Abstract
The potato is a root vegetable native to the Americas; it consists of the starchy tuber of the plant Solanum tuberosum. There are many varieties, and the flesh can have different colour ranging from yellow to red and purple. Coloured varieties have a denser texture and slightly nuttier, earthier flavour than other potatoes. The desirable quality characteristics of potatoes depends on the intended use, and the acceptability of raw potatoes is determined by size, shape, colour, and the quality of can be evaluated in terms of colour, flavour, and texture. Deep-frying is the century-old and it is among the most common cooking processes, still being used to prepare a variety of food products on both industrial and domestic scales. Frying the potatoes is among the tastiest and appreciated way to cook this vegetable. Purple fleshed potatoes are widely considered one of the best-tasting purple potatoes varieties, they have a nice taste and add colour to a meal. They are a source of beneficial health compounds which makes them interesting as functional food. The anthocyanins present in the Purple Majesty variety are interesting for their health promoting abilities, anti-oxidative activity, and even other health beneficial effects, e.g. anti-influenza virus activity, and anti-stomach cancer activity. The aim of this study has been to assess the effect of deep-frying of purple potato Purple Majesty using sunflower oil on the polyphenols, anthocyanins and to evaluate the antioxidant activity of the cooked matrix compared to the fresh one. The results seem to suggest that the healthy characteristics of this functional food are retained after the cooking by frying.
Keywords: Purple potato “Purple Majesty”, Deep-frying, Polyphenols, Anthocyanins, Antioxidant activity, Sunflower oil
Graphical abstract
Highlights
-
•
Purple potato “Purple Majesty” is rich in antioxidants and anthocyanins.
-
•
Deep frying in sunflower oil affects positively the antioxidant activity.
-
•
The anthocyanins increase their amount in the frying time range.
-
•
Polyphenols amount decreases during frying time.
-
•
The ferulic and gallic acids seem to increase their amount after 48 h of frying.
Purple potato “Purple Majesty”; Deep-frying; Polyphenols; Anthocyanins; Antioxidant activity; Sunflower oil.
1. Introduction
Potatoes are among the most widespread crops consumed worldwide (Gebhardt, 2016; Zaheer and Akhtar, 2016), and represent a relevant source of carbohydrates (starch), but are also at the same time a source of proteins, vitamin C, vitamin B6, minerals, and fibers (Andre et al., 2007, 2010; Camire et al., 2009). Like all plant products, they also contains secondary metabolites, such as polyphenols (Perla et al., 2012; Visvanathan et al., 2016), and this seems to make the potatoes appropriate to be considered as a functional food (Burgos et al., 2020; Santini and Cicero, 2020).
Polyphenolic compounds include a large group of phytonutrients which, based on their chemical structure, can be divided into different classes: flavonoids, phenolic acids, tannins, stilbenes, and lignans (Durazzo and Lucarini, 2019a, Durazzo and Lucarini, 2019b). Polyphenols are known for their antioxidant, antimutagenic, anticarcinogenic, and antimicrobial properties (Durazzo et al., 2020). Several studies have shown that their consumption can decrease the risk of chronic diseases (Daliu et al., 2018, 2019; Santini et al., 2017; Santini et al., 2018; Santini and Novellino, 2018). Within the European market, yellow-fleshed potatoes are widespread, but in recent years, varieties of coloured-fleshed potatoes have appeared. These varieties include different types of potatoes: a red skin and a yellow flesh; red skin and red pulp; purple skin and marbled pulp and potatoes with purple skin and purple flesh. Purple Majesty in particular, is a cultivar registered in the British Potato Variety Database (https://varieties.ahdb.org.uk). The interest for this cultivar, as for the rest of the coloured paste potatoes varieties, is given by the quantity of polyphenols contained in it, especially due to the high level of anthocyanins, which are mainly responsible for the peculiar colour of these potatoes’ varieties. In fact, several studies show that average content of polyphenolic compounds in coloured and especially purple fleshed potatoes is two to three times higher as compared to yellow or white potatoes (Kalita et al., 2013; Kita et al., 2015; Lachman et al., 2008; Rytel et al., 2014). Specifically, in coloured potato varieties, as reported by Kita et al. (2015) the polyphenol content varies from 250 to 526 mg/100 g DM, while the anthocyanin amount ranges from 16 to 57 mg/100 g DM.
Although there are several studies that confirm the effect that these compounds have on health, it should be taken into consideration that the amount of polyphenols varies when comparing the raw product with those subjected to a heat treatment (Lachman et al., 2012; Bellumoria et al. 2017). Indeed, it is known that the phenolic content and its stability depend not only on the agronomic and genotypic factors, but also on the post-harvest storage conditions, processing, and cooking processes (Amarowicz et al., 2009). In particular, physicochemical conditions such as pH, temperature, light, oxygen availability, metal ions, chemical modification, enzymes, proteins, nitrite salt and sulfur dioxide should be taken into consideration to evaluate the stability of polyphenols in cooking processes (Deng et al., 2018). Deep fat frying or deep frying is one of the most widespread and oldest cooking processes in the world, in fact there are historical hints dating back to 1600 B.C. documenting its use (Firestone, 2007). It is defined as a cooking and dehydration process which consists in the immersing of a food in a bath of oil, or another other type of fat, kept at a temperature between 150 and 190 °C. The typical flavor and texture of fried food are generated by chemical-physical transformations influenced by numerous factors, such as: the nature of the food and the frying oil, time, temperature, continuous or discontinuous heating, oil refilling, model of the fryer and the use of filters (Kalogianni et al., 2010; Rotondo et al., 2020). The nutraceutical stability is affected especially by length of time and method of frying (Da Silva and Moreira, 2008).
Therefore, in this study it was investigated how the deep-frying process using a microwave drying treatment affects parameters such as the content of polyphenols, anthocyanins, and the antioxidant activity in purple potato Purple Majesty.
Only few studies (Lemos et al., 2015; Tian et al., 2016; Xu et al., 2016) have examined the effects of cooking methods on the bioactive compound content in purple potato, but to the best of our knowledge there are no works that have evaluated the combination of microwaving and deep-frying.
