Summary
Sepsis is defined as a dysregulated host-response to infection, across all ages and pathogens. What defines a dysregulated state remains intensively researched but incompletely understood. Here, we dissect the meaning of this definition and its importance for the diagnosis and management of sepsis. We deliberate on pathophysiological features and dogmas that range from cytokine storms and immune paralysis to dormancy and altered homeostasis setpoints. Mathematical reasoning, used to test for plausibility, reveals three interlinked cardinal rules governing host-response trajectories in sepsis. Rule one highlights that the amplitude of the immune response while important is not sufficient and is strictly dependent on rule two, specifying bioenergetic capacity and are together dynamically driven by rule three, delineating stability and alterations in setpoints. We consider these rules and associated pathophysiological parameters for guiding data-science and artificial intelligence mining of multi-omics and big-data for improving the precision of diagnostic and therapeutic approaches to sepsis.
Funding
PG funded by the European Regional Development Fund and Welsh Government (Ser Cymru programme – Project Sepsis).
Keywords: Sepsis, Host-response, Cytokine storm, Immunosuppression, Inflammation, Infection, Immune-paralysis, Homeostasis, Setpoint, Non-resolving, Dysregulation, Mathematical model, Dormancy, Artificial intelligence, Big-data, Multi-omics, Genomics, Innate-immune, Hyperinflammatory, Immuno-metabolism
Introduction
Since the time of Hippocrates definitions of sepsis have been discussed, concurring that it is caused by infection. Yet even in the late 1890’s the medical profession had remarked on the infrequent occurrence of detecting an offending microbe from culture tests making diagnosis and treatment imprecise.1 Clinically, this situation has not changed.
The definition for sepsis advanced to “the pathophysiological alterations and life-threatening consequences of microorganisms or their products invading the blood stream from a focus of infection; resulting in an initial multi-organ insufficiency and progressing to severe multiple system organ failure”.2 About 30 years ago, an intensivist, Roger Bone, suggested adding to this definition a clause, “along with the organism's reaction against this invasion”.2,3 This insightful modified definition has essentially remained unchanged except for a greater emphasis on the host response and organ failure over the invading microbe. The most current consensus definition of adult sepsis is “a life‐threatening organ dysfunction caused by a dysregulated host response to infection”.4,5 However, clinical management of sepsis varies in different age populations, especially for neonates and children, a high-risk age group accounting for 4 million global deaths due to sepsis.6,7 Notably, the variability of physiology and organ system maturation from birth to adolescence leads to challenges in the identification of sepsis using tools based on the current definition.8,14 In children, guidance for managing sepsis takes a pragmatic approach using in part the IPSCC 2005 consensus criteria for Systemic Inflammatory Response Syndrome (SIRS) and presence of suspected or confirmed infection5 as well as considering organ function.6, 7, 8 A recent systematic review for sepsis criteria in children has highlighted organ function and notably metabolic failure as relevant paediatric markers of sepsis.9 For neonates, there remains a lack of a consensus definition of sepsis10 and often a combination of risk factors, clinical signs and laboratory values have been suggested for identifying patients with clinical sepsis.11
Nevertheless, across all ages the following causal pathway for developing the condition emerges from these consensus definitions: upon or during an infection (of any kind) the host response deviates from a normal healthy response to infection and explicitly, develops a fulminant dysregulated state. The extent of dysregulation, or uncontrollability, of the host response consequently inflicts multi-system damage that is unsustainable for maintaining tissue and organ function, leading to a state of shock that becomes life-threatening as consequence of vital organ failure. Hence it is neither the infective agent, nor the usual relatively benign inflammatory condition of infection that underlies sepsis, but rather a pernicious host-response affecting multiple systems, organs, and pathways. In particular, the cardiovascular, gastrointestinal, neuronal systems and their associated organs, and critically metabolic and coagulation pathways.12, 13, 14, 15, 16 It is also implicit that an individual's state of frailty and immune health, comprising the very young and old and those compromised either through iatrogenic or natural causes would have a marked impact on the susceptibility of sepsis.
These implicit and explicit axioms remain largely consistent with current investigations and clinical observations but lack biological mechanism. A gap in mechanistic understanding opens the door for molecular dogmas to be established without formal verification or rejection. Dogmas surrounding sepsis also risk conceptual oversimplification of the inherent multifactorial complexity driving the maladapted systemic and organ system pathway dynamics and may have negative consequences toward our understanding. Despite dogmas the consensus definitions of sepsis have greatly helped toward stimulating considerable research effort to identify the molecular, cellular, and pathophysiological changes that occur in sepsis, including from the most recent genomic and multi-‘omic’ revolution. Detailed and insightful reviews on the molecular and cellular pathophysiology and challenges in sepsis research have been published elsewhere and quoted throughout as points of reference.
We shall concern ourselves with the first principles of our understanding of how sepsis evolves. These first principles must account for the known empirical gains in outcome from early recognition and antibiotic intervention, and vaccination, and the prospect for reversibility of the condition. We seek to critically describe the underlying dogmas and assumptions that have arisen from these definitions (Figure 1) through applying mathematical reasoning supported by evidence from experimental and clinical studies. The dogmas and phenomena we deliberate on are cytokine storms (inclusive of genomic storms), endotoxin tolerance, non-resolving infection, immune paralysis, setpoint changes in homeostasis, inclusive of dormancy, as well as hyper-inflammatory and immune suppressed states.
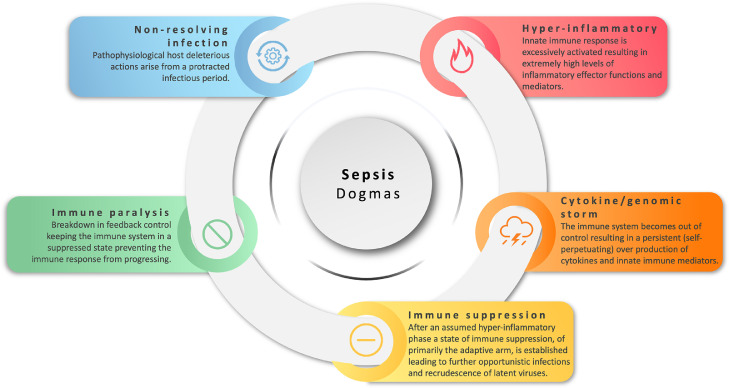
Figure 1.
Hallmark of molecular dogmas in sepsis. The illustration depicts the various dogmas surrounding sepsis.
Sepsis: reconciling past and present dogmas with emerging pathophysiological features
In this section, we outline the molecular dogmas (summarised in Figure 1) and the key pathophysiological features (summarised in Figure 2) of sepsis with a critical eye on whether the dogmas are biologically plausible and if pathophysiological features can be informative for providing early warning signals.
