Abstract
Rationale & Objective:
Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) have been found to have many benefits for patients with type 2 diabetes (T2D). However, whether SGLT-2i increase the risk of acute kidney injury (AKI) remains unknown. We examined the association of AKI hospitalization with prior initiation of an SGLT-2i compared to initiation of a dipeptidyl peptidase 4 inhibitor (DPP-4i) or a glucagon-like peptide 1 receptor agonist (GLP-1RA) among older adults with T2D in routine practice.
Study Design:
Population-based cohort study.
Setting and Participants:
Older adults aged ≥ 66 years with T2D enrolled in Medicare fee-for-service and who were new users of SGLT-2i, DPP-4i, or GLP-1RA agents in the interval from March 2013 to December 2017.
Exposures:
New use of SGLT-2i versus new use of DPP-4i or GLP-1RA.
Outcome:
The primary outcome was hospitalization for AKI, defined as a discharge diagnosis of AKI in the primary or secondary position.
Analytical Approach:
New users of SGLT-2i were 1:1 matched to new users of DPP-4i or GLP-1RA using propensity scores in two pairwise comparisons. Cox proportional hazards regression models generated hazard ratios (HRs) with 95% confidence intervals (CIs) in propensity score matched groups.
Results:
A total of 68,130 and 71,477 new users of SGLT-2i were matched to new users of DPP-4i or GLP-1RA, respectively. Overall, the mean age of study participants was 72 years. The risk of AKI was lower in the SGLT-2i group than the DPP-4i group (HR 0.71, 95% CI 0.65–0.76) or the GLP-1RA group (HR 0.81, 95% CI 0.75–0.87).
Limitations:
Residual confounding and lack of laboratory data.
Conclusions:
Among older adults with T2D, initiation of an SGLT-2i was associated with a reduced risk of AKI compared to initiation of a DPP-4i or GLP-1RA.
Index words: acute kidney injury, SGLT-2i, DPP-4i, GLP-1RA, type 2 diabetes, older adults
Plain-Language Summary
SGLT-2i, DDP-4i, and GLP-1RA are three newer classes of glucose-lowering medications to treat type 2 diabetes. The beneficial effects of SGLT-2i extend beyond glycaemic control, and include reduction in cardiovascular events, kidney disease progression, and mortality. However, there was a concern that SGLT-2i might increase the risk of acute kidney injury. In this nationwide study using Medicare data, we found that the initiation of a SGLT-2i was associated with a lower risk of acute kidney injury compared to initiation of a DPP-4i or a GLP-1RA among matched older adults with type 2 diabetes. Our results add to the available evidence on the safety profile of SGLT-2i in older adults.
INTRODUCTION
Type 2 diabetes (T2D) is a major public health problem with high risk of complications and excess risk of death.1 In 2017, 8.5% (27.6 million) of U.S. adults were estimated to have T2D, with higher prevalence—almost one in five—among adults aged 65 years or greater.2
Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) are a novel class of oral diabetes medications that reduce the risk of hospitalization for heart failure, kidney disease progression, kidney failure, death from renal or cardiovascular causes, and all-cause mortality in large randomized controlled trials (RCTs).3–11 Consequently, guidelines recommend SGLT-2i as one of the add-on diabetes medications to metformin in patients with atherosclerotic cardiovascular disease or high risk, clinical heart failure, and chronic kidney disease (CKD).12 Despite the renoprotective effects with long-term treatment, there is an acute drop in glomerular filtration rate with SGLT-2i initiation and concern that SGLT-2i may lead to acute kidney injury (AKI) due to hypovolemia, excessive decline in transglomerular pressure through tubuloglomerular feedback, uricosuric action, and renal medullary hypoxia.13–15 From March 2013 to October 2015, the Food and Drug Administration (FDA) received reports of 101 confirmable cases of AKI related to SGLT-2i, 58 of them developed AKI within one month of SGLT-2i initiation. Ninety-six of the 101 patients required hospitalization, and 15 required hemodialysis. While the majority recovered, 11 were left with CKD and four died during hospitalization. Based on these postmarketing reports, the FDA issued an initial warning in December 2015 and then strengthened the warning in June 2016 that SGLT-2i might cause AKI.16
In contrast, RCTs have found that the incidence of AKI in SGLT-2i treated patients does not increase6–8,10 and may even be attenuated5,9,17 compared to placebo, and a recent network meta-analysis of RCTs indicated that SGLT-2i may have a lower AKI risk compared with both dipeptidyl peptidase 4 inhibitors (DPP-4i) and glucagon-like peptide 1 receptor agonist (GLP-1RA).18 However, in these trials AKI was documented as an adverse event, rather than a pre-specified outcome, and the included populations were not representative of the patients at the greatest risk for AKI, e.g., routine care patients older than 65 years with multiple co-morbidities and polypharmacy. To address the current knowledge gap, we investigated the association of SGLT-2i initiation with the risk of AKI hospitalization in older adults with T2D.
METHODS
Study Design and Data Sources
We conducted a population-based, longitudinal cohort study using Medicare fee-for-service data. Medicare is a nationwide U.S. federal health insurer for eligible individuals primarily aged 65 and older and provides coverage for inpatient services (Part A), outpatient services (Part B), and prescription medications (Part D). We leveraged Medicare claims data from Parts A, B, and D, which include dates and place of service, International Classification of Disease, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM and ICD-10-CM) codes, Current Procedural Terminology, Fourth Edition (CPT-4) codes, provider type, National Drug Codes (NDCs), and prescription drug days supplied. This study was based on deidentified patient data, so individual-level informed consent was not obtained. This study was approved by the Brigham and Women’s Institutional Review Board (approval number 2011P002580), and an appropriate data use agreement was in place.
Study Population
We used an active comparator, new-user design, where the initiation of SGLT-2i was compared to the initiation of DPP-4i or GLP-1RA in two pair-wise comparisons. DPP-4i and GLP-1RA were chosen as active comparators to SGLT-2i, since any of these three novel diabetes classes may have been selected as a second-line treatment for T2D, per clinical guidelines recommended at that time.19
We included patients aged ≥ 66 years with T2D who were newly filled a prescription for an SGLT-2i or the comparator, i.e., either DPP-4i or GLP-1RA, between March 29, 2013 (date of the first SGLT-2i approval in the U.S.) and December 31, 2017 (latest available data). For each pair-wise comparison, the cohort entry date was the date of the first prescription of an SGLT-2i or the specific comparator during the study period. Eligible patients must have had at least 365 days of Medicare Part A, B and D enrollment before cohort entry. We excluded patients with prior use of SGLT-2i or the specific comparator (depending on the pair-wise comparison) in the 365-day period, as well as those who received more than one medication of interest on the cohort entry date. We also excluded those who had a diagnosis of any of the following during the 365-day period prior to cohort entry: type 1 diabetes, secondary or gestational diabetes, malignancy, kidney failure, human immunodeficiency virus infection, organ transplant, or nursing home care. Patients with missing demographic information (age, gender, race, or region) or who were disenrolled on the cohort entry day were excluded. Individuals meeting the inclusion criteria could contribute to each comparison only once.
Follow-up and Outcomes
Follow-up started on the day after cohort entry until the occurrence of any of the following: an outcome event, death, switching to a comparator class, discontinuation of index therapy, end of study period, or end of healthcare or pharmacy enrollment. Medication use was evaluated by prescription refill date and supply. A medication was considered to be discontinued if 60 days elapsed after expiration of the last prescription’s supply without the prescription being refilled. We considered patients to be at risk for an event up to 60 days after their last prescription would be expected to run out based on supply to allow for intermittent adherence.
