVOT had no significant difference between concordant and discordant groups, CT revealed larger normalized LVOT area in re-classified moderate AS patients, which probably contribute to discordance. In classic LF-LG AS group, approximately one third of the patients were re-classified to moderate AS. There was no significantly difference of LVOT, of which either measured by echocardiography or CT, in between concordant and discordant groups. Instead, AVC was lower in the AVACT ≥ 1.2 cm2 compared to that of AVACT < 1.2 cm2 group, and in this group, moderate AS patients might be misclassified as severe AS and vice versa. This can also be applied to paradoxical LF-LG patients, despite 14.5% of these patients presenting AVACT ≥ 1.2 cm2. Although we could not derive the role of AVACT in diagnosing LF-LG AS patients, we consider that further studies with large population of LF-LG AS might reveal different prognosis or post-surgical outcome in patients who reclassified to moderate AS based on AVACT.
The outcome of AS after AVR was associated with preoperative high BNP levels, AF, classic LF-LG AS, and small aortic root. The plasma BNP level was associated with LV dysfunction in AS, and was a well-known predictor of poor outcome in patients with AS overall and after AVR15–17. AF is also a dominant predictor in both asymptomatic and symptomatic patients with moderate to severe AS, and after AVR18–20. Classic LF-LG AS was associated with worse outcomes after AVR compared those observed in high-gradient AS patients, although LF-LG AS patients have displayed survival benefits with AVR21. Finally, small aortic root measured on CT was an independent prognostic factor. This finding should be interpreted cautiously. When AS severity progresses, the increased LV cavity volume may increase the size of the aortic annulus and sinus of Valsalva. However, a small aortic root has also been associated with increased ischemic cardiovascular events and mortality in patients with AS22, possibly reflecting impaired root remodelling process and atherosclerotic changes.
Our study has several limitations. Because this is a retrospective study using a patient cohort that underwent AVR, patients not indicated for surgery due to poor general conditions or comorbidities or who declined operation were not included. The selection bias may affect the outcome assessment, and AVR itself was not used as an outcome parameter. Instead, we used MACCE after AVR. Therefore, the outcomes of this study may not directly infer the outcomes of AS population managed with diverse treatment options. Further studies with AS managed by conservative treatment, surgical AVR, and transcatheter AVR could be of value to evaluate overall outcomes of AS patients. Second, we were not able to consider the reverse dynamism of LVOT which could affect the discrepancy between AVAecho and AVACT. Dynamic changes of the diameter of LVOT can result variability of AVAecho, but unfortunately it was not routinely evaluated in our institution and the LVOT diameter measured on mid-systolic phase was used for AVA calculation. Third, we showed the CT characteristics of LF-LG AS: AVACT and aortic annulus were larger in classic LF-LG AS compared to those in high-gradient severe AS. This finding may be explained by the aortic root remodelling which is associated with the dilated LV. However, because of the small number of LF-LG AS patients, we could not generalize the CT findings of LF-LG AS. Further study with larger number of LF-LG AS would be of value. Finally, although classic LF-LG patients showed higher all-cause mortality and a large aortic annulus, a small aortic root was one of the factors associated with MACCE. Both decreased LV function in classic LF-LG AS and impaired aortic root remodelling may contribute to the outcome, respectively, but further studies are necessary to provide more evidence.
In conclusion, AVAecho and AVACT showed high concordance rate (89.0%) to classify severe AS, however, 56 patients who were re-classified as moderate AS by AVACT. AVC and aortic root size on CT were different among the AS subtypes, high-gradient severe AS, classic LF-LG AS and paradoxical LF-LG AS. Old age, high BNP, AF, classic LF-LG AS and small aortic root on CT were associated with MACCE after AVR. These findings suggest the potential role of cardiac CT in classification and outcome assessment of severe AS.