Thus, our research may have great potential from a technological and nutritional point of view in the food industry because it could lead to the production and consumption of purple fries. Even if consumers are accustomed to potato varieties with traditional yellow or white flesh, they could be driven to the consumption of coloured potatoes as a product to add to salads, or as a raw material to produce coloured chips and French fries, not only for the colour, flavor and texture, but also for the higher nutritional value conferred by the presence of phenolic compounds.
2. Material and methods
2.1. Materials and frying protocol
Purple Majesty cultivar purple fresh potatoes (obtained from Albert Bartlett USA, Inc. Greenwood Village, CO, United States) and McCain yellow pulp frozen pre-fried potatoes (purchased from local market) have been used for the proposed study. The oily matrix used in the discontinuous and prolonged heat treatment was a high oleic (brand) sunflower oil (84.11% oleic acid).
All reagents were of analytical or spectroscopic grade and were supplied by Sigma Aldrich (St. Louis, MO, Unites States).
Setting up of the pre-treatment phase consisted in homologate fresh purple potatoes to a pre-fried commercial product. A pre-treatment of the fresh Purple Majesty was adopted using a microwave drying treatment to homologate them to the pre-fried McCain yellow pulp potatoes. Before reaching the final parameters as described in this work, the fresh purple potatoes were subjected to various treatments varying the microwave parameters, with the aim of achieving a pre-fried product with suitable texture characteristics for the subsequent treatment phases.
2.1.1. Sample preparation and frying process
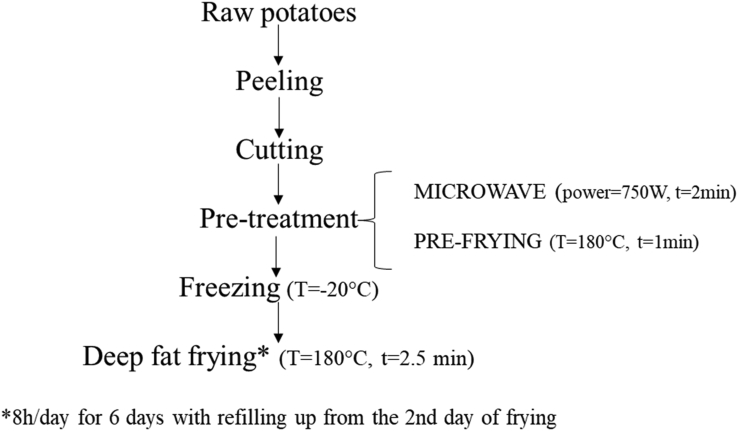
The fresh potatoes were peeled and cut using a domestic potato cutter into the shape of sticks (4 × 1 × 1 cm) (Figure 1). One hundred fifty grams of sticks were subjected to a microwave drying pre-treatment (using a Samsung ME711K device) for 2 min at 750 W in order to remove large part of the water content. The potatoes were then subjected to a pre-frying process for 1 min at 180 ± 3 °C (2.75 L). After drying the excess of oil with the use of absorbent paper, the potatoes were allowed to cool and then frozen at a temperature of - 20 °C until ready for use. The frozen pre-fried potatoes were then subjected to frying for 2.5 min at 180 ± 3 °C. With the aim to simulate a typical fast food frying, a discontinuous and prolonged method was adopted. The frying process of potatoes (FPOP) was carried out according to the procedure described by Romano et al. (2012). The procedure steps can be summarized as follow. Each batch of potatoes was fried for 8 h/day for 6 days, on a total of 48 aliquots of fried potatoes. Starting from the second day of treatment, before starting the heating of the oil, fresh oil (60 mL) was added in order to restore the initial oil level. The behaviour of high oleic sunflower oil in deep frying process of purple potato Purple Majesty has been studied in a previous work (Romano et al., 2021), where free fatty acids, peroxide value, total polar compounds, fatty acid composition, and volatile organic compounds have been evaluated. The flow chart for the experimental design preparation of purple potatoes is shown in Figure 2.
Figure 1.
Purple Majesty potatoes and pre fried sticks.
Figure 2.
Experimental design of purple potato sample preparation.
2.2. Analysis
The purple potato samples at the different frying times were subjected to the following analytical determinations:
-
•
qualitative and quantitative determination of polyphenols;
-
•
determination of the antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay;
-
•
quantitative determination of anthocyanins.
2.2.1. Quantitative determination of total polyphenols in potatoes
Total polyphenols were extracted following the method reported by Burgos et al. (2013). Briefly, 1 g of freeze-dried potatoes were extracted using 10 mL of a mixture of methanol and water (80:20) for 10 min in an ultrasonic bath (Sonorex SUPER RK 31 H, Bandelin Electronic GmbH & Co. KG, Berlin, Germany). The sample was subsequently subjected to centrifugation at the conditions of 4000 rpm for 10 min at 4 °C using a refrigerated centrifuge (ALC PK121R, ALC International, Cologno Monzese, Italy). Subsequently, the supernatant was taken and transferred into a flask after filtration on filter paper. The extraction operation was repeated three times and the final extract was brought to a volume of 50 mL with a solution of methanol and water (80:20). The sample was diluted in a ratio of 1: 5 before being subjected to the determination of the total polyphenols.
Total polyphenols were determined by the Folin-Ciocalteau method (Lemos et al., 2015; Rytel et al., 2014). Briefly, 500 μL of the extract were mixed with 500 μL of Milli Q® ultra-pure water and with 1 mL of the Folin-Ciocalteau reagent and the mixture was reacted for 5 min. Subsequently 5 mL of a 5% Na2CO3 solution were added, and the whole brought to a volume of 10 mL with Milli Q® ultra pure water. The mixture was left to stand in the absence of light for 1 h at room temperature. After the dwell time, the absorbance of the solution was recorded at the wavelength of 765 nm using a spectrophotometer (UV-1601, Shimadzu, Japan). The total polyphenol concentration was calculated using gallic acid as standard whose calibration curve points ranged from concentrations of 5–100 ppm (5, 10, 25, 50, 100 ppm). The results have been expressed as mg of gallic acid/g of dry matter.