The primary outcome was a hospitalization for AKI, defined as a hospitalization with a discharge diagnosis of AKI in the primary or secondary position (first two positions). Secondary outcomes included AKI hospitalization within 30 days after medication initiation, AKI hospitalization with an AKI discharge diagnosis in the primary position, in any position, AKI hospitalization requiring dialysis, and hypovolemia (Table S1). Hypovolemia was defined as a hospitalization with a discharge diagnosis of hypovolemia in the primary or secondary position. In prior investigations, the use of claims data to identify AKI has been validated against hospital records (with AKI defined as a ≥ 0.5 mg/dl increment of serum creatinine). It was found that an AKI discharge diagnosis in any position had a positive predictive value of 86% and a specificity of 97%.20 A claims-based definition of AKI requiring dialysis has also been previously validated with a positive predictive value of 94% and a specificity of 93%.20
Statistical Analyses
To mitigate risk of confounding by indication, we used 1:1 propensity score matching. Propensity scores were calculated using a multivariable logistic regression that modeled the probability of initiating an SGLT-2i versus a DPP-4i or a GLP-1RA as a function of more than 100 pre-defined baseline characteristics. These baseline covariates were selected based on previous studies21 and clinical experience, and were assessed in the 365-day period before cohort entry unless otherwise specified. Covariates included demographics (such as age, gender, race, or region), comorbid conditions (for example, CKD, history of AKI, heart failure, or claims-based frailty index22,23), drugs (including metformin, insulin, or diuretics), and health care utilization (such as serum creatinine test). For each of the two pair-wise comparisons, we created a 1:1 propensity score matched cohort using a nearest-neighbor matching approach within a maximum caliper width of 0.01 on the propensity score.24 We assessed covariate balance among the matched cohorts by using standardized differences, with values less than 0.1 suggesting negligible differences between matched groups.25 Cox proportional hazards models were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs). Kaplan-Meier plots were generated to visualize the cumulative incidence of AKI over time in each group. All analyses were performed using Aetion Evidence Platform® (2020) version 4.12, software26 for real-world data analysis, which has been previously validated for a range of studies.27,28
We performed several sensitivity analyses on the primary outcome to assess the robustness of the study findings. We changed the grace period and risk periods from 60 days to 30 days. In addition to the primary as-treated analysis, we carried the index exposure forward to 365 days, disregarding treatment discontinuation or switching, to mimic an intention-to-treat (ITT) approach. We also evaluated potential residual confounding by quantifying the association between SGLT-2i with two control outcomes shown previously to be positively or negatively associated with this drug class: diabetic ketoacidosis and heart failure hospitalization.28–32
We also quantified the association of SGLT-2i and AKI hospitalization in several relevant subgroups: (1) patients aged < 72 versus ≥ 72 years; (2) female versus male; (3) baseline non-metformin users versus metformin users; (4) no history of heart failure versus a history of heart failure; (5) no history of CKD versus a history of CKD; (6) no history of AKI versus a history of AKI. A separate propensity score calculation and matching procedure was performed for each subgroup analysis using the same methods as in the primary analysis.33
RESULTS
Study Cohort and Patient Characteristics
A total of 417,304 patients met study inclusion and exclusion criteria for the SGLT-2i versus DPP-4i cohort (74,161 SGLT-2i users and 343,143 DPP-4i users) and 211,235 met inclusion and exclusion criteria for the SGLT-2i versus GLP-1RA cohort (111,685 SGLT-2i users and 99,550 GLP-1RA users) (Figures S1 and S2). Propensity score distribution before and after matching for both cohorts were shown in Figure S3. Compared to patients initiating DPP-4i or GLP-1RA, patients initiating SGLT-2i had less kidney disease burden as reflected by lower rates of CKD, history of AKI, diabetic nephropathy, and hypertensive nephropathy (Tables S2 and S3). After 1:1 propensity score matching, we identified 136,260 patients (68,130 pairs) initiating either SGLT-2i or DPP-4i and 142,954 patients (71,477 pairs) initiating either SGLT-2i or GLP-1RA. In both matched cohorts, approximately 65% of SGLT-2i patients were prescribed canagliflozin, followed by empagliflozin (20%) and dapagliflozin (15%). The mean age was 72 years, about half were male, and the majority of them were White. On average, patients were taking more than two diabetes agents at baseline: approximately three-quarters of them were prescribed metformin and almost one-third were prescribed insulin. After matching, baseline characteristics were well balanced between SGLT-2i and DPP-4i or GLP-1RA groups with standardized differences < 0.1 (Table 1). The median (interquartile range, IQR) duration of follow-up was 181 (88–370) days amongst SGLT-2i users and 197 (103–397) days amongst DPP-4i users in the matched SGLT-2i versus DPP-4i cohort, and 180 (88–377) days amongst SGLT-2i users and 164 (88–341) days amongst GLP-1RA users in the matched SGLT-2i versus GLP-1RA cohort. Overall, more than half of the patients discontinued the index medication during the follow-up period (Table S4).
Table 1.
Selected baseline characteristics of SGLT-2i versus DPP-4i and SGLT-2i versus GLP-1RA cohorts after 1:1 propensity score matching.
| SGLT-2i versus DPP-4i (n=68,130 pairs) | SGLT-2i versus GLP-1RA (n=71,477 pairs) | |||||
|---|---|---|---|---|---|---|
| Characteristic | SGLT-2i | DPP-4i | St. D | SGLT-2i | GLP-1RA | St. D |
| Age, mean (SD), years | 71.9 (5.1) | 71.8 (5.2) | 0.02 | 71.9 (5.1) | 71.9 (5.2) | 0.00 |
| Male | 34,244 (50.3%) | 34,157 (50.1%) | 0.00 | 33,174 (46.4%) | 33,190 (46.4%) | 0.00 |
| Race | ||||||
| White | 56,252 (82.6%) | 56,245 (82.6%) | 0.00 | 59,366 (83.1%) | 59,070 (82.6%) | 0.01 |
| Black | 4,952 (7.3%) | 4,929 (7.2%) | 0.00 | 5,373 (7.5%) | 5,458 (7.6%) | 0.00 |