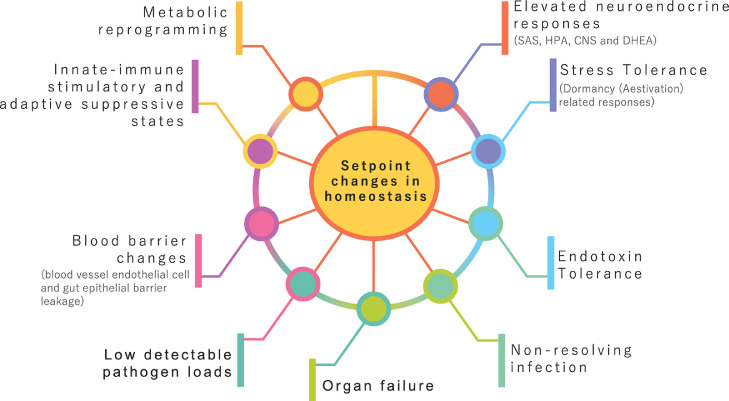
Figure 2.
Systemic pathophysiological characteristics of sepsis. Maladapted setpoint changes underlie the complex interplay of multiple systems in sepsis.
The dysregulation of the host response driving sepsis has been considered for over 40 years to be a persistent and overwhelming hyper-inflammatory state.17,18 In this scenario, the innate immune system is thought to be caught in a self-perpetuating cytokine activation mechanism. This mechanism for sepsis was readily accepted and by the late 1990s adopted the name of “a cytokine storm”, a name initially evoked not from the infectious disease community but from the field of transplantation medicine that later discounted such terminology19,20 (Figure 1). It is perceived that in such a maelstrom, the immune system becomes uncontrollably impaired in terms of both defending against infection, and in protecting the host from immune damage, adding at that time to an emerging clinical understanding of sepsis.3 In contrast to transplantation medicine, the concept of a cytokine storm rapidly became accepted dogma and has even recently re-emerged in the guise of a “genomic storm” from gene-expression profiling of leukocytes in critical trauma and bacterial sepsis21,22 (Figs. 1 and 2). This concept persists even today, contrary to both experimental and human studies, that show highly variable inflammatory responses but not necessarily hyper-inflammatory levels of cytokines. Yet attempts are made to explain this inconsistency, for example, by suggesting these lower levels are due to the short-half-lives of cytokines.23 Kinetically such argumentation is flawed as the half-life is a product of the rate of production and rate of decay and therefore, either the rate of production is low, or decay rate is high, (or both), either way you do not arrive in a position of excessively high levels of cytokines that are out of control (“storm”). The fact remains that most sepsis patients including children have high variability with a few that show extremely high levels of cytokines.24, 25, 26
Moreover, there are many experimental and clinical human studies, from both in vitro and in vivo investigations, spanning many decades of research, that have repeatedly demonstrated against the possibility for the occurrence of a hyper-inflammatory storm arising from continuously repeated activation. These studies go as far back as 194627 and they all show quite the opposite biological response, which is a non-responsiveness or hypo-inflammatory state induced after initial activation and which, led to many decades before the “cytokine-storm” dogma to the term of “endotoxin tolerance”28, 29, 30 (Figure 2).
So, if not a storm, then what is observable? Figure 2 shows the key characteristic immune features of the systemic host response in sepsis, across all ages, from pre-term infants to adults. This includes a heightened yet regulated innate immune response involving negative and positive feedback loops, and a predominately suppressed adaptive (T- and B-cell) immune arm.31,32 Further, there is also a marked catabolic change in metabolism.33,34 It should be noted, however, that in severe trauma, a very similar profile is also observed that is also characterized by immunosuppression, primarily involving the adaptive immune system with T lymphocyte populations being the most markedly affected cell population.35 Therefore, these pathophysiological features suggest this characteristic is an intrinsically driven regulated, rather than unregulated, consequence of the immune response following an excessively acute challenge. Moreover, the relationship between trauma and metabolic response is also well documented, including paediatric patients.36,37 An elegant paediatric study showed that mitochondrial respiration is lower in children with signs of immunosuppressive phenotype and inversely related to the systemic inflammatory response.38 At a physiological level the body responds to trauma with tachycardia (increase in heart rate), an increase oxygen consumption, an increased in respiratory rate and negative nitrogen balance; these are all signs of catabolism and sepsis.39,40 Nevertheless, over the last decade a new dogma of ‘immune paralysis’ (Figure 1) has emerged to account for the observed sustained suppression of the immune system following or at the same time as the inflammatory activation phase of a severe infection.41 A paralysis mechanism would require a lesion or breakdown in feedback after initial activation.
There is good clinical and experiential evidence to show a state of suppression in the adaptive arm of the immune system, as functionally evidenced by the increase in reactivation of latent herpes viruses in adult septic patients.42,43 Many studies show that not only stimulatory but also inhibitory responses occur very early and indeed are observable at the same time.14,22,44 In neonatal sepsis, it was shown that for every activator of innate immunity the corresponding cognate inhibitor was also increased.14 The innate immune system communicates directly with the adaptive arm through bi-directional signalling via the immunological synapse, an exchange that is tightly regulated by co-stimulators or co-inhibitors and is termed immune checkpoint control.45 Thus, the immune system response in sepsis exhibits multiple states of immunosuppression spanning a wide range of innate and adaptive processes. In particular, with co-inhibitory factors for the expansion of regulatory T cells and myeloid‐derived suppressor cell populations, and down‐regulation of co-stimulatory molecules and HLA‐DR‐mediated activation pathways.14,34,35,41,46 These altered expression levels are not exclusive to adult sepsis cases and in pre-term infants, the same immune phenotypic changes have also been observed, including more specifically to this age group increased levels of non-classical HLAs, in particular immune suppressive HLA-G are observed.14 Accordingly, immune checkpoint control plays a vital role in the balance of resistance and the resolution of an immune response, as well as in the homeostasis of tolerance.47 This certainly raises questions about the balance between innate and adaptive effectiveness of the host response and metabolism toward defending against infection. Mechanistically these regulatory changes do not indicate paralysis or an uncontrolled immune system. On the contrary, these features point to a regulated host response as part of an acute feedback reaction to an insult. Thus, therapeutic intervention moderating the type of immune reaction from a challenge, by targeting in particular, immune checkpoint inhibitors as well as reducing complement activation levels, may hold future promise for the management of sepsis.48
Of course, mild immunosuppression can be protective helping the host to tolerate an infection but too much will impact on host resistance to infection. From an evolutionary perspective, a central tenet of infection biology is the fine balance between host-defensive (resistance) and host-protective (tolerance) pathways.49,50 (Figure 2). In agreement with an impaired defence against infection in sepsis, several studies have pointed out the high numbers of unresolved opportunistic infections associated with patients who die.51, 52, 53 Hence, the immune response in sepsis may functionally be regulated toward a tolerance response that is intended to be host protective but consequentially results in reduced resistance to infection and a prolonged non-resolving state (Figs. 1 and 2). Again, in this scenario changes in the tipping point of the regulatory balance between tolerance and resistance does not necessarily imply a state of immune paralysis. It has been argued from a clinical perspective that the host deleterious actions arise from the observed protracted non-resolving infection that ultimately leads to organ dysfunction.54 Critically a non-resolving response retains the ability to switch to a resolving phase and hence cannot be unregulated or out of control.