2.2.2. Qualitative determination of phenolic acids by HPLC-DAD (liquid chromatography - diode array spectrophotometric detector)
Phenolic acids of purple potatoes were analyzed by high performance liquid chromatography (HPLC) using an Agilent 1100 chromatograph (Agilent, Santa Clara, CA, United States) equipped with Diode Array UV/VIS detector) using a Spherisorb Eclipse XDB C-18 5 μm (4.6 × 150 mm) reverse phase analytical column (Agilent, Santa Clara, CA, United tates). The HPLC analytical system used was monitored and checked using the HP Chem Station (Agilent, Santa Clara, CA, United States) software. Mobile phases consisted of a 2% acetic acid solution in ultra-pure water (solution A), and a solution of formic acid, acetonitrile, and ultra-pure water (2:30:68 v/v) (solvent B). The process was carried out with a gradient run as follows: 5% B, 0 min; 50% B, 18 min; 70% B, 35 min; 80% B, 40 min; 100 B, 45 min. Mobile phase flow was 0.5 mL/min and injection volumes were 20 μL for standards and sample extracts. The wavelength used at the detector was 280 nm.
The sample preparation method for the chromatographic analysis followed the procedure used by Rytel et al. (2014) with some modifications. Briefly, 3 g of lyophilized sample was homogenized with 20 mL of methanol (HPLC grade, Merck Millipore, United States) for 10 min at 75 °C with frequent stirring, in a constant temperature bath. After cooling, the samples were centrifuged at 4000 rpm for 10 min at room temperature and subsequently the supernatant was collected and filtrated on filter paper. The extraction process was repeated under the same conditions three times. The final extract volume was filtered with the aid of a 0.20 μm filter and transferred into a 100 mL flask, and then subjected to rotating evaporator to remove the solvent. The dry residue was recovered in a known volume of 1 mL of methanol. Before injection into the column, the samples were filtered again using hydrophilic microfilters.
Phenolic acids were identified and quantified using the following phenolic acid calibration lines: caffeic, chlorogenic, gallic, ferulic, p-hydroxy benzoic, syringic, p-coumaric acids. The identification has been made based on the retention times and the UV spectrum of the aforementioned standards, see Table 1.
Table 1.
Standards used for the determination of phenolic acids.
| Standard | λmax | Equation linearity | R2 |
|---|---|---|---|
| Caffeic acid | 280 | 13.887x-6.0066 | 1 |
| Chlorogenic acid | 280 | 2.9963x+1.1789 | 0.9998 |
| Gallic acid | 280 | 22.279x-2.1165 | 0.9998 |
| p-hydroxybenzoic acid | 280 | 26.879x+6.102 | 0.9985 |
| p-coumaric acid | 280 | 53.748x-17.437 | 0.9994 |
| Syringic acid | 280 | 46.283x+2.674 | 1 |
| Ferulic acid | 280 | 14.481–5.4053 | 1 |
2.2.3. Quantitative determination of anthocyanins
For the determination of the total anthocyanin content, the spectrophotometric method of the pH differential described by Lemos et al. (2015) with some modifications was used. The method has been adapted by Ribereau-Gayon and Stonestreet (1965), and it is based on the specific property of anthocyanins to change colour according to pH. An amount of 0.1 g of freeze-dried potatoes was added to 20 ml of a 50% solution of methanol: water (v/v), re-suspended with the help of a vortex mixer (Velp Scientifica, Usmate Velate, MB, Italy) and subsequently filtered with the use of filter paper. One mL of the filtrate was transferred into a 10 mL volume of a 2% HCL solution (pH 0.8) and 1 mL, on the other hand, was transferred into a 10 mL volume of a buffer solution (pH 3.5), which was prepared by mixing 0.2 M Na2HPO4 and 0.1 M citric acid. Both solutions were stirred thoroughly and subsequently their absorbance was measured at the wavelength of 520 nm, using a spectrophotometer (UV-1601, Shimadzu, Japan). The samples absorbance was read against a 50% methanol blank. The total anthocyanin content (TAC) was calculated using the following equation:
| TAC = (A1-A2) x f |
where:
A1 = Sample absorbance in 2% HCl solution.
A2 = Absorbance of the sample in the buffer solution solution.
f = 396.598.
The total anthocyanin content was expressed as mg of cyanidin/Kg of dry matter.
2.2.4. Determination of antioxidant activity
For the preparation of the extract, 0.1 g of lyophilized sample was dissolved in 10 mL of a 50% solution of ethanol in water, stirred for 10 s and then centrifuged at 6000 rpm for 10 min using a centrifuge ALC PK121R, (ALC International, Cologno Monzese, Italy). The supernatant was taken, filtered on a filter paper and then collected in a flask. The extraction process was repeated 3 times. Samples were stored at –20 °C before being analysed.
The antioxidant activity of the extract was determined using the DPPH method, as indicated by Ravichandran et al. (2014). The stock solution (1 × 10−3 M) was prepared by dissolving 22 mg of DPPH in 50 mL of methanol and stored at a temperature of -20 °C before use. The working solution (6 × 10−5 M) was prepared by mixing 6 mL of the stock solution with 100 mL of methanol, to obtain a solution with an absorbance value of 0.8 ± 0.02 at the wavelength of 515 nm, using a spectrophotometer UV-1601 (Shimadzu, Japan). A volume of 0.1 mL of the extract was reacted with 3.9 mL of the working solution for 30 min, after which the absorbance value was read at a wavelength of 515 nm. The absorbance of the DPPH solution without the addition of the extract was used as a control. The autozero was obtained with methanol. The antioxidant activity was calculated using Trolox as standard whose points on the calibration curve were between the concentrations of 2 and 150 μM (5, 10, 25, 50, 100, 150 μM). The results were expressed as mmol of Trolox/100 g of dry matter.