| Other | 6,926 (10.2%) | 6,956 (10.2%) | 0.00 | 6,738 (9.4%) | 6,949 (9.7%) | −0.01 |
| Diabetes related comorbidities | ||||||
| Diabetic nephropathy | 7,155 (10.5%) | 7,046 (10.3%) | 0.01 | 9,561 (13.4%) | 9,647 (13.5%) | 0.00 |
| Diabetic neuropathy | 16,994 (24.9%) | 16,995 (24.9%) | 0.00 | 19,682 (27.5%) | 19,767 (27.7%) | 0.00 |
| Diabetic retinopathy | 7,231 (10.6%) | 7,128 (10.5%) | 0.00 | 8,258 (11.6%) | 8,317 (11.6%) | 0.00 |
| Hyperglycemia | 4,253 (6.2%) | 4,284 (6.3%) | 0.00 | 4,273 (6.0%) | 4,249 (5.9%) | 0.00 |
| Hypoglycemia | 5,194 (7.6%) | 5,219 (7.7%) | 0.00 | 5,871 (8.2%) | 5,847 (8.2%) | 0.00 |
| Other comorbid conditions | ||||||
| Acute kidney injury prior 90 days | 673 (1.0%) | 676 (1.0%) | 0.00 | 931 (1.3%) | 993 (1.4%) | −0.01 |
| Acute kidney injury prior 91–365 days | 1,552 (2.3%) | 1,576 (2.3%) | 0.00 | 2,046 (2.9%) | 2,064 (2.9%) | 0.00 |
| Chronic kidney disease stages 1–2 | 2,598 (3.8%) | 2,593 (3.8%) | 0.00 | 3,180 (4.4%) | 3,195 (4.5%) | 0.00 |
| Chronic kidney disease stages 3–5 | 5,525 (8.1%) | 5,498 (8.1%) | 0.00 | 8,237 (11.5%) | 8,306 (11.6%) | 0.00 |
| Hypertensive nephropathy | 4,178 (6.1%) | 4,261 (6.3%) | −0.01 | 5,748 (8.0%) | 5,823 (8.1%) | 0.00 |
| Hypertension | 62,857 (92.3%) | 62,760 (92.1%) | 0.01 | 66,464 (93.0%) | 66,512 (93.1%) | 0.00 |
| Hyperlipidemia | 60,060 (88.2%) | 60,020 (88.1%) | 0.00 | 63,266 (88.5%) | 63,357 (88.6%) | 0.00 |
| Ischemic heart disease | 22,793 (33.5%) | 22,711 (33.3%) | 0.00 | 24,171 (33.8%) | 24,205 (33.9%) | 0.00 |
| Heart failure | 7,481 (11.0%) | 7,520 (11.0%) | 0.00 | 8,771 (12.3%) | 8,788 (12.3%) | 0.00 |
| Cardiac arrhythmia | 14,239 (20.9%) | 14,093 (20.7%) | 0.00 | 15,392 (21.5%) | 15,350 (21.5%) | 0.00 |
| Stroke/transient ischemia attack | 8,445 (12.4%) | 8,384 (12.3%) | 0.00 | 9,215 (12.9%) | 9,284 (13.0%) | 0.00 |
| Peripheral vascular disease | 8,129 (11.9%) | 8,014 (11.8%) | 0.00 | 9,324 (13.0%) | 9,378 (13.1%) | 0.00 |
| Smoking | 12,439 (18.3%) | 12,506 (18.4%) | 0.00 | 13,457 (18.8%) | 13,324 (18.6%) | 0.01 |
| Overweight/obesity | 20,396 (29.9%) | 20,439 (30.0%) | 0.00 | 23,320 (32.6%) | 23,443 (32.8%) | 0.00 |
| Dehydration/hypovolemia | 1,810 (2.7%) | 1,826 (2.7%) | 0.00 | 2,142 (3.0%) | 2,199 (3.1%) | −0.01 |
| Dementia | 3,088 (4.5%) | 2,999 (4.4%) | 0.00 | 3,652 (5.1%) | 3,666 (5.1%) | 0.00 |
| Claims-Based Frailty Index | ||||||
| Non-frail | 21,376 (31.4%) | 21,505 (31.6%) | 0.00 | 19,225 (26.9%) | 19,290 (27.0%) | 0.00 |
| Pre-frail | 39,576 (58.1%) | 39,520 (58.0%) | 0.00 | 43,278 (60.5%) | 43,183 (60.4%) | 0.00 |
| Frail | 7,178 (10.5%) | 7,105 (10.4%) | 0.00 | 8,974 (12.6%) | 9,004 (12.6%) | 0.00 |
| Combined comorbidity index (CCI), mean (SD) | 1.2 (2.0) | 1.2 (2.0) | 0.00 | 1.5 (2.1) | 1.5 (2.1) | 0.00 |
| Diabetes drugs | ||||||
| Metformin | 53,142 (78.0%) | 53,319 (78.3%) | −0.01 | 53,957 (75.5%) | 53,925 (75.4%) | 0.00 |
| Sulfonylureas | 31,937 (46.9%) | 32,353 (47.5%) | −0.01 | 35,278 (49.4%) | 35,297 (49.4%) | 0.00 |
| Insulin | 19,153 (28.1%) | 18,988 (27.9%) | 0.00 | 22,904 (32.0%) | 22,818 (31.9%) | 0.00 |
| GLP-1RA | 8,137 (11.9%) | 7,595 (11.1%) | 0.03 | |||
| DPP-4i | 26,527 (37.1%) | 26,787 (37.5%) | −0.01 | |||
| Number of antidiabetic medications at index date, mean (SD) | 2.4 (0.9) | 2.4 (0.9) | 0.00 | 2.5 (1.0) | 2.5 (1.0) | 0.00 |
| Other drugs | ||||||
| ACEI/ARBs | 54,088 (79.4%) | 53,823 (79.0%) | 0.01 | 57,506 (80.5%) | 57,846 (80.9%) | −0.01 |
| Aldosterone antagonists | 2,849 (4.2%) | 2,852 (4.2%) | 0.00 | 3,321 (4.6%) | 3,286 (4.6%) | 0.00 |
| Beta blockers | 32,972 (48.4%) | 32,844 (48.2%) | 0.00 | 35,676 (49.9%) | 35,712 (50.0%) | 0.00 |
| Calcium channel blocker | 22,568 (33.1%) | 22,285 (32.7%) | 0.01 | 24,501 (34.3%) | 24,547 (34.3%) | 0.00 |
| Loop diuretics | 12,385 (18.2%) | 12,283 (18.0%) | 0.01 | 14,657 (20.5%) | 14,736 (20.6%) | 0.00 |
| Thiazide | 10,749 (15.8%) | 10,604 (15.6%) | 0.01 | 11,818 (16.5%) | 11,858 (16.6%) | 0.00 |
| Statins | 52,340 (76.8%) | 52,295 (76.8%) | 0.00 | 55,889 (78.2%) | 55,884 (78.2%) | 0.00 |
| Antiplatelet | 11,185 (16.4%) | 11,284 (16.6%) | −0.01 | 12,099 (16.9%) | 12,231 (17.1%) | −0.01 |
| NSAIDs | 16,770 (24.6%) | 17,000 (25.0%) | −0.01 | 18,423 (25.8%) | 18,561 (26.0%) | 0.00 |
| Number of total medications, mean (SD) | 13.1 (5.9) | 13.1 (6.0) | 0.00 | 14.1 (6.3) | 14.1 (6.0) | 0.00 |
| Health care utilization | ||||||
| Any hospitalization prior 90 days | 2,381 (3.5%) | 2,362 (3.5%) | 0.00 | 2,565 (3.6%) | 2,595 (3.6%) | 0.00 |
| Any hospitalization prior 91–365 days | 6,058 (8.9%) | 6,061 (8.9%) | 0.00 | 6,839 (9.6%) | 6,834 (9.6%) | 0.00 |
| Number of office visits, mean (SD) | 10.9 (7.6) | 10.9 (7.8) | −0.01 | 11.6 (8.0) | 11.6 (7.9) | 0.00 |
| Internal medicine/family medicine visits | 60,102 (88.2%) | 60,200 (88.4%) | −0.01 | 63,057 (88.2%) | 63,166 (88.4%) | −0.01 |
| Endocrinologist visit | 12,949 (19.0%) | 12,520 (18.4%) | 0.02 | 15,744 (22.0%) | 15,871 (22.2%) | 0.00 |
| Nephrologist visit | 2,955 (4.3%) | 2,947 (4.3%) | 0.00 | 4,280 (6.0%) | 4,341 (6.1%) | 0.00 |
| Iodinated contrast exposure prior 30 days | 1,177 (1.7%) | 1,196 (1.8%) | −0.01 | 1,240 (1.7%) | 1,216 (1.7%) | 0.00 |
| Number of HbA1c tests ordered, mean (SD) | 2.8 (1.3) | 2.8 (1.4) | 0.00 | 2.9 (1.4) | 2.9 (1.4) | 0.00 |
| Serum creatinine tests ordered | 6,759 (9.9%) | 6,656 (9.8%) | 0.00 | 7,401 (10.4%) | 7,434 (10.4%) | 0.00 |
Values are numbers (percentages) unless stated otherwise, all standardized differences in post-matching cohorts were less than 0.1
Abbreviations: SGLT-2i, sodium-glucose cotransporter-2 inhibitors; DPP-4i, dipeptidyl peptidase 4 inhibitors; GLP-1RA, glucagon-like peptide 1 receptor agonist; St. D, standardized difference; SD, standard deviation; ACEI/ARBs, angiotensin converting enzyme inhibitor/ angiotensin II receptor blockers; NSAIDs, nonsteroidal anti-inflammatory drugs; HbA1c, hemoglobin A1c
Primary Outcome
After propensity score matching, there were 1,060 AKI hospitalization events amongst SGLT-2i users and 1,560 events amongst DPP-4i users in the SGLT-2i versus DPP-4i cohort (19.6 vs 27.8 per 1000 person-years, HR 0.71, 95% CI 0.65–0.76), and 1,254 events amongst SGLT-2i users and 1,438 events amongst GLP-1RA users in the SGLT-2i versus GLP-1RA cohort (21.7 vs 27.1 per 1000 person-years, HR 0.81, 95% CI 0.75–0.87), as shown in Table 2.