Therefore, while immune suppressive pathways contribute toward host tolerance to infection, sustained depression would reduce host resistance and consequently any change in equilibrium between these suppressive and activation pathways would reflect changes in the homeostatic setpoint of the system50,55, 56, 57 (Figure 2). In a resolving infection, the immune setpoint normalises pathogen resistant and host protective pathways such that the response is balanced towards minimising unwanted immune reactions and damage while preventing pathogen growth. Vaccination also selectively changes the immune setpoint to a specific pathogen through the rapid deployment of precision targeted host-defensive pathways. So, can we better understand the meaning of a dysregulated host response through alterations in normal setpoints of homeostasis. In sepsis, shifts in normal homeostatic setpoints was first put forward from a systems biology investigation of neonatal sepsis as the underlying mechanism for reconciling concurrent regulatory pathways governing innate immune activation, adaptive immune suppression, and changes in the homeostasis of sugar and lipid metabolism.14,55 The setpoint hypothesis can also explain the relative immune tolerance and hence, susceptibility to bacterial sepsis, in newborns to conserve energy required for growth and development.58
This new concept of changes in homeostatic setpoints relates to extreme physiological adaptive responses to damage that are reversible and, hence, if correct offers hope for therapeutic intervention. A pre-clinical study where the feasibility of resetting the immune setpoint by memory reactivation reduced morbidity and mortality in antibiotic-treated sepsis has been tested.59 Manipulating the setpoint of the immune system is not a new concept, indeed, vaccines function by resetting the homeostatic setpoint of the immune system for a specific pathogen and effectively work by promoting a defensive pathway (rather than a tolerance trajectory). The setpoints of the immune system will also adaptively change with age such as in early life and in old age with the onset of immune senescence. Reactivation of viremia in paediatric sepsis is different from adults where the occurrence is infrequent60; and comparing children and adult immunity in SARS-CoV-2 infection, a pre-existing higher innate immune type I interferon state is observed in children indicating a notable setpoint difference in innate-immunity.61,62
However, it is not immediately apparent how or what part of the immune state directly impacts organ failure in sepsis. Indeed, from the so-called genomic storm observed in critical trauma the severity of injury and magnitude of physiological derangement did not correlate with genomic immune patterns.22 It is noteworthy, however, that other systems are also markedly affected in sepsis. In particular, metabolic and neuroendocrine systems as well as the central nervous system (CNS). These systems are key in determining immune functional states and maintaining healthy organ function.16 Failing organs, metabolic systems and immunity in sepsis all retain the capacity to recover arguing for a host response that is reversible and therefore cannot have lost control or be dysregulated. Indeed, the reversibility of a septic condition is perhaps the strongest argument against an uncontrolled dysregulation of the host response and organ function. Rather this suggests an adaptive regulation toward extreme physiological and pathophysiological states. In this connection, the ability to recover, especially organ and metabolic systems, has previously evoked the idea that the host response is altered to a dormancy (aestivation) metabolic response, evolutionarily linked to protecting organs during low nutritional reserves and periods of hibernation63 (Figure 2). The switching to a dormancy response to cope with energy availability for cells and tissues must also be mechanistically related to changes in homeostasis setpoints.64,65 These mechanisms of extreme homeostatic regulation may help provide molecular answers for how a life‐threatening organ dysfunction is caused by a dysregulated host response to infection. Energetic demands are high in neonates and children, especially in periods of active growth, and this may contribute a key molecular determinant for the type of immune host response.
Examining the dogmas of sepsis through mathematical reasoning
Mathematics offers a concise language in which to describe our understanding. If our understanding is correct then a mathematical model should reproduce observations, or at least be consistent with the real-world. Importantly, this not only tests our current understanding, but provides a way of generating predictions in the behaviour of the system.
The generalizable mathematical equations of excitable systems,66 offers a relevant modelling tool for any excitable response, regardless of the system. The mathematical equations describing this non-linear behaviour were developed over 65 years ago, known as the Fitzhugh-Nagumo equations67,68 (Figure 3). The models were initially developed for understanding neuronal activation but have been used to describe many different types of excitable systems, even a forest fire. Using this as an example, a burning tree ignites (excites) neighbouring trees, generating a flame front (active wave) that propagates until all the trees are burnt in its path. This active, or travelling, wave can be transferred to other types of ignitable fuel if in the path of the wave. Notably the active wave leaves behind a non-excitable refractory state whereby a new excited state (burn) cannot happen until there is new regenerated excitable media. In sepsis, the host response can be described as an excitable system activated by infection. Here we use the modelling of excitable systems to test the first principles of our understanding of sepsis and to help delineate a set of rules for guiding future predictive algorithms.
Figure 3.
A description of the mathematical ODEs used for testing the host response in sepsis adapted from the Fitzhugh-Nagumo equations. This is a simplification but the form of ODE system (1) and (2) matches the general form of Fitzhugh-Nagumo equations. Namely, each response is generated and resolved due to the interactions of the host and infection signals. Specifically, the infection-inflammatory regulation means that the infection is directly coupled to the inflammatory response and is generated proportionally to how much infection there already is (i.e., higher infection tends to give higher inflammation). However, as the infection-inflammatory response grows there is a limiting factor and competition keeps the infection-inflammatory level from growing without bound. The immune response in Eq. (1) is −u, due to this term being negative the immune response suppresses the infection-inflammatory reaction. Finally, the additional parameter S is a constant and represents a source of infection. Initially, we set S = 0, so that there is no source beyond the initial infection. Note, Figure 4 demonstrates by altering S provides a means of achieving a recurring infection-inflammatory response.
If we apply this approach then we gain a lot in terms of simplicity and understanding, but we lose the specificity of interpretation. Namely, instead of linking outputs to a specific cytokine, or cellular actions, we are simply going to consider the time evolution of the defensive immune response, u, and the infection-inflammatory response, v. The interaction is then defined by the ordinary differential equation (ODE) system shown in Figure 3, which matches the general form of the Fitzhugh-Nagumo equations, in that each response is generated and resolved due to the interactions of the host and infection signals. Eq. (1) describes the immune response in relation to the initial trigger (infection source, S) (Figure 3). In terms of Eq. (2) (Figure 3) the immune response occurs in proportion to the level of infection-inflammation, i.e. a larger infection-inflammation leads to a larger immune response. The resolution of the immune response is proportional to itself. The constant, M, is a positive parameter representing the extent of metabolic demand to produce the infection-inflammatory reactions. Namely, M will affect the amplitude and how quickly the body can respond to the infection by producing an immune response. In this context, this equation could be further extended to include other coupled systems such as the neuroendocrine or cardiovascular systems. Finally, the initial conditions represent how the infection is started. We assume that we start from no immune response, u0 = 0, and an infection response appears due to some initial pathogen load, v0 > 0.
The first point to note is that two first-order autonomous equations are minimally required to derive a model of excitable state behaviour and that just modelling the immune response in isolation (Eq. (1) alone, Figure 3) is not sufficient. The second point, highlighted in Figure 3 by Eq. (2), is that there is the requirement for energetic, or metabolic resources, which places a demand on the system. Hence, the excitability of the immune system cannot be understood without linking it to metabolic demand. In the last decade, the importance of how immunity is coupled and cross-regulated by metabolism has become well established.69,70 The linked immune and metabolic changes are clearly observable at the genomic level during neonatal sepsis.14
We use this simple model to understand all possible dynamics of an excitable system through graphical methods. This aspect illustrates the power and elegance of mathematical consideration in that we can understand, without bias, the qualitative results of a system and without regard for the quantitative results of a system. Hence, without fitting any data we can know if an equation system can produce a realistic output. To visually display the dynamics, we need to determine the lines along which one of the population dynamics is zero, namely either dv/dt = 0, or du/dt = 0. These lines are called nullclines (one for each equation). When the nullclines cross both dynamics disappear, and we are at a “steady state”. Further depending on the system's interactions these steady states can either be stable, or unstable. The stable steady states are the “setpoints” of the system, namely, these are the homeostatic states that the system will evolve to due to feedback-regulation. This raises a third point that the dynamical behaviour of an excitable system can have multiple setpoints depending on initial conditions and the setting of these will determine the overall behaviour. We next summarise the various behaviours possible in the system.