2.3. Statistical analysis
All analyses were performed in triplicate. Significant differences between the different samples were determined by the unilateral statistical analysis ANOVA and by the PCA (Principal Component Analysis). Tukey's test was used to discriminate between the means of the variables. Differences with p < 0.05 were considered significant. Data processing was carried out using XLStat version 2009.3.02 (Addinsoft Corp., Paris, France).
3. Results and discussion
3.1. Total polyphenols
Potatoes are a good source of phenolic compounds, presenting a surprising variety in terms of profile and content. Phenolic compounds are mainly present in the peel, but also in the pulp of the potato (Ezekiel et al., 2013). Table 2 shows the values related to the total polyphenol content, expressed as gallic acid mg/g dry matter (DM).
Table 2.
Trend of total polyphenols in purple potatoes, at different treatment times.
| Total polyphenols | |
|---|---|
| Time (h) | mg gallic acid/g DM |
| Fresh | 2.60a ± 0.11 |
| F1 | 1.58b,c ±0.22 |
| 8 | 1.30c ± 0.07 |
| 16 | 1.63b,c±0.22 |
| 24 | 1.77b,c±0.25 |
| 32 | 1.91b,±0.00 |
| 40 | 1.70b,c±0.01 |
| 48 | 1.18d ± 0.14 |
a-d: Different letters indicate significant differences (p ≤ 0.05) between treatment times.
The results obtained indicate a reduction, during the heat treatment, of the total polyphenol content, bringing out a significant difference between the fresh sample and the samples subjected to the frying process. In particular, after the 48th hour of heat treatment there was a variation of -120% of total polyphenols compared to the fresh. Statistical analysis showed significant reductions between time F1 (the time measured after the pre-treatment phase, regarding as the starting ime of the frying process) and time 48 h, with values of 1.58 mg gallic acid/g DM and 1.18 mg gallic acid/g DM, respectively. The reduction of total polyphenols observed in this study was in agreement with the results published by Perla et al. (2012) and Tian et al. (2016). In fact, the total polyphenol content in potatoes generally decreases substantially during cooking. Heating during home cooking has been shown to damage the cellular structure of potatoes; this facilitates the extractability and bioaccessibility of chemicals, promotes hydrolysis of bound phytochemicals and forms soluble low molecular weight molecules, which can be easily degraded by heat treatment, inducing a decrease in the total phenolic content (Ruiz-Rodriguez et al., 2008). Furthermore, the increase in cooking time and temperature could cause a loss of phenolic compounds, due to the degradation induced by heat (Palermo et al., 2014). Another explanation of the trend of the results obtained in this study could be given by the chemical composition of the potato, rich in polysaccharides and starch. In fact, Faller and Fialho (2009) reported that polyphenols become unavailable for extraction because they could be encapsulated by polysaccharides with mechanisms which have been hypothesized by Pinelo et al. (2006) only for grape skins but not yet for potatoes.
3.2. Phenolic acids
Phenolic acids exhibit various biological activities in the human body, taking an active part in the removal of free radicals, the chelation of metal ions and influence enzymatic activity (Tian et al., 2016). They represent about one third of the phenols in the diet and can be present in plants in free and bound forms (Robbins, 2003; Durazzo, 2018; Durazzo and Lucarini, 2019a; Durazzo et al., 2019a). Phenolic acids consist of two subgroups: hydroxybenzoic (gallic, p-hydroxybenzoic, protocatechoic, vanillic, and syringic acids), and hydroxycinamic (caffeic, ferulic, p-coumaric, and synapic acids) (Bravo, 1998). Table 3 shows the values relating to the content of phenolic acids, expressed as mg/100 g DM.
Table 3.
Trend of phenolic acids in purple potatoes, at different treatment times.
| Phenolic acids (mg/100 g DM) | Raw | F1 | 8 | 16 | 24 | 32 | 40 | 48 |
|---|---|---|---|---|---|---|---|---|
| Gallic acid | 0.07e ± 0.00 | 2.36b ± 0.02 | 2.52a ± 0.06 | 2.27c ± 0.02 | 2.18c ± 0.02 | 2.23c ± 0.01 | 1.59d ± 0.01 | 1.57d ± 0.01 |
| p-hydroxybenzoic acid | 0.45a ± 0.02 | 0.21d ± 0.00 | 0.24c,d±0.01 | 0.22d ± 0.01 | 0.27c ± 0.01 | 0.33b ± 0.00 | 0.32b ± 0.01 | 0,27c ± 0,01 |
| Chlorogenic acid | 30.5a ± 0.13 | 8.44b ± 0.04 | 7.49c ± 0.06 | 5.89d ± 0.02 | 5.3e,f±0.00 | 5.43e ± 0.11 | 5.33e,f±0.05 | 5.08f ± 0.03 |
| Caffeic acid | 1.04a ± 0.02 | 0.55b ± 0.01 | 0.46c ± 0.00 | 0.45c ± 0.01 | 0.4d ± 0.01 | 0.4d ± 0.02 | 0.37d,e±0.00 | 0.33e ± 0.01 |
| p-coumaric acid | 0.04a ± 0.00 | 0.07c ± 0.00 | 0.08c ± 0.00 | 0.08c ± 0.00 | 0.09b ± 0.00 | 0.09b ± 0.00 | 0.08c ± 0.00 | 0.09c ± 0.00 |
| Syringic acid | 0.04f ± 0.00 | 0.02e ± 0.00 | 0.02d ± 0.00 | 0.02c ± 0.00 | 0.02a ± 0.00 | 0.02a,b±0.00 | 0.02b,c±0.00 | 0.02a,b±0.00 |
| Ferulic acid | 0.02c ± 0.00 | 2.5a ± 0.20 | 1.66b ± 0.26 | 1.72b ± 0.04 | 1.82b ± 0.02 | 1.61b ± 0.21 | 1.66b ± 0.08 | 1.81b ± 0.01 |
a-e: Different letters indicate significant differences (p ≤ 0.05) between treatment times.