Table 2.
Number of events, incidence rate, hazard ratios for primary and secondary outcomes after 1:1 propensity score matching
| SGLT-2i | DPP-4i | SGLT-2i vs DPP-4i (n=68,130 pairs) | SGLT-2i | GLP-1RA | SGLT-2i vs GLP-1RA (n=71,477 pairs) | |
|---|---|---|---|---|---|---|
| Primary Outcome | N events (IR) | N events (IR) | HR (95% CI) | N events (IR) | N events (IR) | HR (95% CI) |
| AKI hospitalization* | 1,060 (19.6) | 1,560 (27.8) | 0.71 (0.65, 0.76) | 1,254 (21.7) | 1,438 (27.1) | 0.81 (0.75, 0.87) |
| Secondary Outcomes | ||||||
| AKI hospitalization* within 30 days | 137 (25.0) | 180 (32.7) | 0.76 (0.61, 0.95) | 170 (29.5) | 211 (36.6) | 0.81 (0.66, 0.99) |
| AKI hospitalization (primary position) | 287 (5.3) | 537 (9.5) | 0.56 (0.48, 0.64) | 350 (6.0) | 510 (9.5) | 0.64 (0.56, 0.73) |
| Hospitalization with AKI (any position) | 2,043 (38.1) | 3,055 (55.1) | 0.69 (0.65, 0.73) | 2,458 (42.9) | 2,723 (51.9) | 0.83 (0.79, 0.88) |
| AKI hospitalization requiring dialysis | 57 (1.1) | 156 (2.7) | 0.39 (0.28, 0.52) | 79 (1.4) | 130 (2.4) | 0.56 (0.42, 0.73) |
| Hypovolemic event# | 51 (0.9) | 50 (0.9) | 1.1 (0.72, 1.57) | 52 (0.9) | 60 (1.1) | 0.82 (0.56, 1.18) |
Abbreviations: SGLT-2i, sodium-glucose cotransporter-2 inhibitors; DPP-4i, dipeptidyl peptidase 4 inhibitors; GLP-1RA, glucagon-like peptide 1 receptor agonist; N events, number of events; IR, incidence rate in 1000 patient years; HR, hazard ratio; CI, confidence interval; AKI, acute kidney injury.
AKI hospitalization, defined as a hospitalization with a discharge diagnosis of AKI in the primary or secondary position.
Hypovolemic event was defined as a hospitalization with a discharge diagnosis of hypovolemia in the primary or secondary position.
The cumulative incidence of AKI hospitalization over time in the matched groups are shown in the Kaplan-Meier plots (Figures 1A and 1B). The graphs were truncated at four years of follow-up and the curves began to separate within one year.
Figure 1A. Kaplan-Meier plots for cumulative incidence of AKI hospitalization in 1:1 propensity score matched SGLT-2i versus DPP-4i cohort.
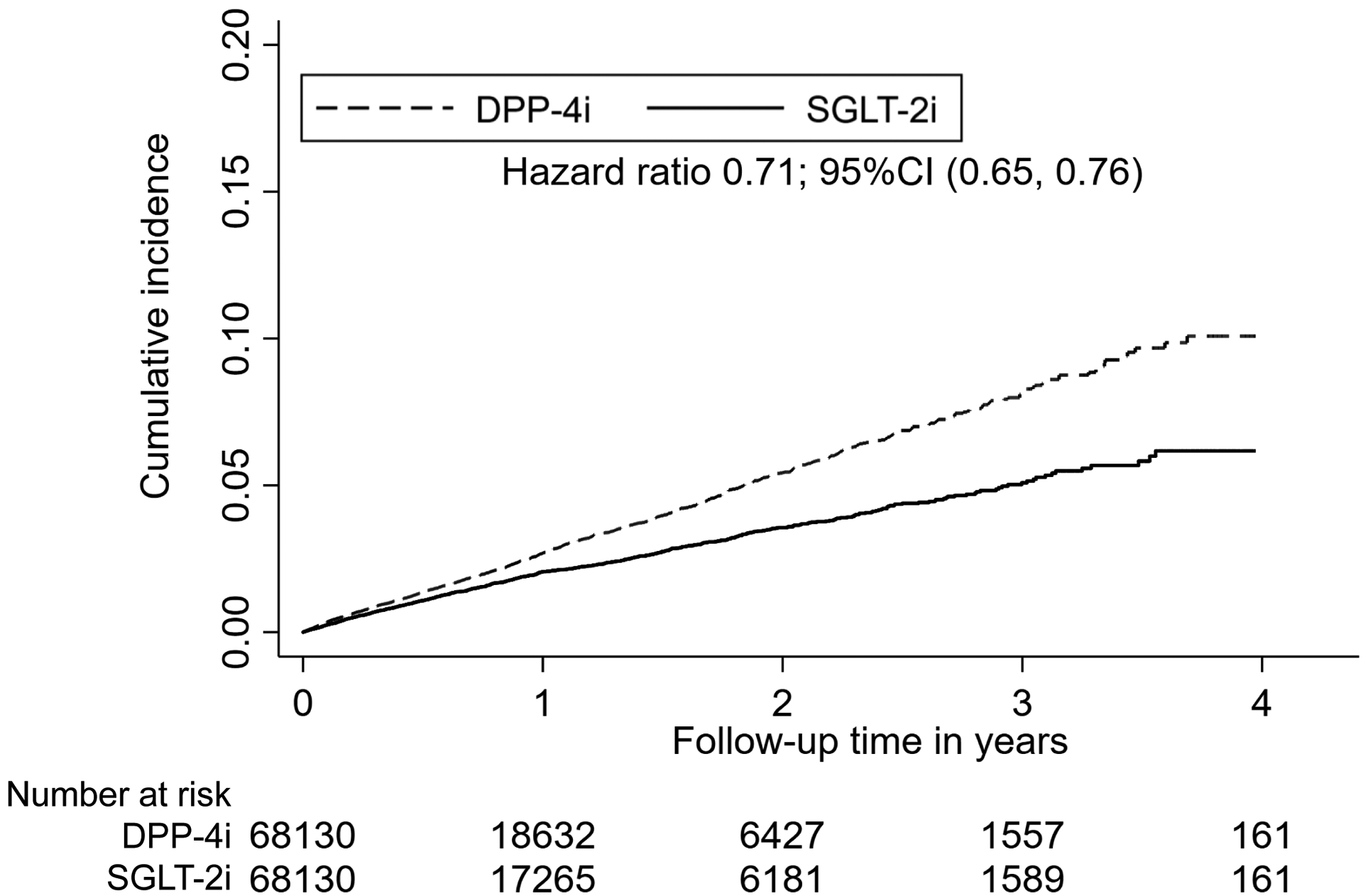
Abbreviations: AKI, acute kidney injury; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; DPP-4i, dipeptidyl peptidase 4 inhibitors; CI, confidence interval.