In the next series of figures two types of graphs are shown for each case scenario; the left panel shows a black line representing the evolution of the solution in the (v,u) coordinates. This is known as a phase plane, and it enables us to see what happens without considering time. The right panel represents the time trajectory of these responses.
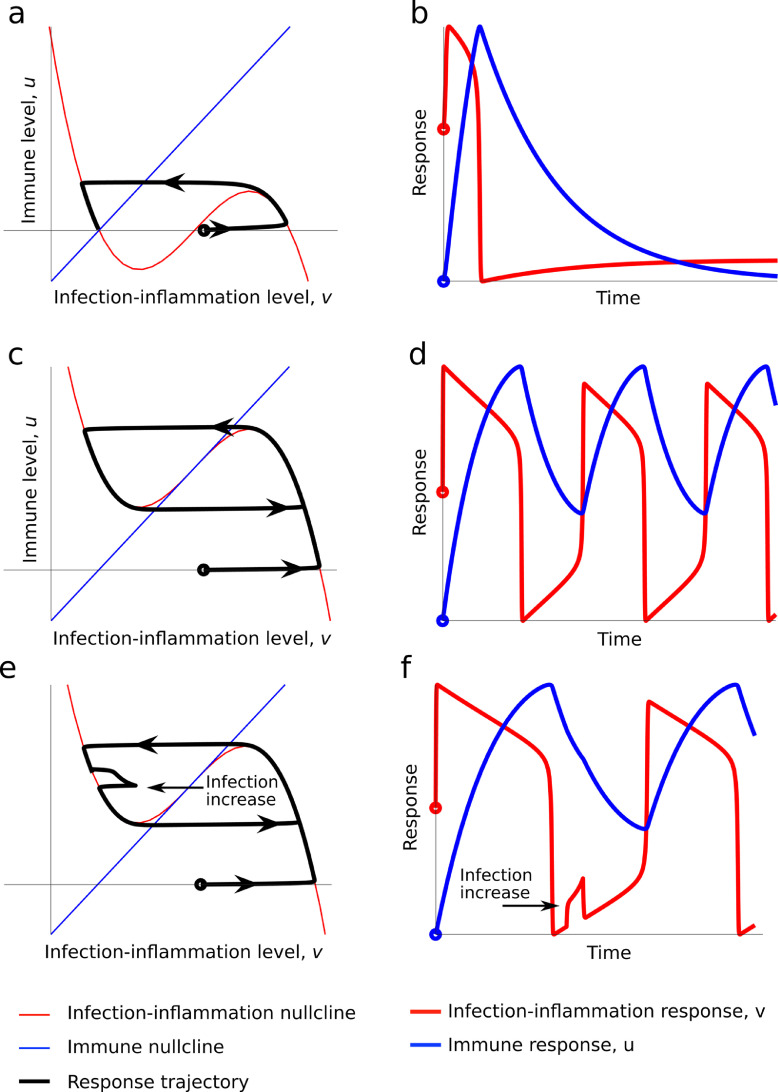
Host-response dynamics leading to a resolving or a non-resolving infection:Figure 4a and b shows what happens when we observe a normal homeostasis response to infection with consequences of mild or no disease symptoms. A small initial infection (the initial point is marked by a hollow circle in all figures) produces a rapid growth in the infection-inflammatory response. This is followed by a slower growth of the immune response, which clears and resolves the infection-inflammatory response. The immune response then falls back to a new resting state. This state can accommodate a low level of inflammatory, latent, or persistent infection states after an initial resolving infection. There are two key features to note. First the immune setpoint is low, triggering a rapid inflammatory and infection clearance with a relatively slower immune resolution phase. Second, the model predicts infection and/or state of inflammation that does not return to zero but is kept at a low setpoint by the immune system. This is consistent with our biological understanding, as the immune system has had little if any selective pressure to evolve sterilising immunity. It has evolved, however, to accommodate commensals such as the microbiome and forces of positive selection to clear pathogen load to asymptomatic disease levels, but not unnecessarily to sterilise against a pathogen. Further, the immune system for both innate and adaptive immunity is well established not to return to baseline because of the development of memory in the case of the adaptive and trained or primed immunity for the innate system, respectively.
Figure 4.
Mathematical simulation of case scenarios for resolving, non-resolving and endotoxin tolerance-hypo-inflammation. Panels a and b: Resolving infection simulation. The left panel shows the “phase plane” plot of the solution trajectory in the (v,u) coordinates, which is the black line. The arrows on the black line represent the evolution of the solution and, we observe that when the black line terminates it does so at a point where the nullclines (red and blue lines) cross. The right panel represents the trajectories of the (t,v) (red line) and (t,u) (blue line) solutions, respectively. (v0,u0) = (1.1,0). Panels c and d show non-resolving infection simulation. By providing a constant source of infection, i.e. S > 0, we cause the u-nullcline to cut only the central branch of the v-nullcline and, therefore, results in an unresolved immune-infection response. To achieve this the infection source has been increased to S = 10. (v0,u0) = (1.1,0). Panels E and F show Endotoxin tolerance (hypo-inflammatory) simulation. Phase plane (left) and simulated trajectories (right) of Eqs. (1) and (2). In the right plot the red line illustrates the infection level whilst the blue represents the immune response. This is the same simulation as shown in panels c and d, except an additional constant infection source between the first and second oscillation. This is seen and the perturbation in the black and red lines in the left and right plots, respectively. The initial conditions are (v0,u0) = (1.1,0) and S = 10.
The system can also probe what happens to the system during an unresolved immune-infection response under the realistic conditions of allowing a constant source of infection, i.e. S > 0 (Figure 4c and d). Here, the system oscillates between having a high infection-inflammatory load and a low infection-inflammatory load. The oscillations occur because an increase in the infection load leads to an increase in the immune response. In turn, the growth of the immune response drives the infection-inflammatory level rapidly down and resulting in the immune response to decrease. However, as the immune response falls the higher level of infection source allows the infection to grow once again, causing the immune response to grow again and, thus, we are left with a cycle, as shown by the black trajectory in Figure 4c. The model satisfies the possibility of a non-resolving infection.
The key features to note are that the immune setpoint is set much higher as a response to a relatively high initial pathogen load and the pattern established is oscillatory. It is known that in several sepsis cases that an infection can persist for protracted periods, however, the mechanisms underlying such cases are not clearly defined and time series data from sepsis patients are sparse. This makes the detection of any rhythmic or oscillatory behaviour forming an infection setpoint difficult to discern. Nevertheless, this type of behaviour in infection biology has been documented for certain persistent viruses, but notably all outside sepsis. In particular, for human immunodeficiency virus (HIV), where the infection setpoint is clinically defined as the viral load in the blood (HIV RNA) that settles at within a few weeks to months after the initial infection. For HIV, it is known that immediately after infection, a higher initial viral load will lead to a higher infection setpoint and is therefore consistent with the model.71 Arguably, the same principle would apply for an infection-inflammatory response, where the source of infection is not identified. Note the higher setpoint for inflammatory response does not imply a cytokine storm although inflammatory cytokine levels will be oscillating between high and low levels.