Among the phenolic acids identified in the fresh potato sample, chlorogenic acid is the most abundant (30.5 mg/100 g DM), followed by caffeic acid (1.04 mg/100 g DM) and p-hydroxybenzoic acid (0.45 mg/100 g DM). For the other phenolic acids, quantities ranging from 0.02 (ferulic acid) to 0.07 (gallic acid) mg/100 g DM have been detected. The results relating to the effect of the heat treatment show a different trend for each type of phenolic acid.
In particular, considering the starting of the heat processing (time 0) and 48-hour values, a decrease of 500% was recorded for chlorogenic acid, with values that gradually decreased. For caffeic acid, on the other hand, there was a decrease of 215%. For the p-hydroxybenzoic acid, the decrease was 66%, with a variable quantity trend, which touched values during the frying process between 0.21 (time 0) and 0.33 mg/100 g DM (time 32 h). For syringic acid, the decrease was 100%, with values which do not present significant differences during the frying process. Only three of seven phenolic acids showed an increase. For gallic acid, the trend showed a value in fresh potatoes equal to 0.07 mg/100 g DM and then reached 2.36 mg/100 g DM at time 0. During the subsequent treatment times the value decreased with the increase of the frying time. For p-coumaric acid, the increase showed a minimum value of 0.04 mg/100 g DM (fresh) and a maximum of 0.09 mg/100 g DM (time 48 h), showing a non-linear increase during the time of treatment. For ferulic acid, the trend showed a value in fresh potatoes equal to 0.02 mg/100 g dm up to reach 2.50 mg/100 g DM at time 0. During the subsequent treatment times the value decreases with the advance the hours of frying. The mechanisms of formation and destruction of phenolic acids are not well understood as reported in the literature (Tian et al., 2016). The reduction in the content of chlorogenic acid and other phenolic acids, in contrast to the increase of the other acids, could be explained by the fact that these compounds have a different stability during treatment, depending on their chemical structure (Burgos et al., 2013). In a heat treatment, high temperatures could destroy the cell walls of the food matrix and stimulate the release of phenolic compounds. As a consequence, the variation of these compounds could be due to the fact that, after the heat treatment, some reactions, such as the breaking of the glycosidic bond between the phenol and the sugar, favour the formation of phenolic aglycones. On the other hand, the opposite situation could occur, in which the reduction of phenolic compounds could be motivated by the isomerization of some compounds that are more sensitive to high temperatures (Perla et al., 2012). In fact, as reported by Tian et al. (2016), phenolic acids belonging to the same class behave differently even if subjected to the same cooking method.
3.3. Total anthocyanins
Anthocyanins belong to a class of molecules called flavonoids which occur in all plant tissues, including leaves, stems, roots, flowers, and fruits. Anthocyanidins (or aglycones) are the basic structures of anthocyanins and consist of an aromatic ring A bonded to a heterocyclic ring C that contains oxygen, which is also bonded by a carbon-carbon bond to a third aromatic ring B (Konczak and Zhang, 2004; Wallace and Giusti, 2015). When anthocyanidins are found in their glycosylated form (linked to a sugar fraction), they are known as anthocyanins. Anthocyanins are highly unstable compounds, and their stability can be influenced by parameters such as: temperature, pH, oxygen, and light (Tian et al., 2016). They are responsible for the colour of potatoes and of other vegetables and exert an important antioxidant activity (Jansen and Flamme, 2006). The values of total anthocyanins expressed as mg of cyanidin/kg of dry matter, for fresh purple potato samples and those subjected to different heat treatment times, are shown in Table 4.
Table 4.
Trend of total anthocyanins in the purple potato, at different treatment times.
| Total anthocyanins | |
|---|---|
| Time (h) | mg cyanidin/kg DM |
| Raw | 644.35b ± 4.32 |
| F1 | 1114.64a ± 46.27 |
| 8 | 1044.84a ± 58.61 |
| 16 | 1033.93a ± 24.68 |
| 24 | 1090.64a ± 129.56 |
| 32 | 1047.02a ± 49.36 |
| 40 | 1095.01a ± 92.54 |
| 48 | 1129.91a ± 12.34 |
a-b: Different letters indicate significant differences (p ≤ 0.05) between treatment times.
The results indicate a significant difference between the fresh sample and the samples subjected to different heat treatment times, with an increase of approximately 75%. This increase could be attributed to the fact that cooking or pre-treatment systems, such as those used in this study, could destroy the cellular structure of the potato and, therefore, have a positive effect on the extraction of these components. Furthermore, the high temperatures could favour the inactivation of the polyphenol oxidase enzyme, responsible for the degradation of anthocyanins (Lemos et al., 2015). There are no significant differences between the samples at time F1 and time 48, with an average total anthocyanin value of 1050 mg cyanidin/100 dm. The results obtained are in agreement with Lachman et al. (2013) and Lemos et al. (2015), studies in which it was shown that the heat treatments to which purple potatoes were subjected cause an increase in total anthocyanins when compared with fresh samples. Although there may be positive feedback with the other authors, albeit with different values, there is no lack of results in the literature that contradict the present work (Perla et al., 2012). The discrepancies could be attributed to the different methods of cooking and extraction of the anthocyanins used, without neglecting that the content of the starting anthocyanins could vary with factors such as: the cultivar, pedoclimatic conditions and post-harvest conservation (Lachman et al., 2012).
3.4. Antioxidant activity
Antioxidants are compounds that, in the presence of an oxidizable substrate and an oxidizing agent, significantly delay or inhibit the oxidation of the substrate (Wolfe et al., 2008). They also play an important role in the diet, contributing to the reduction of the risk of various chronic diseases (Vinson et al., 2012). Daily consumption and affordable economic value could make the purple potato one of the most important sources of antioxidants in the diet (Liu, 2013). The evaluation of the antioxidant properties as expression of combined and concerted action of biologically active compounds and nutrients can be viewed as a first step of quality and beneficial properties of a food matrix (Durazzo, 2017; Durazzo, 2018; Durazzo and Lucarini, 2019a, Durazzo and Lucarini, 2019b). Moreover, as remarked in previous works, it is needed to have data on antioxidant properties in cooked products, processed food and also in complex food matrices (Durazzo et al., 2017, 2019b). Table 5 shows the values relating to the antioxidant activity, expressed as mmol Trolox/100 g DM.
