Abstract
Nosocomial pneumonia caused by carbapenem-resistant gram-negative bacteria (CRGNB) is a growing threat due to the limited therapeutic choices and high mortality rate. The aim of this study was to evaluate the prognostic factors for mortality in patients with nosocomial pneumonia caused by CRGNB and the impact of colistin-based therapy on the outcomes of intensive care unit (ICU) patients. We conducted a retrospective study of the ICUs in five tertiary teaching hospitals in Taiwan. Patients with nosocomial pneumonia caused by CRGNB from January 2016 to December 2016 were included. Prognostic factors for mortality were analyzed using multivariate logistic regression. The influence of colistin-based therapy on mortality and clinical and microbiological outcomes were evaluated in subgroups using different severity stratification criteria. A total of 690 patients were enrolled in the study, with an in-hospital mortality of 46.1%. The most common CRGNB pathogens were Acinetobacter baumannii (78.7%) and Pseudomonas aeruginosa (13.0%). Significant predictors (odds ratio and 95% confidence interval) of mortality from multivariate analysis were a length of hospital stay (LOS) prior to pneumonia of longer than 9 days (2.18, 1.53–3.10), a sequential organ failure assessment (SOFA) score of more than 7 (2.36, 1.65–3.37), supportive care with vasopressor therapy (3.21, 2.26–4.56), and escalation of antimicrobial therapy (0.71, 0.50–0.99). There were no significant differences between the colistin-based therapy in the deceased and survival groups (42.1% vs. 42.7%, p = 0.873). In the subgroup analysis, patients with multiple organ involvement (> 2 organs) or higher SOFA score (> 7) receiving colistin-based therapy had better survival outcomes. Prolonged LOS prior to pneumonia onset, high SOFA score, vasopressor requirement, and timely escalation of antimicrobial therapy were predictors for mortality in critically ill patients with nosocomial CRGNB pneumonia. Colistin-based therapy was associated with better survival outcomes in subgroups of patients with a SOFA score of more than 7 and multiple organ involvement.
Subject terms: Bacterial infection, Antimicrobial therapy
Introduction
Nosocomial pneumonia is one of the most common hospital-acquired infections and is associated with significant morbidity and mortality in critically ill patients1. Carbapenem-resistant gram-negative bacteria (CRGNB), including Acinetobacter baumannii (CRAB), Pseudomonas aeruginosa (CRPA), and Enterobacteriaceae (CRE), have become an increasingly significant cause of nosocomial pneumonia in many countries2,3. When comparing with carbapenem-susceptible gram-negative bacteria (CSGNB), infections caused by CRGNB are associated with more than 2 times the risk of death, both globally and regionally in Taiwan4–6. In previous studies, prognostic factors for mortality in patients with nosocomial pneumonia such as presence of septic shock, high sequential organ failure assessment (SOFA) score, and inappropriate empiric antimicrobial therapy have been reported7,8; however, there is still minimal data to discuss nosocomial CRGNB pneumonia in intensive care units (ICU).
Due to the minimal effectiveness of antimicrobial agents, nosocomial pneumonia caused by CRGNB presents a major challenge to delivering adequate therapy and is associated with high mortality, ranging from 41 to 53%9,10. Despite the recent development of novel antimicrobial agents11, polymyxins, most commonly colistin (polymyxin E), remain the most important and frequently used agents against CRGNB12. Several issues have been raised around colistin-based therapy to enhance its activity and efficacy, including loading dose13,14, maintenance dose15, combination therapy16,17, and adjunctive inhalation therapy18,19. While colistin-based therapy is considered the backbone of the antimicrobial therapy against CRGNB, there is still controversy regarding its mortality benefit over other antimicrobial agents10,20,21.
The association of colistin-based therapy with improved survival and its influence on different severity stratification criteria remains to be clarified. The aim of this study was to evaluate the prognostic factors associated with in-hospital mortality in critically ill patients with nosocomial CRGNB pneumonia and the impact of colistin-based therapy on patient outcomes.
Methods
Study design
We conducted a retrospective multicenter study of the ICUs in five tertiary teaching hospitals in Taiwan. All consecutive patients who were admitted to the ICU with microbiologically documented nosocomial pneumonia caused by CRGNB between January 2016 and December 2016. Chart review was performed. Patients were excluded if (1) they were younger than 20 years of age, (2) they had lung cancer with post-obstructive pneumonia, (3) they had human immunodeficiency virus infection with a CD4 cell count of less than 200 cells/mm3, (4) they did not receive any antimicrobial therapy with curative intent.
Data collection technique
The data were collected from the medical records of patients, which contained demographic data (age, gender, smoking status, alcohol consumption, source of admission, and type of ICU and supportive care), comorbidities (malignancy, liver disease, including chronic hepatitis or cirrhosis, heart failure, hypertension, cerebral vascular disease, neurodegenerative disorder, chronic kidney disease, chronic lung disease, diabetes mellitus, immunocompromised due to long-term use of steroid or other immunosuppressants, autoimmune disease), clinical manifestations, laboratory findings, severity of illness on the day of pneumonia diagnosis evaluated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score and SOFA score, microbiological profile, antimicrobial therapy, and outcome evaluation.