Host-response dynamics leading to states of hypo-inflammation and tolerance: The model can also be used to probe the refractory (hypo-inflammatory) response observed in the endotoxin tolerance challenge. This refractory case is shown in Figure 4e and f, which is a modified version of Figure 4c and d. Critically, we are in the case of a non-resolving infection-inflammatory response, where the immune-infection response cycles between high and low levels. If we then challenge the system with a small increase in infection, or endotoxin, during one of the refractory periods (see the red fluctuation in the middle of the right graph of Figure 4e and f) we observe that we do not get an additional infection-inflammatory response back. Rather, the extra infection-endotoxin challenge is rapidly inhibited from developing and we must wait until the immune response has relaxed to a low enough level before the next spike in infection-inflammation can occur. This is strikingly consistent with biological and clinical evidence and counters the evolution of a cytokine storm. Innate immune cells such as macrophages and neutrophils are clear biological examples of an excitable cellular system, whereby pathogen and danger associated molecular patterns (PAMPS/DAMPS) acts as a stimulus to trigger an excitable response . With respect to experimental sepsis this first came to light when animals were found to survive a lethal dose of bacterial endotoxin if previously administered with a sub-lethal challenge and the phenomenon was given the name of endotoxin tolerance.29,30,72,73 This has also been shown to occur in vivo in humans.28,30 Further, numerous studies reported hypo-reactive states for antigen presenting cells (APCs), in particular monocytes/macrophage cells, that had received repeated endotoxin challenges in cell culture require a recovery period (refractoriness) before they can be stimulated again.28 Whether this mechanism is at play at birth for a newborn immune state, where the birthing process involves maternal driven inflammatory responses during labour is not known.
Critically, a given population density of excitable cells will rapidly propagate an active wave, a property akin to the original concept of a self-perpetuating cytokine storm. However, the refractory period is not taken into consideration by the concept of a cytokine storm and therefore argues against such a scenario. Further, mechanistically, and out of the scope of the present models, the density of refractory state cells described for APC when in contact with T helper and B-cells will propagate an immune suppressive regulatory state. This will result in increasing proportions of regulatory cells keeping the immune system in a suppressed state. The evolution of this immune state is a hall mark of sepsis across all ages and has been described as a state of immune paralysis. This conceptual notion for a hyper-inflammatory period and a protracted immunosuppressive phase has, in more recent times, become the accepted dogma.23 Immune paralysis implies an immune response blocked from progressing. Notably, T and B-cell immune-suppression is often observed concurrently with elevated innate immunity. An alternative explanation to explain the occurrence of immune suppression in the presence of inflammatory activation proposed changes in the regulatory (homeostatic) setpoint of the immune system.14, 55 This explanation is consistent with the mathematical model that demonstrates the requirement for increased setpoint activity to develop this response. At a molecular level, it is likely the immune setpoint to infection is, therefore, established when the excitable amplitude and refractory frequency of APCs, determined by pathogen antigen/PAMP/DAMP levels, stimulate, or inhibit, specific T and B cell functions.
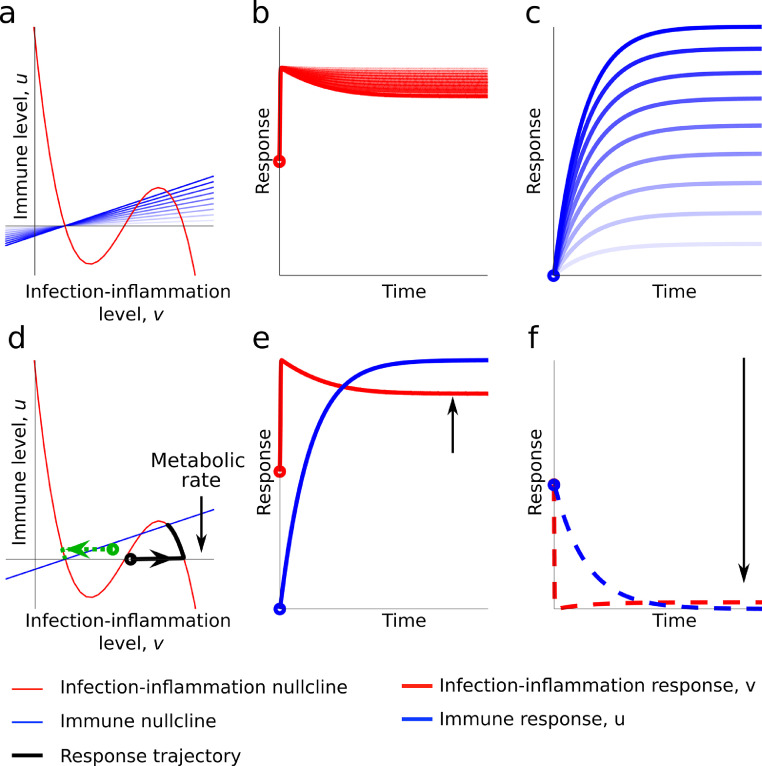
Host-response dynamics of tolerance and resistance under bioenergetic insufficiency: The above cases have been simulated under conditions that have not made metabolic resources rate limiting. When M is moderately reduced in the model, we find the gradient of the immune response is reduced. Figure 5 shows, in this case, the black line of the left plot terminates at a high setpoint, which we observe in the right plot to be a situation in which both the infection-inflammatory response and immune response are fixed to high levels. Here, we can use the interpretability and generality of the mathematical model to describe what happens in an energetically demanding infection-inflammatory reaction. Namely, the production of an immune response depends critically on the metabolism producing energy to enable the response. However, if the immune response is fixed to a high setpoint the metabolism will eventually burn out. This can be included in the model by slowly lowering M. Figure 5a–c presents this case of metabolism burnout and we see that as the immune response drops the infection-inflammatory response increases. Notably, this energetic demand has a direct link to vital organ function, and thus mechanistically connects the host response to organ failure. In the real-world this cannot continue unimpeded. This may be argued to be close to the cytokine storm but the critical parameter here is not the “cytokine” (infection-inflammatory reaction product) but metabolism that determines the outcome.
Figure 5.
Simulation of case scenarios of tolerance and resistance and simulation under increased metabolic demand. Panel d-f shows a bifurcated case in which the infection leads to a constant high infection-inflammation and immune response (Black line trajectory) or a low infection-inflammatory and immune response (dash green line). The intersections of the upper and lower nullclines show two bifurcated setpoints. That is there can be two possible stable states or setpoints for the infection response. The green line setpoint lies on a zero-immune response level and with a low infection-inflammatory reaction and this trajectory predicts a tolerant host response. While the black line shows the trajectory for a higher setpoint for the immune and infection-inflammatory response leading to a resistant host response. In the case, the metabolic rate parameter, M, has been reduced from 10 to 2. Panel a shows the phase plane and simulated trajectories (panels b and c) of Eqs. (1) and (2), respectively. Here, M is reduced stepwise from 2 to 0, which reduces the gradient in the left plot, causing the infection response to increase in the central plot and the immune response to drop in the right plot. In all cases (v0,u0) = (1.1,0). This shows the production of an immune response depends critically on the metabolism producing energy to enable the response.