Table 5.
Trend of antioxidant activity in the purple potato, at different treatment times.
| Antioxidant activity | |
|---|---|
| Time (h) | mmol Trolox/100 g DM |
| Raw | 1.82c ± 0.03 |
| F1 | 3.89a ± 0.18 |
| 8 | 3.64a,b ±0.15 |
| 16 | 3.08b ± 0.22 |
| 24 | 3.49a,b ±0.05 |
| 32 | 3.90a ± 0.23 |
| 40 | 3.67a,b ±0.07 |
| 48 | 3.32a,b ±0.12 |
a-c: Different letters indicate significant differences (p ≤ 0.05) between treatment times.
The antioxidant activity value of the samples subjected to the frying process varied significantly when compared with the fresh potato sample, showing an increase between the fresh sample and time 48 h of +82.4%. The phytonutrient content of potatoes changes with the cooking processes, and consequently, the value of the antioxidant activity also changes. However, the results obtained are in agreement with Blessington et al. (2010), Burgos et al. (2013), and Yang et al. (2016), where a positive correlation is reported between the cooking method (steam, microwave, and frying) and the increase in antioxidant activity in purple potatoes has been observed. Furthermore, as Xu et al. (2016) observed, the combination of different cooking systems, with appropriate operating conditions, contributes to positively increasing this value. This finding is in line with most of the studies that address the topic, even if there are contradictory literature data affirming the opposite (Perla et al., 2012).
During the frying process, the antioxidant activity varied from 3.89 mmol Trolox/100 g DM to 3.32 mmol Trolox/100 g DM with not significant differences. Although phytonutrients play a very important role in the antioxidant characteristics of potatoes subjected to heat treatment, they are not the only compounds that contribute to the antioxidant activity (Nicoli et al., 1999). In particular, Kita et al. (2013), determining the antioxidant activity of purple potatoes during the frying process, found a decrease in the value of anthocyanins and polyphenols in potato chips, but an increase in antioxidant activity. Xu et al. (2009) found in his study no significant correlation between the decrease in phytonutrients and antioxidant power by testing different cooking systems. This could be due to the fact that, although the high treatment temperatures (100–180 °C) can lead to a decrease in the value of natural antioxidants, they can also promote numerous reactions such as: caramelization, Maillard reaction, Strecker degradation, hydrolysis of esters and glycosides, of the antioxidants and the oxidation of phenolic groups to quinones and their polymers, which may produce new substances with antioxidant activity (Nicoli et al., 1999).
4. Conclusions
Deep-frying process of purple potato Purple Majesty variety using a microwave drying treatment affects the polyphenols, anthocyanins, and antioxidant activity. It can be observed that the antioxidants present in these potatoes are capable of significantly delay or inhibit the oxidation of the substrate and play an important role also in the diet, contributing to the reduction of the risk of various chronic diseases. This seems to suggest that the regular consumption of purple potato could be an interesting source of antioxidants in the diet and add beneficial properties to a food matrix which can be considered low cost and affordable for consumers. The frying treatment seems to affect positively the antioxidant activity compared to the fresh (raw) potatoes (from 1.82 to 3.32 mmol Trolox/100 g DM). The polyphenols experience a diminution in quantity while the ferulic acid and gallic acid seem to increase in quantity (from 0.07 to 1.57 and from 0.02 to 1.81 mg/100 g DM, respectively after 48 h of heat treatment). The anthocyanins show an increase in their amount almost linear in the frying time range from 644.35 to 1129.91 mg cyanidin/kg DM. These data suggest that the deep frying in discontinuous mode simulating the “fast food” processing allows to maintain acceptable amounts of polyphenols and anthocyanins in the food matrix evaluated and at the same time to maintain a good antioxidant activity after the heat treatment. Further studies are necessary to evaluate the effect of deep frying, cutting and microwaving coloured potato on other compounds, such as starch and sugars.
Declarations
Author contribution statement
Raffaele Romano, Fabiana Pizzolongo, Alessandra Durazzo, Massimo Lucarini, Patricia Severino, Eliana B. Souto, Antonello Santini: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Alessandra Aiello, Lucia De Luca: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Amarowicz R., Carle R., Dongowski G., Durazzo A., Galensa R., Kammerer D., Maiani G., Piskula M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009;53(Suppl 2):S151–S183. doi: 10.1002/mnfr.200700486. [DOI] [PubMed] [Google Scholar]
- Andre C.M., Oufir M., Guignard C., Hoffmann L., Hausman J.F., Evers D., Larondelle Y. Antioxidant profiling of native Andean potato tubers (Solanum tuberosum L.) reveals cultivars with high levels of β-carotene, alpha-tocopherol, chlorogenic acid, and petanin. Agric. Food Chem. 2007;55:10839–10849. doi: 10.1021/jf0726583. [DOI] [PubMed] [Google Scholar]
- Andre C.M., Larondelle Y., Evers D. Dietary antioxidants and oxidative stress from a human and plant perspective: a review. Curr. Nutr. Food Sci. 2010;6(1):2–12. [Google Scholar]
- Bellumoria M., Innocenti M., Michelozzi M., Cerretani L., Mulinacci N. Coloured-fleshed potatoes after boiling: promising sources of known antioxidant compounds. J. Food Compos. Anal. 2017;59:1–7. [Google Scholar]
- Blessington T., Nzaramba M.N., Scheuring D.C., Hale A.L., Reddivari L., Miller J.C. Cooking methods and storage treatments of potato: effects on carotenoids, antioxidant activity, and phenolics. Am. J. Potato Res. 2010;87(6):479–491. [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998;56(11):317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- British potato variety database. https://varieties.ahdb.org.uk