Semi-quantitative and quantitative cultures were obtained either from bronchoalveolar lavage, endotracheal aspirate, or qualified sputum specimens. Antimicrobial susceptibility tests were performed using either the VITEK® 2 system (bioMérieux, Marcy-l'Etoile, France) or the BD Phoenix™ system (Becton Dickinson Diagnostic Systems, Sparks, MD, USA). Minimum inhibitory concentration (MIC) breakpoints were established based on the Clinical Laboratory and Standards Institute guidelines22. All patients received antimicrobial therapy with curative intent. Information about antimicrobial therapy was recorded only if the prescription duration was longer than or equal to 3 days.
Definitions
Carbapenem-Resistant Gram-Negative Bacteria was defined as A. baumannii, P. aeruginosa, or Enterobacteriaceae with resistance to any carbapenem (intermediately susceptible results were reported as resistant). Pneumonia was considered nosocomial when onset occurred > 48 h after hospitalization. The diagnosis required radiographic images of a new and persistent pulmonary infiltrate and at least one of the following criteria: body temperature > 38 °C or < 36 °C; leukocytosis > 12,000 cells/mm3, leukopenia < 4000 cells/mm3 or band form > 10%; cough or purulent sputum. Nosocomial pneumonia was considered to be ventilator-associated when it occurred > 48 h after the initiation of mechanical ventilation.
Escalation of therapy was defined as a switch to or addition of any antimicrobial agent with additional coverage after definitive identification of CRGNB. Definitive therapy was defined as antimicrobial therapy that was continued or initiated after antimicrobial susceptibilities were available. Due to the possibility of in vivo activity of carbapenem despite in vitro resistance, we did not adopt the term “inappropriateness of empirical therapy,” which has been well defined and discussed in other publications.
Clinical outcome was classified into three categories: cure (complete resolution of symptoms and signs with no further requirement for antimicrobial therapy), improvement (partial resolution of symptoms and signs with requirement for antimicrobial therapy), and failure (persistence of symptoms and signs or death). Cure and improvement were considered as effective therapeutic outcomes. Microbiological outcome was classified into four categories: eradication (absence of the baseline pathogen in at least two sets of respiratory specimens), persistence (persistence of the baseline pathogen in respiratory specimens), recurrence (re-detection of the baseline pathogen in respiratory specimens within 14 days of eradication), and undetermined (culture of respiratory specimens not available during follow-up).
Outcome evaluation
We used in-hospital mortality of any cause as the main outcome measure in the study. Other outcome measures included 90-day mortality, clinical cure/improvement, and microbiological eradication at day 14 following pneumonia diagnosis.
Statistical analysis
Normally- and non-normally distributed continuous data were expressed as mean with standard deviation (SD) and median with interquartile range (IQR), respectively. Categorical variables were reported as number (%). Student’s t-test was used for analysis of continuous variables, and the Chi-square (χ2) test was used for categorical variables. Multivariate analysis using multiple logistic regression was performed to evaluate the prognostic factors associated with in-hospital mortality. Multicollinearity was measured by variance inflation factors, and the fitness of the model was assessed using Hosmer and Lemeshow test. To explore the pattern of organ involvement, k-means clustering was used to derive subtypes based upon individual SOFA subscore data. Internal validation was performed by assessing the silhouette width and Davies-Bouldin score.
We used logistic regression to evaluate the influence of colistin-based therapy on in-hospital mortality and clinical and microbiological outcomes among subgroups with different severity stratification criteria. Kaplan–Meier statistics were used to estimate 90-day mortality of the entire study population first stratified by subgroups followed by colistin-based therapy assignment. Statistical analysis was considered to be significant when the p value was < 0.05. All statistical analyses and visualization were performed using MedCalc Statistical Software version 19.0.7 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019) and Python packages Panda, NumPy, Matplotlib, and Scikit-learn (Python Software Foundation).
Ethics approval and consent to participate
The study was approved by the ethics committee of each participating institution (Institutional Review Board [IRB] of Taipei Veterans General Hospital, IRB number: 2018-03-001CC; IRB of Tri-Service General Hospital, IRB number: 1-107-05-054; IRB of China Medical University Hospital, IRB number: CMUH107-REC3-052; IRB of Taichung Veterans General Hospital, IRB number: IRB CE18100A; IRB of Kaohsiung Medical University Hospital, IRB number: KMUHIRB-E(I)-20180141). Informed consent was waived because of the retrospective nature of the study. The study was performed in accordance with the Declaration of Helsinki concerning the ethical principles for medical research.
Results
Demographic characteristics
Of the 690 critically ill patients with nosocomial pneumonia caused by CRGNB, 458 were male and 232 were female, with a median age of 73.7 years. The most common comorbidity was hypertension (54.3%), followed by diabetes mellitus (35.9%), chronic lung disease (18.4%), chronic kidney disease (16.4%), and cerebral vascular disease (16.2%). With respect to pathogen identified in the study, 543 (78.7%) were A. baumannii, 90 (13%) were P. aeruginosa, 41 (5.9%) were Klebsiella pneumoniae, and 16 (2.3%) were Enterobacter species. The median length of stay (LOS) in the hospital prior to pneumonia onset was 13 days (IQR, 6–26 days). The median APACHE II and SOFA score on the day of pneumonia diagnosis were 22.0 (IQR, 17.0–27.0) and 7.0 (IQR, 5.0–10.0), respectively. Regarding the type of supportive care, most patients received mechanical ventilation (n = 650, 94.2%) and about half of the patients required vasopressor therapy (n = 320, 46.4%). A total of 293 (42.5%) patients received definitive therapy with colistin, of which only 46 (15.7%) patients received a combination of intravenous and inhaled forms.