Changes in energy metabolism is a well-established hallmark of sepsis and causally is the most likely functional link to organ dysfunction.74 Critically, redox homeostasis and mitochondrial functions are reset in sepsis.75 Closely linked to host metabolism is the role of the neuro-endocrine axis, with vasopressin and corticosteroids of particular interest in the pathophysiology and management of septic shock.76, 77, 78 Targeting metabolic enzymes involved in oxidative bioenergetics has shown promise, for example, the pyruvate dehydrogenase complex that serves as a gatekeeper for glucose oxidation is a druggable target that can be used to reset mitochondrial respiration.79 Moreover, the importance of metabolic control is observationally seen in septic shock where metabolism resembles starvation, and there is an overall attenuation of metabolic demand.80 Immunometabolism directly governs dendritic cell and macrophage function.69 Notably, mitochondrial respiration is lower in septic children with signs of an immunosuppressive phenotype and inversely related to systemic inflammation.38
A key feature of the metabolic simulations is that there exist two possible stable states or setpoints for the system upon infection. This is shown by the green and black lines (Figure 5d–f). The green line setpoint lies on a zero-immune response level and with a low infection-inflammatory reaction and this trajectory predicts a tolerant host response. While the black line shows the trajectory for a higher setpoint for the immune and infection-inflammatory response leading to a resistant host response. This means the source of infection can be bifurcated, one leading to a setpoint in the immune response that leads to tolerance and the other to resistance. And satisfies a cardinal tenet of infection biology.57 The metabolic rate changes also reproduce certain features of the dormancy response and provides a mechanism for altering the setpoint of the immune response.
In all cases the model highlights the significance of explaining the host-response as a trajectory to a number of different homeostatic setpoints in the system, governed by the initial starting parameters. The model also elegantly explains the connection of the host response to metabolism and indeed the metabolic bioenergetic state provides the principal force that most closely explains what might be happening in sepsis. It is conceivable that the immune setpoint may be set or re-set to a different threshold for each individual, through genetic, physiological and dietary states or environmental determinants. It is also conceivable that an individual's setpoint(s) may be initially set normal but due to acute rapid excitability, through a large pathogen load or, rapid proliferation or, barrier breakdown the setpoint of the immune response is adaptively reset.
Cardinal rules from modelling: application to big data and multi-omics, and the use of artificial intelligence (AI)
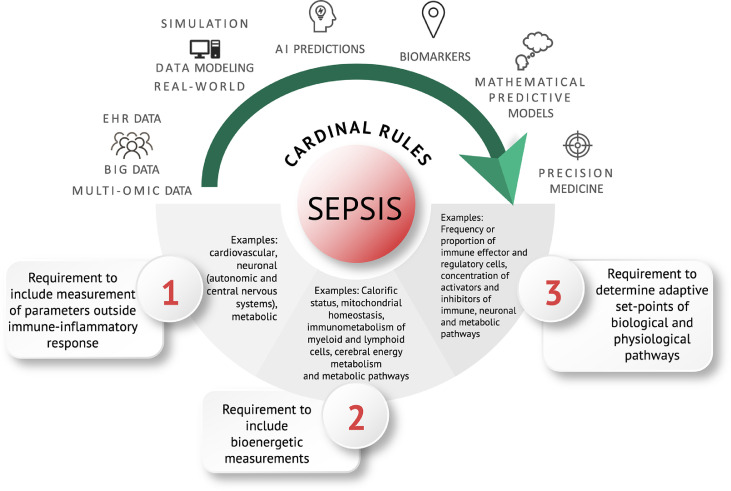
Three cardinal rules drop out from the above mathematical consideration of the host response in sepsis (Figure 6). Rule 1 is that considering the immune response in isolation is insufficient and that other interdependent physiological systems, metabolic and neuroendocrine systems, should be included. Rule 2 is that bioenergetic demand is a key force governing the type of host response. Rule 3 is that alterations in homeostatic setpoints of a system predict the behaviour of the response in sepsis. Therefore, improving precision for diagnostic and therapeutic strategies should consider identifying parameters that underpin these rules. Machine learning, in particular AI approaches, and large-scale statistical inference methodology are ideally suited for this task. The challenge is the collection of data for selecting parameters. These parameters need to account for the often-subtle presentation and be highly discriminative against a considerable number of individuals who will have a physiological response of the immune system to a non-serious infection or trauma that will overlap with a septic infection, although they will not suffer from sepsis.43
Figure 6.
Cardinal rules of sepsis: A roadmap for data-driven precision diagnosis of sepsis. The mathematical testing of first principles of our understanding of sepsis delineate a set of cardinal rules for guiding future predictive algorithms. For each rule examples of pathophysiological features and biomarkers are shown.
How can these “cardinal rules” help to identify and define key pathophysiological markers of sepsis? Table 1 provides examples for each rule in terms of physical and regulatory features. Rule 1 is to integrate other physiological systems with the immune response such as the autonomic and central nervous systems, while Rule 2 requires measurement of, for example, mitochondrial biomarkers and immunometabolism and Rule 3 points to determinants of homeostasis setpoints such as the frequency of effector and regulatory immune cells and inhibitory and stimulatory receptors and ligands. The clinical need is that these should be easily recorded and provide a readout within the first hours of suspected sepsis. In this context the emerging medical tricorder technology and point-of-care diagnostic systems may provide future opportunities to change the current syndromic diagnosis.81 These cardinal rules therefore have the potential to define parameters of a maladapted “dysregulated” host response more precisely to infection. Thus, we envision with a formalized mathematical model that faithfully reproduces sepsis trajectories, this could be parameterized on an individual patient, allowing testable predictions on the behaviour of an individual's response. Figure 6 schematically outlines this pathway or road map.
Concluding remarks
In summary, while there has been considerable advancement in our understanding of sepsis the complex nature of the host-response has thwarted a more a clear definition of sepsis, underestimating its true dynamical nature. We propose the term “dysregulation” is too ambiguous and mechanistically is open to being misleading. On the contrary, the prevailing evidence suggests against the dogma that the host response is regulated in sepsis, just in a different manner. The regulated state in sepsis is mediated by changes in homeostatic thresholds from a normal host-response, resulting in trajectories that are maladaptive and which pivot between tolerance and resistance. We find, the concepts of “cytokine storms” and “immune paralysis” while conceptually attractive notions can be misleading and holds the risk for misguided diagnosis and intervention. The evidence for protracted non-resolving states and alterations in homeostatic regulatory setpoints of the system, that drive a dormancy host-response better reflect the extreme physiological/pathophysiological states in sepsis.
Mathematical considerations identified three cardinal rules: Rule 1 is that the immune system alone is not sufficient to explain the host response in sepsis; Rule 2 is that metabolic demand is a vital determinant in how the response evolves, and rule 3 is that changes in setpoints of the system explain the behaviour and mechanism underlying the pathophysiological response. Finally, we propose that the application of setpoint theory for understanding sepsis and the critical importance of metabolism in driving the response will have a key informative role in guiding future sepsis definitions and, as part of a global health strategy, the development of precision medicine approaches for both diagnostics and therapeutics.