- Burgos G., Amoros W., Muñoa L., Sosa P., Cayhualla E., Sanchez C., Díaz C., Bonierbale M. Total phenolic, total anthocyanin and phenolic acid concentrations and antioxidant activity of purple-fleshed potatoes as affected by boiling. J. Food Compos. Anal. 2013;30:6–12. [Google Scholar]
- Burgos G., Zum Felde T., Andre C., Kubow S. In: The Potato Crop. Campos H., Ortiz O., editors. Springer Cham; Hillerod, Denmark: 2020. The potato and its contribution to the human diet and health. [Google Scholar]
- Camire M.E., Kubow S., Donnelly D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009;49(10):823–840. doi: 10.1080/10408390903041996. [DOI] [PubMed] [Google Scholar]
- Da Silva P.F., Moreira R.G. Vacuum frying of high-quality fruit and vegetable-based snacks. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2008;41(10):1758–1767. [Google Scholar]
- Daliu P., Santini A., Novelino E. From pharmaceuticals to nutraceuticals: bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019;12(1):1–7. doi: 10.1080/17512433.2019.1552135. [DOI] [PubMed] [Google Scholar]
- Daliu P., Santini A., Novellino E. A decade of nutraceutical patents: where are we now in 2018? Expert Opin. Ther. Pat. 2018;2018(12):875–882. doi: 10.1080/13543776.2018.1552260. [DOI] [PubMed] [Google Scholar]
- Deng J., Yang H., Capanoglu E., Cao H., Xiao J. Polyphenols: Properties, Recovery, and Applications. Woodhead Publishing Books, Elsevier; Sawston, United Kingdom: 2018. Technological aspects and stability of polyphenols; pp. 295–323. [Google Scholar]
- Durazzo A. Study approach of antioxidant properties in foods: update and considerations. Food. 2017;6(3):17. doi: 10.3390/foods6030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A. Extractable and Non-extractable polyphenols: an overview. Non-extractable Polyphenols and Carotenoids: Imp. Human Nutr. Health. 2018;2018:37–45. Editors: Fulgencio Saura-Calixto, Jara Pérez-Jiménez. RSC publishing., Cambridge, UK. [Google Scholar]
- Durazzo A., Lucarini M. Extractable and non-extractable Antioxidants. Molecules. 2019;24(10):1933. doi: 10.3390/molecules24101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A., Lucarini M. Current shot and Re-thinking of antioxidant research strategy. Br. J. Anal. Chem. 2019;5(20):9–11. [Google Scholar]
- Durazzo A., Lisciani S., Camilli E., Gabrielli P., Marconi S., Gambelli G., Aguzzi A., Lucarini M., Maiani G., Casale G., Marletta L. Nutritional composition and antioxidant properties of traditional Italian dishes. Food Chem. 2017;218:70–77. doi: 10.1016/j.foodchem.2016.08.120. [DOI] [PubMed] [Google Scholar]
- Durazzo A., Lucarini M., Souto E.B., Cicala C., Caiazzo E., Izzo A.A., Novellino E., Santini A. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33(9):2221–2224. doi: 10.1002/ptr.6419. [DOI] [PubMed] [Google Scholar]
- Durazzo A., Lucarini M., Santini A., Camilli E., Gabrielli P., Marconi S., Lisciani S., Aguzzi A., Gambelli L., Novellino E., Marletta L. Antioxidant properties of four commonly consumed popular Italian dishes. Molecules. 2019;24(8):1543. doi: 10.3390/molecules24081543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A., Lucarini M., Santini A. Nutraceuticals in human health. Foods. 2020;9(3):370. doi: 10.3390/foods9030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel R., Singh N., Sharma S., Kaur A. Beneficial phytochemicals in potato - a review. Food Res. Int. 2013;50:487–496. [Google Scholar]
- Faller A.L.K., Fialho E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 2009;42(1):210–215. [Google Scholar]
- Firestone D. In: Deep Frying. second ed. Erickson Michael D., editor. AOCS Press; Urbana, IL, United States: 2007. Regulation of frying fat and oil; pp. 373–385. [Google Scholar]
- Gebhardt C. The historical role of species from the Solanaceae plant family in genetic research. Theor. Appl. Genet. 2016;129(12):2281–2294. doi: 10.1007/s00122-016-2804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G., Flamme W. Coloured potatoes (Solanum tuberosum L.)–anthocyanin content and tuber quality. Genet. Resour. Crop Evol. 2006;53(7):1321–1331. [Google Scholar]
- Kalita D., Holm D.G., Jayanty S.S. Role of polyphenols in acrylamide formation in the fried products of potato tubers with colored flesh. Food Res. Int. 2013;54(1):753–759. [Google Scholar]
- Kalogianni E.P., Karastogiannidou C., Karapantsios T.D. Effect of potato presence on the degradation of extra virgin olive oil during frying. Int. J. Food Sci. Technol. 2010;45:765–775. [Google Scholar]
- Kita A., Bakowska-Barczak A., Hamouz K., Kułakowska K., Lisińska G. The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum tuberosum L.) J. Food Compos. Anal. 2013;32(2):169–175. [Google Scholar]
- Kita A., Bąkowska-Barczak A., Lisińska G., Hamouz K., Kułakowska K. Antioxidant activity and quality of red and purple flesh potato chips. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2015;62(1):525–531. [Google Scholar]
- Konczak I., Zhang W. Anthocyanins—more than nature's colours. J. Biomed. Biotechnol. 2004;2004(5):239. doi: 10.1155/S1110724304407013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman J., Hamouz K., Orsák M., Pivec V., Dvořák P. The influence of flesh colour and growing locality on polyphenolic content and antioxidant activity in potatoes. Sci. Hortic. 2008;117(2):109–114. [Google Scholar]
- Lachman J., Hamouz K., Orsák M., Pivec V., Hejtmánková K., Pazderu K., Dvořákb P., Čeplc J. Impact of selected factors – cultivar, storage, cooking and baking on the content of anthocyanins in coloured-flesh potatoes. Food Chem. 2012;133(4):1107–1116. [Google Scholar]