Figure 1B. Kaplan-Meier plots for cumulative incidence of AKI hospitalization in 1:1 propensity score matched SGLT-2i versus GLP-1RA cohort.
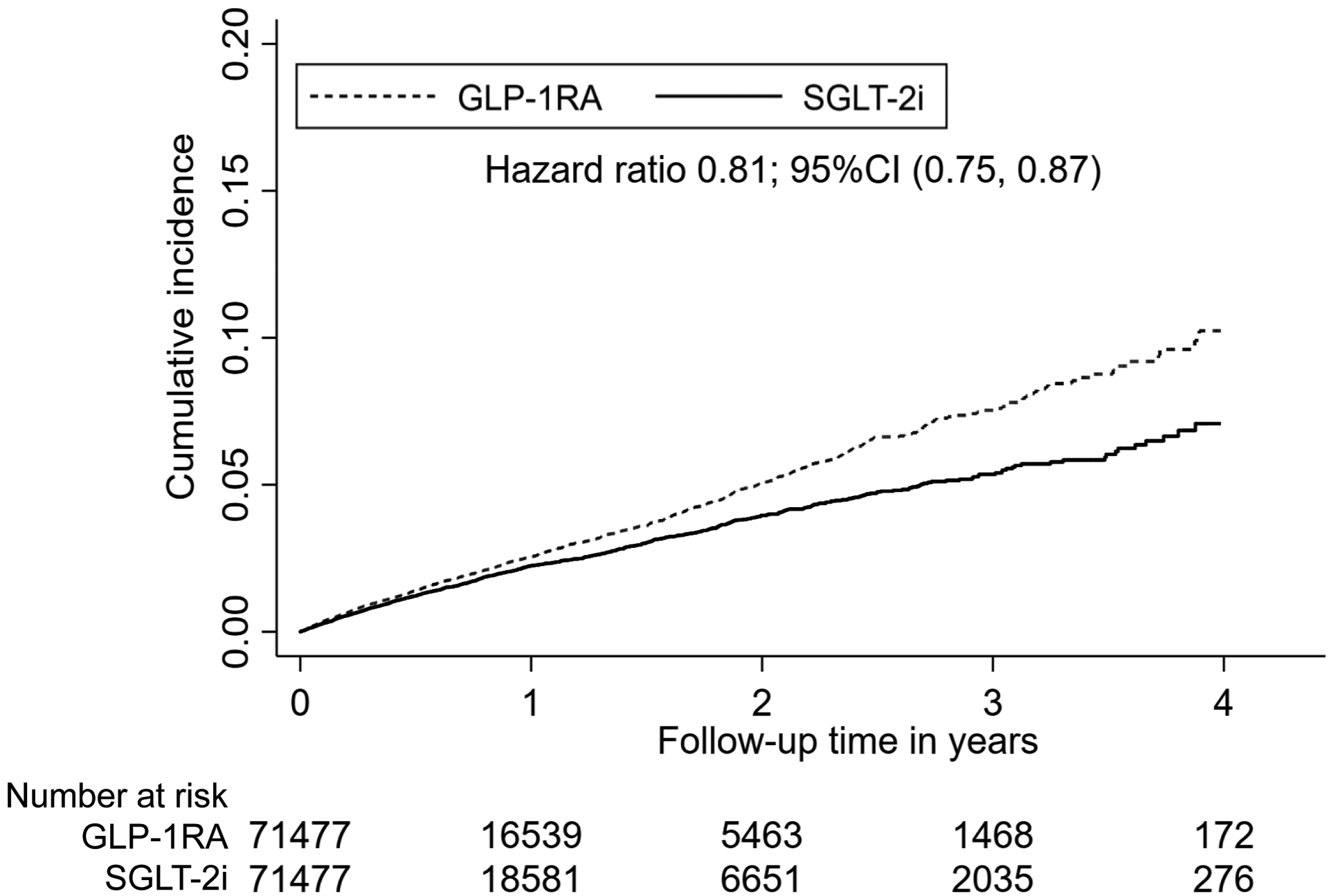
Abbreviations: AKI, acute kidney injury; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; GLP-1RA, glucagon-like peptide 1 receptor agonist; CI, confidence interval.
Secondary Outcomes
The rate of AKI hospitalization amongst SGLT-2i users was consistently lower compared to matched DPP-4i users and GLP-1RA users within 30 days of medication initiation, or when AKI hospitalization was defined using a primary discharge diagnosis or a discharge diagnosis in any position (Table 2). SGLT-2i users had a reduced risk of AKI hospitalization requiring dialysis compared to DPP-4i users (HR 0.39, 95% CI 0.28–0.52) and GLP-1RA users (HR 0.56, 95% CI 0.42–0.73). There was no significant difference in the incidence of hypovolemia across matched groups.
Sensitivity and Subgroup Analyses
We performed several sensitivity analyses to assess the robustness of our primary study findings. The results were consistent across all sensitivity analyses (Table S5). The known associations between SGLT-2i and an increased risk of DKA and between SGLT-2i and a reduction in the risk of hospitalization for heart failure were replicated (Table S5).28–32
There was no evidence of effect heterogeneity in the association between SGLT-2i and AKI hospitalization by age, gender, baseline metformin use, history of heart failure, or history of CKD (Figures 2A and 2B). The initiation of SGLT-2i among patients with history of AKI was associated with a similar risk of subsequent AKI hospitalization compared to DPP-4i (103.2 vs 103.3 per 1000 person-years, HR 0.98, 95% CI 0.78–1.23) and GLP-1RA (102.2 vs 105.6 per 1000 person-years, HR 0.97, 95% CI 0.79–1.18).
Figure 2A. Subgroup analyses for AKI hospitalization in 1:1 propensity score matched SGLT-2i versus DPP-4i groups.