Outstanding questions
There remains an urgent need for a more precise understanding of the behaviour and evolution of pathophysiological processes that drive severity and sepsis outcomes.
Sepsis research in neonates and children in LMICs needs to be urgently addressed as a global health priority, accounting for the highest sepsis-associated morbidity and mortality burden.
Establishing data-driven and context-specific management guidelines, promoting creative clinical interventions will allow these barriers to be overcome and contribute to a more balanced and wide-reaching approach to sepsis.
Mechanistic studies delineating control, in particular metabolic and neuroendocrine systems, in the dynamics of pathophysiological pathways associated with sepsis.
A more accurate consensus definition for the host response in sepsis is urgently required.
Contributors
PG conceived, designed, wrote and edited. TEW designed, wrote and mathematically constructed Figure 3, Figure 4, Figure 5. MC and SO edited. PRSR wrote, edited and constructed Figs. 1, 2 and 6. All authors read and approved the final version of the manuscript.
Declaration of interests
None.
Acknowledgments
We would like to thank Noemi Pico for helpful discussions. PG funded by the European Regional Development Fund and Welsh Government (Ser Cymru programme – Project Sepsis).
Contributor Information
Peter Ghazal, Email: GhazalP@cardiff.ac.uk.
Thomas E. Woolley, Email: WoolleyT1@cardiff.ac.uk.
References
- 1.White F.W. Cultures from the blood in septicaemia, pneumonia, meningitis and chronic diseases. J Exp Med. 1899;4(3–4):425–450. doi: 10.1084/jem.4.3-4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balk R.A., Bone R.C. The septic syndrome. Definition and clinical implications. Crit Care Clin. 1989;5(1):1–8. [PubMed] [Google Scholar]
- 3.Bone R.C., Balk R.A., Cerra F.B., et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 4.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein B., Giroir B., Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 6.Lawn J.E., Cousens S., Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 7.Wiens M.O., Kumbakumba E., Kissoon N., Ansermino J.M., Ndamira A., Larson C.P. Pediatric sepsis in the developing world: challenges in defining sepsis and issues in post-discharge mortality. Clin Epidemiol. 2012;4:319–325. doi: 10.2147/CLEP.S35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss S.L., Parker B., Bullock M.E., et al. Defining pediatric sepsis by different criteria: discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012;13(4):e219–ee26. doi: 10.1097/PCC.0b013e31823c98da. [DOI] [PubMed] [Google Scholar]
- 9.Menon K., Schlapbach L.J., Akech S., et al. Criteria for pediatric sepsis-a systematic review and meta-analysis by the pediatric sepsis definition taskforce. Crit Care Med. 2022;50(1):21–36. doi: 10.1097/CCM.0000000000005294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynn J.L. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28(2):135–140. doi: 10.1097/MOP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modi N., Doré C.J., Saraswatula A., et al. A case definition for national and international neonatal bloodstream infection surveillance. Arch Dis Child Fetal Neonatal Ed. 2009;94(1):F8–12. doi: 10.1136/adc.2007.126458. [DOI] [PubMed] [Google Scholar]
- 12.van Dissel J.T., van Langevelde P., Westendorp R.G., Kwappenberg K., Frölich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351(9107):950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 13.Rigato O., Salomao R. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock. 2003;19(2):113–116. doi: 10.1097/00024382-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Smith C.L., Dickinson P., Forster T., et al. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat Commun. 2014;5(1):4649. doi: 10.1038/ncomms5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delano M.J., Ward P.A. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126(1):23–31. doi: 10.1172/JCI82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutschman C.S., Tracey K.J. Sepsis: current dogma and new perspectives. Immunity. 2014;40(4):463–475. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Beutler B., Milsark I.W., Cerami A.C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 18.Tracey K.J., Fong Y., Hesse D.G., et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara J.L. Cytokine dysregulation as a mechanism of graft versus host disease. Curr Opin Immunol. 1993;5(5):794–799. doi: 10.1016/0952-7915(93)90139-j. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara J.L., Abhyankar S., Gilliland D.G. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25(1 Pt 2):1216–1217. [PubMed] [Google Scholar]
- 21.DiGiandomenico A., Veach R.A., Zienkiewicz J., et al. The "genomic storm" induced by bacterial endotoxin is calmed by a nuclear transport modifier that attenuates localized and systemic inflammation. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0110183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao W., Mindrinos M.N., Seok J., et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angurana S.K., Bansal A., Muralidharan J., Aggarwal R., Singhi S. Cytokine levels in critically ill children with severe sepsis and their relation with the severity of illness and mortality. J Intensive Care Med. 2022;36(5):576–583. doi: 10.1177/0885066620912989. [DOI] [PubMed] [Google Scholar]
- 25.Fioretto J.R., Martin J.G., Kurokawa C.S., et al. Interleukin-6 and procalcitonin in children with sepsis and septic shock. Cytokine. 2008;43(2):160–164. doi: 10.1016/j.cyto.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Thomson J., Chavan A. Handbook IMCI: integrated management of childhood illness. Arch Dis Child. 2007;92(2):187. [Google Scholar]
- 27.Beeson P.B. Development of tolerance to typhoid bacterial pyrogen and its abolition by reticulo-endothelial blockade. Proc Soc Exp Biol Med. 1946;61(3):248–250. doi: 10.3181/00379727-61-15291p. [DOI] [PubMed] [Google Scholar]
- 28.Cavaillon J.M., Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10(5):233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draisma A., Pickkers P., Bouw M.P., van der Hoeven J.G. Development of endotoxin tolerance in humans in vivo. Crit Care Med. 2009;37(4):1261–1267. doi: 10.1097/CCM.0b013e31819c3c67. [DOI] [PubMed] [Google Scholar]
- 30.West M.A., Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30(1 Suppl):S64–S73. [PubMed] [Google Scholar]
- 31.Kasten K.R., Tschöp J., Adediran S.G., Hildeman D.A., Caldwell C.C. T cells are potent early mediators of the host response to sepsis. Shock. 2010;34(4):327–336. doi: 10.1097/SHK.0b013e3181e14c2e. [DOI] [PubMed] [Google Scholar]
- 32.Monserrat J., de Pablo R., Diaz-Martín D., et al. Early alterations of B cells in patients with septic shock. Crit Care. 2013;17(3):R105. doi: 10.1186/cc12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biolo G., Toigo G., Ciocchi B., et al. Metabolic response to injury and sepsis: changes in protein metabolism. Nutrition. 1997;13(9):52–57. doi: 10.1016/s0899-9007(97)00206-2. Supplement 1. [DOI] [PubMed] [Google Scholar]
- 34.Delano M.J., Ward P.A. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274(1):330–353. doi: 10.1111/imr.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keel M., Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 36.McHoney M., Eaton S., Pierro A. Metabolic response to surgery in infants and children. Eur J Pediatr Surg. 2009;19(5):275–285. doi: 10.1055/s-0029-1241192. [DOI] [PubMed] [Google Scholar]
- 37.Cuthbertson D.P. Post-shock metabolic response. Lancet N Am Ed. 1942;239(6189):433–437. [Google Scholar]