Prognostic factors for in-hospital mortality
Table 1 summarizes the comparison of demographic characteristics for patient mortality and survival. The overall in-hospital mortality was 46.1%. When compared with surviving patients, the deceased had a higher incidence of liver disease (11.3% vs. 7.0%, p = 0.048), chronic kidney disease (19.5% vs. 13.7%, p = 0.041), vasopressor therapy (65.7% vs. 29.8%, p < 0.001), continuous renal replacement therapy (24.8% vs. 9.7%, p < 0.001), longer LOS prior to pneumonia onset (17.0 days vs. 10.0 days, p < 0.001), and higher SOFA score (9.0 vs. 6.0, p < 0.001). Furthermore, smoking habits (13.8% vs. 20.7%, p = 0.018), cerebral vascular disease (13.2% vs. 18.8%, p = 0.047), and escalation of therapy (43.1% vs. 51.9%, p = 0.021) were significantly less frequent among the patients with mortality than the surviving patients. Overall, the multivariate analysis showed that in-hospital mortality was significantly associated with a LOS longer than 9 days prior to pneumonia onset (OR 2.18, 95% confidence interval (CI) 1.53–3.10, p < 0.001), a SOFA score on day of pneumonia more than 7 (OR 2.36, 95% CI 1.65–3.37, p < 0.001), supportive care with vasopressor therapy (OR 3.21, 95% CI 2.26–4.56, p < 0.001), and escalation of antimicrobial therapy (OR 0.71, 95% CI 0.50–0.99, p = 0.045), as depicted in Table 2.
Table 1.
Demographic characteristics of the 690 analyzed patients according to in-hospital mortality.
| Total (n = 690) | Mortality (n = 318) | Survival (n = 372) | p value | |
|---|---|---|---|---|
| Age, median (IQR), y | 73.7 (61.4–83.5) | 73.7 (62.1–85.1) | 73.6 (60.8–82.5) | 0.142 |
| Male, No. (%) | 458 (66.4) | 212 (66.7) | 246 (66.1) | 0.882 |
| Body mass index, median (IQR), kg/m2 | 23.0 (20.1–26.2) | 22.5 (20.0–25.8) | 23.4 (20.3–26.3) | 0.061 |
| Personal history, No. (%) | ||||
| Smoking habit | 121 (17.5) | 44 (13.8) | 77 (20.7) | 0.018 |
| Alcohol consumption | 131 (19.0) | 58 (18.2) | 73 (19.6) | 0.644 |
| Comorbidities, No. (%) | ||||
| Malignancy | 93 (13.5) | 50 (15.7) | 43 (11.6) | 0.111 |
| Liver disease | 62 (9.0) | 36 (11.3) | 26 (7.0) | 0.048 |
| Heart failure | 82 (11.9) | 42 (13.2) | 40 (10.8) | 0.321 |
| Hypertension | 375 (54.3) | 167 (52.5) | 208 (55.9) | 0.372 |
| Cerebral vascular disease | 112 (16.2) | 42 (13.2) | 70 (18.8) | 0.047 |
| Chronic kidney disease | 113 (16.4) | 62 (19.5) | 51 (13.7) | 0.041 |
| Chronic lung disease | 127 (18.4) | 63 (19.8) | 64 (17.2) | 0.379 |
| Diabetes mellitus | 248 (35.9) | 107 (33.6) | 141 (37.9) | 0.246 |
| Ventilator-associated, No. (%) | 484 (70.1) | 220 (69.2) | 264 (71.0) | 0.610 |
| Type of ICU, No. (%) | 0.164 | |||
| Medical | 441 (63.9) | 215 (67.6) | 226 (60.8) | |
| Cardiovascular | 34 (4.9) | 15 (4.7) | 19 (5.1) | |
| Surgical | 215 (31.2) | 88 (27.7) | 127 (34.1) | |
| LOS prior to pneumonia onset, median (IQR), days | 13.0 (6.0–26.0) | 17.0 (8.0–32.0) | 10.0 (5.0–20.0) | < 0.001 |
| Microbiological organisms, No. (%) | 0.579 | |||
| Acinetobacter baumannii | 543 (78.7) | 249 (78.3) | 294 (79.0) | |
| Pseudomonas aeruginosa | 90 (13.0) | 43 (13.5) | 47 (12.6) | |
| Klebsiella pneumoniae | 41 (5.9) | 21 (6.6) | 20 (5.4) | |
| Enterobacter species | 16 (2.3) | 5 (1.6) | 11 (3.0) | |
| Severity score, median (IQR) | ||||
| APACHE II score | 22.0 (17.0–27.0) | 23.0 (17.0–28.0) | 22.0 (17.0–27.0) | 0.194 |
| SOFA score | 7.0 (5.0–10.0) | 9.0 (7.0–11.0) | 6.0 (4.0–9.0) | < 0.001 |
| Supportive care, No. (%) | ||||
| Vasopressor therapy | 320 (46.4) | 209 (65.7) | 111 (29.8) | < 0.001 |
| Mechanical ventilation | 650 (94.2) | 296 (93.1) | 354 (95.2) | 0.244 |
| Hemodialysis | 103 (14.9) | 49 (15.4) | 54 (14.5) | 0.743 |
| Continuous renal replacement therapy | 115 (16.7) | 79 (24.8) | 36 (9.7) | < 0.001 |
| Extracorporeal membrane oxygenation | 16 (2.3) | 11 (3.5) | 5 (1.3) | 0.066 |
| Antimicrobial therapy, No. (%) | ||||
| Escalation of therapy, No. (%) | 330 (47.8) | 137 (43.1) | 193 (51.9) | 0.021 |
| Definitive therapy with colistin, No. (%) | 293 (42.5) | 134 (42.1) | 159 (42.7) | 0.873 |
| Intravenous alone | 96 (13.9) | 51 (16.0) | 45 (12.1) | 0.136 |
| Inhaled alone | 151 (21.9) | 61 (19.2) | 90 (24.2) | 0.113 |
| Combination | 46 (6.7) | 22 (6.9) | 24 (6.5) | 0.807 |
APACHE II Acute Physiology and Chronic Health Evaluation II, ICU intensive care unit, IQR interquartile range, LOS length of stay, SOFA Sequential Organ Failure Assessment.