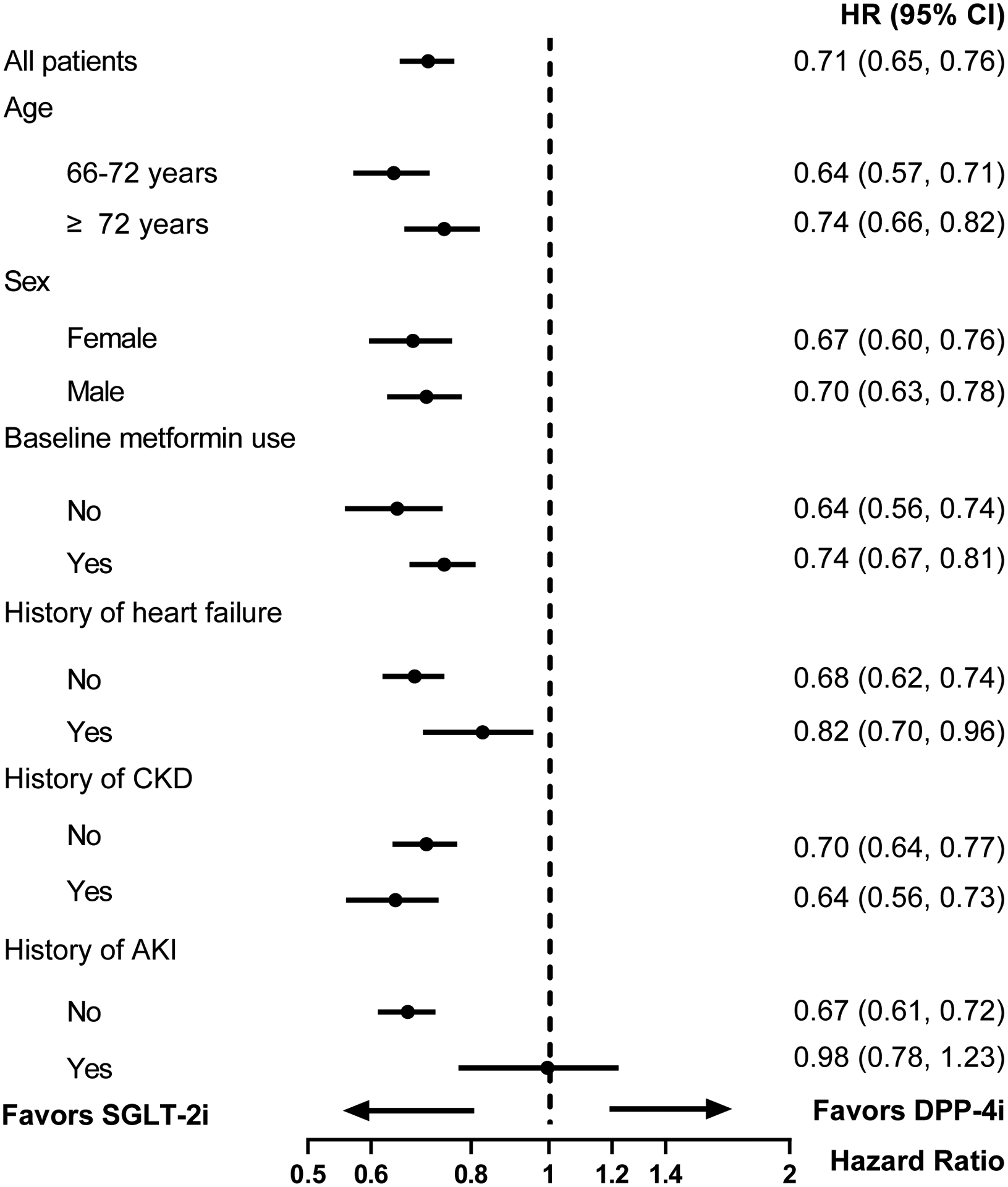
Abbreviations: AKI, acute kidney injury; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; DPP-4i, dipeptidyl peptidase 4 inhibitors; HR, hazard ratio; CI, confidence interval; CKD, chronic kidney disease.
Figure 2B. Subgroup analyses for AKI hospitalization in 1:1 propensity score matched SGLT-2i versus GLP-1RA groups.
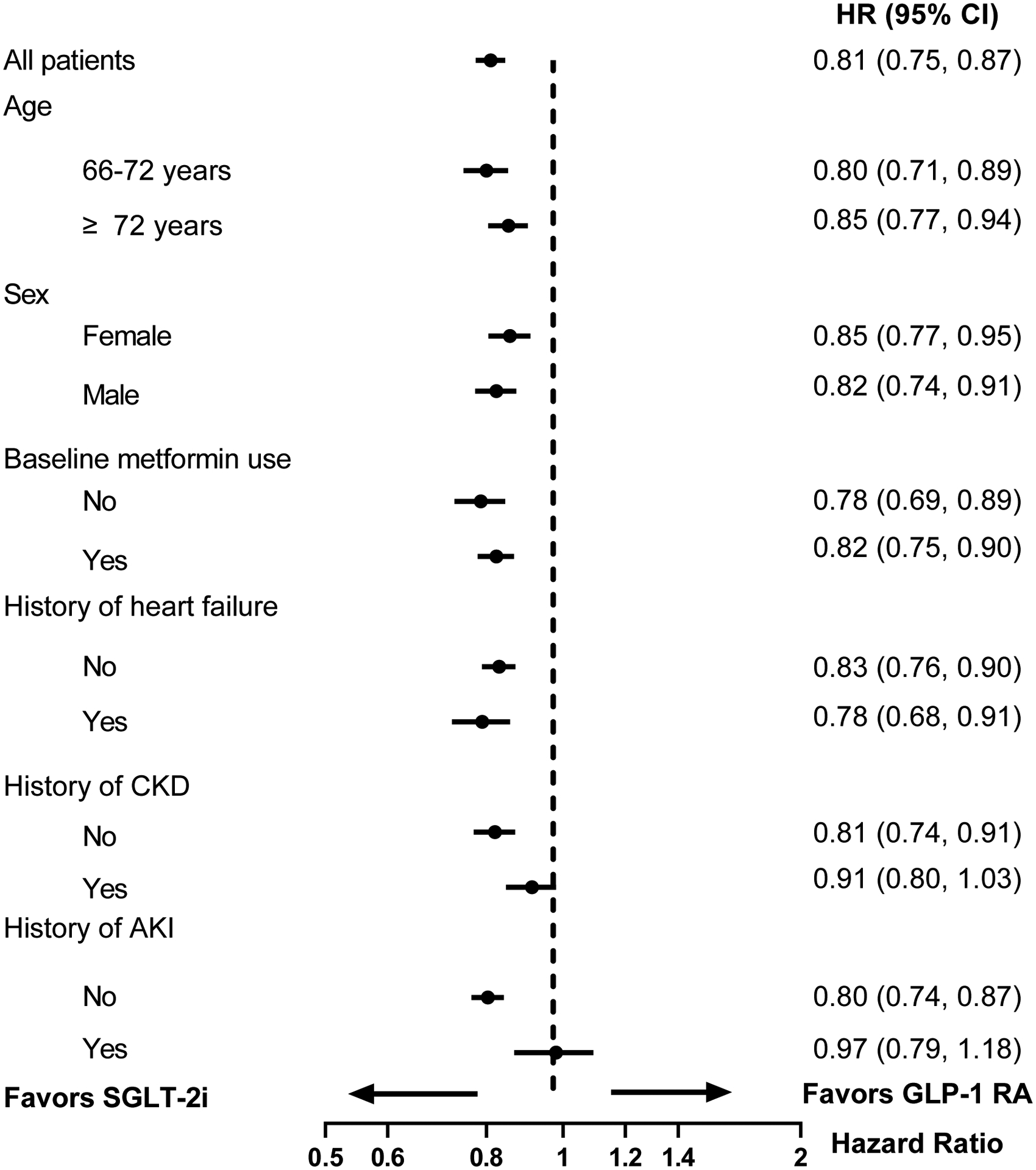
Abbreviations: AKI, acute kidney injury; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; GLP-1RA, glucagon-like peptide 1 receptor agonist HR, hazard ratio; CI, confidence interval; CKD, chronic kidney disease.
DISCUSSION
In this nationwide population-based study of older adults, after propensity score matching for more than 100 covariates, we found that the initiation of SGLT-2i was associated with a nearly 30% reduction in the risk of AKI hospitalization compared to DPP-4i and a nearly 20% reduction compared to GLP-1RA. Study findings were robust to a range of predefined sensitivity analyses. Patients initiating SGLT-2i also experienced a decreased risk of AKI hospitalization requiring dialysis. Among patients with a history of AKI, SGLT-2i initiation was not associated with an increased risk for subsequent AKI hospitalization compared to DPP-4i or GLP-1RA initiation.