- 38.Weiss S.L., Zhang D., Bush J., et al. Mitochondrial dysfunction is associated with an immune paralysis phenotype in pediatric sepsis. Shock. 2020;54(3):285–293. doi: 10.1097/SHK.0000000000001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobi J. Pathophysiology of sepsis. Am J Health Syst Pharm. 2002;59(Suppl 1):S3–S8. doi: 10.1093/ajhp/59.suppl_1.S3. [DOI] [PubMed] [Google Scholar]
- 40.Michie H.R. Metabolism of sepsis and multiple organ failure. World J Surg. 1996;20(4):460–464. doi: 10.1007/s002689900072. [DOI] [PubMed] [Google Scholar]
- 41.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 43.Walton A.H., Muenzer J.T., Rasche D., et al. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9(2):e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 46.Boomer J.S., To K., Chang K.C., et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boussiotis V.A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375(18):1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodrigues P.R.S., Picco N., Morgan B.P. Ghazal P. Sepsis target validation for repurposing and combining complement and immune checkpoint inhibition therapeutics. Expert Opin Drug Discov. 2021;16(5):537–551. doi: 10.1080/17460441.2021.1851186. [DOI] [PubMed] [Google Scholar]
- 49.Schneider D.S., Ayres J.S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8(11):889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soares M.P., Teixeira L., Moita L.F. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol. 2017;17(2):83–96. doi: 10.1038/nri.2016.136. [DOI] [PubMed] [Google Scholar]
- 51.Torgersen C., Moser P., Luckner G., et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth Analg. 2009;108(6):1841–1847. doi: 10.1213/ane.0b013e318195e11d. [DOI] [PubMed] [Google Scholar]
- 52.Otto G.P., Sossdorf M., Claus R.A., et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15(4):R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong H.R. Genome-wide expression profiling in pediatric septic shock. Pediatr Res. 2013;73(4 Pt 2):564–569. doi: 10.1038/pr.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howman A., Chapman T.L., Langdon M.M., et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet N Am Ed. 2013;381(9868):744–751. doi: 10.1016/S0140-6736(12)61566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghazal P., Dickinson P., Smith C.L. Early life response to infection. Curr Opin Infect Dis. 2013;26(3):213–218. doi: 10.1097/QCO.0b013e32835fb8bf. [DOI] [PubMed] [Google Scholar]
- 56.Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335(6071):936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soares M.P., Gozzelino R., Weis S. Tissue damage control in disease tolerance. Trends Immunol. 2014;35(10):483–494. doi: 10.1016/j.it.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Harbeson D., Francis F., Bao W., Amenyogbe N.A., Kollmann T.R. Energy demands of early life drive a disease tolerant phenotype and dictate outcome in neonatal bacterial sepsis. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nowill A.E., Fornazin M.C., Spago M.C., et al. Immune response resetting in ongoing sepsis. J Immunol. 2019;203(5):1298–1312. doi: 10.4049/jimmunol.1900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han M., Roberts A.L., Migliore B.A., Cárdenas A.M., Weiss S.L. Reactivation viremia in pediatric sepsis. Pediatr Crit Care Med. 2020;21(4):e152–e1e9. doi: 10.1097/PCC.0000000000002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida M., Worlock K.B., Huang N., et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature. 2022;602(7896):321–327. doi: 10.1038/s41586-021-04345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loske J., Röhmel J., Lukassen S., et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat Biotechnol. 2021;40(3):319–324. doi: 10.1038/s41587-021-01037-9. [DOI] [PubMed] [Google Scholar]
- 63.Singer M. Critical illness and flat batteries. Crit Care. 2017;21(Suppl 3):309. doi: 10.1186/s13054-017-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morrison M.L., Iwata A., Wick M.L., et al. Iodine redistribution during trauma, sepsis, and hibernation: an evolutionarily conserved response to severe stress. Crit Care Explor. 2020;2(10):e0215. doi: 10.1097/CCE.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singer M. Cellular dysfunction in sepsis. Clin Chest Med. 2008;29(4):655–660. doi: 10.1016/j.ccm.2008.06.003. viii-ix. [DOI] [PubMed] [Google Scholar]
- 66.Rocsoreanu A.G.C., Giurgiteanu N. Springer Science & Business Media; 2012. The FitzHugh-Nagumo Model: Bifurcation and Dynamics. [Google Scholar]
- 67.FitzHugh R. Mathematical models of threshold phenomena in the nerve membrane. Bull Math Biophys. 1955;17(4):257–278. [Google Scholar]
- 68.Nagumo J., Arimoto S., Yoshizawa S. An active pulse transmission line simulating nerve axon. Proc IRE. 1962;50(10):2061–2070. [Google Scholar]
- 69.O'Neill L.A., Pearce E.J. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213(1):15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robertson K.A., Ghazal P. Interferon control of the sterol metabolic network: bidirectional molecular circuitry-mediating host protection. Front Immunol. 2016;7:634. doi: 10.3389/fimmu.2016.00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pontesilli O., Klein M.R., Kerkhof-Garde S.R., et al. Kinetics of immune functions and virus replication during HIV-1 infection. Immunol Lett. 1997;57(1):125–130. doi: 10.1016/s0165-2478(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 72.López-Collazo E., del Fresno C. Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit Care. 2013;17(6):242. doi: 10.1186/cc13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greisman S.E., Hornick R.B., Wagner H.N., Woodward W.E., Woodward T.E. The role of endotoxin during typhoid fever and tularemia in man. IV. The integrity of the endotoxin tolerance mechanisms during infection. J Clin Invest. 1969;48(4):613–629. doi: 10.1172/JCI106020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Preau S., Vodovar D., Jung B., et al. Energetic dysfunction in sepsis: a narrative review. Ann Intensive Care. 2021;11(1):104. doi: 10.1186/s13613-021-00893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willems P.H., Rossignol R., Dieteren C.E., Murphy M.P., Koopman W.J. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015;22(2):207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Landry D.W., Oliver J.A. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345(8):588–595. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- 77.Holmes C.L., Patel B.M., Russell J.A., Walley K.R. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120(3):989–1002. doi: 10.1378/chest.120.3.989. [DOI] [PubMed] [Google Scholar]
- 78.Rochwerg B., Oczkowski S.J., Siemieniuk R.A.C., et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Crit Care Med. 2018;46(9):1411–1420. doi: 10.1097/CCM.0000000000003262. [DOI] [PubMed] [Google Scholar]
- 79.McCall C.E., Zabalawi M., Liu T., et al. Pyruvate dehydrogenase complex stimulation promotes immunometabolic homeostasis and sepsis survival. JCI Insight. 2018;3(15) doi: 10.1172/jci.insight.99292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kreymann G., Grosser S., Buggisch P., Gottschall C., Matthaei S., Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit Care Med. 1993;21(7):1012–1019. doi: 10.1097/00003246-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 81.Iregbu K., Dramowski A., Milton R., et al. Global health systems’ data science approach for precision diagnosis of sepsis in early life. Lancet Infect Dis. 2021;22(5):e143–e152. doi: 10.1016/S1473-3099(21)00645-9. Online ahead of print. [DOI] [PubMed] [Google Scholar]