Table 2.
Multivariate analysis of prognostic factors for in-hospital mortality.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Smoking habit | 0.62 (0.41–0.92) | 0.019 | ||
| Comorbidities | ||||
| Liver disease | 1.70 (1.00–2.88) | 0.049 | ||
| Cerebral vascular disease | 0.66 (0.43–1.00) | 0.047 | ||
| Chronic kidney disease | 1.52 (1.02–2.29) | 0.042 | ||
| LOS prior to pneumonia onset > 9 days | 2.39 (1.74–3.28) | < 0.001 | 2.18 (1.53–3.10) | < 0.001 |
| SOFA score on day of pneumonia > 7 | 3.52 (2.57–4.82) | < 0.001 | 2.36 (1.65–3.37) | < 0.001 |
| Supportive care | ||||
| Vasopressor therapy | 4.51 (3.27–6.21) | < 0.001 | 3.21 (2.26–4.56) | < 0.001 |
| Continuous renal replacement therapy | 3.09 (2.01–4.73) | < 0.001 | ||
| Escalation of therapy | 0.70 (0.52–0.95) | 0.021 | 0.71 (0.50–0.99) | 0.045 |
CI confidence interval, LOS length of stay, SOFA Sequential Organ Failure Assessment.
The pattern of organ involvement
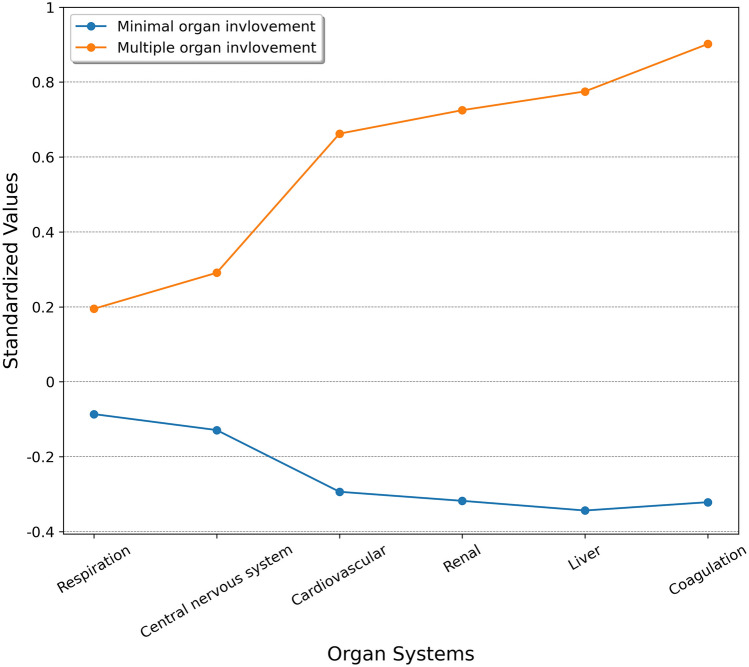
All patients had complete SOFA subscore data and were included in the analysis. The evaluation of k-means clustering performance suggested that a two-cluster model was the best option (Additional file 1: eFigure 1). As shown in Fig. 1, patients in cluster 1 had more organ dysfunction (more coagulation and liver, cardiovascular, and renal dysfunction) compared with patients in cluster 2. Therefore, we denoted the clusters as the multiple organ involvement subtype (cluster 1) and the minimal organ involvement subtype (cluster 2). Cluster 1 had higher in-hospital mortality, lower clinical cure, and lower microbiological eradication rate at day 14 than cluster 2. The detailed SOFA subscore data and outcome evaluation of the two clusters are presented in Table 3.
Figure 1.
Differences in standardized values of each SOFA subscore by subtype. The y-axis represents standardized values, in which all means were scaled to 0 and standard deviations to 1. SOFA Sequential Organ Failure Assessment.
Table 3.