- Lachman J., Hamouz K., Musilová J., Hejtmánková K., Kotíková Z., Pazderů K., Domkářová J., Pivec V., Cimr J. Effect of peeling and three cooking methods on the content of selected phytochemicals in potato tubers with various colour of flesh. Food Chem. 2013;138:1189–1197. doi: 10.1016/j.foodchem.2012.11.114. [DOI] [PubMed] [Google Scholar]
- Lemos M.A., Aliyu M.M., Hungerford G. Influence of cooking on the levels of bioactive compounds in Purple Majesty potato observed via chemical and spectroscopic means. Food Chem. 2015;173:462–467. doi: 10.1016/j.foodchem.2014.10.064. [DOI] [PubMed] [Google Scholar]
- Liu R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013;78(1):18–25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- Nicoli M.C., Anese M., Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999;10(3):94–100. [Google Scholar]
- Palermo M., Pellegrini N., Fogliano V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014;94(6):1057–1070. doi: 10.1002/jsfa.6478. [DOI] [PubMed] [Google Scholar]
- Perla V., Holm D.G., Jayanty S.S. Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2012;45(2):161–171. [Google Scholar]
- Pinelo M., Arnous A., Meyer A.S. Upgrading of grape skins: significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006;17(11):579–590. [Google Scholar]
- Ravichandran R., Rajendran M., Devapiriam D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014;146:472–478. doi: 10.1016/j.foodchem.2013.09.080. [DOI] [PubMed] [Google Scholar]
- Ribéreau-Gayon P., Stonestreet E. Determination of anthocyanins in red wine. Bull. Soc. Chim. Fr. 1965;9:2649–2652. [PubMed] [Google Scholar]
- Robbins R.J. Phenolic acids in foods: an overview of analytical methodology. J. Agric. Food Chem. 2003;51(10):2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- Romano R., Giordano A., Vitiello S., Le Grottaglie L., Spagna Musso S. Comparison of the frying performance of olive oil and palm superolein. J. Food Sci. 2012;77(5):C519–C531. doi: 10.1111/j.1750-3841.2012.02663.x. [DOI] [PubMed] [Google Scholar]
- Romano R., Filosa G., Pizzolongo F., Durazzo A., Lucarini M., Severino P., Souto E.B., Santini A. Oxidative stability of high oleic sunflower oil during deep-frying process of purple potato Purple Majesty. Heliyon. 2021;7(3) doi: 10.1016/j.heliyon.2021.e06294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotondo A., La Torre G.L., Dugo G., Cicero G., Santini A., Salvo A. Oleic acid is not the only relevant mono-unsaturated fatty ester in olive oil. Foods. 2020;9:384. doi: 10.3390/foods9040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Rodriguez A., Marín F.R., Ocaña A., Soler-Rivas C. Effect of domestic processing on bioactive compounds. Phytochem. Rev. 2008;7(2):345–384. [Google Scholar]
- Rytel E., Tajner-Czopek A., Kita A., Aniołowska M., Kucharska A.Z., Sokół-Łętowska A., Hamouz K. Content of polyphenols in coloured and yellow fleshed potatoes during dices processing. Food Chem. 2014;161:224–229. doi: 10.1016/j.foodchem.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Santini A., Cicero N. Development of food chemistry, natural products, and nutrition research: targeting new frontiers. Foods. 2020;9:482. doi: 10.3390/foods9040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini A., Cammarata S.M., Capone G., Ianaro A., Tenore G.C., Pani L., Novellino E. Nutraceuticals: opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018;84(4):659–672. doi: 10.1111/bcp.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini A., Novellino E. Nutraceuticals: shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018;11(6):545–547. doi: 10.1080/17512433.2018.1464911. [DOI] [PubMed] [Google Scholar]
- Santini A., Tenore G.C., Novellino E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharmacol. Sci. 2017;96:53–61. doi: 10.1016/j.ejps.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Tian J., Chen J., Lv F., Chen S., Chen J., Liu D., Ye X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016;197:1264–1270. doi: 10.1016/j.foodchem.2015.11.049. [DOI] [PubMed] [Google Scholar]
- Vinson J.A., Demkosky C.A., Navarre D.A., Smyda M.A. High-antioxidant potatoes: acute in vivo antioxidant source and hypotensive agent in humans after supplementation to hypertensive subjects. J. Agric. Food Chem. 2012;60(27):6749–6754. doi: 10.1021/jf2045262. [DOI] [PubMed] [Google Scholar]
- Visvanathan R., Jayathilake C., Chaminda Jayawardana B., Liyanage R. Health-beneficial properties of potato and compounds of interest. J. Sci. Food Agric. 2016;96(15):4850–4860. doi: 10.1002/jsfa.7848. [DOI] [PubMed] [Google Scholar]
- Wallace T.C., Giusti M.M. Anthocyanins. Adv. Nutr. 2015;6(5):620–622. doi: 10.3945/an.115.009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K.L., Kang X., He X., Dong M., Zhang Q., Liu R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008;56(18):8418–8426. doi: 10.1021/jf801381y. [DOI] [PubMed] [Google Scholar]
- Xu X., Li W., Lu Z., Beta T., Hydamaka A.W. Phenolic content, composition, antioxidant activity, and their changes during domestic cooking of potatoes. J. Agric. Food Chem. 2009;57(21):10231–10238. doi: 10.1021/jf902532q. [DOI] [PubMed] [Google Scholar]
- Xu Y., Chen Y., Cao Y., Xia W., Jiang Q. Application of simultaneous combination of microwave and steam cooking to improve nutritional quality of cooked purple sweet potatoes and saving time. Innovat. Food Sci. Emerg. Technol. 2016;36:303–310. [Google Scholar]
- Yang Y., Achaerandio I., Pujola M. Effect of the intensity of cooking methods on the nutritional and physical properties of potato tubers. Food Chem. 2016;197:1301–1310. doi: 10.1016/j.foodchem.2015.11.028. [DOI] [PubMed] [Google Scholar]
- Zaheer K., Akhtar M.H. Potato production, usage, and nutrition--A review. Crit. Rev. Food Sci. Nutr. 2016;56(5):711–721. doi: 10.1080/10408398.2012.724479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.