Methods
Patients
This retrospective study was approved by the institutional review board committee of the Asan Medical Center, University of Ulsan College of Medicine (approval number: 2018-0233) and informed consent was waived by the institutional review board due to the retrospective nature of observational study. This study was performed in accordance with the Helsinki Declaration. Between June 2011 and Mar 2016, 781 patients underwent surgical AVR. The use of CT was determined mainly by clinician’s decision, but in our hospital, cardiac CT examination is generally performed in most of the patients who have performed planned surgical AVR for evaluation of AV and root morphology based on the guidelines for the appropriate use of cardiac CT23–26. After excluding patients with moderate AS (n = 24), moderate degree of concomitant aortic regurgitation or other valvular heart disease (n = 177), patients not subjected to preoperative cardiac CT (n = 47) or CT without multiphase data (n = 21), and a patient with quadricuspid AV (n = 1), 511 patients were finally included. High-gradient severe AS was defined as AVAecho < 1 cm2 and a mean trans-valvular gradient ≥ 40 mmHg with LVEF < 50%. Classic LF-LG severe AS was defined as AVAecho < 1 cm2, but with a low-gradient (< 40 mmHg). Low-gradient severe AS with preserved LVEF was defined as paradoxical LF-LG AS. We classified patients with AS into three groups: (1) high-gradient severe; (2) classic LF-LG; and (3) paradoxical LF-LG AS. Clinical findings including age, BSA, hypertension, AF, BNP, echocardiography parameters, and cardiac CT data were collected. Postoperative echocardiography findings and reported clinical outcomes were comprehensively reviewed. Clinical outcomes included all-cause mortality and MACCE (major arrhythmias requiring treatment, composite of cardiac death, cerebrovascular accident or stroke, coronary artery revascularization or myocardial infarction, and redo-AVR) were evaluated. Major arrhythmias included sick sinus syndrome, ventricular fibrillation, and AF/flutter.
Echocardiography
Preoperatively, all patients underwent transthoracic echocardiography using commercially available ultrasound machines with 3–5 MHz real-time transducers (iE33, EPIC; Philips Medical Systems, Andover, MA; Vivid 7, E9, General Electric Healthcare, Waukesha, WI, USA). Comprehensive two-dimensional and Doppler images were obtained by expert cardiologists according to American Society of Echocardiography recommendations27. End-systolic volume, end-diastolic volume, and LVEF were obtained with the biplane Simpson method. The maximal aortic jet velocity was recorded with the apical, right parasternal, or suprasternal window that yielded the highest-velocity signal. The maximal and mean PG across the AV were estimated using a modified Bernoulli equation, and the AVA was calculated from the continuity equation. LV mass and LV mass indexed to BSA calculated by LV cavity dimension and LV wall thickness at end-diastole. SAC was calculated as the ratio of SV index (SVI)/pulse pressure28, and valvulo-arterial impedance (Zva), which is a parameter for global LV load, was defined as (systolic blood pressure + mean net aortic gradient)/SVI29.
Cardiac CT protocol and image analysis
Preoperative cardiac CT was performed using a second-generation dual-source CT scanner (Somatom Definition Flash; Siemens Medical Solutions, Forchheim, Germany). Detailed CT protocol is described in Supplementary File 1. Post-processing was conducted using an external workstation (AquariusNet; TeraRecon, Foster City, CA, USA) using multiphase CT data sets reconstructed by a 10% R–R interval. CT analysis methods are described in Supplementary Fig. 1. CT characteristics such as AV morphology (tricuspid, bicuspid with raphe, and bicuspid without raphe), AVACT, AVA obtained by planimetry (AVAplani), aortic annulus diameter, perimeter, and area, circularity (minimum annulus diameter/maximum annulus diameter × 100), and diameters of sinus of Valsalva, ST junction, and ascending aorta tubular portion were measured by two experienced radiologists in consensus (S.J.C. and H.J.K.). AVACT was calculated by using the LVOT area measured on CT in the continuity equation with VTI at LVOT and transaortic flow:
AVC was defined as a CT density of 130 Hounsfield units or greater confined to AV on non-enhanced cardiac gated images and measured using the methods suggested by Agatston et al.30. The AVC was measured using a commercially available software (Syngo.via Siemens Healthcare, Berlin, Germany). For stratification by sex, AVCratio was calculated by dividing AVC with sex-specific thresholds (Male, 2000; Female, 1250)31.
Systolic phase with largest AVA (20–30% RR) was selected and thick multiplanar reconstruction images were used to demarcate the tips of the aortic cusps for measuring AVAplani. To evaluate reliability of CT measurements, a third experienced radiologist (Y.A.) measured CT parameters in 100 randomly selected cases and interobserver agreement was determined. Observers were blinded to clinical data including echocardiography findings and operation records.