SOFA subscore data and clinical outcomes of the two clusters.
| Cluster 1: Multiple organ involvement (n = 212) | Cluster 2: Minimal organ involvement (n = 478) | p value | |
|---|---|---|---|
| SOFA score, mean (SD) | 12.0425 (2.7577) | 5.9435 (2.2070) | < 0.001 |
| Respiration, mean (SD) | 1.7547 (1.3085) | 1.3891 (1.2834) | < 0.001 |
| Coagulation, mean (SD) | 1.9434 (1.1547) | 0.4665 (0.7756) | < 0.001 |
| Liver, mean (SD) | 1.2736 (1.3942) | 0.1464 (0.4664) | < 0.001 |
| Central nervous system, mean (SD) | 3.1321 (0.8662) | 2.7176 (1.0124) | < 0.001 |
| Cardiovascular, mean (SD) | 1.6179 (1.5517) | 0.4414 (0.8366) | < 0.001 |
| Renal, mean (SD) | 2.3208 (1.5117) | 0.7824 (1.1775) | < 0.001 |
| Outcomes | |||
| In-hospital mortality, No. (%) | 137 (64.6) | 181 (37.9) | < 0.001 |
| Cure/improvement at day 14, No. (%) | 99 (46.7) | 285 (59.6) | 0.002 |
| Eradication at day 14, No. (%) | 39 (18.4) | 125 (26.2) | 0.027 |
SD standard deviation, SOFA Sequential Organ Failure Assessment.
Antimicrobial therapy
Most patients received combination therapy (n = 500, 72.5%) throughout the study. A total of 330 (47.8%) patients received escalation therapy. Of the 465 antimicrobial prescriptions for escalation therapy, the most common agents were inhaled colistin (n = 126, 27.1%), followed by tigecycline (n = 82, 17.6%), intravenous colistin (n = 79, 17.0%), and cefoperazone-sulbactam (n = 48, 10.3%). Furthermore, of the 1084 antimicrobial prescriptions for definitive therapy, the most common agents were inhaled colistin (n = 197, 18.2%), followed by tigecycline (n = 144, 13.3%), intravenous colistin (n = 142, 13.1%), cefoperazone-sulbactam (n = 90, 8.3%), and meropenem (n = 90, 8.3%). Overall, a total of 293 (42.5%) patients received colistin-based therapy (including definitive or escalated therapy with either intravenous or inhaled colistin regimen), of which 181 (61.7%) patients were treated for the purpose of escalation of therapy.
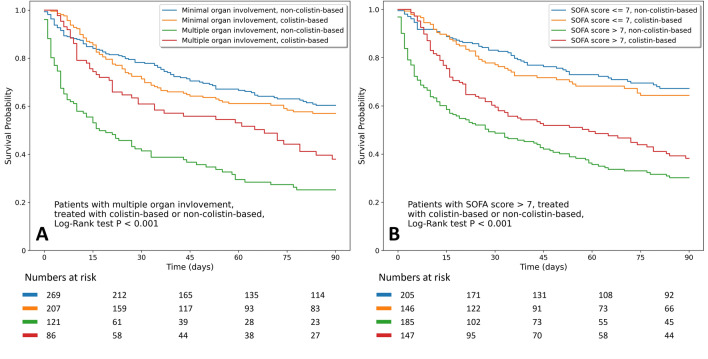
For critically ill patients with nosocomial pneumonia caused by CRGNB, colistin-based therapy did not provide a better survival benefit than non-colistin-based therapy (54.3% vs. 53.7%, p = 0.873). Colistin-based therapy showed different influence on survival outcome in the subgroup analysis, as shown in the Kaplan–Meier curve of 90-day mortality across patients with colistin-based therapy with the pattern of organ involvement (Fig. 2a) and SOFA score (Fig. 2b). Patients with multiple organ involvement and a SOFA score of more than 7 had better survival outcomes with colistin-based therapy than non-colistin-based therapy (adjusted OR 0.38, 95% CI 0.19–0.75, p = 0.006; adjusted OR 0.52, 95% CI 0.32–0.86, p = 0.011, respectively). The influence of colistin-based therapy in patients with different severity stratification criteria is depicted in Fig. 3. Similar findings were also observed regarding the clinical and microbiological outcomes (Additional file 1: eFigure 2).
Figure 2.
(a) Kaplan–Meier survival curves for 90-day mortality stratified by pattern of organ involvement (minimal and multiple) and antimicrobial therapy (colistin-based and non-colistin-based). (b) Kaplan–Meier survival curves for 90-day mortality stratified by SOFA score (> 7 and ≤ 7) and antimicrobial therapy (colistin-based and non-colistin-based). SOFA Sequential Organ Failure Assessment.
Figure 3.
Forest plot of adjusted odds ratios for in-hospital mortality in the colistin-based group versus non-colistin-based group. Multivariate analysis adjusted for age, gender, smoking habit, comorbidities with liver disease, cerebral vascular disease, chronic kidney disease, LOS prior to pneumonia onset, SOFA score, supportive care with vasopressor therapy, and continuous renal replacement therapy. CI confidence interval, LOS length of stay, SOFA Sequential Organ Failure Assessment.
Discussion
Carbapenem-Resistant Gram-Negative Bacteria are a prevalent hospital-acquired infection with poor prognosis and high mortality, especial in the ICU2,3. More data on CRGNB infections in the ICU are required to explore prognostic factors and outcomes to guide therapeutic decisions. In this multicenter ICU study of nosocomial CRGNB pneumonia, significant predictors of in-hospital mortality included LOS in hospital prior to pneumonia of longer than 9 days, a SOFA score of more than 7, supportive care with vasopressor therapy, and escalation of antimicrobial therapy. Subgroup analysis showed that patients with multiple organ involvement or a SOFA score greater than 7 had a better survival outcome when receiving colistin-based therapy.