SGLT-2i, such as canagliflozin, empagliflozin, and dapagliflozin, have led to a paradigm shift in the management of T2D as they substantially prevent heart failure hospitalizations and kidney disease progression and improve survival. However, the FDA Adverse Event Reporting System (FAERS) suggests that SGLT-2i might increase the risk of AKI, which may occasionally be fatal or require renal replacement therapy.16 Notably, reports to FAERS are voluntary and do not require a causal relationship to be established. In large RCTs, there was no evidence to suggest SGLT-2i initiation increased the risk of AKI compared with placebo3–10,17,18, though older adults were underrepresented in these trials. In addition, as hospitalization for AKI was not a common outcome in these populations, RCTs were underpowered to assess AKI outcome robustly. Our study is complementary to clinical trials.
In order to assess the association between SGLT-2i and the risk of AKI in older adults, we leveraged U.S. Medicare data and included millions of older individuals at high risk for AKI. The large number of events, the magnitude of the effect, and the robustness of our findings across several predefined sensitivity analyses suggest that SGLT-2i are not associated with an increased risk of AKI in older adults, and potentially offer protection against AKI risk. The association between SGLT-2i use and the risk for AKI in routine practice has been primarily evaluated among patients younger than 65 years. Two studies based on nationwide registers from Sweden and Denmark found that SGLT-2i were associated with a lower risk of AKI compared with DPP-4i (HR 0.41, 95% CI 0.32–0.52) and GLP-1RA (HR 0.69, 95% CI 0.45–1.05),21,30 and a small cohort study from Canada showed that SGLT-2i were associated with a numerical decrease in risk of AKI (HR 0.64, 95% CI 0.40–1.03) compared with other diabetes agents.34 Findings from this large population-based study, which specifically focuses on older adults (≥ 66 years of age), are in line with a recent smaller population-based study from Canada, which showed that the initiation of SGLT-2i compared to DPP-4i was associated with a lower 90-day risk of a hospital encounter with AKI as defined by laboratory data in older adults (risk ratio 0.79, 95% CI 0.64–0.98).35 We also found that among patients with a history of AKI, SGLT-2i initiation was associated with a similar risk of subsequent AKI hospitalization compared to DPP-4i or GLP-1RA initiation. Further research is needed to guide diabetes medication initiation among older adults with a history of AKI.
To date, the mechanisms through which SGLT-2i could prevent AKI are still under investigation. In addition to potential AKI protection through heart failure and CKD risks reduction,3–11 it has been postulated that SGLT-2i reduce sodium and glucose reabsorption in the proximal tubule, which may lead to reduced oxygen consumption and increased resistance to ischemia perfusion injury.15 As SGLT-2i increase sodium delivery to the macula densa, they could decrease intraglomerular pressure and reduce podocyte stress through tubuloglomerular feedback.36 Furthermore, SGLT-2i could increase renal hypoxia-inducible factor expression, erythropoietin production, suppression of peritubular inflammation and fibrosis, and increased use of ketone bodies as an alternative fuel source.37,38 Biomarker studies in patients treated with SGLT-2i showed a reduction of several tubular damage markers, suggesting SGLT-2i might decrease tubular susceptibility to AKI.39 Further research is needed to better elucidate the mechanisms of protection against AKI risk associated with SGLT2iin older adults with T2D.
Our study has important clinical implications. The latest clinical guidelines for the treatment of T2D recommend SGLT-2i, DPP-4i, and GLP-1RA as second-line therapies for T2D following metformin failure or intolerance, and suggest the choice among these medications should be based on patient-specific characteristics, e.g., history of cardiovascular disease.12 Notably, T2D per se increases the risk of AKI by three to five fold.40 Older age is also a significant risk factor for AKI.41 Therefore, a glucose-lowering medication reducing the risk of AKI would be advantageous for older adults. Our study, which is based on a large population of routine care patients older than 65 years,42 provides reassurance on the safety of SGLT-2i with respect to the risk of AKI, and suggests SGLT-2i may actually prevent AKI events compared to alternative diabetes treatments.
Our study has several limitations. First, residual confounding by unmeasured factors cannot be ruled out. Notably our Medicare dataset lacked laboratory results, which limited our ability to match for baseline serum creatinine and albuminuria levels. To address this limitation, we adjusted our analyses by baseline kidney function as measured by history of AKI and baseline CKD stage as indicated by diagnostic codes in claims data. We also performed subgroup analyses to assess the association between SGLT-2i and AKI hospitalization among metformin users, who were assumed to have adequate baseline kidney function since metformin is contraindicated in patients with CKD stage 4 or above. Second, since we used claims data to identify AKI, we evaluated only AKI events that required hospitalization to maximize specificity. As such, milder cases of AKI that did not require hospitalization were undetected. Third, our study had a median follow-up of six months and more than half of patients were censored by one year. However, more than half of the AKI cases reported to FAERS (58 cases) occurred within one month of SGLT-2i initiation. The large size of our study population allowed us to generate results with high precision despite the relative short follow-up duration comparing to RCTs. Fourth, in the subgroup of patients with a history of AKI, we observed a comparable risk for subsequent AKI in users of SGLT-2i compared to DPP-4i and GLP-1RA. However, the small number of patients in this subgroup resulted in a wide confidence interval and we could not classify the history of AKI by severity or by cause. Fifth, we could not study the association between each SGLT-2i agent and the risk of AKI due to the limited sample size of patients treated with each individual SGLT-2i. Finally, our findings may be generalizable only to older adults with T2D.
In this large, population-based cohort including more than 200,000 propensity score matched older adults with T2D, initiating treatment with SGLT-2i, as compared with DPP-4i and GLP-1RA, was associated with a 29% and 19% reduction in the risk of AKI hospitalization, respectively. Our data provide substantive evidence supporting the safety of initiation of SGLT-2i in older adults with T2D.
Supplementary Material
Table S1. Outcome definitions
Fig S1. Flowchart of patients included in SGLT-2i versus DPP-4i cohort.
Fig S2. Flowchart of patients included in SGLT-2i versus GLP-1RA cohort.
Fig S3. Propensity score distribution for SGLT-2i versus DPP-4i and SGLT-2i versus GLP-1RA cohorts before and after 1:1 propensity score matching.
Table S2. Baseline characteristics of SGLT-2i versus DPP-4i initiators before and after 1:1 propensity score matching.
Table S3. Baseline characteristics of SGLT-2i versus GLP-1RA initiators before and after 1:1 propensity score matching.