Statistics
Continuous variables were expressed as mean ± standard deviation or median with IQR and categorical variables are presented as numbers and percentages. Interobserver agreement of CT findings was determined using a two-way random model ICC with consistency assumption. Comparison of AVAecho, AVACT, and CT-derived AVAplani was performed using Pearson correlations and Bland–Altman plots were graphed. One way ANOVA with post-hoc (Tukey) test or Kruskall–Wallis test and Chi-square test were used to compare baseline clinical and radiological findings among high-gradient severe AS, classic LF-LG AS, paradoxical LF-LG AS, and moderate AS groups. Bonferroni correction was applied to control the type I error for multiple comparison, and P-value 0.05/4 = 0.0125 was used for comparing the three groups. Student t test and Chi-square test were performed to compare two subgroups among the three groups. In LF-LG AS patients, clinical and CT findings for AVACT < 1.2 cm2 and AVACT ≥ 1.2 cm2 were compared using the Student t test and Chi-square test or Fisher’s exact test. For the stratification of risk factors for MACCE after AVR, cox proportional hazard models were used. Kaplan–Meier survival curves were drawn for statistically significant factors to predict MACCE. The 95% CIs were calculated and factors with P < 0.10 were included for multivariable cox regression analysis with enter method. To avoid multicollinearity, one of the aortic root parameters was included in the multivariable analysis among the CT parameters significantly associated with MACCE in univariate analysis. For BNP analysis, a continuous parameter was used and a cut-off of 700 pg/mL32 was set for outcome analysis using Kaplan–Meier curves17. P < 0.05 was considered statistically significant, except for multiple dependant variable analyses. Statistical analysis was performed using commercial software (SPSS, version 20; SPSS, Chicago, IL, USA).
Supplementary Information
Author contributions
Conception: H.J.K. and D.K., Data curation: H.J.K., S.L., J.B.K. J.-M.S. D.-H.K, J.-K.S., J.-W.K., D.H.Y., Statistical analyses, H.J.K., and H.J.K., Manuscript writing: S.J.C., Y.A., H.J.K., Revising the manuscript: H.J.K. and D.K. All authors approved the final version of this manuscript.
Funding
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI18C0022).
Data availability
All data used during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yura Ahn and Se Jin Choi.
Contributor Information
Dae-Hee Kim, Email: daehee74@amc.seoul.kr.
Hyun Jung Koo, Email: radkoo@amc.seoul.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11491-3.
References
- 1.Adda J, et al. Low-flow, low-gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle-tracking echocardiography: A multicenter study. Circ. Cardiovasc. Imaging. 2012;5:27–35. doi: 10.1161/CIRCIMAGING.111.967554. [DOI] [PubMed] [Google Scholar]
- 2.Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012;60:1845–1853. doi: 10.1016/j.jacc.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 3.Dumesnil JG, Pibarot P. Low gradient severe aortic stenosis with preserved ejection fraction: Don't forget the flow! Rev. Esp. Cardiol. (Engl. Ed.) 2013;66:245–247. doi: 10.1016/j.rec.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Minners J, et al. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur. Heart J. 2008;29:1043–1048. doi: 10.1093/eurheartj/ehm543. [DOI] [PubMed] [Google Scholar]
- 5.Minners J, et al. Inconsistent grading of aortic valve stenosis by current guidelines: Haemodynamic studies in patients with apparently normal left ventricular function. Heart (British Cardiac Society) 2010;96:1463–1468. doi: 10.1136/hrt.2009.181982. [DOI] [PubMed] [Google Scholar]
- 6.Oh JK, et al. Prediction of the severity of aortic stenosis by Doppler aortic valve area determination: Prospective Doppler-catheterization correlation in 100 patients. J. Am. Coll. Cardiol. 1988;11:1227–1234. doi: 10.1016/0735-1097(88)90286-0. [DOI] [PubMed] [Google Scholar]
- 7.de Filippi CR, et al. Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. Am. J. Cardiol. 1995;75:191–194. doi: 10.1016/S0002-9149(00)80078-8. [DOI] [PubMed] [Google Scholar]
- 8.Annabi MS, et al. Dobutamine stress echocardiography for management of low-flow, low-gradient aortic stenosis. J. Am. Coll. Cardiol. 2018;71:475–485. doi: 10.1016/j.jacc.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 9.Koos R, et al. Aortic valve calcification as a marker for aortic stenosis severity: Assessment on 16-MDCT. AJR Am. J. Roentgenol. 2004;183:1813–1818. doi: 10.2214/ajr.183.6.01831813. [DOI] [PubMed] [Google Scholar]
- 10.Pawade T, et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ. Cardiovasc. Imaging. 2018;11:e007146. doi: 10.1161/circimaging.117.007146. [DOI] [PubMed] [Google Scholar]
- 11.Cueff C, et al. Measurement of aortic valve calcification using multislice computed tomography: Correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart (British Cardiac Society) 2011;97:721–726. doi: 10.1136/hrt.2010.198853. [DOI] [PubMed] [Google Scholar]