Through nationwide surveillance in Taiwan since 2011, an increased resistance to carbapenem has been demonstrated in A. baumannii, K. pneumoniae, and P. aeruginosa from 69.8 to 75.3%, 10.5 to 44.4% and 17.7 to 22.6%, respectively23. Previous studies have demonstrated that risk factors, such as carbapenem consumption, LOS, diabetes mellitus, multiple lobar involvement, and prior exposure to antimicrobial therapy, are associated with the acquisition of CRGNB infection24–27. Moreover, the mortality rate of nosocomial pneumonia caused by CRGNB is also high. By pooling data from previous clinical studies, the mortality rate was in excess of 40%, even in the subgroup of patients receiving colistin-based combination therapy9,10. As the only prognostic factor amenable to intervention, appropriateness of empirical antimicrobial therapy has been extensively evaluated. Despite the promising results from early observational studies7,8,28, a more recent randomized control trial failed to demonstrate any benefit from empiric treatment with colistin in an attempt to improve its appropriateness for ventilator-associated pneumonia29. There are concerns regarding the potential for developing nephrotoxicity and the emergence of antimicrobial resistance following colistin treatment30,31. Considering the poor prognosis and controversial role of colistin-based therapy for nosocomial pneumonia caused by CRGNB, our study aimed to investigate prognostic factors to improve therapeutic decisions.
In this study, we present a multicenter retrospective study of 690 critically ill patients with nosocomial pneumonia caused by CRGNB. Consistent with previous studies9,10,22, about 80% of patients had late-onset nosocomial pneumonia (defined as pneumonia after five or more days of hospitalization) with the median LOS prior to pneumonia onset of 13 days. Approximately 46% of patients eventually died in hospital. Moreover, we noted that LOS in hospital following pneumonia diagnosis was prolonged, with a median of 28 days. These results reinforce the importance that while treating patients with nosocomial pneumonia caused by CRGNB, clinicians should be aware of the high mortality and economic burden. Every effort should be made to prevent nosocomial cross-transmission between patients.
Using multivariate logistic regression analysis, we identified that a LOS in hospital longer than 9 days prior to pneumonia onset, a SOFA score of more than 7, and supportive care with vasopressor therapy negatively affected the prognosis. Escalation of antimicrobial therapy positively influenced patient survival. Timely escalation of therapy is an important factor since it is currently the only intervening factor shown to improve clinical outcome.
Previous studies demonstrated that prolonged LOS prior to pneumonia onset, with a cut-off point ranging from 4 to 7 days, was associated with the risk of acquisition of potentially resistant pathogens, which resulted in increased mortality1,32,33. In our study, which focused on patients with nosocomial pneumonia caused by CRGNB, we found that a prolonged hospital stay of more than 9 days was an independent prognostic factor, regardless of comorbidity and disease severity, with a sensitivity, specificity, and area under the receiver-operation characteristics curve (AUROC) of 71.4%, 48.9%, and 0.63, respectively. This phenomenon might be explained by the fact that patients with a prolonged hospital stay tend to be frailer and have greater disease complexity, resulting in elevated mortality34.
Septic shock and multiple organ failure are considered to be common and deadly complications of pneumonia35, especially in cases caused by CRGNB8. Consistent with previous studies, we noted that a high SOFA score and vasopressor requirement were significant risk factors for mortality. The present study demonstrated that a SOFA score of more than 7 was associated with increased mortality with a sensitivity, specificity, and AUROC of 65.4%, 65.1%, and 0.70, respectively. To identify the pattern of organ involvement, we applied a k-means algorithm using SOFA subscore data. K-means is one of the most widely used clustering techniques in unsupervised learning36. Regarding the complex and heterogeneous nature of critically ill patients, phenotype identification has been well introduced in previous studies, especially in the field of sepsis37,38 and acute respiratory distress syndrome39. In our study, by using a simple algorithm and six parameters, without other clinical and biological characteristics, we identified two distinct subtypes with different disease trajectories and outcomes. This finding suggests that evaluating individual SOFA subscores rather than the total score alone could be helpful in clinical practice for severity stratification.
In the present study, escalation of therapy was recognized as the only modifiable factor to decrease in-hospital mortality. Due to the high prescription rate for escalation therapy, we further evaluated the influence of colistin-based therapy on patient outcomes. Although colistin is often considered to be a reliable option for the treatment of CRGNB infection, previous meta-analyses only showed significant effects for clinical cure and microbiological eradication but not for mortality10,20, which was similar to our findings. As different severity stratification criteria may be a factor that influences therapeutic response and clinical outcome, we further analyzed and demonstrated that colistin-based therapy was associated with better survival outcomes in patient subgroups with a SOFA score of more than 7 and multiple organ involvement. These results reinforce that while treating critically ill patients with nosocomial pneumonia in the setting of high prevalence of CRGNB, high SOFA scores and the presence of multiple organ involvement should not be ignored by clinicians. In this condition, timely escalation of colistin-based therapy may improve patient outcomes.