Table S4. Reasons for censoring in 1:1 propensity score matched cohorts
Table S5. Number of events, incidence rate, hazard ratios for sensitivity analyses in 1:1 propensity score matched cohorts
Support:
This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. Dr. Zhuo is supported by a NIH NIDDK T32 award DK007199. Dr. Patorno is supported by a career development grant K08AG055670 from the National Institute on Aging. Dr. Bonventre is supported by the NIH grants R37D039773 and R01DK072381. These funders had no role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Financial Disclosure:
Dr. Wexler reports serving on Data Monitoring Committees for Novo Nordisk (oral and subcutaneous semaglutide), not directly related to the topic of the submitted work. Dr. Bonventre is cofounder and holds equity in Goldfinch Bio. He is an advisor for Janssen, Seattle Genetics, Praxis and Sarepta. His interests were reviewed and are managed by BWH and MGB HealthCare in accordance with their conflict-of-interest policies. Dr. Kim has received research support to the Brigham and Women’s Hospital from Pfizer, AbbVie, Roche and Bristol-Myers Squibb for unrelated studies. Dr. Patorno is investigator of an investigator-initiated grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not directly related to the topic of the submitted work. Dr. Zhuo declares that he has no other relevant financial interests. Dr. Paik declares that he has no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. doi: 10.1056/NEJMoa1504347 [DOI] [PubMed] [Google Scholar]
- 2.Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497. doi: 10.1136/bmj.k1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020:1–12. doi: 10.1056/nejmoa2022190 [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):1–12. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 5.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 6.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 7.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019:1–13. doi: 10.1056/nejmoa1911303 [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2018:NEJMoa1812389. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020:1–11. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2020:NEJMoa2030186. doi: 10.1056/NEJMoa2030186 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. ADA Standards of Diabetes Care 2021. Diabetes Care. 2021;44(Supplement1). [Google Scholar]
- 13.Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711–712. doi: 10.1038/nrneph.2016.159 [DOI] [PubMed] [Google Scholar]
- 14.Szalat A, Perlman A, Muszkat M, Khamaisi M, Abassi Z, Heyman SN. Can SGLT2 Inhibitors Cause Acute Renal Failure? Plausible Role for Altered Glomerular Hemodynamics and Medullary Hypoxia. Drug Saf. 2018;41(3):239–252. doi: 10.1007/s40264-017-0602-6 [DOI] [PubMed] [Google Scholar]
- 15.O’Neill J, Fasching A, Pihl L, Patinha D, Franzén S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol. 2015;309(3):F227–34. doi: 10.1152/ajprenal.00689.2014 [DOI] [PubMed] [Google Scholar]
- 16.The United States Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin. Accessed November 10, 2020.
- 17.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019. doi: 10.1016/S2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
- 18.Zhao M, Sun S, Huang Z, Wang T, Tang H. Network Meta-Analysis of Novel Glucose-Lowering Drugs on Risk of Acute Kidney Injury. Clin J Am Soc Nephrol. 2020;16:CJN.11220720. doi: 10.2215/cjn.11220720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. ADA Standards of Diabetes Care 2015. Diabetes Care. 2015;38(January):S1–S2. doi: 10.2337/dc15-S001 [DOI] [Google Scholar]
- 20.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol. 2006;17(6):1688–1694. doi: 10.1681/ASN.2006010073 [DOI] [PubMed] [Google Scholar]
- 21.Pasternak B, Wintzell V, Melbye M, et al. Use of sodium-glucose co-transporter 2 inhibitors and risk of serious renal events: Scandinavian cohort study. BMJ. 2020;369:1–9. doi: 10.1136/bmj.m1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the U.S. Medicare Data. J Gerontol A Biol Sci Med Sci. 2020;75(6):1120–1125. doi: 10.1093/gerona/glz224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripollone JE, Huybrechts KF, Rothman KJ, Ferguson RE, Franklin JM. Implications of the Propensity Score Matching Paradox in Pharmacoepidemiology. Am J Epidemiol. 2018;187(9):1951–1961. doi: 10.1093/aje/kwy078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med. 2014;33(10):1685–1699. doi: 10.1002/sim.6058 [DOI] [PubMed] [Google Scholar]
- 26.Aetion Evidence Platform (2020). Software for real-world data analysis. http://aetion.com.
- 27.Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of Health Care Databases to Support Supplemental Indications of Approved Medications. JAMA Intern Med. 2018;178(1):55–63. doi: 10.1001/jamainternmed.2017.3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the Risk of Heart Failure Hospitalization in Routine Clinical Care: A First Analysis from the EMPRISE Study. Circulation. 2019;139(25):2822–2830. doi: 10.1161/CIRCULATIONAHA.118.039177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: A real-world meta-analysis of 4 observational databases (OBSER. Diabetes, Obes Metab. 2018;20(11):2585–2597. doi: 10.1111/dom.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: Nationwide register based cohort study. BMJ. 2018;363(December 2016):1–10. doi: 10.1136/bmj.k4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasternak B, Ueda P, Eliasson B, et al. Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. BMJ. 2019;366. doi: 10.1136/bmj.l4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YG, Jeon JY, Han SJ, Kim DJ, Lee KW, Kim HJ. Sodium-glucose co-transporter-2 inhibitors and the risk of ketoacidosis in patients with type 2 diabetes mellitus: A nationwide population-based cohort study. Diabetes, Obes Metab. 2018;20(8):1852–1858. doi: 10.1111/dom.13297 [DOI] [PubMed] [Google Scholar]
- 33.Wang SV, Jin Y, Fireman B, et al. Relative Performance of Propensity Score Matching Strategies for Subgroup Analyses. Am J Epidemiol. 2018;187(8):1799–1807. doi: 10.1093/aje/kwy049 [DOI] [PubMed] [Google Scholar]
- 34.Rampersad C, Kraut E, Whitlock RH, et al. Acute Kidney Injury Events in Patients With Type 2 Diabetes Using SGLT2 Inhibitors Versus Other Glucose-Lowering Drugs: A Retrospective Cohort Study. Am J kidney Dis Off J Natl Kidney Found. 2020;76(4):471–479.e1. doi: 10.1053/j.ajkd.2020.03.019 [DOI] [PubMed] [Google Scholar]
- 35.Iskander C, Cherney DZ, Clemens KK, et al. Use of sodium-glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: a population-based cohort study. CMAJ. 2020;192(14):E351–E360. doi: 10.1503/cmaj.191283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anders H-J, Davis JM, Thurau K. Nephron Protection in Diabetic Kidney Disease. N Engl J Med. 2016;375(21):2096–2098. doi: 10.1056/NEJMcibr1608564 [DOI] [PubMed] [Google Scholar]
- 37.Mudaliar S, Alloju S, Henry RR. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care. 2016;39(July):dc160542. doi: 10.2337/dc16-0542 [DOI] [PubMed] [Google Scholar]
- 38.Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation. 2019;139(17):1985–1987. doi: 10.1161/CIRCULATIONAHA.118.038881 [DOI] [PubMed] [Google Scholar]
- 39.Dekkers CCJ, Petrykiv S, Laverman GD, Cherney DZ, Gansevoort RT, Heerspink HJL. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes, Obes Metab. 2018;20(8):1988–1993. doi: 10.1111/dom.13301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hapca S, Siddiqui MK, Kwan RSY, et al. The Relationship between AKI and CKD in Patients with Type 2 Diabetes: An Observational Cohort Study. J Am Soc Nephrol. 2021;32(1):138–150. doi: 10.1681/ASN.2020030323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell S, James MT, Farmer CKT, Tan Z, de Souza N, Witham MD. Development and external validation of an acute kidney injury risk score for use in the general population. Clin Kidney J. 2020;13(3):402–412. doi: 10.1093/ckj/sfaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mues KE, Liede A, Liu J, et al. Use of the Medicare database in epidemiologic and health services research: A valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol. 2017;9:267–277. doi: 10.2147/clep.s105613 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Outcome definitions
Fig S1. Flowchart of patients included in SGLT-2i versus DPP-4i cohort.
Fig S2. Flowchart of patients included in SGLT-2i versus GLP-1RA cohort.
Fig S3. Propensity score distribution for SGLT-2i versus DPP-4i and SGLT-2i versus GLP-1RA cohorts before and after 1:1 propensity score matching.
Table S2. Baseline characteristics of SGLT-2i versus DPP-4i initiators before and after 1:1 propensity score matching.
Table S3. Baseline characteristics of SGLT-2i versus GLP-1RA initiators before and after 1:1 propensity score matching.
Table S4. Reasons for censoring in 1:1 propensity score matched cohorts
Table S5. Number of events, incidence rate, hazard ratios for sensitivity analyses in 1:1 propensity score matched cohorts