- 12.Clavel MA, et al. Aortic valve area calculation in aortic stenosis by CT and Doppler echocardiography. JACC Cardiovasc. Imaging. 2015;8:248–257. doi: 10.1016/j.jcmg.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Halpern EJ, Mallya R, Sewell M, Shulman M, Zwas DR. Differences in aortic valve area measured with CT planimetry and echocardiography (continuity equation) are related to divergent estimates of left ventricular outflow tract area. AJR Am. J. Roentgenol. 2009;192:1668–1673. doi: 10.2214/ajr.08.1986. [DOI] [PubMed] [Google Scholar]
- 14.Stolzmann P, et al. Remodelling of the aortic root in severe tricuspid aortic stenosis: Implications for transcatheter aortic valve implantation. Eur. Radiol. 2009;19:1316–1323. doi: 10.1007/s00330-009-1302-0. [DOI] [PubMed] [Google Scholar]
- 15.Iwahashi N, Nakatani S, Umemura S, Kimura K, Kitakaze M. Usefulness of plasma B-type natriuretic peptide in the assessment of disease severity and prediction of outcome after aortic valve replacement in patients with severe aortic stenosis. J. Am. Soc. Echocardiogr. 2011;24:984–991. doi: 10.1016/j.echo.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Capoulade R, et al. Prognostic value of plasma B-type natriuretic peptide levels after exercise in patients with severe asymptomatic aortic stenosis. Heart (British Cardiac Society) 2014;100:1606–1612. doi: 10.1136/heartjnl-2014-305729. [DOI] [PubMed] [Google Scholar]
- 17.Dahou A, et al. B-type natriuretic peptide and high-sensitivity cardiac troponin for risk stratification in low-flow, low-gradient aortic stenosis: A substudy of the TOPAS study. JACC Cardiovasc. Imaging. 2018;11:939–947. doi: 10.1016/j.jcmg.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Minamino-Muta E, et al. Causes of death in patients with severe aortic stenosis: An observational study. Sci. Rep. 2017;7:14723. doi: 10.1038/s41598-017-15316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thourani VH, et al. Outcomes in 937 intermediate-risk patients undergoing surgical aortic valve replacement in PARTNER-2A. Ann. Thorac. Surg. 2018;105:1322–1329. doi: 10.1016/j.athoracsur.2017.10.062. [DOI] [PubMed] [Google Scholar]
- 20.Bahler RC, et al. Predicting outcomes in patients with asymptomatic moderate to severe aortic stenosis. Am. J. Cardiol. 2018;122:851–858. doi: 10.1016/j.amjcard.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Clavel MA, Magne J, Pibarot P. Low-gradient aortic stenosis. Eur. Heart J. 2016;37:2645–2657. doi: 10.1093/eurheartj/ehw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahlmann E, et al. Small aortic root in aortic valve stenosis: Clinical characteristics and prognostic implications. Eur. Heart J. Cardiovasc. Imaging. 2017;18:404–412. doi: 10.1093/ehjci/jew159. [DOI] [PubMed] [Google Scholar]
- 23.Beck KS, Kim JA, Choe YH, Hian SK, Hoe J, Hong YJ, et al. 2017 Multimodality appropriate use criteria for noninvasive cardiac imaging: Expert consensus of the Asian Society of Cardiovascular Imaging. Cardiovasc. Imaging Asia. 2017;1:156–165. doi: 10.22468/cvia.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YJ, et al. Korean guidelines for the appropriate use of cardiac CT. Korean J. Radiol. 2015;16:251–285. doi: 10.3348/kjr.2015.16.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai IC, et al. ASCI 2010 appropriateness criteria for cardiac computed tomography: A report of the Asian Society of Cardiovascular Imaging Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging Guideline Working Group. Int. J. Cardiovasc. Imaging. 2010;26(Suppl 1):1–15. doi: 10.1007/s10554-009-9577-4. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura RA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017 doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell C, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 28.de Simone G, et al. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999;33:800–805. doi: 10.1161/01.HYP.33.3.800. [DOI] [PubMed] [Google Scholar]
- 29.Briand M, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J. Am. Coll. Cardiol. 2005;46:291–298. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 30.Agatston AS, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 31.Clavel MA, et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler echocardiographic and computed tomographic study. J. Am. Coll. Cardiol. 2013;62:2329–2338. doi: 10.1016/j.jacc.2013.08.1621. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai S, et al. Brain natriuretic peptide facilitates severity classification of stable chronic heart failure with left ventricular dysfunction. Heart (British Cardiac Society) 2003;89:661–662. doi: 10.1136/heart.89.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used during the current study are available from the corresponding author upon reasonable request.