Our multicenter design overcomes potential single-center bias and strengthens the external validity of our findings. In addition, we studied a large critically ill population from five tertiary teaching hospitals covering different regions of Taiwan, supporting its high representative reliability. However, our study has several limitations. First, given the nature of retrospective analysis, missing data were unavoidable, and the prescription pattern of antimicrobial therapy was heterogeneous and complex. Therefore, it is impossible to compare various treatment strategies directly and to determine the most effective therapy for nosocomial pneumonia caused by CRGNB. Second, in vitro susceptibility for colistin was not tested at all the participating hospitals. In addition, reporting the upper limit values of MIC for meropenem/doripenem was not all consistent. Hence, it is impractical to assign patients based on inappropriateness of therapy and evaluate its influence on patient outcomes. Third, as an important complication of pneumonia, acute respiratory distress syndrome was not recorded and could be a confounding factor in this study. Fourth, due to lack of ground-truth reference, k-means clustering performance was only evaluated by internal validation. Further prospective studies should be performed to assess external validity and confirmation of robustness.
Conclusions
Despite advances in critical care, mortality due to nosocomial pneumonia caused by CRGNB remains high. Prolonged LOS prior to pneumonia onset, high SOFA score, vasopressor requirement, and timely escalation of antimicrobial therapy were independent prognostic factors for in-hospital mortality. Despite the lack of benefit in the entire study population, colistin-based therapy was associated with better survival outcomes in patient subgroups with a SOFA score of more than 7 and multiple organ involvement.
Supplementary Information
Acknowledgements
The authors acknowledge all of our collaborators from the T-CARE (Taiwan Critical Care and Infection) Group.
Abbreviations
- ALAT
Asociacion latinoamericana del torax
- AUROC
Area under the receiver-operation characteristics curve
- CI
Confidence interval
- CRGNB
Carbapenem-resistant gram-negative bacteria
- ERS
European Respiratory Society
- ESCMID
European Society of Clinical Microbiology and Infectious Diseases
- ESICM
European Society of Intensive Care Medicine
- ICU
Intensive care unit
- IRB
Institutional Review Board
- MIC
Minimum inhibitory concentration
- SD
Standard deviation
- VAP
Ventilator-associated pneumonia
Author contributions
C.-Y.C., S.-J.L. and Y.-C.L. conceived and designed the experiments. C.-Y.C. and Y.-C.L. analyzed the data. The project was administered by K.-Y.Y., C.-K.P., C.-C.S., M.-C.C. and Y.-C.L. and supervised by K.-Y.Y., C.-K.P., C.-C.S., M.-C.C. and Y.-C.L. C.-Y.C. and Y.-C.L. wrote the manuscript and reviewed and edited by C.-Y.C., J.-Y.F., K.-Y.Y., C.-K.P., S.-H.W., C.-C.S., C.-M.C., M.-C.C., Z.-R.Z. and Y.-C.L.
Funding
This study was financially supported by the grant from the China Medical University Hospital (Grant number: DMR-109-020, DMR-110-027).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Yu-Chao Lin, Email: cmchlyc@yahoo.com.tw.
T-CARE (Taiwan Critical Care and Infection) Group:
Chih-Yu Chen, Bing-Ru Wu, Yi-Cheng Shen, Wei-Cheng Chen, Shinn-Jye Liang, Yu-Chao Lin, Kuang-Yao Yang, Jia-Yih Feng, Chung-Kan Peng, Sheng-Huei Wang, Chau-Chyun Sheu, Chia-Min Chen, Ming-Cheng Chan, and Zhe-Rong Zheng
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11061-7.
References
- 1.Torres A, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT) Eur. Respir. J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 2.Chung DR, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am. J. Respir. Crit. Care Med. 2011;184(12):1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 3.Delle Rose D, et al. Clinical predictors and microbiology of ventilator-associated pneumonia in the intensive care unit: A retrospective analysis in six Italian hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35(9):1531–1539. doi: 10.1007/s10096-016-2694-9. [DOI] [PubMed] [Google Scholar]
- 4.Lemos EV, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin. Microbiol. Infect. 2014;20(5):416–423. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 5.Martin A, Fahrbach K, Zhao Q, Lodise T. association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to enterobacteriaceae: Results of a systematic literature review and meta-analysis. Open Forum Infect. Dis. 2018;5(7):150. doi: 10.1093/ofid/ofy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu PF, et al. High minimum inhibitory concentration of imipenem as a predictor of fatal outcome in patients with carbapenem non-susceptible Klebsiella pneumoniae. Sci. Rep. 2016;6:32665. doi: 10.1038/srep32665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnacho-Montero J, et al. Acinetobacter baumannii ventilator-associated pneumonia: Epidemiological and clinical findings. Intensive Care Med. 2005;31(5):649–655. doi: 10.1007/s00134-005-2598-0. [DOI] [PubMed] [Google Scholar]
- 8.Inchai J, et al. Prognostic factors associated with mortality of drug-resistant Acinetobacter baumannii ventilator-associated pneumonia. J. Intensive Care. 2015;3:9. doi: 10.1186/s40560-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zusman O, et al. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2017;72(1):29–39. doi: 10.1093/jac/dkw377. [DOI] [PubMed] [Google Scholar]
- 10.Lyu C, Zhang Y, Liu X, Wu J, Zhang J. Clinical efficacy and safety of polymyxins based versus non-polymyxins based therapies in the infections caused by carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. BMC Infect. Dis. 2020;20(1):296. doi: 10.1186/s12879-020-05026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2019;69(Suppl 7):S565–S575. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papst L, et al. Antibiotic treatment of infections caused by carbapenem-resistant Gram-negative bacilli: an international ESCMID cross-sectional survey among infectious diseases specialists practicing in large hospitals. Clin. Microbiol. Infect. 2018;24(10):1070–1076. doi: 10.1016/j.cmi.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Bellos I, et al. Efficacy and safety of colistin loading dose: a meta-analysis. J. Antimicrob. Chemother. 2020;75(7):1689–1698. doi: 10.1093/jac/dkaa064. [DOI] [PubMed] [Google Scholar]
- 14.Katip W, Yoodee J, Uitrakul S, Oberdorfer P. Efficacy of loading dose colistin versus carbapenems for treatment of extended spectrum beta lactamase producing Enterobacteriaceae. Sci. Rep. 2021;11(1):18. doi: 10.1038/s41598-020-78098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalfino L, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin. Infect. Dis. 2012;54(12):1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng IL, Chen YH, Lai CC, Tang HJ. Intravenous colistin monotherapy versus combination therapy against carbapenem-resistant gram-negative bacteria infections: Meta-analysis of randomized controlled trials. J. Clin. Med. 2018;7(8):208. doi: 10.3390/jcm7080208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nutman A, et al. Colistin plus meropenem for carbapenem-resistant Gram-negative infections: In vitro synergism is not associated with better clinical outcomes. Clin. Microbiol. Infect. 2020;26(9):1185–1191. doi: 10.1016/j.cmi.2020.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Valachis A, Samonis G, Kofteridis DP. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit. Care Med. 2015;43(3):527–533. doi: 10.1097/CCM.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 19.Feng JY, et al. Efficacy of adjunctive nebulized colistin in critically ill patients with nosocomial carbapenem-resistant Gram-negative bacterial pneumonia: a multi-centre observational study. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Gu WJ, Wang F, Tang L, Bakker J, Liu JC. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2014;44(6):477–485. doi: 10.1016/j.ijantimicag.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Jung SY, et al. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: A systemic review and Bayesian network meta-analysis. Crit. Care. 2017;21(1):319. doi: 10.1186/s13054-017-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Document M100-S24 in Performance standards for antimicrobial susceptibility testing. Twenty-fourth international supplement (2014).
- 23.Taiwan Centers for Disease Control. Nosocomial infections surveillance system. https://www.cdc.gov.tw/Category/MPage/4G8HuDdUN1k4xaBJhbPzKQ (2020).
- 24.Kim T, et al. Risk factors for hospital-acquired pneumonia caused by carbapenem-resistant Gram-negative bacteria in critically ill patients: A multicenter study in Korea. Diagn. Microbiol. Infect. Dis. 2014;78(4):457–461. doi: 10.1016/j.diagmicrobio.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Yang P, et al. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob. Resist. Infect. Control. 2018;7:137. doi: 10.1186/s13756-018-0430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang D, et al. Antibiotic consumption versus the prevalence of carbapenem-resistant Gram-negative bacteria at a tertiary hospital in China from 2011 to 2017. J. Infect. Public Health. 2019;12(2):195–199. doi: 10.1016/j.jiph.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Lima EM, et al. Predictive factors for sepsis by carbapenem resistant Gram-negative bacilli in adult critical patients in Rio de Janeiro: A case-case-control design in a prospective cohort study. Antimicrob. Resist. Infect. Control. 2020;9(1):132. doi: 10.1186/s13756-020-00791-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumbarello M, et al. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med. 2013;39(4):682–692. doi: 10.1007/s00134-013-2828-9. [DOI] [PubMed] [Google Scholar]
- 29.Cisneros JM, et al. Colistin versus meropenem in the empirical treatment of ventilator-associated pneumonia (Magic Bullet study): an investigator-driven, open-label, randomized, noninferiority controlled trial. Crit. Care. 2019;23(1):383. doi: 10.1186/s13054-019-2627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacobbe DR, et al. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: Results from a multicenter case-control-control study. Clin. Microbiol. Infect. 2015;21(12):1106.e1–e8. doi: 10.1016/j.cmi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Falcone M, et al. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin. Microbiol. Infect. 2016;22(5):444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Trouillet JL, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 1998;157(2):531–539. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim EH, Ward S, Sherman G, Kollef MH. A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest. 2000;117(5):1434–1442. doi: 10.1378/chest.117.5.1434. [DOI] [PubMed] [Google Scholar]
- 34.Khandelwal D, et al. Frailty is associated with longer hospital stay and increased mortality in hospitalized older patients. J. Nutr. Health Aging. 2012;16(8):732–735. doi: 10.1007/s12603-012-0369-5. [DOI] [PubMed] [Google Scholar]
- 35.Almirall J, Mesalles E, Klamburg J, Parra O, Agudo A. Prognostic factors of pneumonia requiring admission to the intensive care unit. Chest. 1995;107(2):511–516. doi: 10.1378/chest.107.2.511. [DOI] [PubMed] [Google Scholar]
- 36.Sammut C, Webb GI. Encyclopedia of Machine Learning and Data Mining. Springer; 2017. [Google Scholar]
- 37.Knox DB, Lanspa MJ, Kuttler KG, Brewer SC, Brown SM. Phenotypic clusters within sepsis-associated multiple organ dysfunction syndrome. Intensive Care Med. 2015;41(5):814–822. doi: 10.1007/s00134-015-3764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seymour CW, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson JG, Calfee CS. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24(1):102. doi: 10.1186/s13054-020-2778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.